Abstract
An 8 year old boy with genetically confirmed X-linked myotubular myopathy developed progressively worsening dementia and subclinical seizures at age 5–6 years. Previously seizures or dementia have only been noted in a small number of myotubular myopathy patients and only in association with significant metabolic disturbances. Our patient had no evidence of hypoxemia or other metabolic disturbance, and we suggest that the clinical spectrum of X-linked myotubular myopathy is broader than previously considered and may include mutation-dependent central nervous system (CNS) disease.
Introduction
X-linked Myotubular Myopathy (XLMTM), a congenital disorder occurring in 1 in 50,000 newborn males, causes severe muscle weakness and is often lethal secondary to respiratory compromise. Infants are hypotonic and frequently require ventilatory assistance. However, surviving children typically have normal intelligence, and CNS symptoms have been reported only after significant metabolic disturbances [1]. The disease is due to mutation in the MTM1 gene encoding the myotubularin protein [2]. We report a child with XLMTM who developed seizures and progressive dementia without a documented history of hypoxemia.
Case Report
Pregnancy was complicated by decreased fetal movement and polyhydramnios. The patient was intubated at birth and remained ventilator dependent for life. He had macrocephaly with prominent forehead, a flat nasal bridge, and flat facies, profound hypotonia, and birth length greater than 90th percentile. Neonatal neuroimaging (CT scan) was unremarkable.
Family history revealed six male maternal relatives deceased in infancy from respiratory failure. Diagnosis of XLMTM was initially made by muscle biopsy. Subsequent genetic testing demonstrated a transversion, c.2T>G, which removes the ATG translation start signal from exon 2 of myotubularin. The patient’s mother was a heterozygous carrier of the same mutation.
He made excellent cognitive progress and at age four attended regular school. At 5 – 6 years, however, he experienced loss of cognitive milestones. He lost his ability to use sign language, bring objects to his mouth, and transfer. He also experienced deterioration in vision without funduscopic changes and became increasing irritable and obtunded.
Serial magnetic resonance imaging (MRI) demonstrated progressive white matter and cortical atrophy, as well as a 5 mm partially resolving chronic subdural collection over the left parietal convexity. MR venogram was negative. Cerebrospinal fluid (CSF) examination was unrevealing. The patient underwent an electroencephalogram (EEG) which demonstrated bilateral fronto-temporal slowing, frequent brief bursts of generalized spike and wave activity, and a 20 second subclinical seizure in the right frontal region. Repeat EEG two months later revealed bifrontal subclinical seizures which were confirmed by video-EEG. Treatment with anticonvulsants, including phosphenytoin and lorazepam, altered neither his EEG nor his mental status. Extensive laboratory investigations including CBC, electrolytes, glucose, thyroid functions, BUN, creatinine, liver functions, B12, and folate levels were unremarkable. The patient continued to decline and died of respiratory failure at age 8 years.
Autopsy, which was limited to brain, demonstrated megalencephaly (brain weight 1640 grams), temporal lobes with bilateral anterior-posterior axis elongation, and ependymitis granularis. The leptomeninges showed no evidence of subarachnoid hemorrhage or purulent material. There were no findings suggestive of neuronal migration disorders. The junction between the cortex and white matter was distinct, and no gray matter heterotopia were seen. Neurons in the cerebral cortex were arranged in a normal laminar fashion, and both hippocampi showed normally arranged pyramidal and granular neurons. Demyelination was not observed. In addition, there were no findings consistent with past hypoxia. PAS stain was performed on selected blocks to detect storage material, but no stainable material was noted. On electron microscopy, both neurons and astrocytes exhibited cell swelling but no vacuolization.
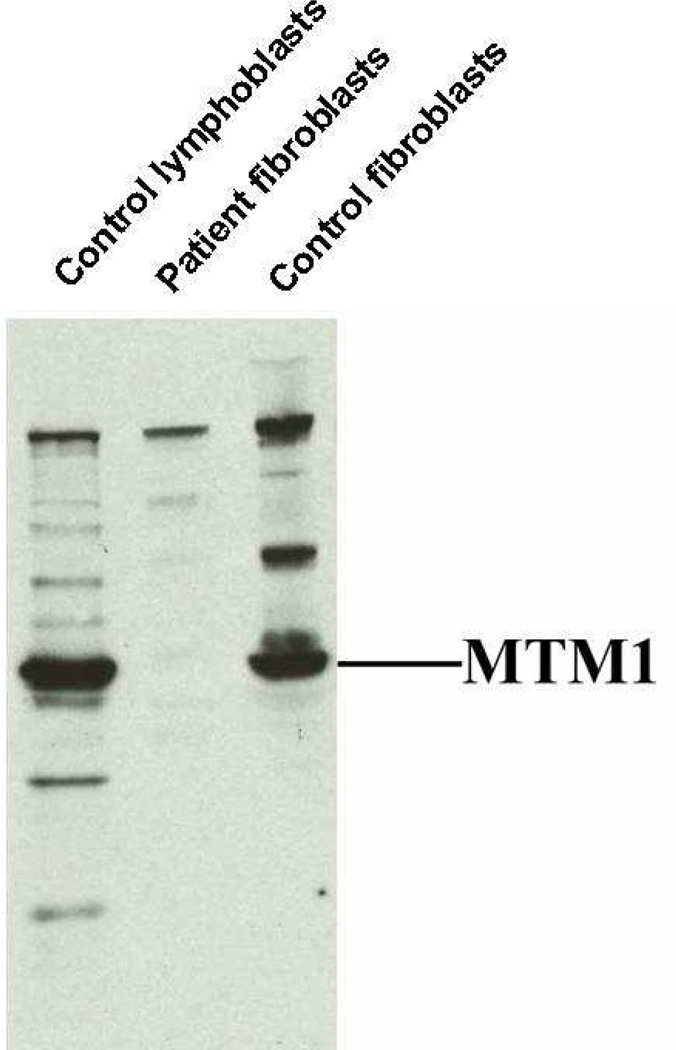
Fibroblast lysates examined via Western blot for the presence of myotubularin revealed a decreased expression, below the level of detection, and no clear evidence of a truncated form of the protein (see Figure).
Anti-myotubularin western blotting of equal amounts of protein from cell lysates of control human lymphoblasts, patient fibroblasts, and control human fibroblasts. (Antibody against C-terminal portion of myotubularin).
Discussion
We present a case of XLMTM with progressive dementia and white matter and cortical atrophy. While many patients die within a few months due to respiratory compromise, the lifespan of XLMTM subjects can be markedly improved with appropriate support. Descriptions of long-term survivors are unfortunately rare. Reporting outcomes of 35 subjects ages 1 – 27 years, Herman noted length greater than 90th percentile in 69% and macrocephaly in 61% (14/23), but weight was greater than 90th percentile in only 11% [1]. The children exhibited marked delay in motor milestones (average age to sit was 30 months with no patients ambulatory), but cognition was normal in 88% (29/33) [1]. Four had clinical seizures with significant metabolic disturbances. Herman concluded that CNS sequelae of XLMTM were secondary to metabolic encephalopathies and not to XLMTM [1]. In contrast, Lerman-Sagie reported an infant with microcranium, cerebral atrophy, abnormal cortical differentiation, and muscle biopsy consistent with XLMTM [3]. Of note, CNS abnormalities have been reported in other congenital myopathies, such as lissencephaly with Fukuyama-type dystrophy, but genetic testing was not performed in Lerman-Sagie’s case.
Published genotype data report disease-causing mutations throughout the MTM1 gene. Although genotype-phenotype correlation suggests that children with mild disease are more likely to have non-truncating mutations of MTM1, many children with such mutations have severe disease [4]. Thus, the use of molecular genetics to predict outcome is currently limited.
Myotubularin is ubiquitously expressed at low levels, including in brain, but expression is highest in muscle and heart. The protein is a 3-phosphatase specific for phosphatidylinositol 3-phosphate (PI(3)P) and PI(3,5)P2 [5]. Phosphoinositides recruit cytosolic proteins to specific membrane compartments and thus have important roles in signaling and intracellular trafficking. While there are no other reports of neurodegenerative changes in XLMTM patients, the role of PI(3,5)P2 misregulation in neurodegenerative disease is emerging [6]. PI(3,5)P2 is critical for proper endosome-lysosome function, and mutations in another enzyme affecting PI(3,5)P2 levels, Fig4, lead to neurodegeneration in mice and a variant of Charcot-Marie-Tooth disease in humans [6]. PI(3,5)P2 reportedly inhibits sphingomyelinase [7], the enzyme mutated in Niemann-Pick Disease, which raises the possibility that our patient’s symptoms could be a downstream effect of increased PI(3,5)P2 levels and secondary downregulation of acid sphingomyelinase. However, the neuropathologic examination of our patient, within the limits of detection, did not demonstrate neuronal vacuoles, a typical finding in both children with Niemann-Pick Disease and Fig4 mice.
It is unclear what led to the megalencephaly of this child or his elongated temporal lobes. Furthermore, given the significant clinical findings of this patient, it is surprising that correlative neuropathologic findings were not observed, suggesting, that such changes were below the level of microscopic detection.
Although there are other possibilities for the neuro-cognitive problems which our patient experienced including a second, unrelated, dementia-producing mutation, we suggest that specific MTM1 mutations may lead to more complex disorders which include involvement of the CNS. Emerging care strategies have permitted the increasing survival of children with XLMTM, and longitudinal cohort studies with serial cognitive evaluations and expanded genetic testing may provide further evidence for the etiology of dementia in subjects with XLMTM.
Acknowledgements
H.J. McCrea, PhD is supported by the NIH (MSTP TG 5T32GM07205). This work was also supported by NIH grant NS36251, INSERM, CNRS, Collège de France and by grants from the Association Française contre les Myopathies and Fondation pour la Recherche Médicale. The authors thank Dr. Alexander Vortmeyer, MD and Dr. Jung Kim, MD for their analysis of the patient’s pathology and Pietro De Camilli, MD for advice and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herman GE, Finegold M, Zhao W, Wei MS, de Gouyon B, Metzenberg A. Medical complications in long-term survivors with X-linked myotubular myopathy. J pediatr. 1999;134:206–214. doi: 10.1016/s0022-3476(99)70417-8. [DOI] [PubMed] [Google Scholar]
- 2.Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, Coy JF, Klauck SM, Poustka A, Dahl N. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet. 1996;13:175–182. doi: 10.1038/ng0696-175. [DOI] [PubMed] [Google Scholar]
- 3.Lerman-Sagie T, Berns L, Tomer A, Glick B, Ariel I, Harel S. Central Nervous System Involvement in X-linked Myotubular Myopathy. J Child Neurol. 1997;12:70–72. doi: 10.1177/088307389701200114. [DOI] [PubMed] [Google Scholar]
- 4.McEntagart M, Parsons G, Buj-Bello A, Biancalana V, Fenton I, Little M, Krawczak M, Thomas N, Herman G, Clarke A, Wallgren-Pettersson C on behalf of the International Consortium on Myotubular Myopathy of the ENMC. Genotype-phenotype Correlations in X-linked Myotubular Myopathy. Neuromuscul Disord. 2002;12:939–946. doi: 10.1016/s0960-8966(02)00153-0. [DOI] [PubMed] [Google Scholar]
- 5.Tronchere H, Laporte J, Pendaries C, Chaussade C, Liaubet L, Pirola L, Mandel JL, Payrastre B. Production of Phosphatidylinositol 5-Phosphate by the Phosphoinositide 3-Phosphatase Myotubularin in Mammalian Cells. J Biol Chem. 2004;279:7304–7312. doi: 10.1074/jbc.M311071200. [DOI] [PubMed] [Google Scholar]
- 6.Volpicelli-Daley L, De Camilli P. Phosphoinositides’ Link to Neurodegeneration. Nat Med. 2007;13:784–786. doi: 10.1038/nm0707-784. [DOI] [PubMed] [Google Scholar]
- 7.Kolzer M, C. Arenz C, Ferlinz K, Werth N, Schulze H, Klingenstein R, Sandhoff K. Phosphatidylinositol-3,5-Bisphosphate is a potent and selective inhibitor of acid sphingomyelinase. Biol Chem. 2003;384:1293–1298. doi: 10.1515/BC.2003.144. [DOI] [PubMed] [Google Scholar]