Abstract
AIM
To explore the feasibility that human amniotic epithelial cells (hAECs) have the potential to differentiate into corneal epithelial-like cells under the microenvironment replicated by spontaneously immortalized human corneal epithelial cells (S-ihCECs).
METHODS
hAECs were isolated by enzyme digestion, and flow cytometry was used to analysis the expression of CD29/90/166/73/34 and HLA-DR. Recovered and cultured S-ihCECs, immunocytochemistry was used to detect the expression of CK3/12. The proliferation of S-ihCECs handled by different concentrations of mitomycin was detected by CCK-8. The proliferation of hAECs cultured by S-ihCECs culture media collected at different time was analyzed by CCK-8. After filtered out the optimal conditions, we collected S-ihCECs culture media for 5 days, then prepared conditioned medium to incubate hAECs, inverted phase contrast microscope and scanning electron microscope were used to observe the change of morphology in hAECs. Quantitative real-time reverse transcription-polymerase chain reaction (QRT-PCR) was carried out to evaluate the expression of Oct-4, NANOG, PAX6, and CK12 in the differentiation period. Immunocytochemistry and western bloting were used to detect the expression of CK3/12.
RESULTS
The culture media collected every 12h, from 20µg/mL mitomycin pretreatment S-ihCECs could significantly promote the proliferation of hAECs. In the period of differentiation, the morphology of differentiated hAECs was obviously different compared with the control group, and the distinctive CK3/12 for corneal epithelial cells was detected.
CONCLUSION
This study showed that hAECs can differentiate into corneal epithelial-like cells by in vitro replication of the corneal epithelial microenvironment, using the culture media collected from S-ihCECs, and it is possible that S-ihCECs culture media could be used in corneal tissue engineering.
Keywords: human amniotic epithelial cells, spontaneously immortalized human corneal epithelial cells, mytomicin, microenvironment, tissue engineering
INTRODUCTION
In the field of regenerative medicine, a reliable cell source also named as seed cells, a suitable microenvironment, and biomaterial scaffolds are equally necessary. Currently, the human perinatal stem cells are increasingly concerned because of its plasticity[1]-[3]. However, human amniotic epithelial cells (hAECs) are considered as an ideal stem cell source because of its easy accessible, inflammation suppression, immune privilege, and no ethical concerns[4]. hAECs which forms a continuous monolayer in contact with the amnion fluid develop from the epiblast by 8d after fertilization and before gastrulation. hAECs can express stem cell surface markers, such as stage-specific embryonic antigen-3 (SSEA-3), SSEA-4, tumor rejection antigen 1-60 (TRA1-60) and TRA1-81[5]. Miki et al[6],[7] have reported that hAECs also can express the pluripotent stem cell-specific key transcription factors octamer-bingding protein 4 (Oct-4) and NANOG, but do not express telomerase, and are no tumorigenic. Under appropriate conditions in vitro, hAECs can be induced to differentiate into cells of three germinal layers, such as neural tissue (ectodermic lineage), pancreatic cells and hepatocytes (endodermal line), as well as cardiac cells (mesodermal line)[6]-[10].
Cornea plays a very important role as the first and most powerful refracting media of the eye. It consists of three cellular layers (stratified epithelium, stroma and monolayer endothelium). The corneal epithelium can regenerate and be maintained by stem cells located at the periphery of the cornea, in a region named as the limbus. If cells in this region suffer large areas of deficiency following trauma, chemical burns, malnutrition, infection or inflammation, the integrity of the corneal epithelium will be destroyed, and the repair is difficult. Concomitantly, the cornea will lose transparency as a result of conjunctiva, neovascularization and scarring. Finally, the visual function will be decreased, even be lost. Keratoplasty is the most efficient treatment method, but the shortage of the cornea from donor limits its carrying out. So it is necessary to find out a safety and available substitute. As reported in recent 10 years, imitating the microenvironment of corneal epithelial cells in vitro can induce pluripotent cells to differentiate into corneal epithelial-like cells[11]-[14]. Compared with undifferentiated cells, corneal reconstruction using the differentiated cells can significantly reduce the neovascularization, and improve the transparency of cornea in the rabbit model of limbal stem cell deficiency[11].
In this study, we explore the feasibility that hAECs have the potential to differentiate into corneal epithelial-like cells under the microenvironment replicated by spontaneously immortalized human corneal epithelial cells (S-ihCECs).
SUBJECTS AND METHODS
Isolation and Culture of Human Amniotic Epithelial Cells
Human amniotic membranes were obtained from health women after 37±1 weeks caesarean sections performed at the First Affiliated Hospital of Jinan University, in Guangzhou, China. Then the tissue was generally processed immediately as follows. Firstly, the amnion was manually separated from chorion and rinsed extensively with the sterile phosphate-buffered saline (PBS) containing 200U/mL gentamicin, to remove the clot, mucus, and debris. Then cut the tissue into small pieces. Amnion fragments were then digested for 4h in Dulbecco's modified Eagle's medium (DMEM; Gibco, USA) containing 2mg/mL collagenase (Invertrogen, USA) and 0.05mg/mL deoxyribonuclease I (DNAse I; Sigma, USA) at 37°C. Next, the digested fragments were centrifuged and the supernatant was discarded. The tissue was then subjected to a second enzymatic digestion containing 0.25% trypsin (Gibao, USA) and 0.02% ethylenediaminetetraacetic acid(EDTA, Sigma, USA) for 6m at 37°C. The resulting cell suspension was then centrifuged for 5m at 1 000r/min. The supernatant was removed, and the cells were resuspended in DMEM with 10% foetal bovine serum (FBS; Gibco, USA) and seeded in 25cm[2] culture flasks. Finally, the cells were incubated in a humidified 5% CO2 atmosphere at 37°C. After 48h, non-adherent cells were removed, and the adherent cells were continually cultured with fresh medium, and then every 3d thereafter.
Flow Cytometry
The hAECs were harvested by trypsinization, washed with sterile PBS and centrifuged for 5m at 800r/min. The supernatant was removed and the cell pellets were resuspended in flourescence-actived cell sorting (FACS) before cytometry. The resultant suspension was incubated for 10m at room temperature. After repeated centrifugation and removal of the superanant, resuspended and incubated with antibodies (Abcam, UK) for 30m at 4°C in darkness. This final suspension was analyzed using a FACSCalibur flow cytometer (Becton Dickinson, USA).
Spontaneously Immortalized Human Corneal Epithelial Cells Thawing and Cryopreservation
Cells were obtained from Prof. Wang Zhichong, Zhongshan Ophthalmic Center, Sun Yat-sen University[15]. For cell thawing, cryovials were immediately immersed at 37°C water as long as taken out from -80°C refrigerator, and then centrifuged for 5m at 800r/min, discarded the supernatant. The cells were resuspended with DMEM containing 10% FBS at the density of 2×104, then placed in 25cm2 culture flasks, incubated in 37°C. Until 80% confluent, cells were harvested by trypsinization, centrifuged for 5m at 1 000r/min, resuspended at a density of 106/mL in a mixture of DMSO (Sigma, USA), FBS and DMEM (1:6:3, v/v/v), then processed as follows: -4°C for 30m, -20°C for 1h, finally -80°C for a long-term storage.
Spontaneously Immortalized Human Corneal Epithelial Cells Mitotically Inactivated by Mitomycin
To determine the optimal concentration of mitomycin, the S-ihCECs were placed into a 96-wells plate at the density of 3 000 cells per well. After adhered, these cells were mitotically inactivated by adding 0µg/mL, 10µg/mL, 20µg/mL, 30µg/mL, 40µg/mL mitomycin and incubated for 2h at 37°C. Then removed mitomycin, cultured with DMEM containing 10% FBS. Cell proliferation assay cell counting kit-8 (CCK-8; Dojindo, Japan) was used to evaluate the proliferation properties at day 3, 5, 7.
Proliferation of Human Amniotic Epithelial Cells Cultured by Spontaneously Immortalized Human Corneal Epithelial Cells Culture Medium
S-ihCECs were mitotically inactivated by 10µg/mL or 20µg/mL mitomycin. Collected the epithelial culture medium every 12h or 24h, saved at -20°C. Cultured hAECs with this medium. CCK 8 was used to evaluate the proliferation properties at day 1, 3, 5, 7, 9.
Conditioned Medium from Spontaneously Immortalized Human Corneal Epithelial Cells
When 60% confluent, S-ihCECss were mitotically inactivated by mitomycin, the epithelial culture medium was collected every 12h. The epithelial medium was stored at -4°C. After 5d of repeated collection, centrifuged all stored epithelial medium at 1 000r/min for 3m. The supernatant was filter-sterilized using a 0.22µm filter to remove from any cell pellets, then stored at -20°C. The epithelial culture medium was added to the normal culture media at a ratio of 1:1, named as conditioned medium (CM), which was used to induce hAECs to differentiate into corneal epithelial-like cells, stored at 4°C.
Immunocytochemistry of Cell Cultures
The medium in the 6-well plate with coverslip was removed and the wells were washed with PBS three times. The wells were then incubated with 4% paraformaldehyde for 15m at room temperature, washed three times. Permeabilized with 0.3% triton X-100 (Sigma, USA) in PBS for 30m, blocked by 5% rabbit serum in PBS for 1h at room temperature, and then stained the cells with goat primary antibody against CK3, CK12 (1:100; Santa Cruz, USA) overnight at 4°C. The following day, the primary antibody was removed, and wells were washed with PBS five times at room temperature. The cells were incubated with fluorescein isothiocyanate (FITC) (or TRITC)-conjugated rabbit anti-goat IgG (ComWin, Beijing, China) for 40m in the dark. After discarded the secondary antibody, each well was irrigated five times with PBS in the dark. Finally, the cells were then incubated with a solution of 4′,6-diamidino-2-phenylindole(DAPI, Sigma, USA ) for 15m in the dark. After removal of the DAPI solution, washed three times with PBS. The cells were then viewed under a fluorescence camera microscope immediately (Leica, Germany).
Quantitative Real-Time Reverse Transcription- Polymerase Chain Reaction
Levels of the indicated mRNA were determined by QRT-PCR with the SYBR Green Master Mix (Toyobo, Japan), following the 2−ΔΔCt method to analyze the data[16]. Total RNA was isolated (TRNzol; Tiangen, Beijing, China) from cells expanded under conditioned medium and normal medium. First-strand cDNA synthesis from 1µg of total RNA and QRT-PCR was performed with a thermal cycler and software (Bio-Rad, Germany) following the protocol of the manufacturer. PCRs were run in duplicate using the following program: 95°C for 1m, and 45 cycles of 95°C for 15s, annealing temperature for 30s, and 72°C for 30s. Glyceraldehyde-3-phosphate dehydro-genase (GAPDH), a housekeeping gene, was used as an internal control. Primer pairs were showed in Table 1.
Table 1. Human primer sequences used for QRT-PCR.
| Gene | Accession No. | Primer(5′→3′) | Annealing temperature(°C ) | Product length (bp) |
| Oct-4 | NM_001173531 | (F)GCAAAGCAGAAACCCTCGTG (R)GAACCACACTCGGACCACAT |
58.8 | 173 |
| NANOG | NM_024865 | (F)AAGGTCCCGGTCAAGAAACAG (R)CTTCTGCGTCACACCATTGC |
55.7 | 237 |
| PAX6 | NM_001604 | (F)AACGATAACATACCAAGCGTGT (R)GGTCTGCCCGTTCAACATC |
62.0 | 120 |
| CK12 | NM_000223 | (F)TTCCATGTTTGGTTCTAGTTCCG (R)TCATTGCCCGAGAGAATACCT |
60.0 | 171 |
| GADPH | NM_002046 | (F)AAGGTGAAGGTCGGAGTCAAC (R)GGGGTCATTGATGGCAACAATA |
56.0 | 102 |
Western blotting
Total protein was isolated from cells expanded under CM and normal medium using radio immunoprecipitation assay(RIPA) buffer [50mmol/L Tris (pH 7.4), 150mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodiumdodecylsulfate, sodium orthovanadate, sodium fluoride, EDTA, leupeptin; Beyotime, Shanghai, China]. Separated total protein by 10% SDS-PAGE 1h at 100V, then transferred the gel proteins to polyvinylidene fluoride memberanes (Millipore, USA) 1h at 180mA. Blocked the membranes with 5% nonfat dry milk in phosphate buffered saline with Tween-20(PBST) containing 0.05% Tween-20 for 1h, incubated with primary antibodies against CK3 (1:200), CK12 (1:200), GADPH (1:2000) at 4°C overnight. After being washed five times with PBST, the membranes were incubated with secondary antibody for 1h. Finally, localization of antibodies was detected by chemiluminescence using ECL kit (Amersham, USA).
Scanning Electron Microscopy
For scanning electron microscopy examination, cells were seeded on coverslips, cultured with CM or normal culture media for 1 week, irrigated with PBS three times, fixed in 2.5% glutaraldehyde, dehydrated with graded alcohols, dried at critical temperature, finally observed and took photos with scanning electron microscope (ESEM-30, Philips, Holland).
Statistical Analysis
All statistical analyses were performed using SPSS13.0 software for windows; P value <0.05 was considered to be statistically significant.
RESULTS
Isolation and Phenotypical Characterization of Human Amniotic Epithelial Cells
Human Amniotic Epithelial Cells (hAECs) were isolated from full-term amnion after two steps enzyme digestion. The amnion contained two kinds of cell populations, one population had a fibroblast-like cell morphology and named as human amniotic mesenchymal stem cells (hAMSC), which was derived from the embryonic mesoderm, the other was hAECs, which was derived from embryonic ectoderm. In this study, we successfully separated hAECs from hAMSC. hAECs were strong three-dimensional cells that grew in a lattice with the triangulate, quadrangular or oval shape. hAECs were density dependence, the low density was disadvantageous to proliferate.
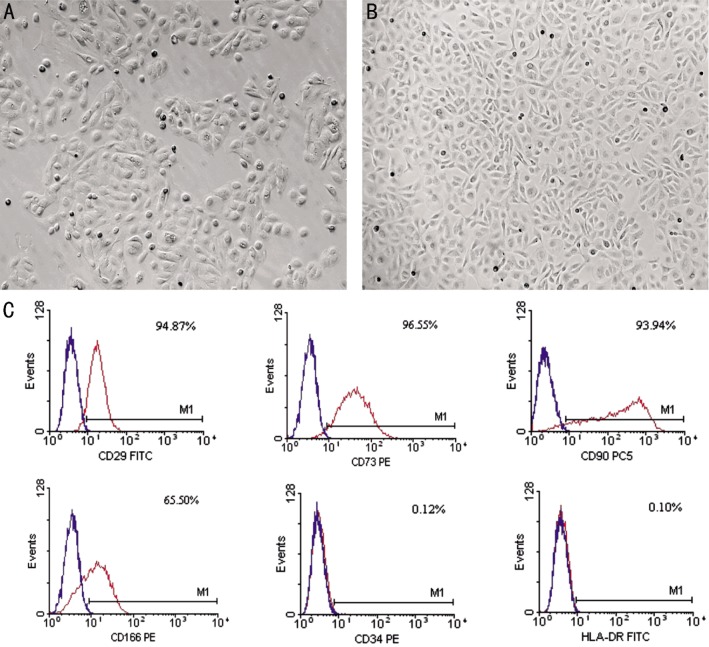
To assess the expression of mesenchymal and hematopoietic markers in isolated hAECs, flow cytometry analysis was performed. The mesenchymal markers CD29, CD90, CD73, CD166 were present in hAECs, the hemotopoietic marker CD34 was absent, and the expression of HLA-DR was negative (Figure 1).
Figure 1. The morphology and phenotypical characterization of hAECs.
A: Cultured hAECs 48h after plating; B: Cultured hAECs confluence at day 7; C: Flow cytometry analysis of confluent hAECs. The results showed that the expression of stem cell markers CD29, CD73, CD90, CD166 was positive, the hemotopoietic marker CD34 was negative, and the expression of HLA-DR was absent. Magnification: 50×(A, B).
Morphology and Identification of Spontaneously Immortalized Human Corneal Epithelial Cells
Phase contrast observation of S-ihCECs initially showed a fusiform appearance after attached to the culture surface, while it was a cobblestone appearance like normal epithelial morphology after reached confluence (Figure 2A, B). However, S-ihCECs had small cell size and high proliferation. When 100% confluent, S-ihCECs were spontaneous apoptosis, with a spherical appearance, and detached from the culture surface. After subculture 10 passages, cells went to senescence with larger cell size, lower adhesivity and colony forming efficiency.
Figure 2. Morphology and characterization of S-ihCECs.
A: Cultured S-ihCECs for 24h after attachment; B: Cultured S-ihCECs near confluence at day 3; C, D: Immunocytochemistry analysis of S-ihCECs for the expression of CK3 and CK12. Magnification: 50×(A, B), 200×(C, D).
S-ihCECs retained the expression of CK3, and CK12, which were the terminally differentiated cell markers for corneal epithelial cells. It was confirmed by immunocytochemistry (Figure 2C, D).
Spontaneously Immortalized Human Corneal Epithelial Cells Mitotically Inactivated by Mitomycin
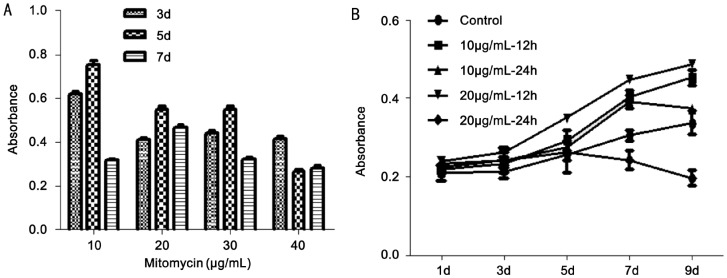
After mitotically inactivated by mitomycin, the proliferation of S-ihCECs was significantly inhibited. The inhibition of cell proliferation was determined by CCK8: 10µg/mL (65.48%±1.03), 20µg/mL (77.01%±0.99), 30µg/mL (75.25%±0.71), 40µg/mL (76.90%±0.97). The inhibition handled by 20µg/mL mitomycin was relatively stable and lasting (P<0.05) (Figure 3A).
Figure 3. Cells proliferation results by CCK8.
A: Proliferation of S-ihCECs mitotically inactivated by mitomycin. The inhibition handled by 20µg/mL mitomycin was relatively stable and lasting (P<0.05); B: Proliferation of hAECs cultured with medium collected from S-ihCECs. The culture medium collected every 12h after inactivated by 20µg/mL mitomycin could significantly promote the proliferation of hAECs (P<0.05). The data represent the mean±SEM from five experiments. The repetitive measurement and analysis of variance were performed.
Proliferation of Human Amniotic Epithelial Cells Cultured by Spontaneously Immortalized Human Corneal Epithelial Cells Culture Medium
To determine the optimal timing to collect S-ihCECs culture medium, we collected culture medium every 12h or 24h after S-ihCECs were inactivated by 10µg/mL and 20µg/mL. The proliferation of hAECs was observed by CCK8 at day 1, 3, 5, 7, 9. Then drew the curve of growth, accessed the optimal timing to collect the culture medium. The result showed that the culture medium collected every 12h after inactivated by 20µg/mL mitomycin could significantly promote the proliferation of hAECs (P<0.05) (Figure 3B).
Morphological Changes in Differentiated Human Amniotic Epithelial Cells
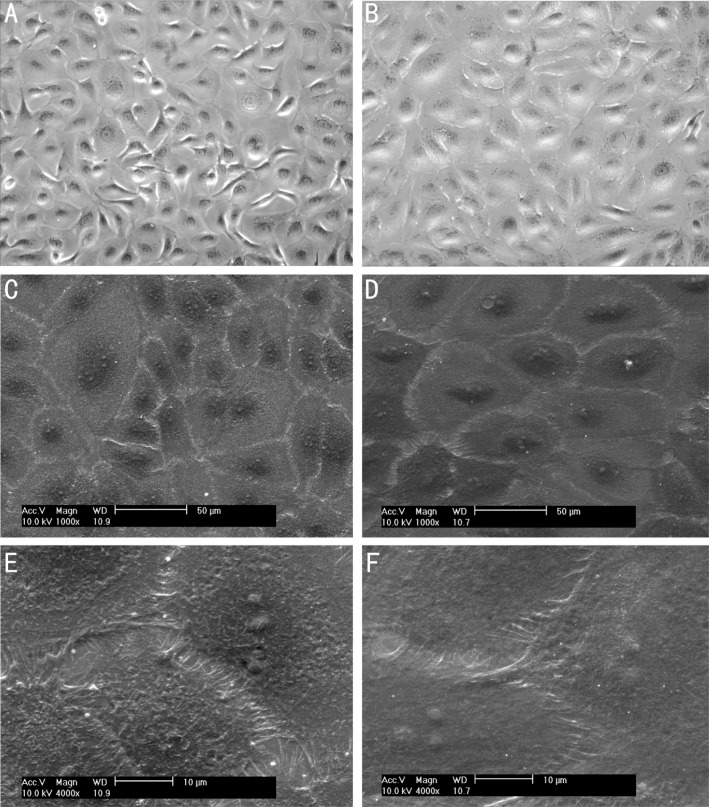
Inverted phase contrast microscope was used to observe the morphology change of the differentiating cultures, the images showed that the undifferentiated cells began to change morphology into much flatter, and spread out. The karyoplasmic declined gradually. Within the first 7d, the proliferation was significant, after that the cells grew slowly (Figure 4A, B).
Figure 4. Bright phase contrast microscopy images and scanning electron microphotographs of the hAECs and differentiated hAECs.
A, C, E: Light and electron microscopic appearance of undifferentiated; B, D, F: Light and electron microscopic appearance of differentiated after 1 week of differentiating. Images showed that the differentiated cells were larger cell size, much more flatter, and the microcilia were less in number and shorter than did those seen on undifferentiated cells. Magnification: 200×(A, B), 1000×(C, D), 4000×(E, F).
Scanning electron microscopy was performed on hAECs for 1 week culturing with normal culture medium and CM. The first significant change was the differentiated hAECs had a larger cell size than undifferentiated cells, the karyoplasmic ratio declined. Secondly, the differentiated hAECs had microcilia, although these cilia were less in number and shorter than undifferentiated cells. We also observed that the differentiated cells were flatter, and the size was much more uniform than those undifferentiated cells, which were more similar to normal corneal epithelial cell morphology (Figure 4C-F).
Differentiation of Human Amniotic Epithelial Cells into corneal epithelial-like cells
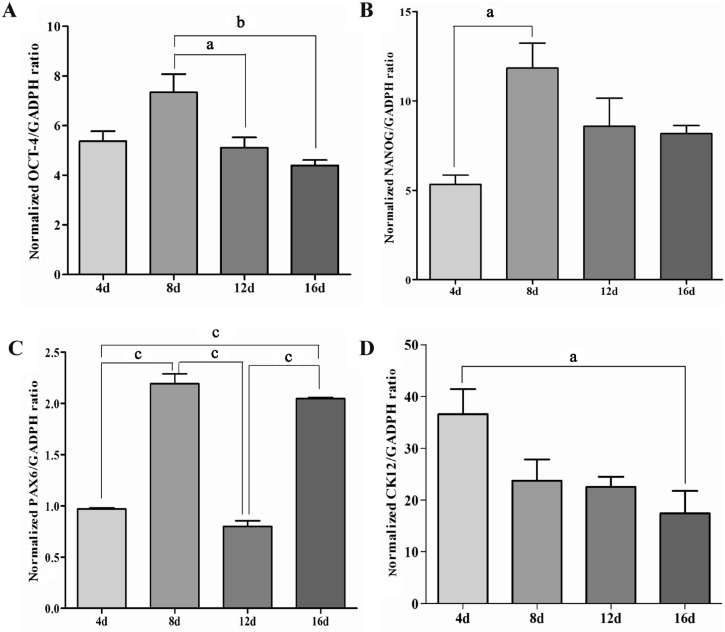
The pluripotency of hAECs during the differentiation process was detected by QRT-PCR analysis. The results showed that the expression of undifferentiated cell markers Oct-4, NANOG increased significantly, especially the NANOG (Figure 5A, B). There was an initial increase both in Oct-4 and NANOG during the first 8d of differentiation, then they decreased gradually.
Figure 5. QRT-PCR analysis for expression of Oct-4(A), NANOG(B), PAX6(C), and CK12(D) during the differentiation of hAECs.
The expression level was normalized against GADPH expression The data represent the mean±SEM from three experiments. Statistical significance was assessed using the Tukey's multiple comparison test. aP<0.05, bP<0.01, cP<0.005.
The differentiation of hAECs into corneal epithelial-like cells was confirmed by QRT-PCR analysis, immunocytochemistry, and western blot analysis. During the differentiation, QRT-PCR showed that the expression of PAX6 was fluctuant (Figure 5C). At the 8th and 16th day of differentiation, the expression increased, then at day 4 and 12, the expression was almost back to the baseline which hAECs originally expressed. QRT-PCR analysis for expression of distinctive keratin CK12 (for corneal epithelial cells) showed an initial peak at the 4th day, then the expression declined gradually during the later stage of the 16-day differentiation time period (Figure 5D). It was interesting to observe that an earlier peak of expression for CK12 compared to Oct-4, NANOG, and PAX6.
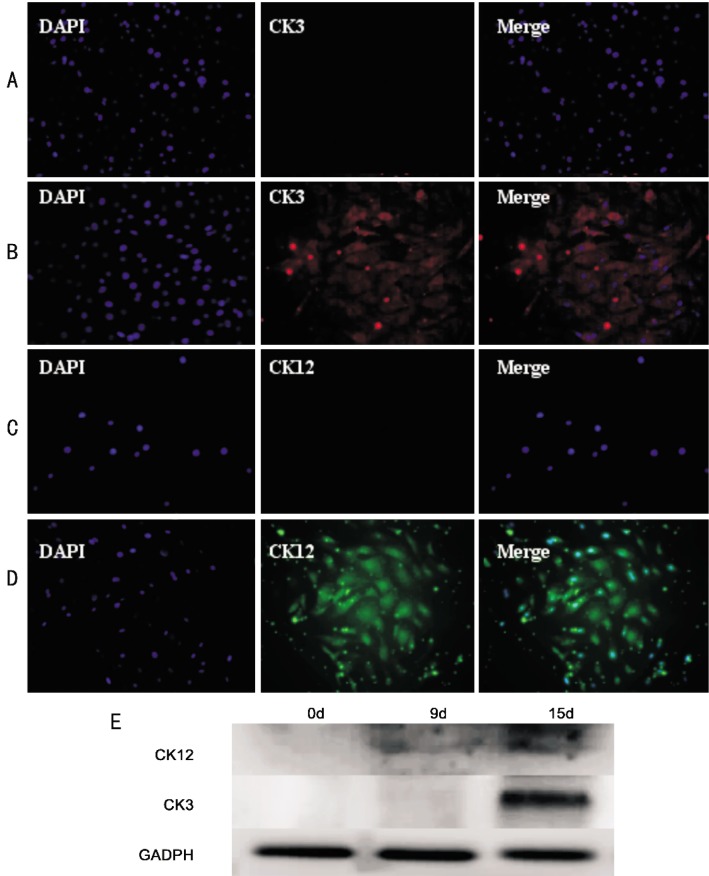
Immunocytochemistry of differentiating cells showed the expression of CK3 and CK12, the terminally differentiated cell markers specific to corneal epithelium[17](Figure 6A-D). Western blot analysis also showed the expression of CK3 and CK12. It was interesting that CK12 expressed weakly both at day 8 and 16 of differentiating, while CK3 was observed only at day 16 of differentiating (Figure 6E).
Figure 6. Immunocytochemistry and western blot analysis for expression of CK12 and CK3 after differentiated.
A, B: The expression of CK3 was positive after 2 weeks of differentiating; C, D: The expression of CK12 was observed at day 10 of differentiating; E: Determination of CK12 and CK3 protein levels in cells at day 0, 9 and 15 of differentiating confirmed by western blot analysis based on a GADPH loading control.
DISCUSSION
Compared with normal cells, immortalized cells are easy to cultivate, saving time, and cost saving. S-ihCECs we used were derived from serial cultivation of cells, which can express corneal epithelial specific phenotypes, and can be continuously subcultured for more than 20 passages[15]. Compared to those cell lines obtained from other experimental approaches, such as the ectopic expression of oncogenes by transfection, viral infection, or via retroviral vectors the inactivation of p16 and p53 pathways and hTERT expression, this cell line is more safety, and the morphology is more similar to normal corneal epithelial cells[18]. Currently, immortalized corneal epithelial cells are mainly used to investigate issues such as gene regulation and tissue development, or for validating alternative methods in toxicology[18]. Because of the possibility of oncogenicity and its barrier dysfunction, immortalized corneal epithelial cells are restricted to be used in the field of tissue engineering.
Studies outlined in this article highlight the feasibility that hAECs can be induced differentiated into corneal epithelial-like cells under the microenvironment replicated by S-ihCECs. Our experiments showed that the culture of hAECs with CM from S-ihCECs resulted in differentiation of these cells into corneal epithelial-like cells, it was confirmed by various detective methods.
In our experiment, S-ihCECss were inactivated by mitomycin to get enough time to collect the medium. The proliferation of hAECs cultured with medium collected from S-ihCECs which was inactivated by 20µg/mL mitomycin is showed in Figure 3B, we can see hAECs got into the logarithmic growth phase at day 3, and then got into the platform growing season at day 7, which was similar to the control group. After cultured with CM, the morphology of hAECs was changed, conformed by inverted phase contrast microscope and scanning electron microscope. Important differences certainly exist before and after differentiated. The scanning electron microscope images showed that the differentiated hAECs were much larger than undifferentiated hAECs. Secondly, cilia on the differentiated hAECs were less in number and much shorter than those of undifferentiated group.
Morphological changes were noted within the period of differentiating, and the changes in the interior of cells were analyzed by QRT-PCR, immunoflurescence, and western blotting. Miki et al[6] reported that the expression of OCT-4 and NANOG increased over the first 12-15d, when hAECs were kept in high-density culture. In our paper, the expression of OCT-4 and NANOG began to downregulated at day 8 in the microenvironment replicated by culture media from S-ihCECs. We speculate that S-ihCECs possibly provide some secreted factors which help induce hAECs to directional differentiation.PAX6 has been recognized as a suite of necessary genes for eye development[19]. The expression levels of PAX6 directly influence corneal epithelial cells, include immune function, vascularization, differentiation, and wound healing[20]-[22]. Garcia-Villegas et al[23] have reported that PAX6 is expressed early in the process of differentiation of corneal epithelial model system. In our experiment, hAECs originally express PAX6. During the period of differentiation, the expression of PAX6 was increased, although it was undulating. However, the expression of CK12 was initially increased to peak then gradually decreased. Western blot analyzed the expression of CK12 and CK3, the results revealed that the expression of CK3 was latter than CK12 during the differentiation, which was correspond to the results reported by Ahmad et al[13].
Our study for the first time reported that the microenvironment replicated by S-ihCECs can induce differentiation of hAECs into corneal epithelial-like cells. Notwithstanding this success, some deficiencies still exist which need to be improved. First, functional researches to certify whether hAECs-derived corneal epithelial-like cells can function in the same way as do corneal epithelia have to be carried out, by reconstructing the corneal epithelium in vivo or in vitro. Second, by the end of the differentiation process, the undifferentiated cell markers OCT-4 and NANOG still remained, which may result in tumor formation on transplantation[24]. The reason is worth to find out. We consider there are some ingredients or soluble factors in CM collected from S-ihCECs are carcinogenic, also may be the period of differentiation process is not enough. Third, in this study, the expression of CK12 was earlier than it was in CK3, so it is necessary to explore that when to transplant can play a big function in vivo. Therefore, steps have to be taken to eliminate this risk.
Acknowledgments
We thank Wang Zhichong for supplying S-ihCECs.
Footnotes
Foundation item: National Natural Science Foundation of China (No.30872808)
REFERENCES
- 1.Fauza D. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):877–891. doi: 10.1016/j.bpobgyn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem cells. 2008;26(2):300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 3.Pozzobon M, Ghionzoli M, De Coppi P. ES, iPS, MSC, and AFS cells. Stem cells exploitation for pediatric surgery: current research and perspective. Pediatr Surg Int. 2010;26(1):3–10. doi: 10.1007/s00383-009-2478-8. [DOI] [PubMed] [Google Scholar]
- 4.Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem cell Res Ther. 2011;2(3):25. doi: 10.1186/scrt66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, Andrews PW. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem cells. 2002;20(4):329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 6.Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 7.Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133–142. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- 8.Elwan MA, Sakuragawa N. Evidence for synthesis and release of catecholamines by human amniotic epithelial cells. Neuroreport. 1997;8(16):3435–3438. doi: 10.1097/00001756-199711100-00004. [DOI] [PubMed] [Google Scholar]
- 9.Wei JP, Zhang TS, Kawa S, Aizawa T, Ota M, Akaike T, Kato K, Konishi I, Nikaido T. Human amnion-isolated cells normalize blood glucose in streptozo-tocin-induced diabetic mice. Cell Transplant. 2003;12(5):545–552. doi: 10.3727/000000003108747000. [DOI] [PubMed] [Google Scholar]
- 10.Sakuragawa N, Enosawa S, Ishii T, Thangavel R, Tashiro T, Okuyama T, Suzuki S. Human amniotic epithelial cells are promising transgene carriers for allogeneic cell transplantation into liver. J Hum Genet. 2000;45(3):171–176. doi: 10.1007/s100380050205. [DOI] [PubMed] [Google Scholar]
- 11.Jiang TS, Cai L, Ji WY, Hui YN, Wang YS, Hu D, Zhu J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;16:1304–1316. [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Ge J, Wang Z. The preliminary experimental study of induced differentiation of embryonic stem cells into corneal epithelial cells. Yan Ke Xue Bao. 2001;17(3):138–143. [PubMed] [Google Scholar]
- 13.Ahmad S, Stewart R, Yung S, Kolli S, Armstrong L, Stojkovic M, Figueiredo F, Lako M. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25(5):1145–1155. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 14.Blazejewska EA, Schlotzer-Schrehardt U, Zenkel M, Bachmann B, Chankiewitz E, Jacobi C, Kruse FE. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem cells. 2009;27(3):642–652. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Song G, Wang Z, Huang B, Gao Q, Liu B, Xu Y, Liang X, Ma P, Gao N, Ge J. Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp Eye Res. 2007;84(3):599–609. doi: 10.1016/j.exer.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Kurpakus MA, Stock EL, Jones JC. Expression of the 55-kD/64-kD corneal keratins in ocular surface epithelium. Invest Ophthalmol Vis Sci. 1990;31(3):448–456. [PubMed] [Google Scholar]
- 18.Castro-Muñozledo F. Corneal epithelial cell cultures as a tool for research, drug screening and testing. Exp Eye Res. 2008;86(3):459–469. doi: 10.1016/j.exer.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Fernald RD. Eyes: variety, development and evolution. Brain Behav Evol. 2004;64(3):141–147. doi: 10.1159/000079743. [DOI] [PubMed] [Google Scholar]
- 20.Kucerova R, Walczysko P, Reid B, Ou J, Leiper LJ, Rajnicek AM, McCaig CD, Zhao M, Collinson JM. The role of electrical signals in murine corneal wound re-epithelialization. J Cell Physiol. 2011;226(2):1544–1553. doi: 10.1002/jcp.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis J, Piatigorsky J. Overexpression of Pax6 in mouse cornea directly alters corneal epithelial cells: changes in immune function, vascularization, and differentiation. Invest Ophthalmol Vis Sci. 2011;52(7):4158–4168. doi: 10.1167/iovs.10-6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucerova R, Dora N, Mort RL, Wallace K, Leiper LJ, Lowes C, Neves C, Walczysko P, Bruce F, Fowler PA, Rajnicek AM, McCaig CD, Zhao M, West JD, Collinson JM. Interaction between hedgehog signalling and PAX6 dosage mediates maintenance and regeneration of the corneal epithelium. Mol Vis. 2012;18:139–150. [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Villegas R, Escamilla J, Sanchez-Guzman E, Pasten A, Hernandez-Quintero M, Gomez-Flores E, Castro-Munozledo F. Pax-6 is expressed early in the differentiation of a corneal epithelial model system. J Cell Physiol. 2009;220(2):348–356. doi: 10.1002/jcp.21771. [DOI] [PubMed] [Google Scholar]
- 24.Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104(10):2092–2098. doi: 10.1002/cncr.21435. [DOI] [PubMed] [Google Scholar]