Abstract
AIM
To establish the rat model of streptozotocin (STZ)-induced diabetic retinopathy (DR), which is the most common cause of visual loss and blindness in patients with diabetes, and observe the gene expression of vascular endothelial growth factor (VEGF) and its receptors during the development of DR.
METHODS
A rat model of diabetes was established by intraperitoneal injection of STZ. The diabetic rats were housed for 2, 3 and 4 months after the development of diabetes. Retinal histopathological observation was performed. The retinal vessels were observed by immunofluorescence staining by CD31. The mRNA expression of VEGF, VEGF receptor 1 and 2 (VEGFR1/2) in rat retina was detected by reverse transcription-polymerase chain reaction (RT-PCR) analysis.
RESULTS
Retinal histopathological observation showed the morphological changes of inner nuclear layer (INL) and outer nuclear layer (ONL) at any time-point, and also demonstrated the increased new vessels at both 3, 4 months after the development of diabetes. The CD31 staining results showed that the number of vessels was increased in the retinas of diabetic rats at both 3 and 4 months after the development of diabetes. As compared to the normal rats, the mRNA expression of VEGF was increased in retinas of diabetic rats at 3 months after the development of diabetes, while VEGFR1 and VEGFR2 mRNA expression was increased at 2, 3 and 4 months after the development of diabetes.
CONCLUSION
Taken together, our results demonstrated that DR was occurred at 3 months after the development of diabetes, and the mRNA expression of VEGF, VEGFR1 and VEGFR2 were increased in the process of DR. The present study further evidenced the involvement of VEGF and its receptors in the process of DR.
Keywords: diabetic retinopathy, vascular endothelial growth factor, vascular endothelial growth factor receptor
INTRODUCTION
The retinal neovascularization caused by excessive proliferation of vessels in retina is a serious ophthalmopathy, which can cause vision loss and blindness. This kind of ophthalmopathy mainly includes diabetic retinopathy (DR), retinopathy of prematurity (ROP) and age-related macular degeneration (AMD). DR is one of the most common complications of diabetes mellitus, and also is the most common reason of blindness in diabetics. It is reported that nearly all people with type 1 and more than half with type 2 diabetes will develop retinopathy [1].
The whole process of DR includes the following key events: loss of retinal capillary pericytes, basement membrane thickening, loss of endothelial barrier function, and breakdown of the blood retinal barrier, which will lead to the ischemia of retina. Further, retinal ischemia will result in the elevation of vascular endothelial growth factor (VEGF), which contributes to neovascularization and fibrosis which is the hallmark of proliferative stage of DR[2]-[4]. Due to its complicated pathogenesis, the study on the process of the development of DR has been a hotspot. There are already some reports about the establishment of the rat model of streptozotocin (STZ)-induced DR[5],[6]. However, the concrete time of the occurrence of DR induced by STZ is not the same in different experiments.
Generally, numerous pro-angiogenic growth factors such as VEGF, basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1) and extracellular matrix components were associated with the pathogenesis of progressive DR[7],[8]. The VEGF family includes VEGF, VEGF-B, VEGF-C, placenta growth factor (PlGF), and viral VEGF proteins [9]. Among which, VEGF has emerged as a central regulator of the angiogenic process in physiological and pathological conditions[10]. VEGF stimulates endothelial cell proliferation, migration, and promotes angiogenesis via binding to two tyrosine kinase receptors: VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1)[11],[12]. The excessive secretion of retinal VEGF will stimulate angiogenesis, which contributes to the development of DR[13]-[15]. There are already various reports about the increased expression of VEGF in retina of STZ-induced diabetic rats[16],[17]. However, there is no much report about the expression of its receptors such as VEGFR1, VEGFR2.
In the present study, we establish the rat model of STZ-induced DR, and observe the progression of DR at 2, 3, 4-month after the development of diabetes, and further observe the gene expression of VEGF and its two receptors in rat retina.
MATERIALS AND METHODS
Materials
Reagents
CD31 antibody and FITC conjugated anti-Rat IgG were purchased from BD Biosciences (Franklin Lakes, NJ, USA). RevertAid first strand cDNA synthesis kit was from Fermentas, Merck Calbiochem (Darmstadt, Germany). Streptozotocin (STZ) and other reagents unless indicated were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Animal
The Sprague Dawley (SD) rats weighting 180g-220g were from the shanghai laboratory animal center of Chinese Academy of Sciences (Shanghai, China). The rats were maintained under controlled temperature (23±2°C), humidity (50%), and lighting (12h light/12h dark). The rats were fed with a standard laboratory diet and given free access to tap water. All animals were received humane care in compliance with the institutional animal care guidelines approved by the Experimental Animal Ethical Committee of Shanghai University of Traditional Chinese Medicine.
Methods
Rat model of diabetic retinopathy
Twenty-two rats were administered intraperitoneally (i.p.) with 65mg/kg STZ, while the other 18 rats were injected (i.p.) with physiological saline and served as control animals. After 7 days, serum glucose concentration was measured, and the rats with high glucose concentration (>16.5mmoL/L) were considered as diabetic rats. In this experiment, the glucose concentration of 18 rats was >16.5mmoL/L, and those rats were randomly divided into three groups: 2-month STZ group, 3-month STZ group, 4-month STZ group. The other control rats were also randomly divided into three groups: 2-month control group, 3-month control group, 4-month control group. At 2, 3, 4 months after the injection of STZ, the rats were anesthetized by sodium pentobarbital (40mg/kg, i.p.), the blood samples were taken from the abdominal aorta, and the eyes were removed immediately.
Retinal immunfluorescence staining
The retinas were incubated with 4% paraformaldehyde solution over night in 4°C, and then were blocked in blocking buffer (5% BSA, 0.5% triton X-100 in PBS) for 1-3h at room temperature, and then were incubated with the CD31 antibody for 1d or 2d at 4°C, and then washed with washing buffer (0.5% triton X-100 in PBS) for every 20min. After washing 6 times, the retinas were incubated with FITC- conjugated anti-Rat IgG antibody for 2h. After washing 6 times again, the retinas were placed on a slide glass, mounted in gelatin, covered with a cover slip, and pictured under the fluorescence microscopy (Oympus, Japan). The quantitative of the vessels was counted as described method[18]. Firstly, two lines, which constitute a cross, were drawn in the central on the pictures. Then, the number of vessels cross these two lines was counted.
Histological assessment
The retina tissues were isolated from the normal rats and diabetic rats, and then fixed in 4% paraformaldehyde solution. Samples were subsequently sectioned (5µmoL/L), stained with haematoxylin and eosin, and examined under the microscopy (Oympus, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from rat retinas using a TRIZOL (Life Technologies, USA) reagent according to the manufacturer’s protocol. The single strand cDNA was synthesized according to the manufacturer's protocol. Transcripts of the gene for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as an internal control. The following primers were used: GAPDH: 5′-TGTTGCCATCAATGACC CCTT-3′ (Forward), and 5′-CTCCACGACGTACTCAGCG-3′ (Reverse) (Gene ID:24383); VEGF: 5′-TTCGTCCAACTTCTGGGCTCTT -3′ (Forward), and 5′-CTC CTCTTCCTTCTCTTTCTCCCC-3′ (Reverse) (Gene ID:83785); VEGFR2: 5′-TA GCACGACAGAGACTGTGAGG-3′ (Forward), and 5′-TGAGGTGAGAGAGATG GGTAGG-3′ (Reverse) (Gene ID:25589). The reaction mixture was subjected to 30 cycle of 30s at 94°C, 30s at 55°C and 45s at 72°C. The PCR products were visualized using ethidium bromide in 1.5% agarose gel. The quantity of PCR products was automatically analyzed by Smart View Bio-electrophoresis Image Analysis system (Version FR-980, FURI Science and Technology Co. Ltd, Shanghai, China).
Statistical Analysis
The results were expressed as means±SEM. The significance of differences between groups was evaluated by one-way ANOVA with LSD post hoc test; and P<0.05 was considered as indicating statistically significant difference.
RESULTS
Glycemia and Body Weight
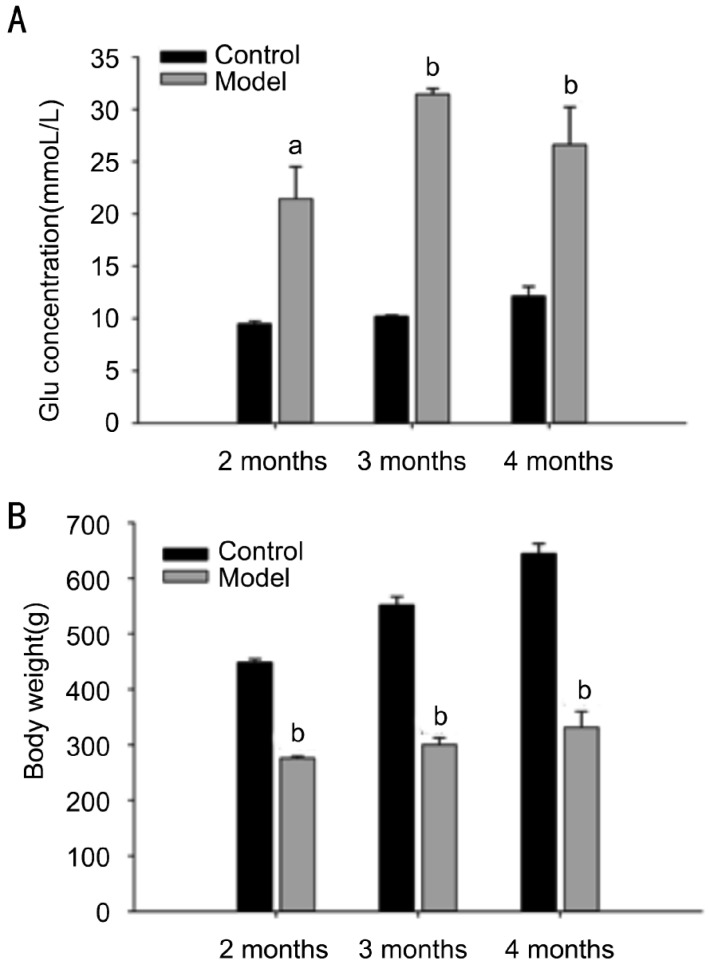
From Figure 1A, we can see that serum glucose values in STZ-treated rats were all higher than 16.5mmoL/L both at 2, 3, 4 months after the development of diabetes. However, serum glucose values were all lower than 16.5mmoL/L in normal rats at different months. Furthermore, the body weight of STZ-treated rat at 2, 3, 4 months after the development of diabetes was lower than normal rat (Figure 1B).
Figure 1. Analysis of serum glucose and body weight.
A: Serum glucose; B: Body weight. Data=Means±SEM (n=6). aP<0.05, bP<0.001 vs control at same age.
Morphological Changes in Retina
Morphological changes were observed in inner nuclear layer (INL) and outer nuclear layer (ONL) in STZ-induced DR for 2, 3, 4 months (Figure 2B, D, F) as compared with respective age matched controls (Figure 2A, C, E). The number of bipolar cells in the INL and ONL of retinas from 3, 4-month diabetic rats was considerably decreased as compared with control. Meanwhile, as the arrows point out that there were new vessels appeared in the junction between ganglion cell layer (GCL) and inner plexiform layer (IPL), and the junction between IPL and INL in retinas at 3, 4-month after the development of diabetes (Figure 2D, F), but there was no new vessels in retinas at 2-month after the development of diabetes (Figure 2B).
Figure 2. Representative pictures of H&E staining of retinas tissues in normal and diabetic rats.
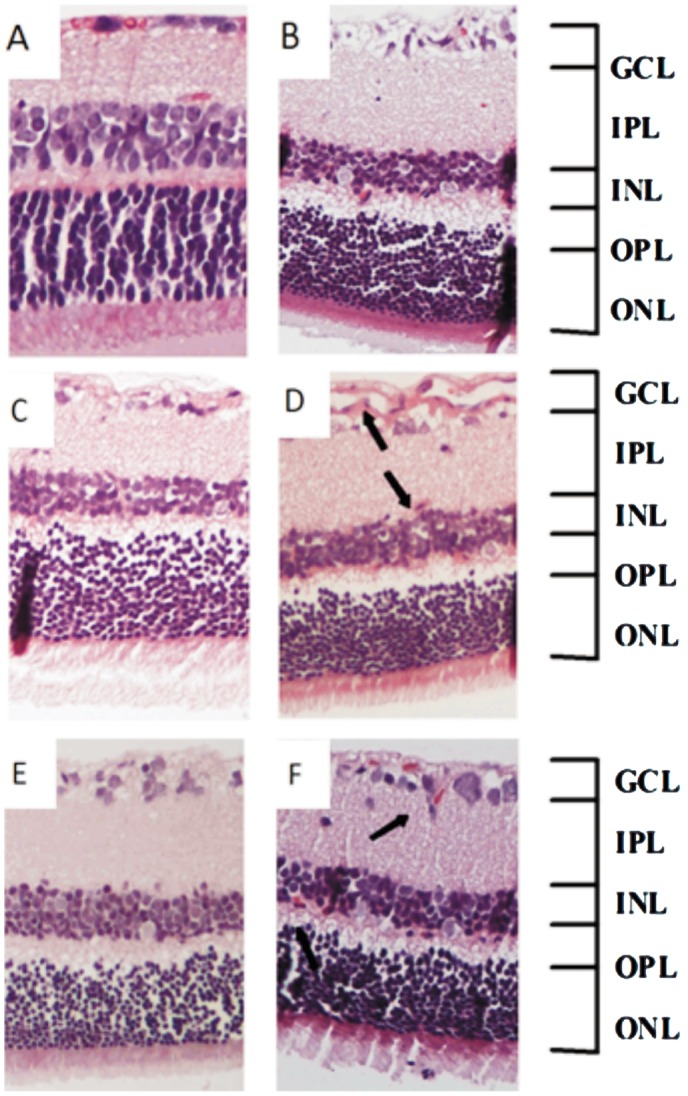
A: Normal rats of 2 months; B: STZ-induced diabetic rats of 2 months; C: Normal rats of 3 months; D: STZ-induced diabetic rats of 3 months; E: Normal rats of 4 months; F: STZ-induced diabetic rats of 4 months. (×200) (GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer). Arrows point out the new vessels.
Immunofluorescence Staining of Retinal Vessels
Platelet endothelial cell adhesion molecule (PECAM-1) also known as cluster of differentiation 31 (CD31) is used primarily to indentify endothelial cells, which represents the presence of vessels. Figure 3 shows that there was no much difference between normal and STZ-treated rat retinas at 2 months after the development of diabetes (Figure 2A, B), but we can see the increased staining of CD31 in STZ-treated rat retinas at 3, 4 months after the development of diabetes (Figure 2C, D, E, F). Further, the quantitative results clearly demonstrate that the vessels were increased at 3, 4 months after the development of diabetes (Figure 2G).
Figure 3. Immunofluorescence staining of retinas for CD31 in normal and diabetic rats.
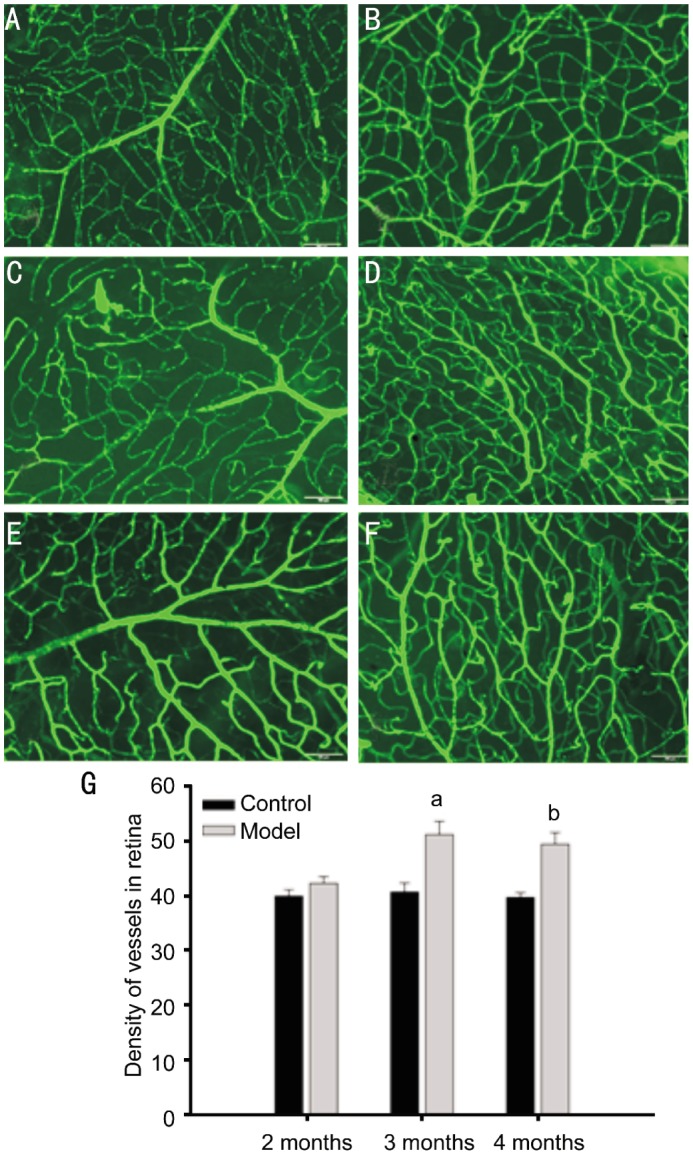
A: Normal rats of 2 months; B: STZ-induced diabetic rats of 2 months; C: Normal rats of 3 months; D: STZ-induced diabetic rats of 3 months; E: Normal rats of 4 months; F: STZ-induced diabetic rats of 4 months. The scale in the pictures represents 50µm. G: Quantitative results of retinal vessels. Data=Means±SEM (n=4). aP<0.05, bP<0.01 vs control at same age.
Expression of Vascular Endothelial Growth Factor (VEGF), VEGFR1 and VEGFR2 in Rat Retinas
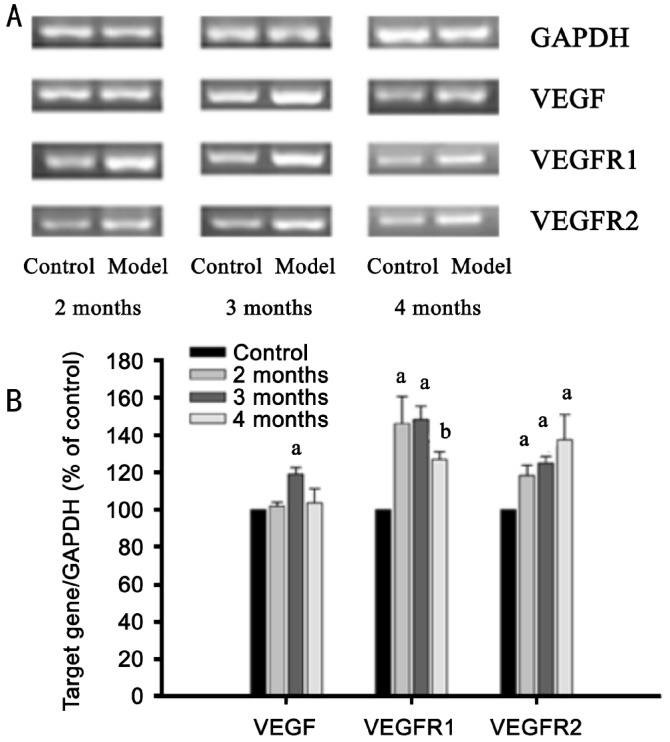
Further, we analyzed the gene expression of VEGF, VEGFR1 and VEGFR2 in rat retinas at 2, 3, 4 months after the development of diabetes. From Figure 4A, B, we can see that the expression of VEGF was increased in rat retina at 3 months after the development of diabetes (P<0.05), but such increase become weak at 4 months after the development of diabetes. The expression of VEGFR1 and VEGFR2 was increased in diabetic rat retina at all 2, 3, 4 months after the development of diabetes (P<0.05).
Figure 4. The mRNA expression of VEGF, VEGFR1 and VEGFR2 in retinas of normal and diabetic rats.
A: The mRNA expression of VEGF, VEGFR1 and VEGFR2 were detected by RT-PCR. The result represents one of three separate experiments; B: The amounts of VEGF, VEGFR1 and VEGFR2 mRNA expression were normalized to the internal control, GAPDH. The results were expressed as percentage of control at the same age. Data=Means±SEM (n=6). aP<0.05, bP<0.01 vs control at the same age.
DISCUSSION
Diabetes mellitus, or simply diabetes, is a chronic disease characterized by hyperglycemic state. STZ is an antibiotic that can cause pancreatic β-cell destruction, so it is widely used in medical research to produce an animal model for type 1 diabetes in large dose as well as type 2 diabetes with multiple low doses[19]-[22]. In the present study, the results of serum glucose level and body weight both proved the successful establishment of the rat model of type 1 diabetes.
As the common complication of diabetes mellitus, DR is present in almost all persons with diabetes for >15 years[23]. According to the longer duration of its development, DR is generally divided into two stages: non-proliferative stage, also named early stage, which is characterized by the leakage of vessels; and proliferative stage or late stage, where proliferation of retinal vessels will be induced by various growth factors[24]. There are already some reports about the establishment of the rat model of STZ-induced DR[5],[6]. In some study, the DR was occurred at 4 months after the development of diabetes, but in other study, the DR was occurred at 6 months after the development of diabetes[5],[6]. Thus, the concrete time of the occurrence of DR is not yet conclusive. In the present study, the results of CD31 immuno-staining and H&E evaluation showed that there were increased retinal vessels at 3, 4 months after the development of diabetes. Our results demonstrated that the late stage of DR was occurred at 3 months after the development of diabetes.
Angiogenesis is the key player in vision loss during the process of DR, and the over-expression of VEGF is reported to be associated with the altered angiogenesis that causes retinal dysfunction[25],[26]. As VEGF/VEGFR receptor signaling plays critical roles in angiogenesis, anti-VEGF therapies are important for the treatment of angiogenesis-related diseases such as cancer, DR etc[27]. Bevacizumab, a full-length antibody against VEGF and generally used for the treatment of cancer, has been also used in clinic for DR and other chorioretinal vascular disorders[28].
There are already various reports about the increased expression of VEGF in retina of STZ-induced diabetic rats[29],[30]. Our present study demonstrated that there was increased expression of retinal VEGF in rats at 3 months after the development of diabetes, which indicate that the increased expression of VEGF may be related with the process of proliferative stage of DR. There is already a report that VEGF is decreased with the progressive of proliferative DR, which may contribute to explain why there was no increase of VEGF at 4 months after the development of diabetes[31]. VEGFR1 and VEGFR2 are receptors of VEGF, whose autophosphorylation will initiate the signaling cascade and induce angiogenesis[32]. Our results also showed that retinal VEGFR1 and VEGFR2 expression was all increased at 2, 3, 4 months after the development of diabetes. The results further evidence the critical role of VEGF/VEGFR signal cascade in regulating the process of DR.
In conclusion, the rat model with DR has been successfully established in this study, and which will be helpful for our further study on the development of therapeutic drugs for DR. Meanwhile, the present study demonstrates the change of angiogenesis-related growth factor VEGF and its receptors VEGFR1 and VEGFR2 during the progression of DR, which will be helpful for the further elucidation of the involvement of angiogenesis in the development of DR and its regulating mechanism.
Footnotes
Foundation item: National Natural Science Foundation of China (No.81173517)
REFERENCES
- 1.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R, American Diabetes Association Diabetic retinopathy. Diabetes Care. 2003;26(1):226–229. doi: 10.2337/diacare.26.1.226. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Kohner EM. Diabetic retinopathy. BMJ. 1993;307(6913):1195–1199. doi: 10.1136/bmj.307.6913.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infeld DA, O'Shea JG. Diabetic retinopathy. Postqrad Med J. 1998;74(869):129–133. doi: 10.1136/pgmj.74.869.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Si YF, Wang J, Guan J, Li Z, Sheng Y, Zhao J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br J Pharmacol. 2013;169(3):619–631. doi: 10.1111/bph.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Rohit S, Jha KA. Hesperetin rescues retinal oxidative stess, neuroinflammtion and apoptosis in diabetic rats. Microvasc Res. 2013;87:65–74. doi: 10.1016/j.mvr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Campochiaro PA. Molecular targets for retinal vascular diseases. J Cell Physiol. 2007;210(3):575–581. doi: 10.1002/jcp.20893. [DOI] [PubMed] [Google Scholar]
- 8.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenci factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2(1):71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 9.Achen MG, Stacker SA. The vascular endothelial growth factor family; proteins which guide the development of the vasculature. Int J Exp Pathol. 1998;79(5):255–265. doi: 10.1046/j.1365-2613.1998.700404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 11.Vaisman N, Gospodarowicz D, Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990;265(32):19461–19466. [PubMed] [Google Scholar]
- 12.Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995;129(4):895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapieha PT, Hamel D, Shao Z, Rivera JC, Zaniolo K, Joyal JS, Chemtob S. Proliferative retinopathies: angiogenesis that blinds. Int J Biochem Cell Biol. 2010;42(1):5–12. doi: 10.1016/j.biocel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 15.Gupta N, Mansoor S, Sharma A, Sapkal A, Sheth J, Falatoonzadeh P, Kuppermann B, Kenney M. Diabetic retinopathy and VEGF. Open Ophthalmol J. 2013;7:4–10. doi: 10.2174/1874364101307010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, Zhang HQ, Zhu J, Liu KY, Cheng H, Li GL, Xu S, Xie ZG. Puerarin enhances superoxide dismutase activity and inhibits RAGE and VEGF expression in retinas of STZ-induced early diabetic rats. Asian Pac J Trop Med. 2012;5(11):891–896. doi: 10.1016/S1995-7645(12)60166-7. [DOI] [PubMed] [Google Scholar]
- 17.Du ZJ, Kamei M, Suzuki M, Tano Y, Wang BR, Hui YN. Coordinated expression of Ets-1, pERK1/2, and VEGF in retina of streptozotocin-induced diabetic rats. Ophthalmic Res. 2007;39(4):224–231. doi: 10.1159/000104831. [DOI] [PubMed] [Google Scholar]
- 18.Huang CX. Experimental study of Fufang XueshuanTong on intervention of retinopathy in diabetic rats. Guangzhou: Sun Yat-Sen University. 2006:13–19. [Google Scholar]
- 19.Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci. 2012;53(13):8067–8074. doi: 10.1167/iovs.12-10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imam MU, Musa SN, Azmi NH, Ismail M. Effects of white rice, brown rice and germinated brown rice on antioxidant status of type 2 Diabetic rats. Int J Mol Sci. 2012;13(10):12952–12969. doi: 10.3390/ijms131012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Yan H. Dysfuction of circulating endothelial progenitor cells in type 1 diabetic rats with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1123–1131. doi: 10.1007/s00417-013-2267-x. [DOI] [PubMed] [Google Scholar]
- 22.Ding Y, Dai XQ, Jiang YF, Zhang ZF, Bao L, Li YJ, Zhang F, Ma XT, Cai XX, Jin LL, Gu JJ, Li Y. Grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in skeletal muscle of low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Mol Nutr Food Res. 2012;57(2):365–369. doi: 10.1002/mnfr.201200463. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy: II. prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 24.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, Nakama T, Ishikawa K. Antiangiogenic shift in vitreous after vitrectomy in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53(11):6997–7003. doi: 10.1167/iovs.12-9671. [DOI] [PubMed] [Google Scholar]
- 26.Aldebasi YH, Rahmani AH, Khan AA, Aly SM. The effect of vascular growth factor in the progression of bladder cancer and diabetic retinopathy. Int J Clin Exp Med. 2013;6(4):239–251. [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 28.Ye X, Xu G, Chang Q, Fan J, Sun Z, Qin Y, Jiang AC. ERK1/2 signlaing pathways involved in VEGF release in diabetic rat retina. Invest Ophthalmol Vis Sci. 2010;51(10):5226–5233. doi: 10.1167/iovs.09-4899. [DOI] [PubMed] [Google Scholar]
- 29.Masuzawa K, Jesmin S, Maeda S, Zaedi S, Shimojo N, Miyauchi T, Goto K. Effect of endothelin dual receptor antagonist on VEGF levels in streptozotocin-induced diabetic rat retina. Exp Biol Med (Maywood) 2006;231(6):1090–1094. [PubMed] [Google Scholar]
- 30.Mima A, Qi W, Hiraoka-Yamomoto J, Park K, Matsumoto M, Kitada M, Li Q, Mizutani K, Yu E, Shimada T, Lee J, Shoelson SE, Jobin C, Rask-Madsen C, King GL. Retinal not systemic oxidative and inflammatory stress correlated with VEGF expression in rodent models of insulin resistance and diabetes. Invest Ophthalmol Vis Sci. 2012;53(13):8424–8432. doi: 10.1167/iovs.12-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum A, Socea D, Ben-Shushan RS, Keinan-Boker L, Naftali M, Segol G, Tamir S. A decrease in VEGF and inflammatory markers is associated with diabetic proliferative retinopathy. Eur Cytokine Netw. 2012;23(4):158–162. doi: 10.1684/ecn.2012.0321. [DOI] [PubMed] [Google Scholar]
- 32.Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63(5):601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]