Abstract
AIM
To measure central corneal thickness (CCT) and pre-corneal tear film thickness using the Galilei dual-Scheimpflug analyzer (GSA) in New Zealand white rabbits.
METHODS
Ten normal New Zealand white rabbits (20 eyes) were included in this study. With the assistance of 0.1% fluorescein, the pre-corneal tear film can be well visualized. Both eyes of each rabbit were scanned once with the GSA pre- and post-instillation of 1µL 0.1% fluorescein. The difference between the two measurements of CCT (4-mm diameter) was recorded as the pachymetric values of the central tear film.
RESULTS
The CCT of pre- and post-instillation was 388.8±9.5µm and 407.0±10.5µm, respectively. After a paired t-test analysis, the central pre-corneal tear film thickness of 4mm diameter was 18.2±5.31µm with a 95% confidence interval of (15.7, 20.6)µm (P<0.001).
CONCLUSION
GSA can be used to measure CCT and analyze central tear film thickness of rabbits with the help of fluorescein.
Keywords: tear film, Scheimpflug, rabbits, central corneal thickness, fluorescein
INTRODUCTION
The pre-corneal tear film, which keeps the corneal and conjunctival surface free from all kinds of damage, such as dust, foreign bodies, microbes, toxic skin lipids and provides a fluid environment for the superficial epithelial cells, is the outermost layer in the eye[1],[2]. Moreover, the tear film, which is composed of three layers including the external lipid layer, an aqueous layer and an internal mucin layer, provides lubrication for the ocular surface as well[3]. Tear film thickness is a demonstration of dynamics and physiology of the tear layer, so it is closely related to the dry eye symptoms, which contains several groups of ocular surface disorders with diverse etiologies[4],[5]. It is believed that dry eye, whose symptoms includes pain and irritation caused by ocular dryness, is one of the most common ocular diseases; its prevalence increases with age and it can impact the quality of life[6]-[8]. Compared with a routine Schirmer Test, which measures aqueous tear volume, a precise and simple measurement of the tear film thickness could provide some information for tear film dysfunction and be important for predicting the stability of it[4]. Rabbits, as a traditional experimental animal species, have been used to develop the dry eye model for many years[9],[10]. Although many kinds of methods, such as laser interferometry, confocal microscopy, optical coherence tomography (OCT) etc, have been used to measure tear film thickness, the precise value largely varies from 3µm to 40µm[11]-[14]. The Galilei dual-Scheimpflug analyzer (GSA), as a high-precision optical system, has been widely used for corneal topography and three dimensional analysis of the ocular anterior segment. It is based on the combination of a revolving dual Scheimpflug camera and a placido disk. It can measure the ocular anterior segment from the anterior corneal surface to the posterior lens surface, but any images located behind the iris were blocked by the pigment. Based on study results of high accuracy and repeatability about GSA, one good quality scan is sufficient to evaluate corneal thickness[15]-[17]. It can accurately detect the anterior and posterior surface and locate the apex of the cornea with high resolution Scheimpflug images. The purpose of this study was to investigate the central corneal thickness (CCT) and the pre-corneal tear film of rabbits using GSA with the help of fluorescein.
MATERIALS AND METHODS
Materials
Experiments were performed on 10 healthy, 4-5 month old New Zealand White rabbits (2.0kg-2.5kg, male) at the Institute of the Sixth People's Hospital Affiliated to Shanghai Jiaotong University and conformed to the Animals in Ophthalmic and Vision Research Statement. The rabbits underwent a complete ophthalmologic examination to exclude any ocular abnormalities. Both eyes of 10 rabbits (20 eyes) were subjected to a GSA (Ziemer Group, Port, Switzerland) corneal thickness measurement in a dark room. Rabbits underwent general anesthesia by intravenous sodium pentobarbital (30mg/kg) before examination. The eyelids were held open by a technician.
Imaging Capture
The GSA measurements were obtained following the manufacturer's instructions. After general anesthesia, the rabbits were put on a comfortable desk, height at chin rest, in order to keep the examined eye in the proper position and distance. Since the rabbits could not maintain stable fixation on the target (red spot), the technician kept the rabbit eyes focusing toward a right angle position by seeing the live image on the screen monitor. The device was brought in focus by aligning the scanning head. When the red-cross passed through the white spots and the single red line touched the external corneal surface, alignment was considered good. The technician was asked to make the rabbit's eye blink once, open the eye wide and keep the eye stable for 5s until the scanning was finished.
According to the online Grewal report, the Scheimpflug camera could not routinely detect pre-corneal tear film thickness[18]. With the instillation of fluorescein, the tear film, with enhanced reflective optical density of different values due to different volumes of fluorescein, can be visualized by the Scheimpflug system (Figures 1, 2). As a result, the differential values of corneal thickness, with and without fluorescein, are identified as the pachymetry values of tear film. At the beginning, we used 1 drop (about 25µL) 0.1% fluorescein to make the tear film visible but the corneal thickness could not be automatically calculated by the software (Figure 1C). One possible reason was that the reflective optical density was so high that it made the posterior corneal boundary unclear to delineate the corneal outline and calculate the corneal thickness. Eventually, we used 1µL as the correct dosage.
Figure 1. Scheimpflug images delineate corneal thickness outline.
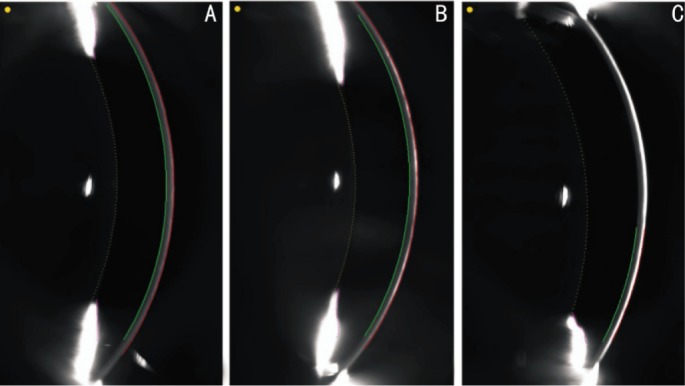
A: Without fluorescein instillation; B: With 1µL 0.1% fluorescein; C: With 1 drop 0.1% fluorescein instillation. The software automatically measures the corneal thickness values between the red and green lines.
Figure 2. Scheimpflug cross-sectional images of rabbit cornea.
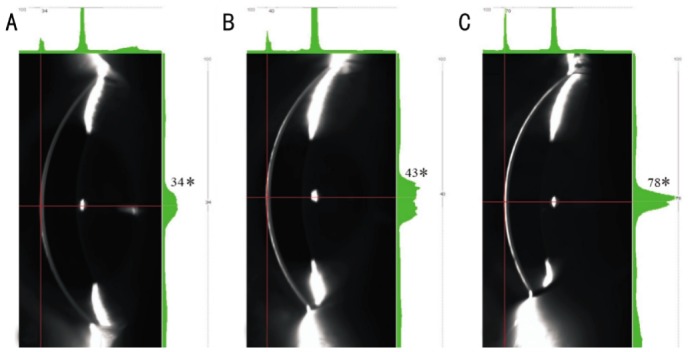
A: Without fluorescein instillation; B: With 1µL 0.1% fluorescein; C: With 1 drop 0.1% fluorescein instillation. The green histograms on the right side indicate different reflective optical intensity (number with asterisk) of tear film pre- and post-fluorescein instillation with different volumes.
Every eye was scanned once to get a baseline corneal pachymetry map. According to the Zhuang et al's[19] study, 1µL 0.1% fluorescein in balanced saline was instilled into the inferior cul-de-sac with a micropipette. The Scheimpflug images were taken every 2s. To avoid interoperator influencing factors, all measurements were performed by the same trained operator.
All the corneal thickness values of central 4-mm diameter were automatically calculated by the Galilei software (Version 5.2.1). The difference between maps was shown by comparing corneal pachymetry with and without fluorescein in each eye (Figure 3). The CCT values of 4-mm diameter, with and without fluorescein, were recorded for analysis.
Figure 3. The software in Galilei shows.
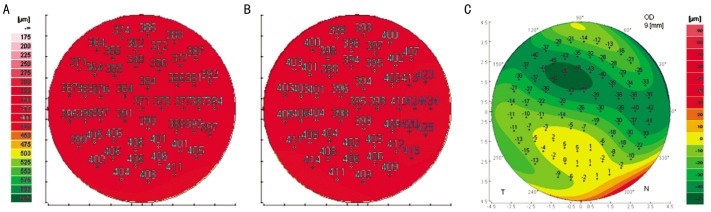
A: Corneal pachymetry map of pre-fluorescein instillation; B: Corneal pachymetry map of post-fluorescein instillation; C: The tear film pachymetric map obtained by substracting map B from map A.
Statistical Analysis
For all statistical analysis, SPSS 13.0 for Windows (SPSS Inc., Chicago IL, USA) was used and a paired t-test was used to analyze the differences between pre- and post-fluorescein instillation. The significance was set at P<0.05.
RESULTS
The CCT pre- and post-instillation was 388.8±9.5µm and 407.0±10.5µm, respectively. After paired t-test analysis, the central pre-corneal tear film thickness of 4mm diameters was 18.2±5.31µm with a 95% confidence interval (15.7, 20.6)µm (P<0.001).
DISCUSSION
Tear film is routinely unevaluated using GSA system. With the aid of fluorescein, it was feasible to calculate the tear film thickness of rabbits. The CCT pre-instillation mean value was close to 385.6±4.47µm found in the Wang and Wu's[20] study on similar age and weight pigment rabbits measured by spectral domain OCT technology. However, the values were less than 407±20µm found in the Chan et al's[21] research on 12 adult New Zealand Albino rabbit eyes performed by ultrasonic pachymetry. This may possibly be due to the age influencing on corneal thickness in both rabbits and humans[22],[23]. Ultrasonic pachymetry was considered as a gold standard tool for corneal thickness measurement, but it is a contact method with necessity of topical anesthetics, which may cause corneal edema resulting in overestimation of the measurements[24]. Moreover, the place of measurement is strongly dependent on the operator's experience[25]. Compared with ultrasonic pachymetry, the GSA can provide comparable and highly repeatable corneal thickness measurements under non-contact condition[16],[17],[26],[27].
According to Cross and Krupin's[28] research, the general anesthesia may produce a depression of basal tear production, which may minimize the measurement values of both tear production and tear film thickness in this study. The central pre-corneal tear film thickness of 4mm diameters was 18.2±5.31µm, which was in the range of 3-40µm reported in previous studies for rabbits and humans[11]-[14]. The different values, compared with previous studies, may be attributed to different measuring technologies and limitations of each method. For example, the classic value of 7µm based on an invasively mechanical and chemical method, 40µm measured by laser interferometry with the difficulty in discerning the definite boundary of tear film, 3µm detected indirectly by optical coherence tomography with the influence of contact lenses[13],[14],[29]. Although the same as Zhuang et al's[19] study method, the value was different from 24.7±3.9µm in his study for humans. There are three possible reasons for the differences: 1) Zhuang's research subjects were human subjects, 2) Zhuang measured the corneal apex whereas we averaged values in the corneal center of the 4-mm diameter, and 3) We used general anesthesia, which may influence the tear secretion and tear film thickness.
It has been demonstrated that there was a relationship between longer examination time and decreased tear film thickness[30]. This correlation may greatly attribute to break-up time and evaporation rate of tear film[31]. Contrary to a dry eye, in a healthy subject with normal lipid layer, the influence of evaporation rate can be negligible in a shorter time[32]. Moreover, Wei et al's[33] study showed that the mean rabbit tear break-up time was approximately 30min using non-invasive method, which is much longer than humans and this time duration is sufficient enough to finish the examination in current study.
Limitations of this study are: 1) a large sample size is a must to more precisely estimate the tear film thickness with fluorescein, 2) 0.1% fluorescein may increase the real tear film thickness, and 3) the fluorescein may cause various degrees of tear secretion, which could also affect the real tear film thickness. We try to avoid the influence of fluoresein with low-dose, but the possibility of a magnification effect cannot be totally prevented even with 0.1% fluorescein. In addition, the inability to stabilize the inner fixation target for rabbits, and general anesthesia may also influence the results of the tear film thickness measurements. However, this technique and results are promising for dry eye animal model research in the future.
In summary, GSA may provide useful information about corneal thickness and the tear film thickness for rabbits with the help of fluorescein. Further studies about a dry eye animal model using this method will be helpful to better understand the pathologic and physiologic aspects of pre-corneal tear film.
REFERENCES
- 1.Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78(3):347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Holly FJ. Tear film physiology. Am J Optom Physiol Opt. 1980;57(4):252–257. doi: 10.1097/00006324-198004000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146(3):350–356. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res. 2004;23(4):449–474. doi: 10.1016/j.preteyeres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Montes-Mico R, Alio JL, Charman WN. Dynamic changes in the tear film in dry eyes. Invest Ophthalmol Vis Sci. 2005;46(5):1615–1619. doi: 10.1167/iovs.05-0017. [DOI] [PubMed] [Google Scholar]
- 6.Begley CG, Caffery B, Nichols K, Mitchell GL, Chalmers R, DREI study group Results of a dry eye questionnaire from optometric practices in North America. Adv Exp Med Biol. 2002;506(Pt B):1009–1016. doi: 10.1007/978-1-4615-0717-8_142. [DOI] [PubMed] [Google Scholar]
- 7.Maissa C, Guillon M. Tear film dynamics and lipid layer characteristics-effect of age and gender. Cont Lens Anterior Eye. 2010;33(4):176–182. doi: 10.1016/j.clae.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Ozdemir M, Temizdemir H. Age- and gender-related tear function changes in normal population. Eye(Lond) 2010;24(1):79–83. doi: 10.1038/eye.2009.21. [DOI] [PubMed] [Google Scholar]
- 9.Burgalassi S, Panichi L, Chetoni P, Saettone MF, Boldrini E. Development of a simple dry eye model in the albino rabbit and evaluation of some tear substitutes. Ophthalmic Res. 1999;31(3):229–235. doi: 10.1159/000055537. [DOI] [PubMed] [Google Scholar]
- 10.Xiong C, Chen D, Liu J, Liu B, Li N, Zhou Y, Liang X, Ma P, Ye C, Ge J, Wang Z. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. 2008;49(5):1850–1856. doi: 10.1167/iovs.07-0720. [DOI] [PubMed] [Google Scholar]
- 11.King-Smith PE, Fink BA, Fogt N, Nichols KK, Hill RM, Wilson GS. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. 2000;41(11):3348–3359. [PubMed] [Google Scholar]
- 12.Palakuru JR, Wang J, Aquavella JV. Effect of blinking on tear dynamics. Invest Ophthalmol Vis Sci. 2007;48(7):3032–3037. doi: 10.1167/iovs.06-1507. [DOI] [PubMed] [Google Scholar]
- 13.Prydal JI, Artal P, Woon H, Campbell FW. Study of human precorneal tear film thickness and structure using laser interferometry. Invest Ophthalmol Vis Sci. 1992;33(6):2006–2011. [PubMed] [Google Scholar]
- 14.Wang J, Fonn D, Simpson TL, Jones L. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Invest Ophthalmol Vis Sci. 2003;44(6):2524–2528. doi: 10.1167/iovs.02-0731. [DOI] [PubMed] [Google Scholar]
- 15.Jahadi Hosseini HR, Katbab A, Khalili MR, Abtahi MB. Comparison of corneal thickness measurements using Galilei, HR Pentacam, and ultrasound. Cornea. 2010;29(10):1091–1095. doi: 10.1097/ICO.0b013e3181cf98e5. [DOI] [PubMed] [Google Scholar]
- 16.Yeter V, Sonmez B, Beden U. Comparison of central corneal thickness measurements by Galilei Dual-Scheimpflug analyzer(R) and ultrasound pachymeter in myopic eyes. Ophthalmic Surg Lasers Imaging. 2012;43(2):128–134. doi: 10.3928/15428877-20120102-08. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mohtaseb ZN, Wang L, Weikert MP. Repeatability and comparability of corneal thickness measurements obtained from Dual Scheimpflug Analyzer and from ultrasonic pachymetry. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1855–1860. doi: 10.1007/s00417-013-2280-0. [DOI] [PubMed] [Google Scholar]
- 18.Grewal SPS. Evaluation of anterior segment pathologies using Pentacam. Highlights Ophthalmology. 2008;36:17–20. [Google Scholar]
- 19.Zhuang H, Zhou X, Xu J. A novel method for pachymetry mapping of human precorneal tear film using Pentacam with fluorescein. Invest Ophthalmol Vis Sci. 2010;51(1):156–159. doi: 10.1167/iovs.08-3265. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Wu Q. Normal corneal thickness measurements in pigmented rabbits using spectral-domain anterior segment optical coherence tomography. Vet Ophthalmol. 2013;16(2):130–134. doi: 10.1111/j.1463-5224.2012.01041.x. [DOI] [PubMed] [Google Scholar]
- 21.Chan T, Payor S, Holden BA. Corneal thickness profiles in rabbits using an ultrasonic pachometer. Invest Ophthalmol Vis Sci. 1983;24(10):1408–1410. [PubMed] [Google Scholar]
- 22.Riau AK, Tan NY, Angunawela RI, Htoon HM, Chaurasia SS, Mehta JS. Reproducibility and age-related changes of ocular parametric measurements in rabbits. BMC Vet Res. 2012;8:138. doi: 10.1186/1746-6148-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Wu Q. Investigation of the human anterior segment in normal Chinese subjects using a dual Scheimpflug analyzer. Ophthalmology. 2013;120(4):703–708. doi: 10.1016/j.ophtha.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mezaine HS, Al-Amro SA, Kangave D, Sadaawy A, Wehaib TA, Al-Obeidan S. Comparison between central corneal thickness measurements by oculus pentacam and ultrasonic pachymetry. Int Ophthalmol. 2008;28(5):333–338. doi: 10.1007/s10792-007-9143-9. [DOI] [PubMed] [Google Scholar]
- 25.Ho T, Cheng AC, Rao SK, Lau S, Leung CK, Lam DS. Central corneal thickness measurements using Orbscan II, Visante, ultrasound, and Pentacam pachymetry after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2007;33(7):1177–1182. doi: 10.1016/j.jcrs.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Amano S, Honda N, Amano Y, Yamagami S, Miyai T, Samejima T, Ogata M, Miyata K. Comparison of central corneal thickness measurements by rotating Scheimpflug camera, ultrasonic pachymetry, and scanning-slit corneal topography. Ophthalmology. 2006;113(6):937–941. doi: 10.1016/j.ophtha.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 27.Ladi JS, Shah NA. Comparison of central corneal thickness measurements with the Galilei dual Scheimpflug analyzer and ultrasound pachymetry. Indian J Ophthalmol. 2010;58(5):385–388. doi: 10.4103/0301-4738.67045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross DA, Krupin T. Implications of the effects of general anesthesia on basal tear production. Anesth Analg. 1977;56(1):35–37. doi: 10.1213/00000539-197701000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Mishima S. Some physiological aspects of the precorneal tear film. Arch Ophthalmol. 1965;73:233–241. doi: 10.1001/archopht.1965.00970030235017. [DOI] [PubMed] [Google Scholar]
- 30.Nichols JJ, Mitchell GL, King-Smith PE. Thinning rate of the precorneal and prelens tear films. Invest Ophthalmol Vis Sci. 2005;46(7):2353–2361. doi: 10.1167/iovs.05-0094. [DOI] [PubMed] [Google Scholar]
- 31.King-Smith PE, Nichols JJ, Nichols KK, Fink BA, Braun RJ. Contributions of evaporation and other mechanisms to tear film thinning and break-up. Optom Vis Sci. 2008;85(8):623–630. doi: 10.1097/OPX.0b013e318181ae60. [DOI] [PubMed] [Google Scholar]
- 32.Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74(1):8–13. doi: 10.1097/00006324-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Wei XE, Markoulli M, Zhao Z, Willcox MD. Tear film break-up time in rabbits. Clin Exp Optom. 2013;96(1):70–75. doi: 10.1111/j.1444-0938.2012.00801.x. [DOI] [PubMed] [Google Scholar]


