Abstract
AIM
To assess the quantitative association between anisometropia magnitude and the losses of resolution and contrast sensitivity; and to exemplify how the function of fusion and stereopsis vary with anisometropia magnitude (AM) in previously untreated anisometropic amblyopes.
METHODS
A total of 57 patients with previously untreated anisometropic amblyopia without strabismus (range: 8-35 years), were measured refractive error, best corrected visual acuity (BCVA), fusion and stereopsis, and 48 patients have completed contrast sensitivity function test. AM was determined by dioptric vector addition model, and the amblyopia depth was determined by the difference of BCVA in logMAR units between the amblyopic and fellow eyes.
RESULTS
AM was significantly correlated with both amblyopia depth (Pearson R=0.728, P<0.001) and the inter-ocular difference of the area under the log contrast sensitivity function (AULCSF) (R=0.505, P<0.001). Depth of amblyopia and the inter-ocular difference of AULCSF was also significantly correlated (R=0.761, P<0.001). The more severity of amblyopia, the poorer levels of contrast sensitivity. Most pure anisometropes with AM was less than 3.0D retain fusion and some stereopsis, but when AM were more than 3.0D, especially for the anisometropes whose AM was more than 6.0D, fusion and stereopsis function were seriously impaired.
CONCLUSION
In the patients with previously untreated anisometropia amblyopia, higher degree of anisometropia is significantly associated with deeper amblyopia, worse contrast sensitivity, fusion and stereopsis functions.
Keywords: anisometropia, amblyopia, area under the log contrast senility function, fusion, stereopsis
INTRODUCTION
Amblyopia, a development visual disorder of spatial vision and binocular function, is always associated with anisometropia, strabismus and both in early childhood[1]. It is customary recognized that anisometropia can cause amblyopia, but the exact mechanism remains unclear. According to Von Noorden and Ciruffreda, anisometropia can result in the defocus of retinal image in one eye, including reduction of retinal image size, contrast and clarity, the span of the defocus spans from occasional to permanent time periods[1],[2]. So for a patient, the signal from one anisometropic eye, which can be sent to the brain to be analyzed, cannot be harmonized with that from the other anisometropic eye. There may be active suppression of the retina fovea to overcome the sensory interaction between the amblyopic eye and non-amblyopic eye. It was usually suggested that the defocus image and active suppression of anisometropia may lead to amblyopia[1],[2].
However, the amblyopia caused by bilateral defocus is milder than that in anisometropia, revealing that the dissimilarity of the visual signals from the eyes, rather than defocus alone, causes the anisometropic amblyopia[3]. Thus, the magnitude of anisometropia was thought to be the most important factor in determining the prevalence and severity of anisometropic amblyopia[3]-[8]. Conversely, in previously untreated anisometropic amblyopia, the quantitative relationships between the magnitude of anisometropia and vision deficits have not been fully discussed. Although Levi et al[3] have analyzed the relationship between the degree of anisometropia and visual function defects, they did not exclude patients who have received amblyopic treatment. Consequently, their results may be influenced by the factor of amblyopic treatment.
Previous studies have demonstrated that anisometropia can be a powerful amblyogenic factor, due to either the decreased resolution by optical defocus at the fovea, or the production of active suppression. This cross-sectional study was therefore set out to assess quantitative association between the degree of anisometropia and the losses of resolution and contrast sensitivity; and to exemplify how the function of fusion and stereopsis vary with anisometropia magnitude in previously untreated anisometropic amblyopias.
SUBJECTS AND METHODS
Subjects
Between January 2011 and October 2011, 57 patients (25 females and 32 males in the age range of 8-35 years, with a mean of 16.73 years) participated in this study. In the present study, anisometropia is defined as the difference in refractive error between the eyes of one or more diopter at the most anisometropia meridians, and the amblyopia was taken to be the difference in best corrected visual acuity (BCVA) of two or more lines on the ETDRS logMAR Test between the eyes. Orthoptic findings and ocular examinations were double checked, and only those subjects in whom no disparity was found (<4 prism), were included. Exclusion criteria were other ocular pathology, previous ocular surgery, and previous ocular trauma. Patients with history of spectacle correction, occlusion or penalization therapy were also excluded from this study.
The research followed the tenets of the Declaration of Helsinki and was approved by the Sciences Ethics Committee of Zhejiang University of China; written consent was obtained from each participant or their guardians after the nature and target results of this research were explained.
Methods
All the cases were referred to a detailed history taking, meticulous slit lamp and fundus examination by direct ophthalmoscopy. Eye alignment was assessed by cover-uncover test at a distance, followed by the same procedure closer to the eye (40cm). Microtropia was excluded using a 4 prism base out test. Distance BCVA was assessed at 4m with ETDRS logMAR Number Charts (Precision vision, Inc. La Salle, IL, USA), cycloplegic refraction (0.5% tropicamide and 0.5% phenylephrine) was performed on all the subjects.
Refractive correction was prescribed by Dr. Song, and applied throughout. Based on the cycloplegic refraction, myopia and astigmatism were fully corrected, and hyperopia was either completely corrected or symmetrically under corrected by no more than 1.50D. Fusion function (Worth 4 dot test at 6m), stereopsis (Lang stereo acuity test at 40cm, based on pantographic presentation of a random-dot pattern), ocular dominant test (hole in hand), and contrast sensitivity function were performed on another day. Contrast sensitivity function was evaluated monocularly with best spectacle correction at five spatial frequencies: 1.5, 3, 6, 12, and 18c/d (cycle per degree) with functional test system OPTECR 6500P (Stereo Optical Co., Inc., Chicago, IL, USA). Only 48/57 of the anisometropic amblyopes have completed the test of contrast sensitivity function, and all the amblyopes have completed the other tests.
The depth of amblyopia is valued to the difference in the initial BCVA in logMAR (logarithm of the minimal angle of resolution) units. The most anisometropia meridian model (MAM), and vector blur anisometropia model were the most commonly used ways to specify the degree of anisometropia[3],[5],[6],[9],[10]. According the results of Levi, the results and conclusions based on these two different methods of specifying anisometropia were similar, but that vector blur anisometropia is about 0.5D less than MAM. The MAM model choose the maximum anisometropia, Whilst the vector blur model specifies spherical and cylindrical refractive errors as a single value using a dioptric vector addition model, computing vector length of blur as: vector length=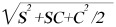 [3],[10]. Where S stands for the spherical refractive error and C represents the cylindrical refractive error. This model was recommended by Rassch to predict visual acuity, and here the analysis is based on the vector blur anisometropia model.
[3],[10]. Where S stands for the spherical refractive error and C represents the cylindrical refractive error. This model was recommended by Rassch to predict visual acuity, and here the analysis is based on the vector blur anisometropia model.
Contrast sensitivity was evaluated monocularly at five spatial frequencies: 1.5, 3, 6, 12, 18c/d with OPTECR 6500, the contrast level of the last correct response was determined as the contrast threshold in logarithmic values for each frequency. From these data, the area under the log contrast sensitivity function (AULCSF) was determined according to the method of Applegate and Gansel[11]. The AULCSF was calculated as the integration of the third-order polynomials fitted function of the log contrast sensitivity units between the fixed limits of log spatial frequency of 0.176 (corresponding to 1.5c/d) and 1.255 (18c/d) on the log spatial frequency scale. This can translate contrast sensitivity data into a single quantity, which can characterize the contrast sensitivity function, representing the function of spatial vision comprehensively.
Statistical Analysis
Pearson correlation coefficients were used to determine whether relationships existed between anisometropia depth, amblyopia magnitude and difference of AULCSF between the eyes. Statistical significance was defined as P<0.05. A Chi-square test and Pearson correlation analysis were conducted with SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA), and graphical analysis was performed using Origin 8.0 (Origin Lab Corp., Northampton, MA, USA).
RESULTS
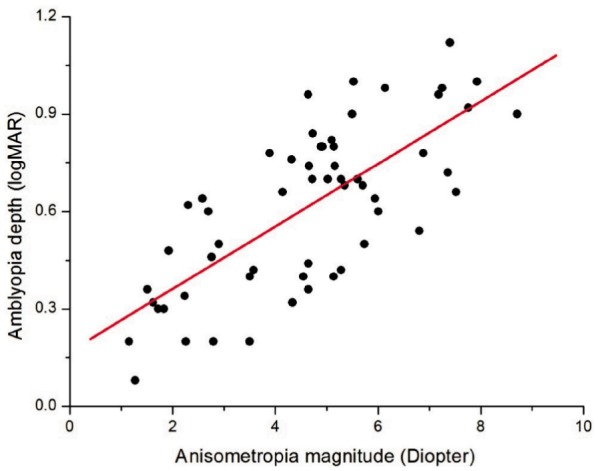
The depth of amblyopia as measured by the difference in logMAR units between the amblyopic eye and fellow eye is significantly related to the anisometropia magnitude as calculated by vector blur anisometropia model (Figure 1). The regression line in the Figure 1 further demonstrated that there was quantitative relationships between magnitude of anisometropia and amblyopia depth (Slope=0.10, Intercept=0.17), with Pearson Correlation Coefficient being 0.728 (P<0.001).
Figure 1. Anisometropia magnitude and amblyopia depth.
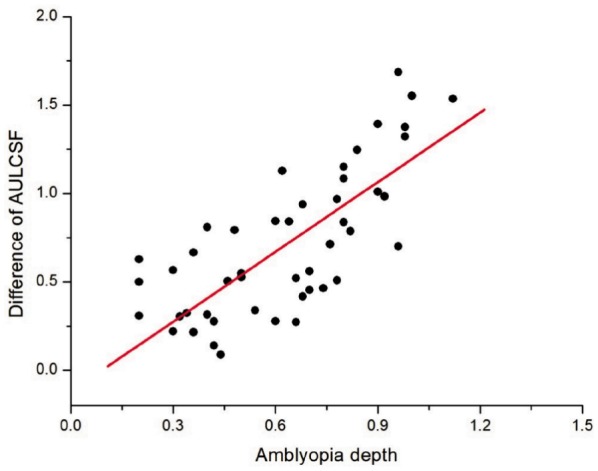
The difference of AULCSF between the amblyopic eyes and fellow eye became progressively greater as the depth of amblyopia increases (Figure 2). There was a strong relationship between the depth of amblyopia and the difference of AULCSF (Slope=1.32, Intercept=-0.12), with Pearson Correlation Coefficient being 0.761 (P<0.001).
Figure 2. Amblyopia depth and difference of AULCSF.
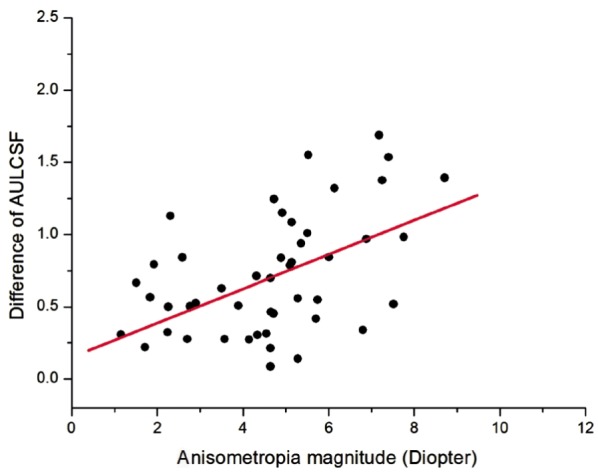
A quantitative assessment showed that there was also a significant correlation between the degree of anisometropia and the difference of AULCSF between the eyes (Slope=0.12, Intercept=0.15), with Pearson Correlation Coefficient being 0.505 (P<0.001, Figure 3).
Figure 3. Anisometropia magnitude and difference of AULCSF.
Binocular functions, including fusion and stereopsis function, decreased remarkably as the level of anisometropia magnitude increased (Table 1). We found that larger degrees of anisometropia resulted in suppression and subnormal stereopsis, especially when the anisometropia magnitude was larger than 3.0D. Furthermore, it had also been documented that when the degree of anisometropia was more than 6.0D, only 16.67% amblyopes had fusion function and about 8.33% persons had stereopsis function. The suppression and stereopsis function will change significantly as the degree of anisometropia varies (χ2=16.29, P=0.770; χ2=20.48, P<0.01).
Table 1. Anisometropia magnitude and binocular function.
| Anisometropia magnitude (AM) | 1.0D<AM<3.0D | 3.0D<AM<6.0D | AM>6.0D | χ2 | P |
| Suppression (worth 4 dot test at 6m) | |||||
| 1- | 14 (93.33) | 15 (50) | 2 (16.67) | ||
| 2+ | 1 (6.67) | 15 (50) | 10 (83.33) | 16.29 | <0.01 |
| Total | 15 (100) | 30 (100) | 12 (100) | ||
| Stereopsis (Lang stereo test at 40cm) | |||||
| Better than 1200” | 12 (80) | 6 (20) | 1 (8.33) | ||
| Poorer than 1200” | 3 (20) | 24 (80) | 11 (91.67) | 20.48 | <0.01 |
| Total | 15 (100) | 30 (100) | 12 (100) |
1-:No suppression; 2+:Suppression.
n (%)
DISCUSSION
There is little doubt that anisometropia is a cause of amblyopia and thought to be due to retinal defocus in the non-preferred eye or the production of active suppression[12]-[14]. Because of the fact that a significant proportion of children with anisometropia lack of noticeable physical abnormalities in childhood, people with anisometropic amblyopia usually are detected later than other types of amblyopia, such as strabismic amblyopia, and for this reason, numerous patients were absent of treatment during the critical period. Therefore, analysis of the pathogenesis of anisometropic amblyopia was especially important. Significant anisometropia present from an early age will persist, so by analyzing the relationship between the anisometropia magnitude and visual function in previously untreated anisometropic amblyopia adolescent patients, we can easily concluded how anisometropia magnitude influenced the amblyopia depth and visual function[15].
The results of our study, in agreement with previous studies, suggest that larger anisometropia magnitude generally can cause more severe amblyopia and lower levels of contrast sensitivity, fusion and stereopsis function[3]-[7]. Anisometropia magnitude was significantly correlated with both amblyopia depth (Pearson R=0.728, P<0.001) and the inter-ocular difference of AULCSF (R=0.505, P<0.001). Which can be also concluded from our results the depth of amblyopia and the deficit of contrast sensitivity function have significantly correlation (R=0.761, P<0.001), the more severe amblyopia, the poorer levels of contrast sensitivity.
The main finding of this study is that the depth of amblyopia is dependent on the degree of anisometropia magnitude, when the anisometropia reached 1.0D, the average difference of visual acuity between eyes reached 0.27logMAR; and when anisometropia magnitude increases 1.0D, the average difference of visual acuity between eyes would increase by approximately 0.1logMAR. Although we do not know to what extent, retinal unilateral defocus can lead to amblyopia in early development, but, we can concluded that the distance of retinal defocus between eyes can predict the degree of severity of amblyopia in previously untreated anisometropic amblyopia.
It has been suggested that the contrast sensitivity function (CSF), which depicts how visual sensitivity varies with spatial frequency, is better than letter acuity in representing. So in this study, we chose contrast sensitivity function to describe the amblyopia's spatial vision deficits. AULCSF, as the area under the log contrast sensitivity function, translated the contrast sensitivity data into a single quantity used to represent the overall contrast sensitivity function. So we can assess quantitative association between anisometropia magnitude and the difference of contrast sensitivity function with AULCSF. The result revealed that the difference of AULCSF between the amblyopic eye and fellow eye becomes progressively greater as the depth of amblyopia increased. The greater was anisometropia, the poorer was spatial vision.
Contrast encoding of primate visual system is mediated by two major processing pathways: the Magnocellular pathway (MC) and the Parvocellular (PC) pathways[16]. The MC stream has high contrast received and reaction at relatively low levels of contrast; whereas the PC stream has lower contrast gain and response at relatively high levels of contrast[17],[18]. The MC stream is thought to be in charge of detection and discrimination that is briefly presented, and the PC stream is thought to be responsible for high spatial vision and chromatic and achromatic processing[16],[19]. In anisometropic amblyopia, contrast sensitivity is traditionally discovered to be deficit at all spatial frequencies, suggesting there may be anomalous processing of MC and PC pathways in the higher visual areas, including the orientation- and spatial frequency-selective cells. In our study, the anisometropia magnitude was proved significantly correlated with AULCSF, suggesting that the retinal defocus caused by the large anisometropia may influence the MC and PC pathways, and decrease the sensitivity of orientation- and spatial frequency-selective cells of the amblyopic eye. Continual retinal defocus may reduce the number of the orientation- and spatial frequency-selective cells, and finally leading to anisometropic amblyopia.
The results of our study also demonstrated that larger degrees of anisometropia can generally induce poor fusion and subnormal stereopsis. When anisometropia magnitude (AM) <3.0D, about 93.33% patients have fusion function with worth 4 dot test, and 80% patients retain stereopsis (smaller than 1 200 sec arc) with Lang stereo acuity test. And when 3.0D<AM<6.0D, about 50% patients retain fusion, and 80% patients have absence of stereopsis function (larger than 1200 sec arc). At last, when AM>6.0D, about 83.33% the patients have absence of fusion (active suppression), and almost 91.67% have lost the function of stereopsis (larger than 1 200 sec arc). Our data support the fact that, binocular functions are relatively normally developed in low anisometropes (AM<3.0D), but when AM>3.0D, especially for the anisometropes whose AM is larger than 6.0D, fusion and stereopsis function are seriously influenced. The suppression and stereopsis function will change significantly as the degree of anisometropia varies (χ2=16.29, P=0.770; χ2=20.48, P<0.01) (Table 1).
This result may agree with experimentally induced optically unilateral anisometropia, but not consistent with several previous studies of real monocular and binocular amblyopia, especially for the patients with anisometropia magnitude more than 3.0D[20],[21]. According to the research of experimentally induced optically unilateral anisometropia, including myopic, hyperopic, and astigmatism, 1D anisometropia can reduce stereo acuity from 40 sec arc to an average of 85 sec arc, and 3D of anisometropia can produce remarkably reduction in stereo acuity for all subjects[22]. But previous studies of real monocular and binocular amblyopia, Rutstein and Corliss[20], Levi et al[3] reported that patients with anisometropic amblyopia tended to react differently and have better binocular function than predicted from studies in which experimental anisometropia is caused by optics in patients with normal binocular vision. But, our results implied that the fusion (26/57) and stereopsis function (38/57) of anisometropic amblyopes are often impaired, the incidence and the extent of damage varies with the anisometropia magnitude closely.
The different results may be caused by the difference of inclusion criteria. Our anisometropic amblyopes have not been treated previously, and the patients' age ranged 8-35 years, and Levi's study has not excluded the patients who have received patching, spectacle correction or penalization therapy. We suspected that the treatment of amblyopia in early childhood may play a critical role in the development and reconstruction of binocular vision, so Levi's anisometropic amblyopes' binocular vision conditions would be better. In addition, the development of binocular vision in anisometropes may be later than other visual function, and the sensitive period would be longer[23],[24]. So the anisometropes of Rutstein' study would have relatively better binocular vision, which may be caused for the inclusion of patients (age <8 years) who may be still in the critical period of the development of fusion and stereopsis function[25]. So they cannot completely reflect the influence of impaired binocular function caused by anisometropia. So only in the previously untreated anisometropic amblyopia, we can comprehensively analyze the influence of visual function caused by anisometropia magnitude.
In summary, in the patients with previously untreated anisometropia amblyopia, higher degree of anisometropia is significantly associated with deeper amblyopia, worse contrast sensitivity, fusion and stereopsis functions.
Footnotes
Foundation item: Zhejiang Province Science Foundation of Health Bureau of China (No.2012KYA102)
REFERENCES
- 1.Ciuffreda KJ, Levi DM, Selenow A. Boston: Butterworth-Heinemann; 1991. Amblyopia: basic and clinical aspects. [Google Scholar]
- 2.Von Noorden GK, Campos EC. Binocular vision and ocular motility: theory and management of strabismus. In: Gunter K, editor. 6th ed. St. Louis: CV Mosby; 2002. [Google Scholar]
- 3.Levi DM, McKee SP, Movshon JA. Visual deficits in anisometropia. Vision Res. 2011;51(1):48–57. doi: 10.1016/j.visres.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanlamai T, Goss DA. Prevalence of monocular amblyopia among anisometropes. Am J Optom Physiol Opt. 1979;56(11):704–715. doi: 10.1097/00006324-197911000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Weakley DR. The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108(1):163–171. doi: 10.1016/s0161-6420(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 6.Cobb CJ, Russell K, Cox A, MacEwen CJ. Factors influencing visual outcome in anisometropic amblyopes. Br J Ophthalmol. 2002;86(11):1278–1281. doi: 10.1136/bjo.86.11.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson V, Miller JM, Clifford-Donaldson CE, Harvey EM. Associations between anisometropia, amblyopia, and reduced stereoacuity in a school-aged population with a high prevalence of astigmatism. Invest Ophthalmol Vis Sci. 2008;49(10):4427–4436. doi: 10.1167/iovs.08-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying GS, Huang J, Maguire MG, Quinn G, Kulp MT, Ciner E, Cyert L, Orel-Bixler D, Vision in Preschoolers Study Group Associations of anisometropia with unilateral amblyopia, interocular acuity difference, and stereoacuity in preschoolers. Ophthalmology. 2013;120(3):495–503. doi: 10.1016/j.ophtha.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jampolsky A, Flom BC, Weymouth FW, Moses LE. Unequal corrected visual acuity as related to anisometropia. AMA Arch Ophthalmol. 1955;54(6):893–905. doi: 10.1001/archopht.1955.00930020899013. [DOI] [PubMed] [Google Scholar]
- 10.Raasch TW. Spherocylindrical refractive errors and visual acuity. Optom Vis Sci. 1995;72(4):272–275. doi: 10.1097/00006324-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Applegate RA, Gansel KA. The importance of pupil size in optical quality measurements following radial keratotomy. Refract Corneal Surg. 1990;6(1):47–54. [PubMed] [Google Scholar]
- 12.Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1981;21(3):467–476. [PubMed] [Google Scholar]
- 13.Legras R, Hornain V, Monot A, Chateau N. Effect of induced anisometropia on binocular through-focus contrast sensitivity. Optom Vis Sci. 2001;78(7):503–509. doi: 10.1097/00006324-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Simpson T. The suppression effect of simulated anisometropia. Ophthalmic Physiol Opt. 1991;11(4):350–358. [PubMed] [Google Scholar]
- 15.Simons K, Preslan M. Natural history of amblyopia untreated owing to lack of compliance. Br J Ophthalmol. 1999;83(5):582–587. doi: 10.1136/bjo.83.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zele AJ, Pokorny J, Lee DY, Ireland D. Anisometropic amblyopia: spatial contrast sensitivity deficits in inferred magnocellular and parvocellular vision. Invest Ophthalmol Vis Sci. 2007;48(8):3622–3631. doi: 10.1167/iovs.06-1207. [DOI] [PubMed] [Google Scholar]
- 17.Lee BB. Receptive field structure in the primate retina. Vision Res. 1996;36(5):631–644. doi: 10.1016/0042-6989(95)00167-0. [DOI] [PubMed] [Google Scholar]
- 18.Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- 19.Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am A. 1990;7(12):2223–2236. doi: 10.1364/josaa.7.002223. [DOI] [PubMed] [Google Scholar]
- 20.Rutstein RP, Corliss D. Relationship between anisometropia, amblyopia, and binocularity. Optom Vis Sci. 1999;76(4):229–233. doi: 10.1097/00006324-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin RT, Romano PE. Stereoacuity degradation by experimental and real monocular and binocular amblyopia. Invest Ophthalmol Vis Sci. 1985;26(7):917–923. [PubMed] [Google Scholar]
- 22.Brooks SE, Johnson D, Fischer D. Anisometropia and binocularity. Ophthalmology. 1996;103(7):1139–1143. doi: 10.1016/s0161-6420(96)30555-1. [DOI] [PubMed] [Google Scholar]
- 23.Daw NW. Critical periods and amblyopia. Arch Ophthalmol. 1998;116(4):502–505. doi: 10.1001/archopht.116.4.502. [DOI] [PubMed] [Google Scholar]
- 24.Kiorpes L. Critical periods in visual development: implications for amblyopia. J Vis. 2004;4:31. [Google Scholar]
- 25.Williams S, Simpson A, Silva PA. Stereoacuity levels and vision problems in children from 7 to 11 years. Ophthalmic Physiol Opt. 1988;8(4):386–389. doi: 10.1111/j.1475-1313.1988.tb01173.x. [DOI] [PubMed] [Google Scholar]


