Abstract
AIM
To determine the relationship between proliferative diabetic retinopathy (PDRP) and plasma coenzyme Q10(CoQ10) concentration.
METHODS
Patients with type 2 diabetes and PDRP were determined to be the case group (n=50). The control group was consist of healthy individuals (n=50). Plasma CoQ10 and malondialdehyde (MDA) levels were measured in both groups.
RESULTS
Ubiquinone-10 (Coenzyme Q10) levels in PDRP and control subjects are 3.81±1.19µmol/L and 1.91±0.62µmol/L, respectively. Plasma MDA levels in PDRP and control subjects were 8.16±2µmol/L and 3.44±2.08µmol/L, respectively. Ratio of Ubiquinol-10/ubiquinone-10 in PDRP and control subjects were 0.26±0.16 and 1.41±0.68, respectively.
CONCLUSION
The ratio of ubiquinol-10/ubiquinone-10 is found lower in patients with PDRP. High levels of plasma ubiquinol-10/ubiquinone-10 ratio indicate the protective effect on diabetic retinopathy.
Keywords: coenzyme Q10, diabetic, retinopathy
INTRODUCTION
Diabetes mellitus (DM) is related to vascular complications resulting in the increase of the morbidity and mortality of the disease. Diabetic complications include macro-vascular diseases that atherosclerosis accelerates and micro-vascular complications such as retinopathy, nephropathy and neuropathy [1].
Retinopathy is one of the frequently observed complications in diabetic patients. Diabetic retinopathy (DRP) develops 20 years after the onset of diabetes in almost all type 1 diabetic patients while it develops in 60% of type 2 diabetic patients [2]. Such hemodynamic changes as increased blood viscosity, increased erythrocyte aggregation, altered erythrocyte permeability and increased adhesion of erythrocytes to endothelial cells are known to play an important role in the pathogenesis of diabetic retinopathy[3],[4]. In addition, higher levels of glucoses are a contributing factor for DRP inducing apoptosis in vascular endothelial cells[5],[6].
Recent studies have focused on oxidative stress on DRP. Oxidative stress is described as the imbalance between pro-oxidant and antioxidants for pro-oxidants[6],[7]. Low levels of serum antioxidant contribute to oxidative stress development in diabetic patients[8],[9]. Oxidative stress results in the generation of Reactive Oxygen Species (ROS) or prevents ROS elimination[10]. Sufficient parts of antioxidant enzymes are necessary for the extraction of free radicals. Over generation of ROS and the lack of their extraction can damage cell proteins, membrane lipids and nucleic acids[10],[11]. Lipid peroxidation products are generated through oxidation of cell membrane lipids via ROS. Lipid peroxidates are used as a sign of oxidative damage to cells and tissues. Lipid peroxides are indecisive and degrade in a series of complexes to generate reactive carbon compounds, most commonly found is malondialdehyde (MDA). Therefore, levels of MDA are used as a sign of lipid peroxidation[11].
Recent studies suggest various data related to the pathogenesis of DRP: Degradation of retinal mitochondrial biogenesis, a decrease in the number of mitochondria and prevention of transmission of mitochondrial DNA transcription factors are considered to be some of the probable mechanisms of DRP[12]. Mitochondrial biogenesis mechanisms play an important role in the development of DRP by controlling superoxide[12]. Regulation of mitochondrial biogenesis by drugs or some molecules could be a potential strategy to slow down the development of DRP[13],[14].
Coenzyme Q10 (CoQ10), also known as ubiquinone 10, is an important component of mitochondria in all animals. Task of CoQ10 is carrying electron between nicotinamide adenine dinucleotide and succinate dehydrogenases in mitochondrial respiratory chain[15]. CoQ10 commonly used for supplementary treatment of some diseases such as Parkinson's disease and diabetes[16],[17]. CoQ10 exists in two forms: oxidized and reduced forms. Reduced form of CoQ10 is known as ubiquinol-10. Ubiqoinol-10 is an important inhibitor of oxidative damage[18]. Ubiquinol-10 is first defense against oxidative damage of low-density lipoproteins[19]. Therefore, the ubiquinol-10/ubiquinone-10 ratio may be regarded as the marker for detection for oxidative damage[20]. Plasma or serum CoQ10 concentrations are often employed for the assessment of CoQ10 status in humans, as it serves as a useful measure of overall CoQ10 status. Although there are conflicting studies, determine the levels of CoQ10 in patients with DRP would be interesting[21].
In the present study, we investigated the levels of CoQ10, ubiquinol-10/ubiquinone-10 ratio and levels of MDA concentration type 2 diabetic patients.
SUBJECTS AND METHODS
Subjects
A total of 50 patients with type 2 DM on stable statin therapy for ≥4 weeks. Fifty control subjects were included in this study. All patients had type 2 diabetes of over 15-20 years' duration. This study was approved by the Ethics Committee (B. 30. 2. ATA.0.01.00/122).
The patients who had history of smoking, alcohol, hypertension, chronic diseases, coronary heart diseases, liver diseases cancer, end-stage renal failure, a history of coronary or cerebrovascular diseases, use of antioxidant supplements and ocular surgery or a history of intraocular inflammation were excluded. The diagnosis of DM was verified by clinical and laboratory examinations. We selected the proliferative DRP subject by random. After mydriasis with tropicamide eye drops, the ophthalmologic examinations were performed by retina specialists. DRP was graded based on ETDRS grading system[22]. The patients with proliferative DRP group included at least new vessels elsewhere, new vessels disc, fibrous proliferations disc, or fibrous proliferations elsewhere.
Methods
Analyses of ubiquinol-10 and ubiquinone-10 were performed according to Mosca et al[23]. This method is performed by forcing the oxidation of CoQ10 in the plasma sample by treating it with para-benzoquinone fol-lowed by extraction with 1-propanol and direct injection into the HPLC (high-performance liquid chromatography) apparatus. Preoxidation of the sample ensures quantification of total CoQ10 by UV detection. This method achieves a linear detector response for peak area measurements over the concen-tration range of 0.05 to 3.47µmol/L. Diode array analysis of the peak level was consistent with the CoQ10 spectrum. Supplementation of the samples with known amounts of CoQ10 yielded a quantitative recovery of 96% to 98.5%; the method showed a level of quantitation of 1.23nmoL per HPLC injection (200µL of propanol extract containing 33.3µL of plasma). A good correlation was found with a reference elec-trochemical detection method (r=0.99, P<0.0001). Within-run precision showed a coefficient of variation of 1.6 for samples approaching normal values (1.02µmol/L). Day-to-day precision was also close to 2%. The reference values of CoQ10 are 0.7 to 1µg/mL[24].
Analysis of plasma for MDA by HPLC MDA concentrations in the blood plasma sample were measured by HPLC with fluorescent detection (HPLC-FLD), as described previously[25]. Briefly, 50µL of plasma sample was mixed with 0.44mol/L H3PO4 and 42mmol/L tiobarbituric acid (TBA), and incubated for 30min in a boiling water bath. After cooling rapidly on ice, an equal volume of alkaline methanol was added to the sample, shaken vigorously, centrifuged (3 000 r/min for 3min) and the aqueous layer removed. Then, 20µL supernatant was then analysed by HPLC (HP, Agilent 1100 modular systems with FLD detector); column, RP-C18 (5µm, 4.6×150mm) (Eclipse VDB-C18; Agilent); elution, methanol (40:60, v/v) containing 50mmol/L KH2PO4 buffer (pH 6.8); flow rate, 0.8mL/min. Fluorometric detection was performed with excitation at 527nm and emission at 551nm. The peak of the MDA-TBA adduct was calibrated as a 1, 1, 3, 3- tetraethoxypropane standard solution, carried out in exactly the same process as with the plasma sample. HbA1c<7.0% indicated that glycemic control was up to normal standard.
Statistical Analysis
All data were presented as mean±standard deviation (SD). Mann-Witney U test was used to evaluate the significance of differences. Spearman's correlation coefficients were used to calculate the association between the changes of groups.
RESULTS
Fifty diabetic subjects (25 men) were included, with a mean age of 61.86±8.03 years. Twenty-three of control subjects were men, a mean age of control subjects was 62.56±9.01 years. There was no significant difference between control and diabetic subjects in years and sex (P>0.05).
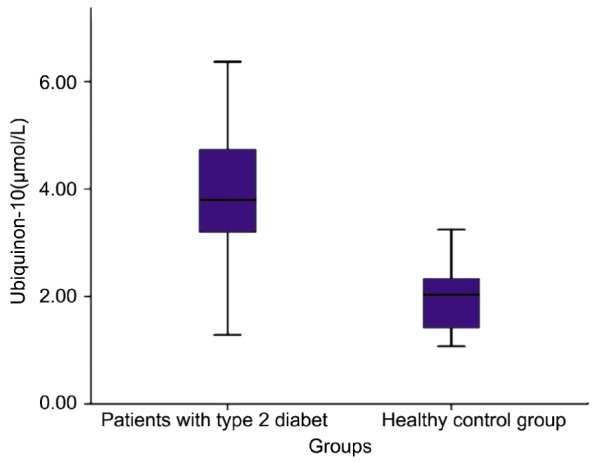
We measured total CoQ10, oxidized CoQ10 (ubiquinone-10), ubiquinol-10/ubiquinone-10 ratio and MDA levels. After measurement of total CoQ10 and oxidized CoQ10, we were calculated reduced CoQ10 (ubiquinol-10) levels and ubiquinol-10/ubiquinone-10 ratio. Ubiquinone-10 levels in PDRP and control subjects were 3.81±1.19µmol/L and 1.91±0.62µmol/L respectively (Table 1). A significant difference was observed between the two groups (Figure 1) (P<0.05). Total CoQ10 levels in patients and control subjects were 4.71±1.23µmol/L and 4.27±1.23 respectively (Table 1). There was no significant difference between the two groups.
Table 1. Ubiquinone-10, MDA levels and Ubiquinol-10/Ubiquinone-10 ratio in patients and control groups.
| Gropus | Patienst groups (mean±SD) n=50 | Control group (mean±SD) n=50 | 95%CID |
P | |
| Lower | Upper | ||||
| Ubiquinone-10 (µmol/L) | 3.81±1.19 | 1.91±0.62 | 1.25 | 2.5 | 0.001 |
| Total coenzyme Q10 (µmol/L) | 4.71±1.23 | 4.27±1.23 | 0.41 | -0.39 | 0.295 |
| Ubiquinol-10/ubiquinone-10 | 0.26±0.16 | 1.41±0.68 | -1.48 | -0.8 | 0.001 |
| MDA (µmol/L) | 8.16±2 | 3.44±2.08 | 0.68 | 3.33 | 0.001 |
95%CID: 95% Confidence interval of the difference.
Figure 1. Levels of ubiquinone-10 in patient and control groups.
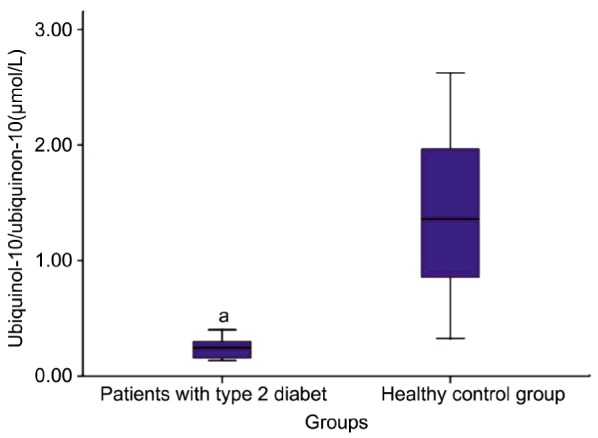
Ubiquinol-10/ubiquinone-10 ratio in proliferative diabetic retinopathy (PDRP) and control subjects were 0.26±0.16 and 1.41±0.68 respectively (Table 1). There was a significant difference between PDRP and control subjects for ubiquinol-10/ubiquinone-10 ratio (Figure 2) (P<0.05).
Figure 2. Ubiquinol-10/Ubiquinone-10 ratio in patient and control groups.
Plasma MDA levels in PDRP and control subjects were 8.16±2µmoL/L and 3.44±2.08µmoL/L respectively (Table 1). There was a significant difference between PDRP and control subjects for plasma MDA levels (Figure 3) (P<0.05). We also confirmed that there was a significant negative correlation between Ubiquinol-10/Ubiquinone-10 ratio and levels of plasma MDA (r=0.555, P<0.001) (Table 2). Median HbA1c was 8.1% (6.9%-10.1%) patients with PDRP. There was also a negative correlation between the levels of HbA1c and ubiquinol-10/ubiquinone-10 ratio (r=0.679, P<0.001).
Figure 3. Levels of MDA in patient and control groups.
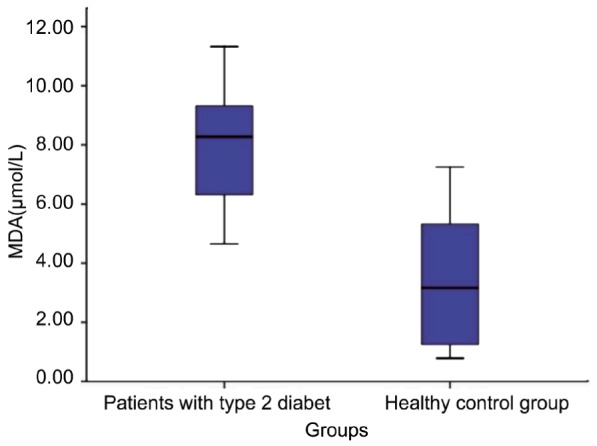
Table 2. Correlation table.
| Coenzyme Q10H/Conezyme Q10 | Conezyme Q10 (µmol/L) | Total Coenzyme Q10 (µmol/L) | ||
| MDA (µmoL/L) | Pearson Correlation (r) | 1-0.555 | 10.557 | 0.150 |
| P | 0.000 | 0.000 | 0.384 | |
| n | 100 | 100 | 100 |
1Correlation is significant at the 0.01 level (2-tailed).
DISCUSSION
Due to degradation of defense mechanisms of antioxidants and the increase in oxygen related radicals, oxidative stress increases in diabetes[26]. Lipid peroxidation in cells and increased free oxygen radicals are thought to play important roles in DM's micro-vascular complications and atherosclerosis development[26],[27]. Antioxidant defence mechanisms in DM are quite contradictory. They are reported to increase in some studies while they decrease in others. High levels of MDA concentrations found in patients with DRP in this study are similar to the findings reported earlier in diabetic patients independent of insulin[28],[29].
Degradation of serum antioxidant may lead to an increase in oxidative stress in diabetic patients. CoQ10 synthesized as endogen in the body is known to play a key role in mitochondrial bioenergetics. Lipophilic CoQ10 is a strong antioxidant and extracts free radicals. The changes in the redox status of CoQ10 may be regarded as a marker of oxidative stress[30]. There were two main findings in our study. First, plasma ubiquinone-10 levels in DRP patients was higher than in control subjects. Second, ubiquinol-10/ubiquinone-10 ratio in DRP patients was lower than in control subjects. The reason of these findings to be extracted may be more than one. One of these reasons can be drugs for the treatment of DM and DRP.
However, some studies have shown that this drugs (for example statins) are not effective on concentration of CoQ10[31]. A difference in dietary intake of CoQ10 could be another important reason of CoQ10 levels in DPR patients. But, only 2%-3% of orally administered CoQ10 is absorbed[32]. In our study, there was not significant difference between of total CoQ10 levels in DRP patients and control groups. This findings showed that total CoQ10 concentration was not affected by dietary difference of individuals or drugs in patient and control groups. However, plasma ubiquinone-10 levels in DRP patients was higher than levels in control subjects. This observation was consistent with existing literature[31],[33]. Conversely, Menke et al[33] in one study measured CoQ10 concentrations in plasma and blood cells and redox conditions in type 1 DM and compared them with healthy children. They found that the level of plasma CoQ10 in children with type 1 DM was higher compared to that of healthy children. This study suggested that specifically in children with poorly controlled diabetes, an increase in antioxidant CoQ10, and intracellular redox capacity and in plasma concentrations may contribute to self-protection of the body during the development of oxidative stress[33].
Lim et al[31] said that profound changes in plasma CoQ10 in individuals with diabetes, suggesting a marked increase in body oxidative burden. They found similar change in prediabetic phase[31]. Similarly, Sourris et al[34] proposed that deficiency of mitochondrial oxidised CoQ10 (ubiquinone) could be a triggering factor for diabetic nephropathy. As a result they suggested that on the condition that CoQ10 supplementation protected mitochondria functions, it could be renoprotective in type 2 diabetes.
This study indicated that plasma MDA, as a marker of oxidative stress, was significantly higher in PDRP patients than that in controls. The level of CoQ10, as antioxidant capacity, was significantly lower in PDRP patients than that in controls. PDRP patients are at increased risk of oxidative stress manifested by increased plasma MDA and decreased CoQ10. We deduced that antioxidant supplementation may be used as adjunctive therapy in patients with PDRP to reduce oxidative stress for diabetic complication protection in type 2 DM.
REFERENCES
- 1.Son SM. Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes Metab J. 2012;36(3):190–198. doi: 10.4093/dmj.2012.36.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Crawford TN, Alfaro DV, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009;5(1):8–13. doi: 10.2174/157339909787314149. [DOI] [PubMed] [Google Scholar]
- 4.Gündüz K, Bakri SJ. Management of proliferative diabetic retinopathy. Compr Ophthalmol Update. 2007;8(5):245–256. [PubMed] [Google Scholar]
- 5.Ishibashi T. Cell biology of intraocular vascular diseases. Nihon Ganka Gakkai Zasshi. 1999;103(12):923–947. [PubMed] [Google Scholar]
- 6.Trudeau K, Molina AJ, Roy S. High glucose induces mitochondrial morphology and metabolic changes in retinal pericytes. Invest Ophthalmol. Vis Sci. 2011;52(12):8657–8664. doi: 10.1167/iovs.11-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Asrar AM. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr J Ophthalmol. 2012;19(1):70–74. doi: 10.4103/0974-9233.92118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7(5):291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- 9.Kasznicki J, Kosmalski M, Sliwinska A, Mrowicka M, Stanczyk M, Majsterek I, Drzewoski J. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep. 2012;39(9):8669–8678. doi: 10.1007/s11033-012-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahnoun Z, Jamoussi K, Zeghal KM. Free radicals and antioxidants: physiology, human pathology and therapeutic aspects (part II) Therapie. 1998;53(4):315–339. [PubMed] [Google Scholar]
- 11.Raffaele M, Donato DP, Chiara V, Angelica C, Alessandra F, Claudio C, Maria Dolores PD, Massimiliano C, Carlo N. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol Vis. 2011;17:1298–1304. [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda M, Muriach M, Roma J, Bosch-Morell F, Genovés JM, Barcia J, Araiz J, Díaz-Llospis M, Romero FJ. Oxidative stress in a model of experimental diabetic retinopathy: the utility of peroxinytrite scavengers. Arch Soc Esp Oftalmol. 2006;81(1):27–32. doi: 10.4321/s0365-66912006000100007. [DOI] [PubMed] [Google Scholar]
- 13.Santos JM, Tewari S, Goldberg AF, Kowluru RA. Mitochondrial biogenesis and the development of diabetic retinopathy. Free Radic Biol Med. 2011;51(10):1849–1860. doi: 10.1016/j.freeradbiomed.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48(8):3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 15.Ostman B, Sjödin A, Michaëlsson K, Byberg L. Coenzyme Q10 supplementation and exercise-induced oxidative stress in humans. Nutrition. 2012;28(4):403–417. doi: 10.1016/j.nut.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Littaru GP, Langsjoen P. Coensyme Q10 and statins: biochemical and clinical implications. Mitochondrion. 2007;7:S168–174. doi: 10.1016/j.mito.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Chinnery P, Majamaa K, Turnbull D, Thorburn D. Treatment for mitochondrial disorders. Cochrane Database Syst Rev. 2006;25(1):CD004426. doi: 10.1002/14651858.CD004426.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Ernster L, Dallner G. Biochemical, physiogical and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;271(1):195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 19.Stocker R, Bowry VW, Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does al-pha-tocopherol. Proc Natl Acad Sci. 1991;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita S, Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal Biochem. 1997;250(1):66–73. doi: 10.1006/abio.1997.2187. [DOI] [PubMed] [Google Scholar]
- 21.Giannubilo SR, Tiano L, Cecchi S, Principi F, Tranquilli AL, Littarru GP. Plasma coenzyme Q10 is increased during gestational diabetes. DiabetesRes Clin Pract. 2011;94(2):230–235. doi: 10.1016/j.diabres.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(5 Suppl):823–833. [PubMed] [Google Scholar]
- 23.Mosca F, Fattorini D, Bompadre S, Littarru GP. Assay of coenzyme Q(10) in plasma by a single dilution step. Anal Biochem. 2002;305(1):49–54. doi: 10.1006/abio.2002.5653. [DOI] [PubMed] [Google Scholar]
- 24.Tomasetti M, Alleva R, Solenghi MD, Littarru GP. Distribution of antioxidants among blood components and lipoproteins: significance of li-pids/CoQ10 ratio as a possible marker of increased risk for atherosclerosis. Biofactors. 1999;9(2–4):231–240. doi: 10.1002/biof.5520090218. [DOI] [PubMed] [Google Scholar]
- 25.Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. Chromatogr B Analyt Technol Biomed Life Sci. 2005;827(1):76–82. doi: 10.1016/j.jchromb.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Soliman GZ. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008;49(2):129–136. [PubMed] [Google Scholar]
- 27.Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26(5):387–392. [PubMed] [Google Scholar]
- 28.Mahboob M, Rahman MF, Grover P. Serum lipid peroxidation and antioxidant enzyme levels in male and female diabetic patients. Singapore Med J. 2005;46(7):322–324. [PubMed] [Google Scholar]
- 29.Palanduz S, Ademoğlu E, Gökkuşu C, Tamer S. Plasma antioxidants and type 2 diabetes mellitus. Res Commun Mol Pathol Pharmacol. 2001;109(5–6):309–318. [PubMed] [Google Scholar]
- 30.El-ghoroury EA, Raslan HM, Badawy EA, El-Saaid GS, Agybi MH, Siam I, Salem SI. Malondialdehyde and coenzyme Q10 in platelets and serum in type 2 diabetes mellitus: correlation with glycemic control. Blood Coagul Fibrinolysis. 2009;20(4):248–251. doi: 10.1097/mbc.0b013e3283254549. [DOI] [PubMed] [Google Scholar]
- 31.Lim SC, Tan HH, Goh SK, Sunramaniam T, Sum CF, Tan IK, Lee BL, Ong CN. (Oxidative burden in prediabtic and diabetic individuals: evidence of plasma Coenzyme Q10. Diabet Med. 2006;23(12):1344–1349. doi: 10.1111/j.1464-5491.2006.01996.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L. Uptake of dietary coenzyme Q supplement is limited in rat. J Nutr. 1995;125(3):446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- 33.Menke T, Niklowitz P, Wiesel T, Andler W. Antioxidant level and redox status of coenzyme Q10 in the plasma and blood cells of children with diabetes mellitus type 1. Pediatr Diabetes. 2008;9(6):540–545. doi: 10.1111/j.1399-5448.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 34.Sourris KC, Harcourt BE, Tang PH, Morley AL, Huynh K, Penfold SA, Coughlan MT, Cooper ME, Nguyen TV, Ritchie RH, Forbes JM. Ubiquinone (coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of type 2 diabetes. Free Radic Biol Med. 2012;52(3):716–723. doi: 10.1016/j.freeradbiomed.2011.11.017. [DOI] [PubMed] [Google Scholar]


