Abstract
IIH is a condition of raised intracranial pressure of unknown pathogenesis, which is most commonly seen in young overweight women. This study was designed to confirm and extend previous reports by our and other groups showing increased inflammatory cytokine expression in patients with IIH. We analyzed the concentrations of 14 cytokines (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-17, IL-22, IL-23, IFNγ, TNFα, TGFβ, and osteopontin) in the serum and cerebrospinal fluid (CSF) of 17 patients with IIH and 53 patients with other neurological conditions. Patients with IIH had highly elevated IL-2, IL-4, IL-10, IL-17 and IFNγ in the CSF compared to patients with multiple sclerosis or non-organic/non-inflammatory neurological conditions. No significant differences were seen between serum cytokine levels in four patient groups (IIH - multiple sclerosis - inflammatory neurological conditions - non-organic/non-inflammatory neurological conditions) and there were no correlations between serum and CSF cytokine levels. In IIH, levels of IL-2, IL-8 and IL-17 were significantly higher in CSF than serum; levels of IL-1β, IL-4, IL-22, IFNγ and TNFα were significantly higher in serum than CSF. For most cytokines, the CSF/serum cytokine ratio was significantly higher than the CSF/serum albumin ratio, indicating intrathecal synthesis of these cytokines in IIH. We conclude that IIH is associated with elevated levels of IL-17 and IL-2 in the CSF, suggesting the involvement of these cytokines in disease pathogenesis.
Keywords: Cerebrospinal fluid, cytokines, idiopathic intracranial hypertension, serum
Background
Idiopathic intracranial hypertension (IIH) is a clinical disorder of unknown etiology, characterized by symptoms and signs of increased intracranial pressure (including headache, transient visual obscurations, papilledema and visual loss), no other abnormalities or impaired level of consciousness, objective elevation of intracranial pressure, and no other demonstrable cause of intracranial hypertension [1]. As yet, the pathogenesis is unknown and treatment is often unsatisfactory [2,3].
Cerebrospinal fluid (CSF) is produced primarily in the choroid plexuses in the ventricles of the brain. From here, it circulates through the ventricular system and passes around the subarachnoid space, from where it is reabsorbed through arachnoid villi and granulations into the venous sinuses and, possibly, through lymphatics as well [4,5].
The leading hypothesis of the defect in IIH is that CSF absorption is impeded at the level of the arachnoid villi [6]. The defects in absorption have been demonstrated by several studies of radioisotopic cisternography, showing delayed clearance of CSF [7,8]. The nature of this blockage is unknown.
The venous sinuses are thought to be a key site for CSF absorption, so that any alteration in their structure or function may interfere with this process. Cerebral venography studies have demonstrated that a majority of IIH patients have stenoses of various degrees in their cerebral venous sinuses, most commonly in the transverse sinus. However, controversy remains as to whether such changes are a cause or a consequence of raised CSF pressure [9,10].
Interestingly, IIH shares many clinical features with venous sinus thrombosis (VST), a clinical condition that needs to be excluded in IIH patients. Some risk factors are indeed common to both IIH and VST, including the presence of pro-thrombotic state [11,12] and obesity [13,14].
Recent studies have examined immunological parameters in the serum and/or CSF of IIH patients. One group has shown elevation of chemokine (C-C motif)-ligand 2 (CCL2) in the CSF of IIH patients compared to controls [15,16], another has shown elevation of CSF leptin and a correlation between CSF IL-6 and waist circumference in IIH patients [17], and a third showed significant elevation of CSF IL-6 levels compared to controls [18].
In studies primarily aimed initially at investigating the pathogenesis of multiple sclerosis (MS), we previously analyzed the levels of four key cytokines, IFNγ, IL-4, IL-10 and IL-17 (focusing on cytokines thought to be secreted by Th1, Th2, T-regulatory and Th17 cells respectively) in patients with IIH, MS, clinically isolated syndrome (CIS) and chronic inflammatory demyelinating polyneuropathy (CIDP). In patients with IIH, we found significantly increased levels of IL-17 in CSF compared to the other neurological conditions [19]. Given the importance of patterns of cytokine secretion in the pathophysiology of T helper responses [20], in the present study, specifically focused on IIH, we analyzed a 244wider range of cytokines in both CSF and serum. The study of a new and larger population of subjects enabled us to validate our previous results and explore central and peripheral cytokine patterns in IIH.
Materials and methods
The study was approved by local ethics committees in the participating sites. Written informed consent was obtained from all patients. Blood and CSF were obtained from patients at Neurology clinics of three separate sites - 1) Nottingham University Hospitals (UK); 2) Sheffield Royal Hallamshire Hospital (UK), and 3) Ulm University Hospital (Germany). All cytokine assays were performed in Nottingham.
Subjects
CSF from 70 subjects was available for analysis; for details, see Table 1.
Table 1.
Details of the 70 subjects included in the CSF analysis
| Diagnosis | No. subjects | Sex | Median age (range) |
|---|---|---|---|
| IIH | 17 | 1M:16F | 34 (21-49) |
| MSa | 40 | 11M:31F | 42.5 (18-70) |
| Other Inflammatoryb | 8 | 5M:3F | 67 (38-72) |
| Functionalc | 5 | 5F | 40 (37-47) |
Multiple sclerosis (MS) group included 7 patients with clinically isolated syndrome (CIS) in remission, 7 patients with PPMS, 12 patients with relapsing remitting MS in remission, 1 patient with progressive relapsing MS in remission, 3 patients with SPMS, in remission, and 10 MS patients in relapse.
The “Other Inflammatory” group included 5 patients with chronic inflammatory demyelinating polyneuropathy (CIDP) and 3 patients with neurosarcoidosis.
Functional - patients with neurologic symptoms for which no organic cause could be found, including non-epileptic attack disorder and fibromyalgia.
Serum from 33 (47%) of the subjects used in the CSF analysis was available for analysis (7 IIH, 22 MS, 4 inflammatory neurological conditions).
Sample processing
Lumbar CSF and serum from peripheral venous blood were collected within 1 hour of each other, between 9 am and 3 pm. CSF was centrifuged at 400 g for 10 min at 10°C. The supernatant was collected and frozen at -80°C until processed. Serum was left to stand at room temperature for 2 h and then centrifuged at 1500 g for 10 min. The supernatant was collected and frozen at -80°C until processed.
Cytokine measurement
The cytokines IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-17, IFNγ and TNFα were measured in CSF and serum using a custom 10-plex assay from BioRad (Hemel Hampstead, UK). Standards were reconstituted in distilled water and serial dilutions were made to provide a standard curve. Samples were diluted 1:3 in sample diluent. Standards and samples were loaded onto a 96-well plate, mixed with cytokine-specific antibody-coated beads and incubated at room temperature for 60 min. The plate was then washed and biotin-conjugated detection antibody was added and incubated for a further 30 min. After washing, streptavidin-PE was added and the plate was incubated for 10 min. After a final wash, the plate was read using a Bioplex system (from BioRad, Hemel Hampstead, UK).
The cytokines IL-22, IL-23, TGFβ and osteopontin (OPN) were assayed using commercial ELISA kits (R&D Systems, Abingdon, UK) according to the manufacturer’s instructions. CSF samples were diluted 1:50 for the OPN assay.
The mean coefficient of variation for the samples assayed using these methods was 6.75%.
Patients’ records were reviewed for diagnosis and other clinical and paraclinical details.
Statistical analysis
Statistical analysis was conducted using “R” (www.r-project.org) and GraphPad Prism (La Jolla, California, USA) software as follows. Groups were combined into “MS” (including clinically definite MS and clinically isolated syndrome [CIS] suggestive of inflammatory demyelination); “other inflammatory” (chronic inflammatory demyelinating polyneuropathy - CIDP - and neurosarcoidosis), non-inflammatory disorders (migraine, non-epileptic attack disorder and fibromyalgia) and “IIH” (diagnosed according to the accepted Friedman-Jacobson diagnostic criteria). Kruskal-Wallis p test was used to compare CSF and serum cytokine levels between the groups. Since 12 cytokines were measured in the CSF, Bonferroni correction was used and the significance was taken as p<(0.05/12) i.e. <0.00417). Mann-Whitney U test was used for further comparisons between two individual groups.
Regression analysis was used to explore the correlation between CSF and serum cytokine levels. The p value taken for statistical significance given multiple comparisons was <0.00417. Mann-Whitney U test was used to compare CSF and serum levels of each cytokine.
In a subgroup of patients in whom both serum and CSF levels of cytokines and serum albumin were available, the CSF/serum albumin index (calculated by dividing CSF albumin by serum albumin concentrations) was compared with the CSF/serum cytokine index (CSF cytokine concentration/serum cytokine concentration) using Wilcoxon matched pairs analysis.
In IIH patients, simple linear regression was used to compare individual CSF cytokine measurements with opening CSF pressure, body mass index (BMI), CSF/serum albumin ratio, CSF protein and CSF cellular composition.
The computer programme “R” (www.r-project.org) was used to perform pairwise Pearson’s correlations between cytokines. Multiple correction analysis was based on an initial significance level of p<0.05 with a total of 12 parameters (12*11/2=66; 0.05/66 giving a significance level set at p<0.00076).
Results
Opening pressure and CSF cellular compositionin IIH patients
Median opening pressure for the IIH patients was 37 cm water (range 24-40), with normal cellular composition of CSF (0-3 white cells, mean 0.9; 0-279 red cells, mean 29).
CSF cytokine levels
Table 2 shows the median values of cytokines detected in the patient groups, measured in pg/ml. Figure 1 shows the distribution of CSF cytokine levels in the patient groups. Statistical corrections were made for multiple comparisons (see Materials and Methods); the statistically significant results are in bold characters in Table 2 and displayed in graphical format in Figure 1.
Table 2.
CSF cytokine concentrations in different neurological conditionsa
| Cytokine | IIH | MS | Other Inflammatory | Functional/Non inflammatory | p value |
|---|---|---|---|---|---|
| IL-1β | 0.13 (0.03-0.54) | 0 (0-0.36) | 0.11 (0-0.51) | 0.06 (0-0.08) | 0.017 |
| IL-2 | 0.89 (0-5.25) | 0 (0-2.35) | 0.65 (0.46-1.98) | 0.54 (0-0.76) | <0.0001 |
| IL-4 | 0.24 (0.17-0.41) | 0.05 (0-0.25) | 0.25 (0.1-0.28) | 0.18 (0.08-0.27) | <0.0001 |
| IL-6 | 3.46 (0.82-10.83) | 2.59 (0.76-31.28) | 5.88 (1.84-14.83) | 2.54 (1.71-4.55) | 0.08 |
| IL-8 | 27.73 (7.16-94.75) | 30.87 (21.14-91.46) | 43.44 (26.33-61.38) | 23.21 (16.92-31.08) | 0.03 |
| IL-10 | 1.25 (0.88-2.07) | 1.10 (0.33-1.61) | 1.63 (1.33-1.71) | 1.01 (0.91-1.23) | 0.0005 |
| IL-12p70 | 2.24 (0-10.67) | 1.11 (0-4.17) | 0.87 (0-4.58) | 0.69 (0-1.7) | 0.0139 |
| IL-17 | 14.96 (10.02-30.27) | 4.86 (0-15.06) | 9.52 (6.14-22.66) | 9.52 (3.35-11.12) | <0.0001 |
| IFNγ | 8.9 (2.34-18.23) | 2.10 (0-17.58) | 9.82 (4.14-16.25) | 2.34 (0-13.2) | 0.0003 |
| TNFα | 2.84 (0-8.53) | 1.14 (0-3.61) | 1.95 (0-8.94) | 0 (0-2.05) | 0.0065 |
| TGFβ | 79.26 (5.88-186.93) | 43.77 (4.29-198.90) | 68.89 (9.87-16.21) | 37.39 (19.44-125.52) | 0.28 |
| OPN | 207.6 (85.13-477.41) | 333.3 (28.51-766.20) | 371.3 (81.86-529.95) | 179.1 (108.44-346.38) | 0.182 |
Concentrations are in pg/ml; “p” denotes p value when groups are compared using Kruskal-Wallis test.
Statistically significant results highlighted in bold text. Range of cytokines values in each group are in parentheses.
Figure 1.
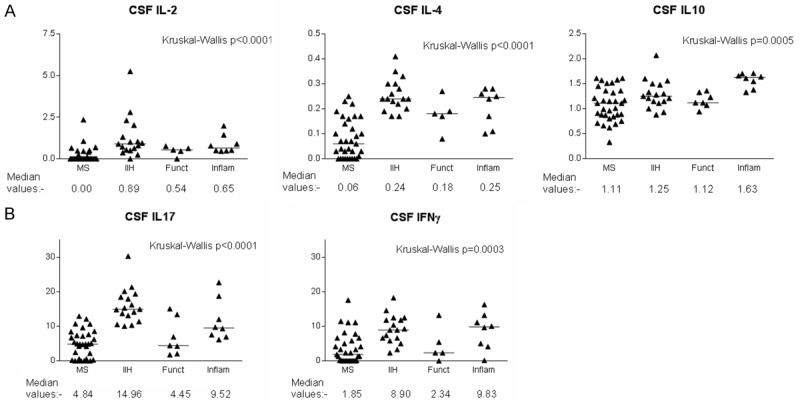
Graphical representation of cytokine levels which were significantly different between the subject groups. Horizontal bars denote median values. Y axes denote cytokine concentrations in pg/ml. “MS” group contains patients in both remission and relapse as well as patients with clinically isolated syndrome.
IL-2 and IL-4 levels were significantly higher in the IIH patients (Mann-Whitney U test; p<0.001 in both cases) and the other inflammatory group (p=0.0006 and 0.0007 respectively) compared to MS patients.
IL-10 was significantly lower in the IIH group than the other inflammatory group (p=0.0043). The CSF IL-10 level in MS patients was significantly lower than in the other inflammatory group (p=0.0003). The latter group had significantly higher levels of IL-10 than the non-inflammatory group (p=0.0006).
Levels of IL-17 were significantly higher in the IIH group than in patients with MS (p<0.0001) and patients with non-inflammatory conditions (p=0.0043). IL-17 levels in the other inflammatory group were also significantly higher than in the MS group (p=0.0029).
IFNγ levels were significantly higher in the IIH group than in the MS group (p<0.0001).
Serum cytokine levels
Details of cytokine levels in the serum of different patient groups are shown in Table 3. After correction for multiple comparisons, there were no statistically significant differences in serum cytokine levels between the patient groups.
Table 3.
Serum cytokine concentrations in different neurological conditionsa
| Cytokine | IIH | MS | Other Inflammatory | p value (K-W) |
|---|---|---|---|---|
| IL-1β | 0.98 (0.05-2.16) | 0.71 (0.02-1.78) | 0.81 (0.63-1.01) | 0.65 |
| IL-2 | 0 | 0 | 0 | U |
| IL-4 | 1.80 (0-4.52) | 1.35 (0-5.18) | 1.88 (1.5-2.38) | 0.40 |
| IL-6 | 4.06 (0-9.45) | 4.14 (0-28.41) | 2.92 (2.23-3.48) | 0.96 |
| IL-8 | 6.12 (3.74-7.28) | 6.77 (3.8-15.67) | 6.78 (6.47-7.09) | 0.76 |
| IL-10 | 1.22 (0.09-3.01) | 1.14 (0-2.69) | 0.82 (0.58-1.24) | 0.59 |
| IL-12p70 | 2.14 (0-4.87) | 2.51 (0-7.18) | 2.84 (0.97-4.5) | 0.52 |
| IL-17 | 0 | 0 | 0 | U |
| IL-22 | 9.92 (0-55.52) | 3.41 (0-18.62) | 272.01 (0-1052) | 0.09 |
| IL-23 | 0 | 0 | 0 | U |
| IFNγ | 87.93 (0-239.66) | 58.45 (0-129.1) | 76.96 (13.2-131.1) | 0.62 |
| TNFα | 17.63 (0-52.54) | 10.84 (0-40.08) | 12.57 (9.22-15.52) | 0.59 |
Concentrations are in pg/ml; range of cytokines values in each group are in parentheses.
KW p denotes Kruskal-Wallis p value for comparisons between groups. U denotes comparison not done due to undetectable cytokine concentrations.
CSF/serum cytokine correlations and differences
No correlations were seen between CSF and serum levels of individual cytokines. There were some significant differences between serum and CSF cytokine levels, with higher levels of IL-1β, IL-4, IL-22 and IFNγ in the serum than the CSF. By contrast, IL-2, IL-8 and IL-17 levels were significantly higher in the CSF than the serum (see Table 4 and Figure 2).
Table 4.
Comparisons between CSF and serum cytokine levels
| Cytokine | CSF/serum correlation r2 | CSF/serum correlation P | MWU CSF vs serum | Location of higher concentration |
|---|---|---|---|---|
| IL-1b | 0.006 | 0.65 | <0.0001 | SERUM |
| IL-2 | U | U | U | CSF |
| IL-4 | 0.00002 | 0.98 | 0.0002 | SERUM |
| IL-6 | 0.021 | 0.41 | 0.55 | - |
| IL-8 | 0.043 | 0.25 | <0.0001 | CSF |
| IL-10 | 0.0008 | 0.88 | 0.15 | - |
| IL-12p70 | 0.093 | 0.08 | 0.44 | - |
| IL-17 | U | U | U | CSF |
| IL-22 | U | U | U | SERUM |
| IL-23 | U | U | U | - |
| IFNγ | 0.006 | 0.67 | <0.0001 | SERUM |
| TNFα | 0.0007 | 0.88 | 0.0003 | SERUM |
CSF/serum correlations: r2 column indicates the r2 value for the CSF and serum values; CSF/serum correlation p value column gives the p value for this correlation. MWU CSF vs serum indicates the Man Whitney U test p value of the comparison of the serum and CSF cytokine concentrations, with the body fluid in which the concentration was higher given in the neighboring column. “U” indicates that statistical analysis could not be done due to undetectable levels in one or more medium.
Figure 2.
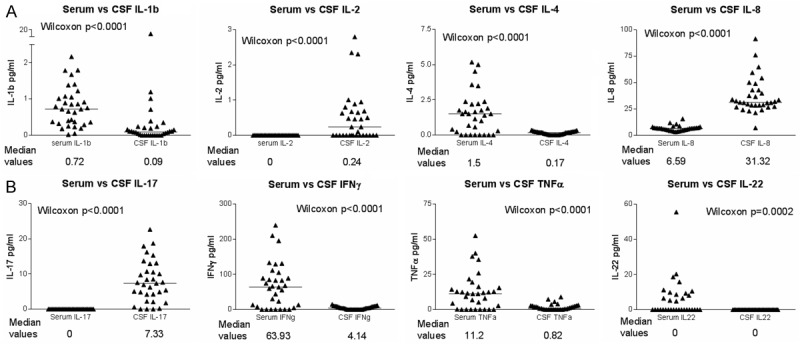
Differences between serum and CSF cytokines; horizontal bars denote median values. Y axes give cytokine concentrations in pg/ml. Comparison of serum/CSF cytokines are paired (i.e. CSF and serum from the same patient). Graph shows all subjects who had both serum and CSF available for analysis (i.e. a mix of diagnostic groups).
CSF/serum albumin and cytokine ratios
In a subgroup of patients, CSF/serum albumin ratios had been measured and were recorded in the medical records. We compared these with the CSF/serum cytokine values when both of these were available (n=19; 27%) and thus assessed the intrathecal cytokine synthesis [21].
CSF/serum albumin ratios did not differ significantly between the patient groups (data not shown). The CSF/serum albumin ratio was lower than the cytokine ratios in all the cytokines where this could be compared, reaching statistical significance with the cytokines IL-1b, IL-4, IL-6, IL-8 and IL-10 (Table 5). It was not possible to calculate ratios of IL-2, IL-17, IL-22 and IL-23 due to the lack of detection in one of either serum or CSF. In a subgroup of the remaining cytokines (1/19 with IL-1b, 6/19 with IL-4, 4/19 with IL-6, 0/19 with IL-8, 1/19 with IL-10, 9/19 with IL-12, 5/19 with IFNγ and 6/19 with TNFα), no serum cytokine was detectable, so CSF/serum cytokine ratio could not be calculated. In each case in Table 5, these individual results have been omitted from the analysis.
Table 5.
CSF/serum ratios for individual cytokines and comparison with the CSF/serum albumin ratio. Bold values denote statistical significance (p<0.05 corrected for 8 comparisons, so p<0.00556)
| Cytokine | CSF/serum ratio | Difference from CSF/serum albumin ratio (overall mean=5.4×10-3) (p value) |
|---|---|---|
| IL-1b | 2.9896 | 0.002 |
| IL-4 | 0.0495 | 0.003 |
| IL-6 | 1.0634 | 0.0006 |
| IL-8 | 5.7019 | 0.0001 |
| IL-10 | 1.3626 | 0.0002 |
| IL-12 | 0.1959 | 0.0751 |
| IFNγ | 0.0758 | 0.00758 |
| TNFα | 0.1311 | 0.00785 |
Differences between the CSF/serum ratios were calculated using Wilcoxon matched pairs test.
Correlation with clinical/paraclinical parameters
In the IIH population, no correlations were seen between individual cytokine measurements and opening pressure, body mass index, CSF/serum albumin ratio, CSF protein or CSF cellular composition (data not shown).
Correlations between CSF cytokine levels
Positive correlations were seen between the following pairs of cytokines (with correlation coefficient and p values shown in parentheses): IL-2 and IL-4 (0.611; 1.94E-08); IL-2 and IL-17 (0.657; 6.28E-10); IL-2 and IFNγ (0.439; 0.0001); IL-2 and TNFα (0.424; 0.0003); IL-4 and IL-17 (0.685; 6.41E-11); IL-8 and OPN (0.602; 0.0002); IL-12p70 and TNFα (0.607; 2.51E-08); IL-17 and IFNγ (0.445; 0.0001); IL-17 and TNFα (0.454; 8.02E-05); (see Figure 3). No correlations were seen between the remaining cytokines.
Figure 3.
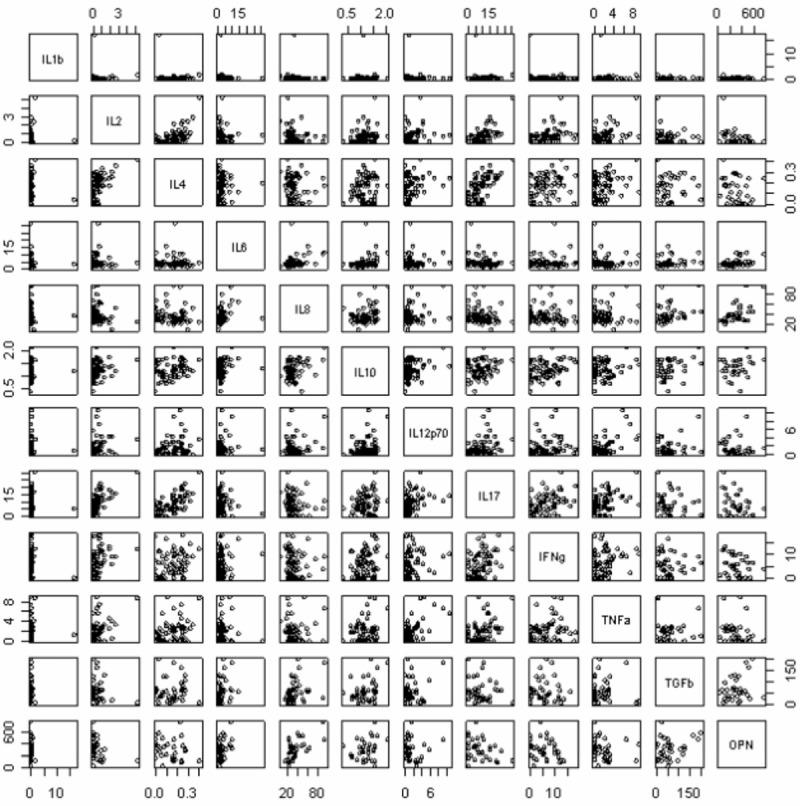
Correlations between pairs of cytokines in the CSF. The computer program “R” was used to perform pair wise correlations between cytokines. The figures show the results of this in graphical form.
Discussion
Our results demonstrate a pro-inflammatory cytokine profile in the CSF of patients with IIH. These findings confirm and expand those of our earlier study [22] which demonstrated a significant upregulation of IL-17 in the CSF of IIH patients compared to patients with CIDP, CIS or MS. The present study has investigated a new and larger group of subjects, a wider range of other neurological conditions and also enabled comparison of serum and CSF cytokine values, allowing us to make inferences on the source of some of the cytokines.
IIH patients had significantly elevated levels of IL-17 and IL-2 compared to the MS, other inflammatory (CIDP and neurosarcoidosis) and functional disorder group. Patients with other inflammatory diseases of the nervous system had higher levels of IL-4, IL-10 and IFNγ than IIH patients and the other two groups. There were also non-significant increases in the levels of IL-1β, IL-12p70, TNFα and TGFβ in IIH patients compared to all other patient groups. In terms of T-helper cell subsets, this may correspond to a significant Th17 (IL-17) and Th1 (IL-2, IFNγ) bias in the IIH patients, with particularly elevated levels of IL-17 and IL-2.
Correlations were also seen between pairs of cytokines in the CSF. IL-12p70, IL-17, IFNγ and TNFα are considered pro-inflammatory cytokines, and their co-expression further supports that they may be stimulated through common pathways.
One explanation for these correlations could be that IIH is a disease with increased production of IL-17, IFNγ and TNFα, and that overproduction of IL-2, IL-4 and IL-10 is a compensatory mechanism [23,24].
Serum was available from just under half of the patients whose CSF was analyzed. This limitation in sample sizes may contribute, at least in part, to the lack of significant differences seen between the groups in terms of their serum cytokine concentration. There were marked differences in concentrations in serum and CSF for several of the cytokines, and the differences between CSF/serum cytokine and CSF/serum albumin ratios (as well as the bidirectional differences in CSF and serum concentrations with different cytokines) argues against simple diffusion between the media [21]. Higher levels of IL-2 and IL-17 in the CSF than in the serum could be explained by selective transport of cytokines or intrathecal synthesis [23]. By contrast, restricted transport could determine lower levels of IL-1β, IL-4, IL-22, IFNγ and TNFα in the CSF than in the serum.
It is well recognized that IIH is strongly associated with obesity, particularly in women of child-bearing age [26,27]. Increasing evidence implicates obesity as a significant contributor to a systemic pro-inflammatory state. Adipose tissue can act as an endocrine organ, secreting cytokines and hormones [28-30]. Obesity has been shown in both mice and humans to be associated with higher levels of IL-17 [31,32]. Weight loss significantly reduces the levels of circulating pro-inflammatory cytokines [33-36] [24] and is an essential component of the treatment for IIH [37-39] In our study, IL-17 was one of the two significantly elevated cytokines in IIH CSF compared to the other groups. We can speculate that adipose-derived cytokines may circulate in the bloodstream and either access the CNS directly or cause local release of cytokines in the CNS [40-42]. The first possibility appears less likely as our data argue against simple diffusion of cytokines from the serum into the CNS. It is difficult to determine, based on our study, whether the increased IL-17 in our IIH patients simply reflect obesity or is directly related to the condition of increased intracranial pressure. Comparing them with a BMI-matched control group without IIH was not possible for ethical reasons related to collecting CSF from healthy volunteers. Nevertheless, the lack of correlation between CSF cytokine and BMI suggests that the increased IL-17 cannot simply be explained on the basis of obesity.
Obesity is a pro-thrombotic state. IIH patients also have pro-thrombotic/anti-fibrinolytic risk factors serologically, and one of the key factors in impaired CSF drainage has been suggested to be microthrombi in the arachnoid villi [11,12,43]. IIH patients who are obese have a higher prevalence of circulating prothrombotic factors than non-obese IIH patients [25]. Pro-inflammatory cytokines such as IL-17 [26,27] and IL-2 [47,48] can mediate thrombosis [49,50]. Therefore, the increased IL-17 could facilitate the formation of microthrombi and reduce CSF drainage. The thrombus itself can stimulate cytokine release [51]. We propose a model in which regardless of whether the inflammatory cytokines are a primary or secondary phenomenon in IIH, their presence can worsen the pathological situation further (Figure 4).
Figure 4.
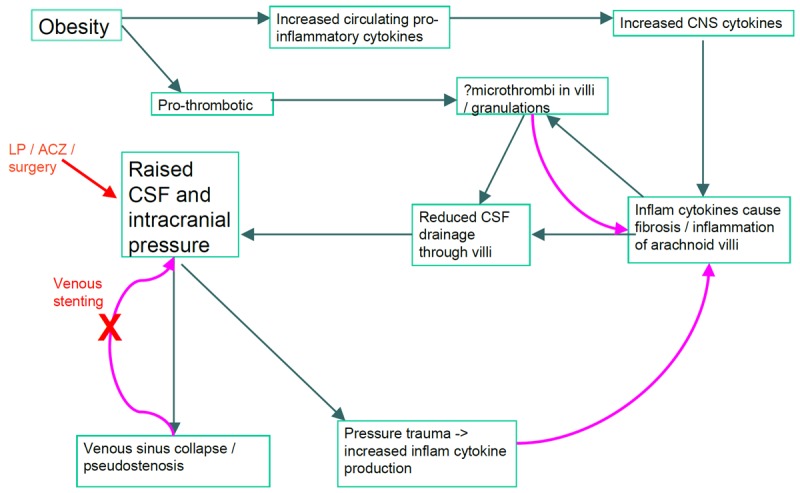
Flow diagram indicates possible mechanisms through which obesity and its associated pro-inflammatory and pro-thrombotic profile may contribute to the initiation and perpetuation of IIH. Curved arrows indicate positive feedback of one process on another; red text indicates sites of current clinical intervention. LP=lumbar puncture; ACZ=acetazolamide.
In conclusion, we report increased IL-17 and IL-2 in the CSF of IIH patients compared to patients with other neurological diseases. Further studies are required to ascertain whether these are causal, subsequent to the disease process, reflective of shared risk factors such as obesity, or chance association. As weight loss, a treatment for IIH, suppresses proinflammatory cytokine production, whether through through dietary/exercise methods [33,35], bariatric surgery [28] or liposuction [24], current treatments may already represent pharmacological interventions on inflammatory responses in this condition.
Acknowledgements
We are grateful to Dr Ian Spendlove in the School of Molecular Medical Sciences at the University of Nottingham for help with Luminex technology and equipment and to Alex Liversage for advice regarding optimizing the assays for CSF measurement. Manjit Braitch, formerly of the University of Nottingham, provided technical assistance. Laura Edwards was supported by a Patrick Berthoud Research Fellowship and an Anne McLaren Fellowship for this work.
Disclosure of conflict of interest
The authors have no competing interests to declare.
References
- 1.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–5. doi: 10.1212/01.wnl.0000029570.69134.1b. [DOI] [PubMed] [Google Scholar]
- 2.Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. 2005:CD003434. doi: 10.1002/14651858.CD003434.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Dhungana S, Sharrack B, Woodroofe N. Idiopathic intracranial hypertension. Acta Neurol Scand. 2010;121:71–82. doi: 10.1111/j.1600-0404.2009.01172.x. [DOI] [PubMed] [Google Scholar]
- 4.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oreskovic D, Klarica M, Bulat M. The formation of cerebrospinal fluid: Nearly a hundred years of interpretations and misinterpretations. Brain Res Rev. 2010;64:241–62. doi: 10.1016/j.brainresrev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Walker RW. Idiopathic intracranial hypertension: any light on the mechanism of the raised pressure? J Neurol Neurosurg Psychiatry. 2001;71:1–5. doi: 10.1136/jnnp.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain. 1974;97:301–12. doi: 10.1093/brain/97.1.301. [DOI] [PubMed] [Google Scholar]
- 8.Orefice G, Celentano L, Scaglione M, Davoli M, Striano S. Radioisotopic cisternography in benign intracranial hypertension of young obese women. A seven-case study and pathogenetic suggestions. Acta Neurol (Napoli) 1992;14:39–50. [PubMed] [Google Scholar]
- 9.Bono F, Giliberto C, Mastrandrea C, Cristiano D, Lavano A, Fera F, Quattrone A. Transverse sinus stenoses persist after normalization of the CSF pressure in IIH. Neurology. 2005;65:1090–3. doi: 10.1212/01.wnl.0000178889.63571.e5. [DOI] [PubMed] [Google Scholar]
- 10.McGonigal A, Bone I, Teasdale E. Resolution of transverse sinus stenosis in idiopathic intracranial hypertension after L-P shunt. Neurology. 2004;62:514–5. doi: 10.1212/wnl.62.3.514. [DOI] [PubMed] [Google Scholar]
- 11.Glueck CJ, Aregawi D, Goldenberg N, Golnik KC, Sieve L, Wang P. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med. 2005;145:72–82. doi: 10.1016/j.lab.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Kesler A, Yatziv Y, Shapira I, Berliner S, Assayag EB. Increased red blood cell aggregation in patients with idiopathic intracranial hypertension. A hitherto unexplored pathophysiological pathway. Thromb Haemost. 2006;96:483–7. [PubMed] [Google Scholar]
- 13.Borch KH, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Anthropometric measures of obesity and risk of venous thromboembolism: the Tromso study. Arterioscler Thromb Vasc Biol. 2010;30:121–7. doi: 10.1161/ATVBAHA.109.188920. [DOI] [PubMed] [Google Scholar]
- 14.Stein PD, Goldman J. Obesity and thromboembolic disease. Clin Chest Med. 2009;30:489–93. viii. doi: 10.1016/j.ccm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Dhungana S, Sharrack B, Woodroofe N. Cytokines and chemokines in idiopathic intracranial hypertension. Headache. 2009;49:282–5. doi: 10.1111/j.1526-4610.2008.001329.x. [DOI] [PubMed] [Google Scholar]
- 16.Dhungana S, Sharrack B, Woodroofe N. IL-1beta, TNF and IP-10 in the cerebrospinal fluid and serum are not altered in patients with idiopathic intracranial hypertension compared to controls. Clin Endocrinol (Oxf) 2009;71:896–7. doi: 10.1111/j.1365-2265.2009.03593.x. [DOI] [PubMed] [Google Scholar]
- 17.Ball AK, Sinclair AJ, Curnow SJ, Tomlinson JW, Burdon MA, Walker EA, Stewart PM, Nightingale PG, Clarke CE, Rauz S. Elevated cerebrospinal fluid (CSF) leptin in idiopathic intracranial hypertension (IIH): evidence for hypothalamic leptin resistance? Clin Endocrinol (Oxf) 2009;70:863–9. doi: 10.1111/j.1365-2265.2008.03401.x. [DOI] [PubMed] [Google Scholar]
- 18.Kermani HR, Faramarzi MS, Ansari M, Ghafarinejad A. Cerebrospinal fluid concentration of Interleukin-6 and interleukin-10 in idiopathic intracranial hypertension. Journal of Medical Science. 2008;8:205–208. [Google Scholar]
- 19.Edwards L, Constantinescu C. Cytokines in idiopathic intracranial hypertension CSF. Headache. 2010;50:323–5. doi: 10.1111/j.1526-4610.2009.01592.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bromander S, Anckarsater R, Kristiansson M, Blennow K, Zetterberg H, Anckarsater H, Wass CE. Changes in serum and cerebrospinal fluid cytokines in response to non-neurological surgery: an observational study. J Neuroinflammation. 2012;9:242. doi: 10.1186/1742-2094-9-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards L, Constantinescu C. Cytokines in Idiopathic Intracranial Hypertension CSF. Headache. 2010;50:323–5. doi: 10.1111/j.1526-4610.2009.01592.x. [DOI] [PubMed] [Google Scholar]
- 23.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 24.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 25.Csuka E, Morganti-Kossmann MC, Lenzlinger PM, Joller H, Trentz O, Kossmann T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol. 1999;101:211–21. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 26.Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988;45:875–7. doi: 10.1001/archneur.1988.00520320065016. [DOI] [PubMed] [Google Scholar]
- 27.Radhakrishnan K, Thacker AK, Bohlaga NH, Maloo JC, Gerryo SE. Epidemiology of idiopathic intracranial hypertension: a prospective and case-control study. J Neurol Sci. 1993;116:18–28. doi: 10.1016/0022-510x(93)90084-c. [DOI] [PubMed] [Google Scholar]
- 28.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–13. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair AJ, Ball AK, Burdon MA, Clarke CE, Stewart PM, Curnow SJ, Rauz S. Exploring the pathogenesis of IIH: an inflammatory perspective. J Neuroimmunol. 2008;201-202:212–20. doi: 10.1016/j.jneuroim.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009;43:157–68. [PubMed] [Google Scholar]
- 31.Pini M, Fantuzzi G. Enhanced production of IL-17A during zymosan-induced peritonitis in obese mice. J Leukoc Biol. 2010;87:51–8. doi: 10.1189/jlb.0309188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–6. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 33.de Mello VD, Kolehmainen M, Schwab U, Mager U, Laaksonen DE, Pulkkinen L, Niskanen L, Gylling H, Atalay M, Rauramaa R, Uusitupa M. Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metabolism. 2008;57:192–9. doi: 10.1016/j.metabol.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Giugliano G, Nicoletti G, Grella E, Giugliano F, Esposito K, Scuderi N, D’Andrea F. Effect of liposuction on insulin resistance and vascular inflammatory markers in obese women. Br J Plast Surg. 2004;57:190–4. doi: 10.1016/j.bjps.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Marfella R, Esposito K, Siniscalchi M, Cacciapuoti F, Giugliano F, Labriola D, Ciotola M, Di Palo C, Misso L, Giugliano D. Effect of weight loss on cardiac synchronization and proinflammatory cytokines in premenopausal obese women. Diabetes Care. 2004;27:47–52. doi: 10.2337/diacare.27.1.47. [DOI] [PubMed] [Google Scholar]
- 36.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, Van Loan MD, Ali MR, Wolfe BM, Havel PJ. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–11. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral JF, Tsiaris W, Morgan T, Thompson WR. Reversal of benign intracranial hypertension by surgically induced weight loss. Arch Surg. 1987;122:946–9. doi: 10.1001/archsurg.1987.01400200096018. [DOI] [PubMed] [Google Scholar]
- 38.Fridley J, Foroozan R, Sherman V, Brandt ML, Yoshor D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg. 2011;114:34–9. doi: 10.3171/2009.12.JNS09953. [DOI] [PubMed] [Google Scholar]
- 39.Wong R, Madill SA, Pandey P, Riordan-Eva P. Idiopathic intracranial hypertension: the association between weight loss and the requirement for systemic treatment. BMC Ophthalmol. 2007;7:15. doi: 10.1186/1471-2415-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banks WA, Kastin AJ. Relative contributions of peripheral and central sources to levels of IL-1 alpha in the cerebral cortex of mice: assessment with species-specific enzyme immunoassays. J Neuroimmunol. 1997;79:22–8. doi: 10.1016/s0165-5728(97)00103-3. [DOI] [PubMed] [Google Scholar]
- 41.Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 42.Licinio J, Wong ML. Pathways and mechanisms for cytokine signaling of the central nervous system. J Clin Invest. 1997;100:2941–7. doi: 10.1172/JCI119846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glueck CJ, Iyengar S, Goldenberg N, Smith LS, Wang P. Idiopathic intracranial hypertension: associations with coagulation disorders and polycystic-ovary syndrome. J Lab Clin Med. 2003;142:35–45. doi: 10.1016/S0022-2143(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 44.Kesler A, Kliper E, Assayag EB, Zwang E, Deutsch V, Martinowitz U, Lubetsky A, Berliner S. Thrombophilic factors in idiopathic intracranial hypertension: a report of 51 patients and a meta-analysis. Blood Coagul Fibrinolysis. 2010;21:328–33. doi: 10.1097/MBC.0b013e328338ce12. [DOI] [PubMed] [Google Scholar]
- 45.Hot A, Lenief V, Cazalis MA, Miossec P. Pathogenic role of IL-17 in endothelial dysfunction, a link between rheumatoid arthritis and atherosclerosis. Annals of Rheumatic Disease. 2010;69:44–45. [Google Scholar]
- 46.Lenief V, Cazalis MA, Miossec P, editors. Pathogenic role of IL-17 in atherogenesis: a cross link between rheumatoid arthritis and atherosclerosis. ACR/ARHP Annual Scientific Meeting; Philadelphia. 2009. [Google Scholar]
- 47.Lentsch AB, Edwards MJ, Miller FN. Interleukin-2 induces increased platelet-endothelium interactions: a potential mechanism of toxicity. J Lab Clin Med. 1996;128:75–82. doi: 10.1016/s0022-2143(96)90115-8. [DOI] [PubMed] [Google Scholar]
- 48.Edwards MJ, Miller FN, Sims DE, Abney DL, Schuschke DA, Corey TS. Interleukin 2 acutely induces platelet and neutrophil-endothelial adherence and macromolecular leakage. Cancer Res. 1992;52:3425–31. [PubMed] [Google Scholar]
- 49.Yoshida H, Russell J, Granger D. Pro-inflammatory cytokines mediate the extra-intestinal thrombus formation associated with inflammatory bowel disease (IBD) FASEB J. 2009;23:593.2. [Google Scholar]
- 50.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740–5. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kranzhofer R, Clinton SK, Ishii K, Coughlin SR, Fenton JW 2nd, Libby P. Thrombin potently stimulates cytokine production in human vascular smooth muscle cells but not in mononuclear phagocytes. Circ Res. 1996;79:286–94. doi: 10.1161/01.res.79.2.286. [DOI] [PubMed] [Google Scholar]


