Abstract
Several lines of evidence suggest that mast cells play a key role in the pathogenesis of Multiple Sclerosis (MS). The contribution of mast cells likely depends upon specific adherence to myelin surface-bound IgE, which triggers degranulation and the release of enzymes that damage central nervous system (CNS) neurons. To block mast cell degranulation, a peptide-based system was developed to neutralize endogenous, myelin-targeting autoantibodies, thus halting the pathological autoimmune process. Development of the MS therapeutic involved: (1) identification of relevant myelin epitopes; (2) estimation of endogenous autoantibody quantities to be neutralized; (3) synthesis of epitope mimicking/autoantibody-neutralizing peptides; (4) subcutaneous administration of the peptides; and (5) assessment, over time, of clinical presentation together with matching, residual autoantibody levels. An open label, interventional study was performed involving a single MS patient and five control subjects as a first step towards a potentially larger, more elaborate investigation. The study encompassed serological testing to confirm the IgE-positive status of the MS patient and negative status of the controls, an eight month course of peptide-based immunotherapy, and assessment of therapeutic efficacy and potentially adverse effects. Treatment of the MS patient with the peptide-based therapy resulted in a reduction in myelin-specific IgE titers and marked clinical improvement. No subjects experienced adverse effects. Thus, peptide-based immunotherapy could provide improved clinical status or life-long remission to MS patients. Substantiation of this premise requires a follow-up examination by other investigators and institutions with larger and more extensive clinical trials.
Keywords: Immunoglobulin E, mast cell degranulation, enzymology, myelin sheath, axons
Introduction
Multiple Sclerosis (MS) has been reported to be an inflammatory autoimmune disease, driven primarily by pro-inflammatory Th1 and Th17 lymphocytes [1,2]. However, we have shown that MS is also marked by a key humoral component, myelin epitope-specific IgE, which, when critically affixed to myelin, could be eliciting degranulation of indrawn mast cells, thus causing and sustaining the MS condition [3,4].
Degranulating mast cells are likely to release damaging proteases and possibly negative immunomodulatory factors. Mast cell enzymes have been postulated to damage or disintegrate myelin and, if diffused beyond an oligodendrocyte surface disruption, may damage uncovered axons as well [3].
Site-specific mast cell degranulation is likely triggered by dimeric IgE coupled to unique myelin-surface epitopes on proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG), and, possibly, myelin basic protein (MBP) [3]. This likely occurs when mast cells make surface contact with the Fc portion of two adjoining IgE molecules that are interspaced by a distance of 40-100 Ångströms. In the sera of previously untreated MS patients, twin, epitope-specific IgE autoantibodies accompanied by insufficient quantities of epitope-matched, competing non-IgE antibodies, appeared to be a disease benchmark [3].
PLP has been shown to have one structurally unique epitope expressed on the myelin surface, ADARM (alanine-aspartic acid-alanine-arginine-methionine) [3]. However, PLP molecules are highly prevalent on myelin and ideally interspaced at 65-71 Ångströms [5], so as to correctly project surface-bound IgE onto IgE receptors present on mast cells, thus inducing degranulation. Mimotopic, ex-vivo-generated ADARM peptide exhibits structural equivalency to its in vivo counterparts and is adequately soluble in aqueous solutions. This peptide was reasoned to be a good candidate to halt PLP-driven mast cell degranulation in MS patients by neutralizing pivotal, specific IgE molecules.
MOG possesses six functional dimer sites: two on its outer oligodendrocyte surface (Figures 1 and 2) and four potential sites that are intracellular (Figures 3 and 4). The latter are amenable to endogenous exposure following oligodendrocyte surface disruption. In order to block MOG-driven mast cell degranulation, in vivo provision of the IgE-neutralizing, mimotopic peptides HSYQE (histidine-serine-tyrosine-glutamine-glutamic acid) and KTGQF (lysine-threonine-glycine-glutamine-phenylalanine) were predicted to suffice, since elimination of one of two dimer points would prevent adequate mast cell coupling followed by degranulation.
Figure 1.
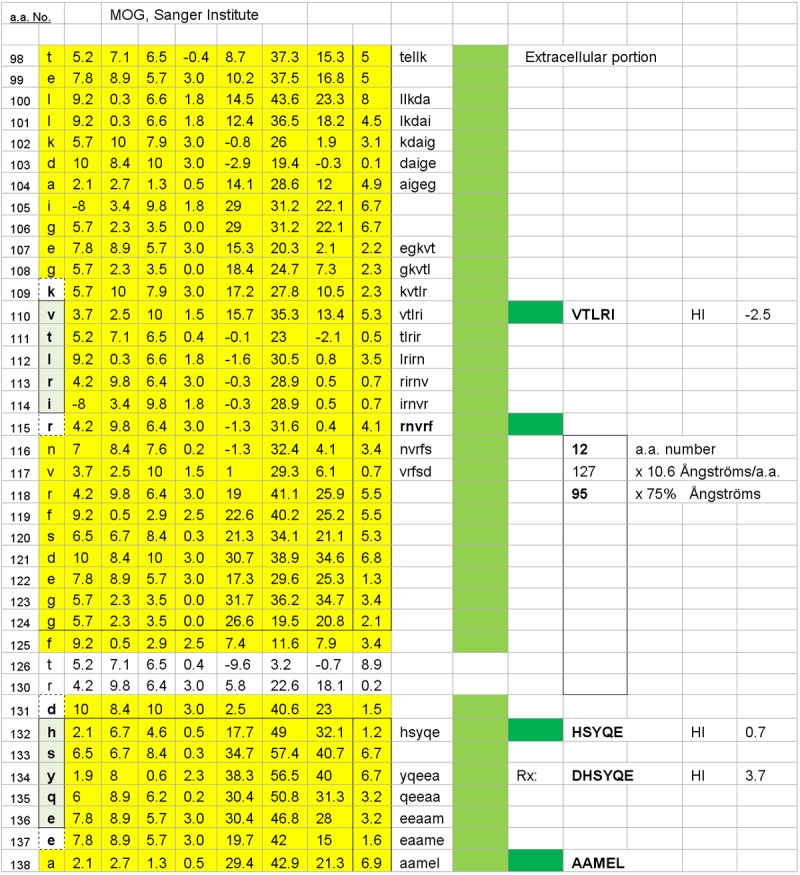
One of two possible MOG surface dimers, HSYQE–VTLRI, is illustrated. The dimer’s inter-epitope interval distance is 95 Ångströms, and it has a target epitope, HSYQE, which is also common to the second potential, oligodendrocyte surface dimer illustrated in Figure 2. The hydrophilic index (HI) of each mimotopic peptide is displayed as it would be if presented alone and not contiguous with the amino acids that preceded or followed it in the overall protein sequence (as per the Hopp and Woods citation listed in the 2012 Calenoff reference).
Figure 2.
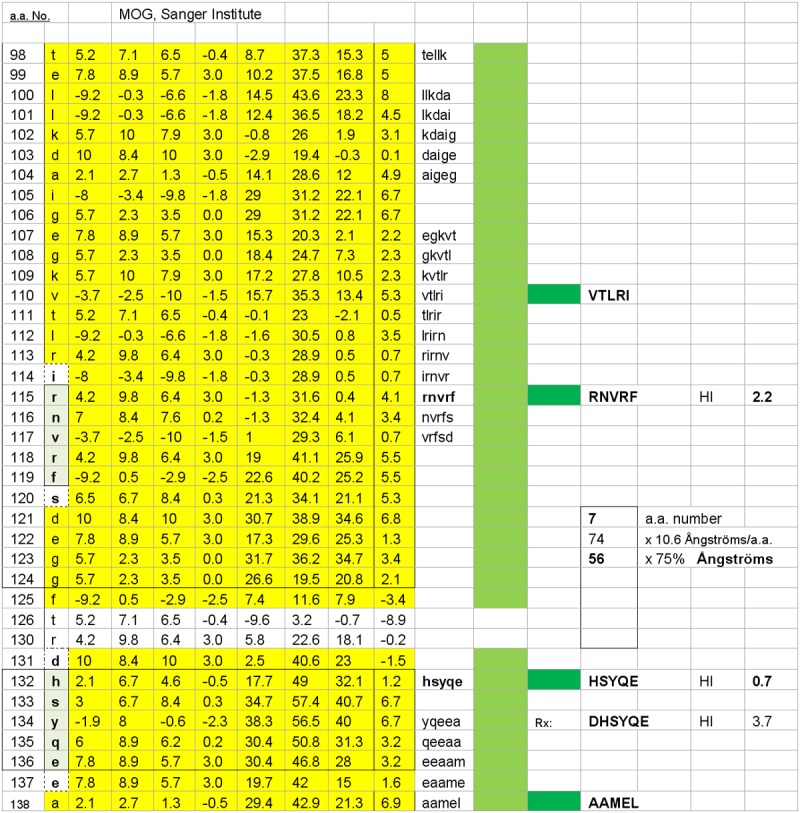
The second of two possible MOG surface dimers, HSYQE–RNVRF is illustrated. The estimated inter-epitope interval distance within the dimer is 56 Ångströms.
Figure 3.
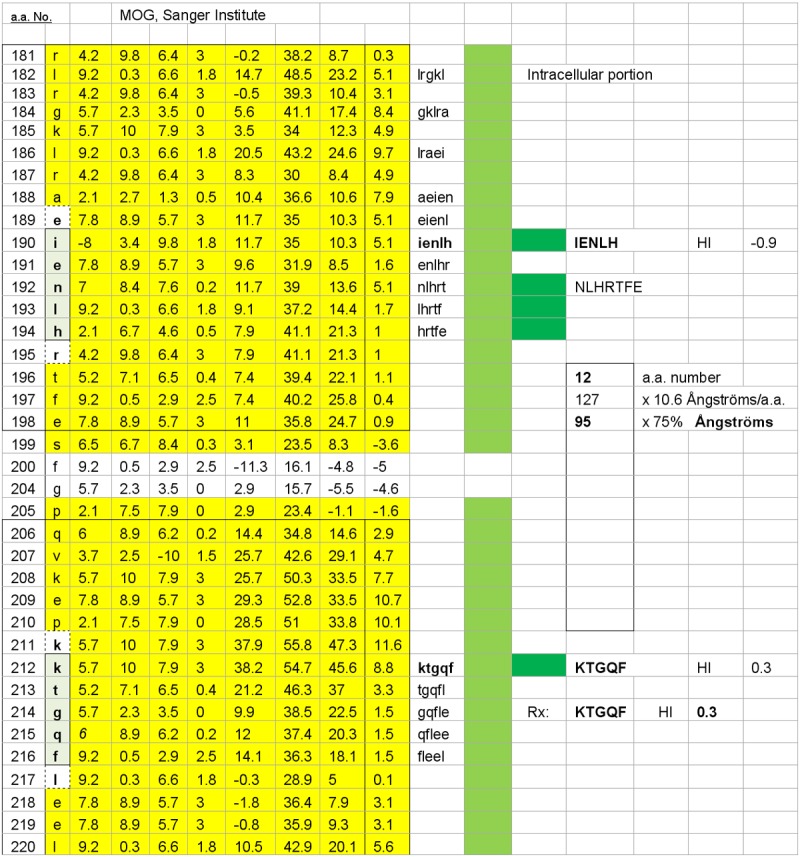
One of four possible MOG subsurface dimers, KTGQF–IENLH is illustrated. The dimers’ accessibility hinges upon disruption of the myelin (oligodendrocyte) cell surface, thus exposing the relevant, subsurface dimer target epitopes. The dimer’s estimated inter-epitope interval distance is 95 Ångströms, and it has a target epitope, KTGQF, which is common to the other three potential sub-surface dimers illustrated in Figure 4.
Figure 4.
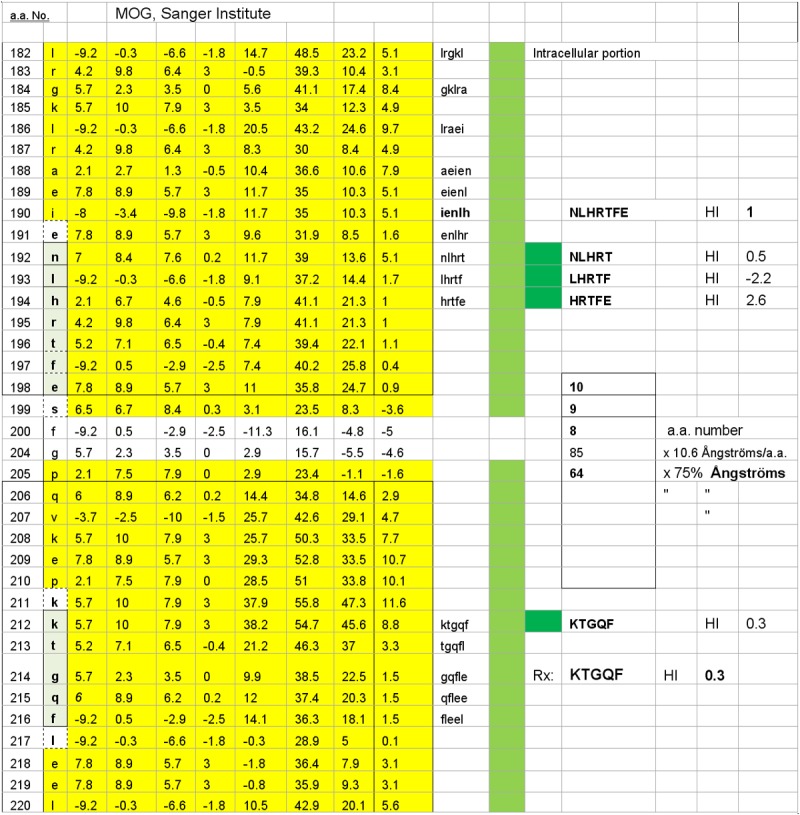
Three of four possible MOG subsurface dimers are illustrated. The dimers’ accessibility hinges upon disruption of the myelin (oligodendrocyte) surface and thus exposing the relevant dimer target epitopes. The dimers’ estimated inter-epitope interval distances are, respectively, 8, 9, and 10 Ångströms, with each having KTGQF as a common target epitope. The potential dimers are KTGQF–NLHRT, KTGQF–LHRTF, and KTGQF–HRTFE.
HSYQE was designed to suppress the outer-surface MOG dimers HSYQE–VTLRI (valine-threonine-leucine-arginine-isoleucine), (Figure 1) and HSYQE–RNVRF (arginine-asparagine-valine-arginine-phenylalanine) (Figure 2). However, the solubility of HSYQE in aqueous media was found to be variable, and, thus, a more soluble peptide substitute, DHSYQE, was synthesized. This peptide was found to retain the mimotopic, structural uniqueness of HSYQE but did not risk triggering an aberrant autoimmune response, because the DHSYQ sequence portion is not likely to be autoimmunogenic, since it is not found on the surface of any other human protein. Elimination of anti-KTGQF epitope-specific IgE autoantibodies would quell a reaction to the intracellular surface MOG dimers KTGQF–IEN-LH, KTGQF–NLHRT, KTGQF–LHRTF, and KTGQF–HRTFE (Figures 3 and 4) should those prove to be pathologically relevant in MS.
Materials and methods
Having identified the key epitopes to which IgE autoantibody binding should be prevented, four consecutive steps were initiated: (1) estimation of the quantity of endogenous antibodies to be neutralized, together with neutralizing peptide quantities; (2) formulation of the epitope-mimicking/ autoantibody-neutralizing peptides for in vivo administration; (3) performance of a murine animal toxicity study to gauge potentially adverse effects; and (4) an MS pilot clinical study monitored by sequential, specific IgE/(kappa + lambda) quantification.
Estimation of the maximum quantity of endogenous antibodies to be neutralized and the required quantities of neutralizing peptide
For patients who were found to be IgE/(kappa + lambda) test-positive against PLP and/or MOG dimer site epitopes (Table 1), the following analytical process was undertaken, in order to estimate a starting therapeutic peptide dose per epitope: (a) determination of the per milliliter quantity of IgA, IgE, IgG, and IgM maximally carried by an average adult Caucasian male and its conversion to grams per milliliter; (c) conversion of the grams per milliliter value to moles per milliliter of serum using the formula, number of moles = gram weight of sample/relative molar mass; (d) multiplication of the individual antibody mole value by the specific antibody valence (antigen binding sites per antibody isotype) to yield valence moles/mL (Table 2); (e) multiplication of each isotype-specific valence mole/mL value by 2,750 mL, which is the average Caucasian adult male’s total serum volume; (f) division of each isotype-specific valence mole/mL value by 1,000,000 potentially expressible humoral epitopes (a subjective starting point) and then summation of the individual isotype values, in order to estimate the required valence mole of mimotopic peptide needed to completely neutralize all endogenous antibodies that bind a single epitope; and (g) determination of the collective molar mass of the three, planned myelin epitope-mimicking peptides ADARM, DHSYQE, and KTGQF, assuming that KTGQF will comprise a two-fold representation because of its tendency to form dimeric molecular aggregates when solubilized.
Table 1.
Pre-Treatment Epitope-specific, Serum IgE/(kappa + lambda) Test Results for MS Patient and Five Control Subjects
| Myelin Mimotopes | Male MS Patient | Female Control 1 | Female Control 2 | Female Control 3 | Male Control 1 | Male Control 2 |
|---|---|---|---|---|---|---|
| ADARM | 7 | 0 | 0 | 0 | 0 | 0 |
| VTLRI | 26 | 0 | 0 | 1 | 0 | 102 |
| HSYQE | 22 | 0 | 0 | 0 | 0 | 0 |
| RNVRF | 0 | 0 | 0 | 0 | 0 | 0 |
| HSYQE | 22 | 0 | 0 | 0 | 0 | 0 |
| IENLH | 0 | 0 | 0 | 0 | 0 | 0 |
| KTGQF | 0 | 0 | 0 | 0 | 0 | 0 |
| NLHRT | 0 | 0 | 0 | 0 | 0 | 0 |
| KTGQF | 0 | 0 | 0 | 0 | 0 | 0 |
| LHRTF | 1 | 0 | 0 | 0 | 0 | 0 |
| KTGQF | 102 | 0 | 0 | 0 | 0 | 0 |
| HRTFE | 0 | 0 | 0 | 0 | 0 | 0 |
| KTGQF | 0 | 0 | 0 | 0 | 0 | 0 |
Depicted are the dimer test-positive results for the MS patient and correspondingly negative results for the three female and two male control subjects. The MS patient was IgE/(kappa + lambda)-positive against the myelin surface PLP dimer epitopes ADARM–ADARM, the myelin surface MOG VTLRI–HSYQE dimer epitopes and the myelin subsurface MOG HRTFE–KTGQF dimer epitopes.
Table 2.
Estimation of Single-epitope Autoantibody Levels, Steps a tdrough d
| Steps: | a | b | c | ||
|
|
|||||
| gm/mL serum | Molar Mass | Mole/mL Serum | Valence No. | Mole/mL Serum x Valence No. | |
|
| |||||
| IgA | 0.0032 | 160,000 | 0.0000000205 | 2 | 0.000000041 |
| IgE | 0.0000000014 | 188,000 | 0.000000000000008 | 2 | 0.00000000000002 |
| IgG | 0.012 | 150,000 | 0.00000008333 | 2 | 0.000000167 |
| IgM | 0.001 | 900,000 | 0.00000000111 | 10 | 0.000000011 |
| Sum of Mole Values => 0.0000002188 | |||||
|
| |||||
| d | Valence Mole per 2,750 mL Serum | ||||
|
| |||||
| IgA | 0.0001127500000 | ||||
| IgE | 0.0000000000432 | ||||
| IgG | 0.0004583333333 | ||||
| IgM | 0.0000305555555 | ||||
| Sum of Mole Values per Epitope => 0.0006016389321 | |||||
|
| |||||
| Gram weight of ADARM peptide required to neutralize endogenous autoantibodies specifically binding tde ADARM epitope = 0.00000038 gram (0.38 μg). | |||||
| Gram weight of DHSYQE peptide required to neutralize endogenous autoantibodies specifically binding tde HSYQE epitope = 0.00000051 gram (0.51 μg). | |||||
| Gram weight of KTGQF peptide needed to neutralize endogenous autoantibodies specifically binding tde KTGQF epitope = 0.00000078 gram (0.78 μg). | |||||
| ADARM + DHSYQE + KTGQF peptides = 1.67 μg | |||||
Approximation of single-epitope autoantibody levels is exhibited wherein tde analytical patd employed in estimating tde valence mole of epitope-specific IgA, IgE, IgG, and IgM antibodies to be found witdin tde serum volume of an average adult Caucasian male is illustrated. tde grams per milliliter of each specific antibody isotype is depicted in step a, and tde corresponding mole/mL in step b. Step c depicts tde mole/mL antibody value multiplied by tde number of each isotope’s antigen binding sites tdat is termed valence mole. Step d illustrates tde per mL valence mole value multiplied by tde average Caucasian, adult male serum volume, 2,750 mL. tde valence mole value per targeted humoral epitope was, tdus, estimated to equal 0.0006016. Mole = Weight of Sample (in grams)/Relative Molar Mass.
Taking into account intracellular diffusion, ongoing hepatic and renal clearance (Table 2), and non-specific endogenous peptide-to-tissue adherence, the targeted peptide quantity required to be constantly and endogenously on hand to maintain near zero specific serum antibody levels was arbitrarily set at 16.7 μg (a 10-fold increase of the statistically estimated 1.67 μg dose).
Of the causative factors, renal clearance of parenterally administered peptides was probably the most significant as per the work of Esposito [11] (Table 3). Sufficient endogenous peptide persistence was confirmed by absence of anti ADARM, anti- HSYQE, and anti-KTGQF epitope-specific serum antibodies.
Table 3.
The endogenous, peptide degradation process, described in the article, assumes a 10 minutes intravascular peptide half-life due to renal clearance and hepatic-enzymatic degradation. 40 mg (4 mL) of the peptide mixture (40,000 μg) was subcutaneously administered weekly. The time-distributed endogenous doses of the ADARM, DHSYQE, and KTGQF peptide mix are reflected below
| μg Peptides | Minutes | Hours | Days | |
|---|---|---|---|---|
| Dilution: | 40,000 | |||
|
| ||||
| ½ | 20,000 | 10 | 0 | 0 |
| ¼ | 10,000 | 20 | 0 | 0 |
| 1/8 | 5,000 | 40 | 1 | 0 |
| 1/16 | 2,500 | 80 | 1 | 0 |
| 1/32 | 1,250 | 160 | 3 | 0 |
| 1/64 | 625 | 320 | 5 | 0 |
| 1/128 | 313 | 640 | 11 | 0 |
| 1/256 | 156 | 1,280 | 21 | 1 |
| 1/512 | 78 | 2,560 | 43 | 2 |
| 1/1,024 | 39 | 5,120 | 85 | 4 |
| 1/2,048 | 20 | 10,240 | 171 | 7 |
| 1/4,096 | 10 | 20,480 | 341 | 14 |
| 1/8,192 | 5 | 40,960 | 683 | 28 |
| 1/16,384 | 2 | 81,920 | 1,365 | 57 |
|
| ||||
| 16.75 μg empirically tittered peptide mix needed to yield zero serum specific IgE level. | ||||
Depicted is the expected, 10 minute intravascular half-life of the PLP epitope-mimicking peptide ADARM and the MOG-mimicking peptides, DHSYQE, and KTGQF. The ongoing, endogenous loss is likely to be caused by renal clearance and enzymatic degradation. The infused peptides would, thereby, complex with and neutralize their corresponding epitope-specific IgE and non-IgE autoantibodies for a 1-2 weeks period. Absence of the specific autoantibodies would bring to a halt the related mast cell degranulation and neuropathology.
Formulation of epitope-mimicking/autoantibody-neutralizing peptides
The MS therapeutic was formulated by dissolving 634.7 mg of ADARM, 845.7 mg of DHSYQE, and 1,303.4 mg of KTGQF (PolyPeptide Laboratories, San Diego, CA USA.) in 278.4 mL of 50% pharmaceutical grade glycerin (Allergy Laboratories, Inc., Oklahoma City, OK, USA; Product Number DG50-100S). The solution of peptides was sterile filtered through a Pall PN 4902 Supor EKV1 0.2 μm filter (Pall Corporation, Ann Arbor. MI, USA). Post-filtration, the solution was aliquoted into sterile 30 mL vials (Allergy Laboratories, Inc., Oklahoma City, OK, USA; product Number SV30S). After vialing, the peptide therapeutic solution was stored at 4°C between uses.
Performance of a murine animal toxicity study to gauge the potential for adverse clinical effects
The IAUAC-approved, in-house animal toxicity study entailed weekly subcutaneous doses that were 10-fold greater, on a per weight basis, than the planned human doses. The murine population was composed of 10 females and 10 males. The starting ages of the mice were 23-26 days, and weights were 13-16 grams. Peptides were administered by subcutaneous injection on the dorsal surface, and the mice were then observed for adverse effects for 6 months.
MS case presentation, investigations and treatment
A 60-year-old, Caucasian male patient with a sixteen-year history of secondary, progressive MS and an EDSS of 6.5 was evaluated and treated. He was still able to walk but exhibited poor balance and required a rolling support to move about. He had lost sensation over his right anterior leg surface since his last relapse ten years before. The patient’s last MRI, obtained six months earlier, had shown enhancing, multifocal cerebral and medullary lesions which were essentially unchanged from earlier studies. He had not previously received long-term treatment with interferon, Glatiramer Acetate (Copaxone), Mitoxantrone (Novantrone) or Natalizumab (Tysabri). He had tried beta-interferon after initial diagnosis, but had experienced a significant adverse reaction and elected to discontinue treatment. He exhibited chronic inhalant and food allergies, the former controlled with desensitization injections and the latter by dietary elimination or rotation.
The study control subjects were five healthy volunteers. The ages of the females were 54, 56, and 64 years, and the ages of the males were 59 and 61 years. All were Caucasian by chance.
Diagnostic peptide construct formulation for use in epitope-specific, IgE/(kappa + lambda) chemiluminescence ELISA
Each 8-amino-3,6-dioxaoctanoic acid2-peptide construct (Mimotopes, Clayton, Victoria, 3168 Australia) was dissolved in pH 7.2 phosphate buffered saline (PBS) immobilization buffer (Product No. 28372, Thermo Scientific, Rockford, IL, USA), in order to yield concentrations of 0.26 μg/mL for ADARM, 0.31 μg/mL for HSYQE, and 0.26 μg/mL for KTGQF.
Diagnostic peptide construct application
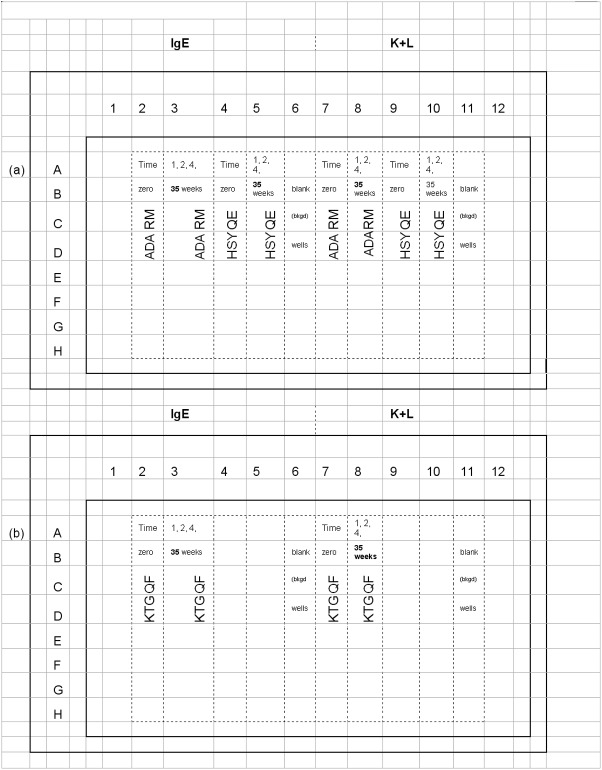
A 100 μL aliquot of each peptide construct solution was applied to vertical 8-well groups of white, 96-well plates (Thermo Scientific, Product No. 15108), as depicted in Figure 5. Eight wells per plate were left blank in order to measure passive, non-specific IgE background and 8 wells were left blank for determining non-specific (kappa + lambda) background values. The coated plates were covered with acetate plate sealers (Thermo Scientific, Product No. 3501) and incubated at 21-26°C for 18-24 h.
Figure 5.
Microplate maps employed in measuring epitope-specific IgE/(lambda + kappa) levels are displayed: Plate map 5a shows the peptide-specific well set positions used to measure date-relevant ADARM and HSYQE-specific IgE/(kappa + lambda) ratio values. Plate map 5b displays plate layout used in measuring date-relevant KTGQF-specific IgE/(kappa + lambda) ratio values.
Microplate blocking procedure
The peptide construct solutions were aspirated and 120 μL of HSA background blocking solution (10 mg of recombinant HSA per mL of immobilization buffer) [3] was applied per well. The plates were covered with acetate plate sealers and incubated at 21-26°C for 18-24 h, washed four times with PBST (Thermo Scientific Product No. 28320), dried, and sealed until the time of use.
Epitope-specific, serum IgE immunoassay
The assay encompassed the following operational steps: (a) application of 100 μL/well of neat subject serum that had been spiked with 1 mg/mL of 8-amino-3,6-dioxaoctanoic acid2 aminated hydrophilic linker (Mimotopes, Clayon, Victoria, 3168 Australia; 50 μL of 1 mg/mL aqueous linker solution per 12 mL of serum), in order to neutralize potential anti- linker antibodies; (b) plate incubation for 2 h at 21-26°C; (c) plate aspiration and washing 4 times using 150 μL/well of PBST; (d) application of 100 μL of 4 μg/mL biotinylated, goat anti-human IgE per well with the anti-IgE solution, comprising 96 μL of BA-3040 (Vector Labs, Burlingame, CA, USA) dissolved in 11.9 mL of conjugate diluent (10 mg/mL recombinant HSA in PBST + 0.25% PEG 4000); (e) plate incubation for 2 h at 21-26°C, followed by aspiration and washing 4 times with 150 μL/well of PBST; (f) application of 100 μL/well of 64 ng/mL streptavidin horseradish peroxidase (Thermo Scientific Product No. 21126 diluted in HSA conjugate diluent); (g) application of 100 μL/well of Thermo Scientific chemiluminescence substrate (Product No. 37074) for 1-3 min; (h) aspirating and washing the plates; and (i) reading the test plate chemiluminescence using a Luminoskan Ascent Microplate Luminometer (Thermo Scientific, Waltham, MA, USA).
Epitope-specific, serum (kappa + lambda) immunoassay
The assay encompassed the following steps: (a) the linker-spiked test serum sample used in the specific IgE assay was diluted 1/25,000 by first making a 1/100 dilution by mixing together 100 μL of linker-spiked serum and 9.9 mL of PBST; and then spiking 11.950 mL of HSA conjugate diluent with 48 μL of the 1/100 diluted serum; (b) test plates were filled with 100 μL/well of diluted serum, sealed, and incubated for 2 h at 21-26°C; (c) equal volumes of biotinylated goat anti-human kappa antibody (Vector Laboratories, BA3060) and biotinylated, goat anti-human lambda antibody (Vector Laboratories, BA-3070) were mixed together to form a 50:50 biotinylated anti-(kappa + lambda) concentrate (500 μg/mL); (d) 96 μL of the anti-(kappa + lambda) concentrate was mixed with 11.9 mL of HSA conjugate diluent, and a 100 μL aliquot of this solution was applied to each well; (e) after a 2 h incubation at 21-26°C, plates were washed and 100 μL of 16 ng/mL streptavidin horseradish peroxidase solution was applied to each well; (f) test plates were incubated at 21-26°C for 30 min, aspirated, and washed; and (g) 100 μL/well of Thermo Scientific chemiluminescence substrate was applied and the plate wells were read at 1-3 min post-application in a microplate lumin-ometer.
Determination of epitope-specific IgE/(kappa + lambda) test point values
Average values corresponding to individual test points were determined by discarding the highest and lowest of each set of eight well values and averaging the remaining six, and the same procedure was followed for the eight corresponding background well values per condition. These values were used to generate six-point averages and standard deviations. Each blank well group’s background value was deemed to be its average value plus twice the standard deviation. The derived background values were subtracted from the respective peptide-coated six-vertical well set averages plus 2 standard deviations to yield net point values. The (kappa + lambda) point values were multiplied by 25,000 in order to determine the corresponding neat serum (undiluted) epitope-specific (ka-ppa + lambda) antibody levels. The IgE/(kappa + lambda) values were calculated and multiplied by 1,000,000, in order to bring each quotient to a positive number wherever possible. Test results with negative values were recorded as being negative.
Results
As depicted in Table 1, the representative MS patient had a positive IgE/(kappa + lambda) test for the polyvalent dimer point of proteolipid protein, ADARM, and two of the six potential dimer point pairs of myelin oligodendrocyte glycoprotein, VTLRI–HSYQE (Figure 1) and LHRTF–KTGQF (Figure 4). None of the control subjects displayed similar dimeric IgE/(kappa + lambda)-positive test results.
Thirty-five week course of peptide-based immunotherapy
As shown in Tables 4, 5, 6,7 and 8, subcutaneous administration of peptides intended to neutralize dimer-point IgE autoantibodies achieved the intended effect, since no anti-ADARM, anti-HSYQE, or anti-KTGQF autoantibodies were detected in the MS patient’s sera following administration of the therapeutic peptides solution. This implied a total and fairly rapid specific IgE and non-IgE antibody elimination. The control subjects were IgE/(kappa + lambda) negative before peptide therapy and remained so after treatment.
Table 4.
1 Week Post-Commencement of MS/Mimotopic Peptide Therapy
| Epitope-Specific Serum IgE Signal: | |||
|
| |||
| ADARM | Pre-Rx | 422 | |
| ADARM | Post-Rx | -93 | |
| HSYQE | Pre-Rx | 809 | |
| HSYQE | Post-Rx | -309 | |
| KTGQF | Pre-Rx | 333 | |
| KTGQF | Post-Rx | -1,353 | |
|
| |||
| Epitope-Specific Serum (kappa + lambda) Signal: | |||
|
| |||
| ADARM | Pre-Rx | 673 | |
| ADARM | Post-Rx | -4,383 | |
| HSYQE | Pre-Rx | 1,458 | |
| HSYQE | Post-Rx | -4,227 | |
| KTGQF | Pre-Rx | 511 | |
| KTGQF | Post-Rx | -4,383 | |
|
| |||
| ADARM before Treatment: | ADARM after Treatment: | ||
|
| |||
| KL | 16,833,501 | KL | -109,577,976 |
| E/KL | 0.000025 | E/KL | -0.000001 |
| x 1 million | 25 | x 1 million | -1 |
|
| |||
| HSYQE before Treatment: | HSYQE after Treatment: | ||
|
| |||
| KL | 36,459,750 | KL | -3,645,975 |
| E/KL | 0.554734 | E/KL | -0.073074 |
| x 1 million | 554,734 | x 1 million | -73,074 |
|
| |||
| KTGQF before Treatment: | KTGQF after Treatment: | ||
|
| |||
| KL | 12,762,509 | KL | -4,383 |
| E/KL | 0.000026 | E/KL | -33,823,947 |
| x 1 million | 26 | x 1 million | -33,823,947,401,145 |
MS activity factor (MSAF) 1 week after commencing therapy is displayed. The MSAF method, IgE/(kappa + lambda, is a numerical formula used to diagnose and monitor multiple sclerosis. The MSAF process distinguishes and integrates: (1) IgE binding onto critically-spaced myelin epitopes; (2) specific IgE targeting and affixation by enzyme-releasing mast cells; and (3) the released enzymes’ degradation of central nervous system tissues. The MSAF, ELISA-based method for distinguishing and quantifying the MS process entails: (a) measurement of specific IgE and non-IgE (kappa + lambda) micro-well, chemiluminescence signals; (b) subtracting background point values from antigenic, peptide-coated well values; (c) multiplying the (kappa + lambda) signals by 25,000 to adjust for required serum dilution; (c) dividing IgE point values by their corresponding (kappa + lambda) values; and (d) multiplying the result by 1,000,000 and seeking the attainment of positive numbers. Derived, positive results indicate the presence of active IgE dimer points and myelin-dimer-generated pathology. Negative values indicate the opposite. The one-week follow-up MSAF analysis of the study showed ADARM-negative, HSYQE-negative and KTGQF-negative results as compared to the positive pre-treatment serum analysis, thus indicating anti-myelin autoantibody neutralization by the administered mimotopic peptides. The absolute ratio quantities, positive or negative, were not disease-severity relevant. Formula to Estimate MS Activity Factor (MSAF) => Net IgE/Net KL x 1,000,000. KL = specific (kappa + lambda) signal x 25,000 (Compensates for 1/25,000 serum dilution).
Table 5.
2 Weeks Post-Commencement of MS/Mimotopic Peptide Therapy
| Epitope-Specific Serum IgE Signal: | |||
|
| |||
| ADARM | Pre-Rx | 57 | |
| ADARM | Post-Rx | -300 | |
| HSYQE | Pre-Rx | 50 | |
| HSYQE | Post-Rx | -235 | |
| KTGQF | Pre-Rx | 184 | |
| KTGQF | Post-Rx | -202 | |
|
| |||
| Epitope-Specific Serum (kappa + lambda) Signal: | |||
|
| |||
| ADARM | Pre-Rx | 4 | |
| ADARM | Post-Rx | -2,983 | |
| HSYQE | Pre-Rx | 124 | |
| HSYQE | Post-Rx | -2,610 | |
| KTGQF | Pre-Rx | 4 | |
| KTGQF | Post-Rx | -2,917 | |
|
| |||
| ADARM before Treatment: | ADARM after Treatment: | ||
|
| |||
| KL | 109,081 | KL | -74,585,187 |
| E/KL | 0.000518 | E/KL | -0.000004 |
| x 1 million | 518 | x 1 million | -4 |
|
| |||
| HSYQE before Treatment: | HSYQE after Treatment: | ||
|
| |||
| KL | 3,108,981 | KL | -310,898 |
| E/KL | 0.399997 | E/KL | -0.089922 |
| x 1 million | 399,997 | x 1 million | -89,922 |
|
| |||
| KTGQF before Treatment: | KTGQF after Treatment: | ||
|
| |||
| KL | 109,081 | KL | -2,917 |
| E/KL | 0.001688 | E/KL | -5,045,294 |
| x 1 million | 1,688 | x 1 million | -5,045,294,317,426 |
Two weeks following initiation of peptide-based MS therapy. Test data end-results were similar to those illustrated in Table 4. Formula to Estimate MS Activity Factor (MSAF) = Net IgE/Net KL x 1,000,000. KL = specific (kappa + lambda) signal x 25,000 (Compensates for 1/25,000 serum dilution).
Table 6.
4 Weeks Post-Commencement of MS/Mimotopic Peptide Therapy
| Epitope-Specific Serum IgE Signal: | |||
|
| |||
| ADARM | Pre-Rx | 701 | |
| ADARM | Post-Rx | -726 | |
| HSYQE | Pre-Rx | 1,836 | |
| HSYQE | Post-Rx | -357 | |
| KTGQF | Pre-Rx | 575 | |
| KTGQF | Post-Rx | -794 | |
|
| |||
| Epitope-Specific Serum (kappa + lambda) Signal: | |||
|
| |||
| ADARM | Pre-Rx | 1,314 | |
| ADARM | Post-Rx | -1,542 | |
| HSYQE | Pre-Rx | 4,798 | |
| HSYQE | Post-Rx | -985 | |
| KTGQF | Pre-Rx | 1,010 | |
| KTGQF | Post-Rx | -1,732 | |
|
| |||
| ADARM before Treatment: | ADARM after Treatment: | ||
|
| |||
| KL | 32,853,058 | KL | -38,542,461 |
| E/KL | 0.000021 | E/KL | -0.000019 |
| x 1 million | 21 | x 1 million | -19 |
|
| |||
| HSYQE before Treatment: | HSYQE after Treatment: | ||
|
| |||
| KL | 119,942,036 | KL | -11,994,204 |
| E/KL | 0.382737 | E/KL | -0.361929 |
| x 1 million | 382,737 | x 1 million | -361,929 |
|
| |||
| KTGQF before Treatment: | KTGQF after Treatment: | ||
|
| |||
| KL | 25,252,754 | KL | -1,732 |
| E/KL | 0.000023 | E/KL | -19,858,822 |
| x 1 million | 23 | x 1 million | -19,858,822,385,775 |
Four weeks following initiation of peptide-based MS therapy. Test data end-results were similar to those illustrated in Table 4. Formula to Estimate MS Activity Factor (MSAF) = Net IgE/Net KL x 1,000,000. KL = specific (kappa + lambda) signal x 25,000 (Compensates for 1/25,000 serum dilution).
Table 7.
35 Weeks Post-Commencement of MS/Mimotopic Peptide Therapy
| Epitope-Specific Serum IgE Signal: | |||
|
| |||
| ADARM | Pre-Rx | 249 | |
| ADARM | Post-Rx | -65 | |
| HSYQE | Pre-Rx | 278 | |
| HSYQE | Post-Rx | -64 | |
| KTGQF | Pre-Rx | 10 | |
| KTGQF | Post-Rx | -145 | |
|
| |||
| Epitope-Specific Serum (kappa + lambda) Signal: | |||
|
| |||
| ADARM | Pre-Rx | 1,766 | |
| ADARM | Post-Rx | -1,100 | |
| HSYQE | Pre-Rx | 2,145 | |
| HSYQE | Post-Rx | -761 | |
| KTGQF | Pre-Rx | 1,898 | |
| KTGQF | Post-Rx | -1,272 | |
|
| |||
| ADARM before Treatment: | ADARM after Treatment: | ||
|
| |||
| KL | 44,146,126 | KL | -27,502,151 |
| E/KL | 0.000006 | E/KL | -0.000002 |
| x 1 million | 6 | x 1 million | -2 |
|
| |||
| HSYQE before Treatment: | HSYQE after Treatment: | ||
|
| |||
| KL | 53,635,961 | KL | -5,363,596 |
| E/KL | 0.129790 | E/KL | -0.084132 |
| x 1 million | 129,790 | x 1 million | -84,132 |
|
| |||
| KTGQF before Treatment: | KTGQF after Treatment: | ||
|
| |||
| KL | 47,448,139 | KL | -1,272 |
| E/KL | 0.000000 | E/KL | -3,629,189 |
| x 1 million | 0.2 | x 1 million | -3,629,188,922,536 |
Thirty-five weeks following peptide-based MS therapy initiation. Test data end-results were similar to those illustrated in Table 4. Formula to Estimate MS Activity Factor (MSAF) = Net IgE/Net KL x 1,000,000. KL = specific (kappa + lambda) signal x 25,000 (Compensates for 1/25,000 serum dilution).
Table 8.
Epitope-specific Serum IgE/(kappa + lambda) Titer Ratios
| Pre-Treatment Serum Tested 1-35 Weeks After Therapy Commencement | |||
|
| |||
| ADARM | HSYQE | KTGQF | |
|
| |||
| 1 Week: | 25 | 555k | 26 |
| 2 Weeks: | 818 | 400 | 1.7k |
| 4 Weeks: | 21 | 283 | 23 |
| 35 Weeks: | 6 | 130k | 0.2 |
|
| |||
| Serum Obtained and Tested 1-35 Weeks After Therapy Commencement | |||
|
| |||
| ADARM | HSYQE | KTGQF | |
|
| |||
| 1 Week: | Less than zero | Less than zero | Less than zero |
| 2 Weeks: | Less than zero | Less than zero | Less than zero |
| 4 Weeks: | Less than zero | Less than zero | Less than zero |
| 35 Weeks: | Less than zero | Less than zero | Less than zero |
The Table depicts a quantitative summary of Table sets 4, 5, 6, and 7, wherein the MS patient’s pretreatment serum sample was test-positive and corresponding post-treatment samples were test-negative.
Commencing in the fourth week after initiating treatment, the MS patient exhibited gradual improvement in clinical status. Specific changes included: (a) improved balance when walking; (b) recovered sensation on the front surface of his right leg that had been lost after his last relapse ten years earlier; and (c) improved auditory discrimination, both subjectively and by audiometry. Prior to initiation of peptide therapy, the patient was able to walk supported for a distance of 60-70 feet (20 meters). This increased to 50 meters by week 8 and to 100 meters using a walking stick for balance (ED-SS 6.0) by week 35. A repeated MRI was unchanged. Neither he nor the control subjects experienced any adverse side effects.
Discussion
The MS pilot study demonstrated that peptide-based immunotherapy can eliminate myelin surface-directed, epitope-specific, autoantibodies, especially IgE. The results described here suggest that there was a cessation, within the MS patient, of myelin-directed mast cell degranulation and ongoing neuronal injury. Consistent with this premise, the subject recovered tactile sensation and exhibited improved ambulatory function, as well as improved auditory discrimination.
While a single therapeutic success is statistically insufficient, it does, nevertheless, establish a positive precedent against which larger, more in-depth clinical trial series might be judged. In order to confirm and build upon these satisfying, but preliminary, findings, a number of sequential tasks must follow. Newly diagnosed and therapeutically unencumbered MS patients of both genders should be tested to confirm that they also are IgE dimer-positive against PLP and/or MOG. Patients not consuming steroids, conventional MS pharmaceuticals, or cognition-enhancing drugs are likely to be dimer test-positive, while MS patients being concurrently treated with such pharmaceuticals are likely to be serologically negative [3].
Table 9 depicts a positive, although not perfect, outcome following peptide therapy onset and can be best described as a good beginning to be followed by enhanced investigation.
Table 9.
Multiple Sclerosis Disease Course Following Onset of Mimotopic Peptide Therapy
| Initial Clinical Presentation: |
| Ambulatory Function: |
| Patient required constant bilateral assistance to walk 20 meters maximum (EDSS 6.5). |
| Sensory Function: |
| Absence of sensation over right anterior leg surface of 10 year’s duration. |
| No other discernible sensory changes. |
| Clinical Presentation 8 Weeks After Commencing Treatment: |
| Ambulatory Function: |
| Patient required constant bilateral assistance to walk 50 meters (EDSS 6.5). |
| Sensory Function: |
| Some return of sensation over right anterior leg surface. |
| Experiencing generalized, non-irritating hyperesthesia. |
| Clinical Presentation 35 Weeks After Commencing Treatment: |
| Ambulatory Function: |
| Patient able to walk to walk 100 meters using walking stick for tactile balance (EDSS 6.0). |
| Sensory Function: |
| Return of sensation over right anterior leg surface. |
| Experiencing: generalized, non-irritating hyperesthesia and improved auditory acuity. |
The Table depicts a qualitative, time-relative summation of the clinical outcome of treating a single MS patient with myelin-matched mimotopic peptides.
MS patients consuming anti-inflammatory or psychotropic pharmaceuticals might be experiencing an ongoing IgE-mediated, neurogenic pathology that is not detectable by conventional serum immunoassays. Thus, MS patients that have elevated anti-myelin serum IgE levels, as well as those that do not, have the potential to benefit from the peptide-based intervention described here. The peptides’ low expected toxic potential, based on this study and an earlier inspection of bacterial and viral genomes for potentially adverse humoral cross-reactivity [3], is also advantageous.
A future clinical study might encompass the following: (1) a repeated, but more in-depth, animal toxicity study which, besides mice or rats, would include dogs and/or monkeys; (2) a peptide-based therapeutic trial of 10-20 patients per MS category (patients exhibiting relapsing remitting, chronic progressive, and primary progressive MS); and (3) a larger, statistically valid number of healthy control subjects.
Pretreatment MRI and cerebrospinal fluid studies should be performed, in order to gauge MS lesion status. After commencing treatment, in-depth neurological examinations of each pat- ient, as well as repeated MRI and cerebrospinal fluid studies, should be carried out at reasonable time intervals to ascertain purposeful improvement or a disease-stabilizing effect. Patients who exhibit resolute radiographic evidence of lesion resolution should continue their weekly peptide therapeutic injections indefinitely with medical evaluation over time.
Patients who lack significant physical improvement but have MRI and/or CSF indications of improvement can be treated with varied doses of the three peptides described in this study. An additional and potentially therapeutic modality, alone or in combination with the above-mentioned peptide therapy, could be comprised of the three key myelin basic protein-derived dimeric peptides, DNTFKD, HGRTQ, and QDTAVT that were described in our initial study [3]. Inclusion of beta interferon, Glatiramer Acetate (Copaxone), Mitoxantrone (Novantrone), or Natalizumab (Tysabri) is yet another approach that might be beneficial.
Conclusion
IgE-mediated mast cell degranulation appears to be an important factor in multiple sclerosis causation and sustenance, and, if validated, peptide-based immunotherapy could engender therapeutic benefit and, potentially, life-long remission.
Convincing proof of this premise requires in-depth investigation and commitment by other investigators and institutions to perform enlarged and substantiated clinical trials.
Acknowledgements
The author wishes to thank Lee Munder, Steve Holland and Larry James for their efforts and dedication to the MS therapy project; and Michael Verlander and Christopher Brooks for their comments and helpful suggestions in composing this article. Funds were provided by Enteron, Dallas, Texas, USA.
Disclosure of conflict of interest
The author has no conflict of interest.
Abbreviations
- CNS
central nervous system
- MS
multiple sclerosis
- HSA
human serum albumin
- PLP
proteolipid protein
- MOG
myelin oligodendrocyte glycoprotein
- MBP
myelin basic protein
- PBST
phosphate buffered saline plus Tweeen20
- PEG
polyethylene glycol
References
- 1.Hania K, Kreymborg K, Hania K, Kreymborg K, Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human Th 17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane IJ, Forester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 3.Calenoff E. Interplaying factors that affect multiple sclerosis causation and sustenance. ISRN Neurol. 2012;2012:851541. doi: 10.5402/2012/851541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai M, Grimbaldeston M, Galli SJ. Mast cells and immunoregulation and immunomodulation. Adv Exp Med Biol. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 5.Brown FR 3rd, Karthigasan J, Singh I, Kirschner DA. X-ray diffraction analysis of myelin lip id/proteolipid protein multilayers. J Neurosci Res. 1989;24:192–200. doi: 10.1002/jnr.490240210. [DOI] [PubMed] [Google Scholar]
- 6.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson SGO. ImmunoCAP specific IgE test: an objective tool for research and routine allergy diagnosis. Expert Rev Mol Diagn. 2004;4:273–279. doi: 10.1586/14737159.4.3.273. [DOI] [PubMed] [Google Scholar]
- 8.Levy J, Barnett EV, MacDonald NS, Klinenberg JR. Altered immunoglobulin metabolism in systemic lupus erythematosus and rheumatoid arthritis. J Clin Invest. 1970;49:708–715. doi: 10.1172/JCI106283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morell A, Skavaril F, Roseda G, Barandun S. Metabolic properties of human IgA subclasses. Clin Exp Immunol. 1973;13:521–528. [PMC free article] [PubMed] [Google Scholar]
- 10.Hellman L. Regulation of IgE homeostasis, and the identification of potential targets for therapeutic intervention. Biomed Pharmacother. 2007;61:34–49. doi: 10.1016/j.biopha.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Esposito P, Barbero L, Caccia P, Caliceti P, D'Antonio M, Piquet G, Veronese FM. PEGylation of growth hormone-releasing hormone (GRF) analogues. Adv Drug Deliv Rev. 2003;55:1279–1289. doi: 10.1016/s0169-409x(03)00109-1. [DOI] [PubMed] [Google Scholar]