Abstract
Introduction
Most patients survive many years following external beam radiotherapy (RT) for nonmetastatic prostate cancer and are therefore at risk for late treatment sequelae. The relationships between RT dose, treatment technique, and late toxicity rates are incompletely understood. Here we perform a meta-analysis and systematic review to characterize those effects.
Materials and methods
We performed a review of published series that report late gastrointestinal (GI) and genitourinary (GU) toxicity rates following definitive RT for prostate cancer using the RTOG Late Radiation Morbidity Scoring Schema. Univariate analyses were performed to test RT technique, RT dose, pelvic irradiation, and androgen deprivation therapy (ADT) as predictors of moderate (grade ≥ 2) and severe (grade ≥ 3) GI and GU toxicity. To isolate the effect of radiotherapy dose on late toxicity, we also performed a meta-analysis restricted to randomized trials that tested RT dose escalation. Statistical analyses were repeated using the subset of studies that utilized escalated RT doses.
Results
Twenty published reports detailing the treatment techniques and toxicity outcomes of 35 patient series including a total of 11,835 patients were included in this analysis. Median rates of moderate late toxicity were 15% (GI) and 17% (GU). For severe effects, these values were 2% (GI) and 3% (GU). Meta-analysis of five randomized trials revealed that an 8–10 Gy increase in RT dose increases the rate of both moderate (OR = 1.63, 95% CI: [1.44 to 1.82], p < 0.001) and severe (OR = 2.03, 95% CI: [1.64 to 2.42], p < 0.001) late GI toxicity. Among 17 series where doses of at least 74 Gy were utilized, use of intensity-modulated radiotherapy (IMRT) or proton beam radiotherapy (PBRT) was associated with a significant decrease in the reported rate of severe GI toxicity compared to 3-D RT.
Conclusion
Meta-analysis of randomized dose escalation trials demonstrates that late toxicity rates increase with RT dose. Series where dose escalated RT is delivered using IMRT or PBRT have relatively short follow up but report lower late GI toxicity rates than those employing 3-D RT.
Keywords: prostate cancer, radiation therapy, late toxicity, IMRT, proton, dose
Introduction
The majority of men diagnosed with nonmetastatic prostate cancer will live longer than 10 years, irrespective of how they are managed.1–3 Patient longevity is more closely tied to age and comorbid conditions than to characteristics of their malignancy or treatment.4–7 Survivorship issues are therefore central to the discussion of management options for prostate cancer.
For prostate cancer patients treated with radiotherapy (RT), dose escalation has been shown to improve biochemical disease control but not overall survival.8–12 Increased RT dose was also linked to increases in late gastrointestinal (GI)8,9,11,12 and genitourinary (GU)8 toxicity in some of those trials. Nonetheless, dose escalation has been adopted by radiation oncologists around the world.13–15
Increasingly, prostate cancer patients are being treated with advanced techniques such as intensity-modulated photon radiotherapy (IMRT) or proton beam radiotherapy (PBRT).16,17 These techniques may improve RT toxicity by limiting the dose delivered to normal tissues such as the rectum and bladder.18–21 Unfortunately, randomized trial data proving the benefits of these resource-intensive treatment modalities are lacking. Published reviews on this subject have been hampered by the inclusion of studies that utilized varying treatment techniques, RT doses, and late toxicity scoring schema.22,23
Here we perform a systematic review and meta-analysis of published reports to determine late toxicity rates following definitive radiotherapy for prostate cancer using a single toxicity scale. We also investigate the statistical relationship between RT technique and late treatment sequelae following dose-escalated RT.
Materials and methods
Search strategy and selection criteria
We performed a PubMed literature search for published series describing the late toxicity of definitive RT for localized prostate cancer. The search was restricted to English-language articles and included the key words “prostate cancer” and “radiation therapy”. In order to permit comparison across studies, only those reports that used the RTOG/EORTC Late Radiation Morbidity Scoring Criteria, Table 1, for grading toxicity were considered in this analysis. Furthermore, series were excluded if they included less than 50 patients, had a median follow up time of less than 36 months, or lacked a description of the RT doses and techniques utilized.
TABLE 1.
RTOG/EORTC late radiation morbidity scoring schema
| Organ | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Bladder | Slight epithelial atrophy, minor telangiectasia (microscopic hematuria) | Moderate frequency, generalized telangiectasia, intermittent macroscopic hematuria | Severe frequency and dysuria, severe generalized telangiectasia (often with petachiae), frequent hematuria, reduction in bladder capacity (< 150 cc) | Necrosis/contracted bladder (capacity < 100 cc), severe hemorrhagic cystitis | Death |
| Small/large intestine | Mild diarrhea, mild cramping, bowel movement 5 times daily, slight rectal discharge or bleeding | Moderate diarrhea and colic, bowel movement > 5 times daily, excessive rectal mucus or intermittent bleeding | Obstruction or bleeding requiring surgery | Necrosis/perforation, fistula | Death |
Data analysis
As patient-level data were not available to us for this review, we performed a study-level meta-analysis. We tabulated the RT doses, RT techniques, and rates of moderate (grade ≥ 2) and severe (grade ≥ 3) GI (bowel) and GU (bladder) toxicity reported in each series that met the criteria described above. We also noted the proportion of patients that received whole pelvic radiotherapy (WPRT) and the portion treated with androgen deprivation therapy (ADT).
In the two series where the rate of ADT use was not described,24,25 we approximated its value as the average rate of ADT use in the other series (where that rate was not 0% or 100%). For series in which fraction sizes other than 1.8 Gy-2.0 Gy were used,25–28 we converted nominal total dose to a bioequivalent dose using a fraction size of 2.0 Gy using the Linear Quadratic Equation with α/β = 3 Gy for late effects.29
Univariate linear regression was utilized to identify factors associated with each type (GI or GU) and severity (moderate or severe) of toxicity. Categorical variables tested included study type (prospective versus retrospective) and treatment technique (2-D RT versus 3-D RT versus IMRT versus PBRT). Continuous variables tested included median RT dose, rate of pelvic radiotherapy use, rate of hormonal therapy use, and timing of toxicity reporting (defined as time point for actuarial toxicity rates or median follow up length for studies that reported crude toxicity incidences).
To isolate the effect of radiotherapy dose on late toxicity, we performed a meta-analysis using randomized trials that tested RT dose escalation. A Q-test was performed to test for heterogeneity across studies; a fixed-effects model was utilized when there was no evidence of heterogeneity.
Additionally, we evaluated the effects of treatment technique on late toxicity in the setting of dose-escalated RT. Multilinear regression was utilized to test both treatment technique and timing of toxicity reporting as predictors of each type and severity of toxicity.
Results
Series
We identified 20 articles that provided satisfactory details, including a total of 11,835 patients treated with definitive radiotherapy for localized prostate cancer, Table 2. We defined a “series” of patients as a group of patients treated with a relatively uniform approach for which late toxicity rates were reported. Therefore, a randomized trial reporting outcomes for two study arms would provide two “series”. The 20 articles utilized for this analysis included five phase III studies of RT dose escalation (ten patient series),8–12 five phase III studies testing the use or timing of ADT (nine series),30–34 three phase I-II trials (seven series),25,27,35 and seven retrospective studies (nine series),24,26,28,36–39 for a total of 35 patient series. Two-dimensional RT was used in nine series, 3-D RT in 17 series, IMRT in six series, and PBRT in three series. Restricting this analysis to studies that used the RTOG/EORTC late toxicity scoring system excluded 8 articles that otherwise would have met all inclusion criteria.
TABLE 2.
Study characteristics
| Author | No. of patients | Median follow up (mths) | RT technique | RT dose (Gy) | Pelvic RT (%) | ADT (%) | Grade 2 GI toxicity (%) | Grade 3 GI toxicity (%) | Grade 2 GU toxicity (%) | Grade 3 GU toxicity (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ataman30 | 377 | 42 | 2D | 70.0 | 17% | 50%+ | 9.8% | 0.3% | 15.9% | 3.4% |
| Beckendorf8 | 256 | 57 | 3D | 70.0 | 0% | 0% | 14.4% | 2.0% | 9.8% | 2.6% |
| 80.0 | 19.6% | 5.9% | 17.6% | 2.0% | ||||||
| Bolla31 | 970 | 77 | 3D | 70.0 | 100% | 100% | NR | 0.6% | NR | 2.2% |
| De Meerleer36 | 133 | 36 | IMRT | 76.0 | 0% | 59% | 17.3% | 0.8% | 21.8% | 3.0% |
| Dearnaley9 | 421 | 60 | 3D | 64.0 | 0% | 100% | 24.0% | 6.0% | 8.0% | 2.0% |
| 422 | 74.0 | 33.0% | 10.0% | 11.0% | 4.0% | |||||
| Horwitz32 | 1521 | 135 | 2D | 70.0# | 100% | 100% | 16.2% | 1.8% | 19.1% | 4.7% |
| Kupelian26 | 770 | 45 | IMRT | 77.0^^^ | 0% | 60% | 6%^ | 2%^ | 7%^ | 0.2%^ |
| Lawton33 | 467 | 97 | 2D | 70.0# | 100% | 0% | NR | 4.3% | NR | 8.8% |
| 477 | 97 | 100% | NR | 3.6% | NR | 7.3% | ||||
| 231 | 83 | 0% | NR | 4.3% | NR | 8.2% | ||||
| 223 | 83 | 100% | NR | 2.2% | NR | 7.6% | ||||
| Michalski35 | 75 | 114 | 3D | 68.4 | 0% | 12% | 9.3% | 1.6% | 24.0% | 1.6% |
| 97 | 103 | 73.8 | 51% | 7.2% | 0.0% | 21.6% | 2.9% | |||
| 103 | 105 | 77.2@ | 44% | 10.7% | 1.8% | 18.4% | 4.2% | |||
| 115 | 88 | 74.0 | 39% | 10.4% | 2.4% | 28.7% | 4.7% | |||
| 118 | 72 | 78.0 | 20% | 25.4% | 5.4% | 22.9% | 5.5% | |||
| Peeters10 | 331 | 51 | 3D | 68.0 | 0% | 22% | 27%^ | 4%^ | 41%^ | 12%^ |
| 333 | 78.0 | 21% | 32%^ | 5%^ | 39%^ | 13%^ | ||||
| Pollack11 | 148 | 60 | 2D | 70.0 | 0% | 0% | 11.5% | 0.7% | 8.8% | 1.4% |
| 151 | 3D | 78.0 | 25.5% | 6.7% | 12.8% | 2.7% | ||||
| Quon27 | 97 | 39 | IMRT | 77.0^^^ | 100% | 100% | 6.5% | 0.0% | 9.7% | 4.3% |
| Roach34 | 638 | 60 | 2D | 70.2 | 0% | 100% | NR | 0.8% | NR | 2.4% |
| 641 | 100% | 1.9% | 2.5% | |||||||
| Slater24 | 643 | 43 | PBRT | 74.5 (GyE) | 50% | NR | 21%^^ | 0%^^ | 5.7%^^ | 0.3%^^ |
| Vesprini25 | 89 | 52 | IMRT | 72.0^^^ | 0% | NR | 7.9% | 1.1% | 14.6% | 0.0% |
| Vora37 | 271 | 62 | 3D | 68.4 | 0% | 18% | 16.2% | 0.7% | 21.4% | 4.8% |
| 145 | 48 | IMRT | 75.6 | 0% | 30% | 24.1% | 1.4% | 28.3% | 5.5% | |
| Zapatero38 | 306 | 64 | 3D | 78.0 | 36% | 95% | 10.1% | NR | 14.7% | NR |
| Zeitman12 | 196 | 66 | PBRT | 70.2 (GyE) | 0% | 0% | 8.2% | 0.5% | 19.4% | 1.5% |
| 195 | 79.2 (GyE) | 17.4% | 0.5% | 20.4% | 0.5% | |||||
| Zelefsky39 | 363 | 42 | 3D | 70.2 | 0% | 29% | 7%^ | 0%^ | 8%^ | NR |
| 380 | 75.6 | 16%^ | 1.6%^ | 15%^ | NR | |||||
| Zilli28 | 82 | 52 | IMRT | 78.4^^^ | 0% | 13% | 4.9% | 1.2% | 7.3% | 1.2% |
RT = radiotherapy; GI = gastrointestinal/bowel; GU = genitourinary/bladder; Gy = gray; GyE = gray equivalent; IMRT = intensity-modulated radiotherapy; PBRT = proton radiotherapy; ADT = androgen deprivation therapy; NR = not reported
median dose;
mean dose;
approximate dose;
actuarial incidence at 5 years;
actuarial incidence at 3 years;
bioequivalent dose;
median PTV dose.
Median follow up ranged from 36 to 135 months (median: 60). Crude toxicity rates were reported in 29 series, actuarial rates of toxicity at 5 years were reported in five series, and actuarial toxicity incidence at 3 years was reported in one series. The time point at which toxicities were reported (median follow up or actuarial reporting time) varied with treatment modality as follows: 3.5 to 11.3 years (median 6.9 years) for 2-D RT, 4.8 to 9.5 years (median 5.0 years) for 3-D RT, 3.0 to 5.0 (median 4.0 years) years for IMRT, and 3.0 to 5.5 years (median 5.5 years) in PBRT series. These differences were statistically significant (p = 0.026, using 1-way ANOVA test).
Median RT dose was 72.0 Gy (range: 64.0 Gy-80.0 Gy). In all nine of the 2-D RT series, the RT dose was 70.0 Gy or 70.2 Gy. All patients received ADT in nine series, and in eight other series no patients received ADT. In the remaining 18 series, the rate of ADT use ranged from 12%–95% (median: 33%). WPRT was utilized uniformly in eight series and for a portion of patients in three series.
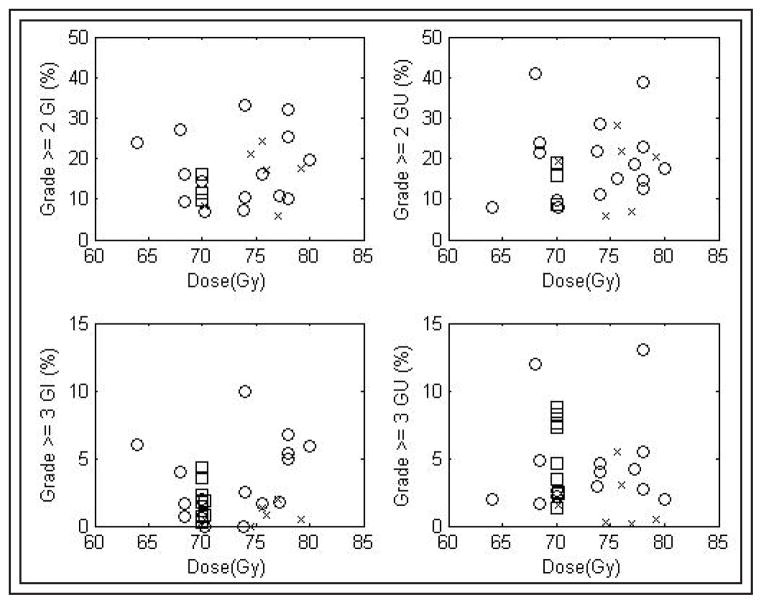
Grade ≥ 2 toxicity rates varied widely between series, Figure 1. The median rate of late GI toxicity ≥ grade 2 was 15% (range: 5%–33%, mean 16%). For late GU toxicity ≥ grade 2, median incidence was 17% (range: 6%–41%, mean 18%). Grade ≥ 3 events were less common. The median rate of late GI toxicity ≥ grade 3 was 2% (range: 0%–10%). For late GU toxicity ≥ grade 3, median incidence was 3% (range: 0%–13%).
Figure 1.
Late toxicity rates.
(□ = 2-D RT, ○ = 3-D RT, × = IMRT, △ = PBRT).
Univariate analyses – all series
Results of univariate analyses are shown in Table 3. There was no significant (p < 0.05) association between RT technique or dose, WPRT use, ADT administration, or the time point at which toxicities were reported and the reported rates of moderate or severe GI or GU toxicity. There was a trend (p = 0.071) suggesting that lower grade ≥ 3 late GU toxicity rates were reported in the 3 PBRT series than in the 17 3-D series.
TABLE 3.
Univariate analyses for late toxicity. Parameters (except for RT technique) were tested as continuous variables.
| Grade ≥ 2 late GI toxicity Coefficient (95% CI, p value) | Grade ≥ 3 late GI toxicity Coefficient (95% CI, p value) | Grade ≥ 2 late GU toxicity Coefficient (95% CI, p value) | Grade ≥ 3 late GU toxicity Coefficient (95% CI, p value) | |
|---|---|---|---|---|
| 3-D RT (n = 17) | reference | reference | reference | reference |
| 2-D RT (n = 9) | −3.5% ([−13.9 to 6.8%], p = 0.488) | −0.2% ([−2.1 to 1.6%], p = 0.791) | −3.3% ([−14.8 to 8.2%], p = 0.556) | 1.6% ([−0.9 to 4.2%], p = 0.202) |
| IMRT (n = 6) | −5.8% ([−13.4 to 1.8%], p = 0.126) | −0.2% ([−3.7 to 0.5%], p = 0.139) | −3.5% ([−12.2 to 5.1%], p = 0.405) | −2.0% ([−4.9 to 1.0%], p = 0.182) |
| PBRT (n = 3) | −0.1% ([−10.6 to 10.3%], p = 0.977) | −2.2% ([−5.1 to 0.6%], p = 0.118) | −2.7% ([−14.2 to 8.8%], p = 0.636) | −3.5% ([−7.4 to 0.3%], p = 0.071) |
| RT dose | 0.1% per Gy ([−0.7 to 0.9%], p = 0.755) | 0.1% per Gy ([−0.2 to 0.3%], p = 0.587) | 0.0% per Gy ([−0.8 to 0.9%], p = 0.966) | −0.1% per Gy ([−0.4 to 0.2%], p = 0.514) |
| Pelvic RT | −4.3% ([−16.1 to 7.4%], p = 0.455) | −0.3% ([−2.3 to 1.7%], p = 0.746) | −6.6% ([−19.4 to 6.3%], p = 0.305) | 2.0% ([−0.6 to 4.6%], p = 0.130) |
| ADT use | 0.1% ([−9.6 to 9.8%], p = 0.977) | 0.0% ([−2.2 to 2.2%], p = 0.981) | −5.8% ([−16.2 to 4.7%], p = 0.269) | −0.2% ([−3.2 to 2.9%], p = 0.910) |
| Reporting time | −0.6% per year ([−2.3 to 1.1%], p = 0.458) | 0.1% per year ([−0.4 to 0.5%], p = 0.682) | −1.2% per year ([−0.6 to 3.0%], p = 0.183) | 0.4% per year ([−0.2 to 1.0%], p = 0.224) |
GI = gastrointestinal; GU = genitourinary; CI = confidence interval; IMRT = intensity-modulation radiation therapy; PBRT = proton beam radiotherapy; RT = radiotherapy; ADT = androgen deprivation therapy.
Meta-analysis of dose escalation trials
Five randomized trials testing dose escalation were utilized for meta-analysis. RT doses in the control groups ranged from 64 Gy to 70.2 Gy, and doses in the experimental arms were 74 Gy to 80 Gy. In each trial, the difference between the low and high dose arms was 8 Gy-10 Gy. Four studies utilized 3-D RT techniques,8–11 and one used PBRT.12
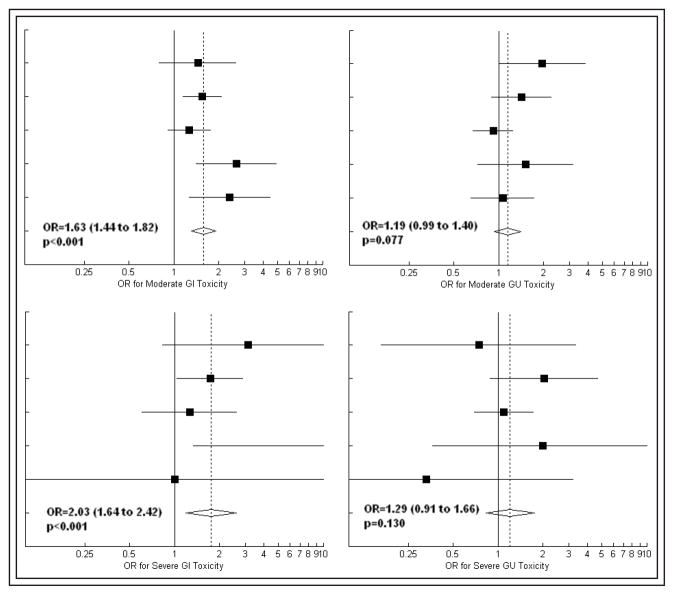
There was no evidence of heterogeneity (p > 0.10) with regards to the effect of dose escalation on any severity (grade ≥ 2 or grade ≥ 3) or type (GI or GU) of toxicity. Forest plots depicting the fixed-effects meta-analysis results are shown in Figure 2. Dose escalation was associated with an increase in late GI toxicity, with odds ratios of 1.63 (95% CI: [1.44 to 1.82], p < 0.001) and 2.03 (95% CI: [1.64 to 2.42], p < 0.001) for moderate and severe effects, respectively. There were also trends for increased late GU complication rates with dose escalation, with odds ratios of 1.19 (95% CI: [0.99 to 1.40], p = 0.077) and 1.29 (95% CI: [0.91 to 1.66], p = 0.130) for moderate and severe toxicity, respectively.
Figure 2.
Forest plots depicting the effect of dose escalation on late toxicity in five randomized trials (Beckendorf et al, Dearnaley et al, Peeters et al, Pollack et al, and Zeitman et al, from top to bottom). Area of solid squares is proportional to sample size. Extent of diamond represents the 95% confidence interval for the pooled estimate of effect size.
OR = odds ratio, GI = gastrointestinal, GU = genitourinary.
Separate analysis of series using escalated RT doses
Seventeen of 35 series utilized RT doses of at least 73.8 Gy. None of those series utilized 2-D RT, 10 used 3-D RT, 5 used IMRT, and 2 used PBRT. Among this subset of high dose studies, there was significant association between treatment modality and the time point at which toxicities were reported; that time point ranged from 4.8 to 8.8 years (median 5.2 years) for 3-D RT series and 3.0 to 5.5 (median 4.0 years) for IMRT and PBRT series. The variation of toxicity reporting time point with treatment modality was statistically significant (p = 0.028) using a 1-way ANOVA test.
The results of a multivariate analysis examining the effects of treatment modality and timing of toxicity reporting on each toxicity endpoint for high dose series are shown in Table 4. Compared with 3-D RT, use of IMRT was associated with a statistically significant (p < 0.05) decline in the reported rates of grade ≥ 2 and grade ≥ 3 late GI toxicity. Use of PBRT was associated with decreased grade ≥ 3 late GI toxicity as well as a trend towards decreased grade ≥ 3 late GU toxicity. There was no evidence that morbidity rates increased with longer follow up before toxicity reporting. In fact, increasing time to toxicity reporting was associated with a statistically significant (p < 0.05) decrease in the incidence of both moderate and severe GI sequelae.
TABLE 4.
Multivariate analyses assessing the impact of treatment technique and time of toxicity reporting on late toxicity rates in series using ≥ 73.8 Gy. Bolded regions indicate a statistically significant effect (p < 0.05).
| Grade ≥ 2 late GI toxicity Coefficient (95% CI, p value) | Grade ≥ 3 late GI toxicity Coefficient (95% CI, p value) | Grade ≥ 2 late GU toxicity Coefficient (95% CI, p value) | Grade ≥ 3 late GU toxicity Coefficient (95% CI, p value) | |
|---|---|---|---|---|
| 3-D RT (n = 9) | reference | reference | reference | reference |
| IMRT (n = 5) | −15.4% ([−26.7 to −4.2%], p = 0.011) | −5.5% ([−8.8 to −2.1%], p = 0.004) | −3.1% ([−16.9 to 10.6%], p = 0.629) | −3.2% ([−8.1 to 1.7%], p = 0.182) |
| PBRT (n = 3) | −6.5% ([−20.4 to 7.3%], p = 0.329) | −5.9% ([−9.9 to −1.8%], p = 0.008) | −5.3% ([−22.1 to 11.5%], p = 0.508) | −5.4% ([−11.3 to 0.4%], p = 0.065) |
| Reporting time | −3.7% per year ([−6.8 to −0.6%], p = 0.023) | −1.0% per year ([−11.3 to 0.4%], p = 0.038) | −1.0% per year ([−2.8 to 4.8%], p = 0.578) | −0.5% per year ([−1.8 to −0.8%], p = 0.440) |
GI = gastrointestinal, GU = genitourinary, CI = confidence interval, IMRT = intensity-modulation radiation therapy, PBRT = proton beam radiotherapy, RT = radiotherapy, ADT = androgen deprivation therapy.
Discussion
In this study, we utilized published data from 35 patient series to investigate the effects of RT dose and technique on late GI and GU toxicity following definitive treatment for prostate cancer. The analysis incorporated the reported results of a total of nearly 12,000 prostate cancer patients who were treated with a variety of RT techniques and evaluated for toxicity using a single instrument (RTOG/EORTC Late Radiation Morbidity Scoring Criteria). Our findings indicate that severe (grade ≥ 3) late GI and GU events are rare, typically occurring in less that 5% of patients. Our meta-analysis of randomized dose-escalation trials revealed that an increase in RT dose from 64–70 Gy to 74–80 Gy may approximately double the rate of severe late GI toxicity. In the setting of dose escalation, reported GI toxicity rates were lower in series employing IMRT or PBRT rather than 3D-RT. Although follow-up tends to be shorter in series using these advanced treatment techniques, we did not find that toxicity rates increase with the interval to toxicity reporting.
Techniques in radiotherapy for localized prostate cancer have changed significantly over the past decade. Level I evidence supports RT dose escalation for localized prostate cancer patients in all risk groups.8–12 This has led to the utilization of higher RT doses around the world.13–15 Over the same time span, there has been a shift towards increasingly advanced and costly RT techniques.13,14,17 While use of high technology, expensive methods for patient immobilization, target visualization, and conformal treatment delivery has become commonplace, there are no randomized data supporting their adoption. Since advanced RT techniques are now considered standard for prostate cancer, it is unlikely that such trials will ever be carried out.
Previous reviews have been unable to define the effects of RT dose and RT technique on late morbidity. An Association for Healthcare Research and Quality report has concluded that there is little or no difference in acute or late GI or GU toxicities between higher and lower dose RT. The ICER review rated IMRT as “unproven with potential” in comparison to 3-D RT.22 Hummel et al performed a systematic review comparing IMRT to 3-D RT and prostatectomy for prostate cancer, and they concluded that “toxicity can be reduced by increasing conformality of treatment… which can be more easily achieved with IMRT than 3-D CRT.”23 Finally, Bekelman et al recently presented a SEER-Medicare analysis of complication rates following prostatic radiotherapy using 3-D RT and IMRT.16 They concluded that the use of IMRT was associated with only a moderate reduction in GI toxicity and that treatment modality did not significantly affect urinary complication rates. As the authors point out, they were unable to account for RT dose. As there have been trends towards both dose escalation and increased utilization of advanced treatment techniques in recent years,13–15, 17 we suspect a correlation between IMRT use and RT dose that may cause the benefits of IMRT to be understated in a population-based study.
The present report addresses some of the shortcomings of previous reviews. To facilitate comparison of outcomes across studies, we restricted our analysis to series that used a single instrument to score toxicity. When all 35 patient series were compared, reported toxicity rates did not seem to vary with treatment technique or RT dose. Meta-analysis of randomized dose escalation trials, however, indicated that the rates of moderate or severe late GI toxicity may increase with RT dose. Thus, we repeated our analysis using only the 17 patient series in which the median RT dose was at least 73.8 Gy. Among those studies, reported rates of severe GI toxicity were lower in series employing IMRT or PBRT than in those using 3-D RT.
We did not detect any relationship between the use of ADT or pelvic RT and the rates of late complications. While this may seem to contradict other reports,33,34 it may be an artifact of the retrospective nature of this study. Pelvic RT, for example, was rarely utilized in series using escalated RT doses. Thus, a modest increase in toxicity caused by pelvic RT might have been overshadowed by the larger effect of total prescription dose. In series where pelvic RT and ADT were not prescribed uniformly, selection bias may also mask potential effects on complication rates.
There are several other limitations to the conclusions that can be drawn from this study. This is a retrospective analysis, and some of the data were extracted from retrospective reports. Scoring late radiotherapy toxicity is somewhat subjective, as prostate cancer patients are at risk for developing GI and GU symptoms from a number of causes. This is particularly important for rare events such as severe toxicity, where a few misattributions can significantly alter the reported incidence rate. Relatively few variables could be tested in this study; advances such as improvements in patient immobilization, image-guided RT techniques, increased use of high energy photons, and improved supportive care may affect treatment toxicity but were beyond the scope of this analysis. Patient-specific factors such as age, comorbidities, and genetic features likely affect the risk of morbidity but could not be accounted for. Of note, median follow up was significantly shorter in series employing IMRT than in those where 2-D or 3-D RT was utilized. While it is known that toxicity can develop many years after RT for prostate cancer,40,41 we could not detect a positive correlation between the time point at which toxicity was reported and incidence of any toxicity. Additional study is necessary to determine if the benefits associated with IMRT and PBRT in this analysis are maintained with longer follow up.
Conclusion
Our meta-analysis of randomized trials, using a uniform toxicity scale, indicates that late toxicity rates increase with RT dose. Published series in which dose escalated RT is delivered using IMRT or PBRT have relatively short follow up but report lower late GI toxicity rates than those employing 3-D RT to deliver similar doses. This analysis supports the role of IMRT or PBRT for the safe delivery of dose escalated RT, and these observations should be further evaluated through prospective studies.
References
- 1.Liu L, Coker AL, Du XL, et al. Long-term survival after radical prostatectomy compared to other treatments in older men with local/regional prostate cancer. J Surg Oncol. 2008;97(7):583–591. doi: 10.1002/jso.21028. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 4.Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 5.Taira AV, Merrick GS, Galbreath RW, et al. Factors impacting all-cause mortality in prostate cancer brachytherapy patients with or without androgen deprivation therapy. Brachytherapy. 2010;9(1):42–49. doi: 10.1016/j.brachy.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Davies BJ, Smaldone MC, Sadetsky N, et al. The impact of obesity on overall and cancer specific survival in men with prostate cancer. J Urol. 2009;182(1):112–117. doi: 10.1016/j.juro.2009.02.118. discussion 117. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, Chen MH, Beard CJ, et al. Radiation with or without 6 months of androgen suppression therapy in intermediate- and high-risk clinically localized prostate cancer: a postrandomization analysis by risk group. Int J Radiat Oncol Biol Phys. 2010;77(4):1046–1052. doi: 10.1016/j.ijrobp.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Beckendorf V, Guerif S, Le Prise E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–1063. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 10.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 12.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa K, Nakamura K, Sasaki T, et al. Radical external beam radiotherapy for clinically localized prostate cancer in Japan: changing trends in the patterns of care process survey. Int J Radiat Oncol Biol Phys. 2011;81(5):1310–1318. doi: 10.1016/j.ijrobp.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Martin J, Rodrigues G, Malone S, et al. Changing management of localized prostate cancer: a comparison survey of Ontario radiation oncologists. Can J Urol. 2006;13(Suppl 2):226–233. [PubMed] [Google Scholar]
- 15.Zelefsky MJ, Moughan J, Owen J, et al. Changing trends in national practice for external beam radiotherapy for clinically localized prostate cancer: 1999 Patterns of Care survey for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59(4):1053–1061. doi: 10.1016/j.ijrobp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Bekelman JE, Mitra N, Efstathiou J, et al. Comparative effectiveness of intensity modulated (IMRT) versus 3D conformal (CRT) radiotherapy for non-metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(3 Suppl):S77. [Google Scholar]
- 17.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S 2004. Cancer. 2005;104(6):1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 18.De Meerleer GO, Vakaet LA, De Gersem WR, et al. Radiotherapy of prostate cancer with or without intensity modulated beams: a planning comparison. Int J Radiat Oncol Biol Phys. 2000;47(3):639–648. doi: 10.1016/s0360-3016(00)00419-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee CT, Dong L, Ahamad AW, et al. Comparison of treatment volumes and techniques in prostate cancer radiation therapy. Am J Clin Oncol. 2005;28(6):618–625. doi: 10.1097/01.coc.0000172281.32437.d4. [DOI] [PubMed] [Google Scholar]
- 20.Luxton G, Hancock SL, Boyer AL. Dosimetry and radiobiologic model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2004;59(1):267–284. doi: 10.1016/j.ijrobp.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Chera BS, Vargas C, Morris CG, et al. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75(4):994–1002. doi: 10.1016/j.ijrobp.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 22.Ollendorf DA, Hayes J, McMahon P, et al. Management options for low-risk prostate cancer: a report on comparative effectiveness and value. Boston, MA: Institute for Clinical and Economic Review; 2009. [Google Scholar]
- 23.Hummel S, Simpson EL, Hemingway P, et al. Intensity-modulated radiotherapy for the treatment of prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2010;14(47):1–108. iii–iv. doi: 10.3310/hta14470. [DOI] [PubMed] [Google Scholar]
- 24.Slater JD, Yonemoto LT, Rossi CJ, Jr, et al. Conformal proton therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 1998;42(2):299–304. doi: 10.1016/s0360-3016(98)00225-9. [DOI] [PubMed] [Google Scholar]
- 25.Vesprini D, Sia M, Lockwood G, et al. Role of principal component analysis in predicting toxicity in prostate cancer patients treated with hypofractionated intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81(4):e415–e421. doi: 10.1016/j.ijrobp.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68(5):1424–1430. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 27.Quon H, Cheung PC, Loblaw DA, et al. Hypofractionated concomitant intensity-modulated radiotherapy boost for high-risk prostate cancer: late toxicity. Int J Radiat Oncol Biol Phys. 2012;82(2):898–905. doi: 10.1016/j.ijrobp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Zilli T, Jorcano S, Rouzaud M, et al. Twice-weekly hypofractionated intensity-modulated radiotherapy for localized prostate cancer with low-risk nodal involvement: toxicity and outcome from a dose escalation pilot study. Int J Radiat Oncol Biol Phys. 2010;81(2):382–389. doi: 10.1016/j.ijrobp.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 29.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 30.Ataman F, Zurlo A, Artignan X, et al. Late toxicity following conventional radiotherapy for prostate cancer: analysis of the EORTC trial 22863. Eur J Cancer. 2004;40(11):1674–1681. doi: 10.1016/j.ejca.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26(15):2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 33.Lawton CA, Bae K, Pilepich M, et al. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02. Int J Radiat Oncol Biol Phys. 2008;70(2):437–441. doi: 10.1016/j.ijrobp.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roach M, 3rd, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21(10):1904–1911. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3D conformal radiation therapy for prostate cancer from the RTOG 9406 phase I/II dose escalation study. Int J Radiat Oncol Biol Phys. 2010;76(1):14–22. doi: 10.1016/j.ijrobp.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Meerleer GO, Fonteyne VH, Vakaet L, et al. Intensity-modulated radiation therapy for prostate cancer: late morbidity and results on biochemical control. Radiother Oncol. 2007;82(2):160–166. doi: 10.1016/j.radonc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Vora SA, Wong WW, Schild SE, et al. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68(4):1053–1058. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Zapatero A, Garcia-Vicente F, Martin de Vidales C, et al. Long-term results after high-dose radiotherapy and adjuvant hormones in prostate cancer: how curable is high-risk disease? Int J Radiat Oncol Biol Phys. 2011;81(5):1279–1285. doi: 10.1016/j.ijrobp.2010.07.1975. [DOI] [PubMed] [Google Scholar]
- 39.Zelefsky MJ, Cowen D, Fuks Z, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85(11):2460–2468. doi: 10.1002/(sici)1097-0142(19990601)85:11<2460::aid-cncr23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 40.Gardner BG, Zietman AL, Shipley WU, et al. Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol. 2002;167(1):123–126. [PubMed] [Google Scholar]
- 41.Giordano SH, Lee A, Kuo YF, et al. Late gastrointestinal toxicity after radiation for prostate cancer. Cancer. 2006;107(2):423–432. doi: 10.1002/cncr.21999. [DOI] [PubMed] [Google Scholar]