SUMMARY
Synaptic contacts are largely established during embryogenesis and are then maintained during growth. To identify molecules involved in this process we conducted a forward genetic screen in C. elegans and identified cima-1. In cima-1 mutants, synaptic contacts are correctly established during embryogenesis, but ectopic synapses emerge during post-developmental growth. cima-1 encodes a solute carrier in the SLC17 family of transporters that includes Sialin, a protein that when mutated in humans results in neurological disorders. cima-1 does not function in neurons but rather functions in the nearby epidermal cells to correctly position glia during post-larval growth. Our findings indicate that CIMA-1 antagonizes the FGF receptor (FGFR), and does so most likely by inhibiting FGFR’s role in epidermal-glia adhesion rather than signaling. Our data suggest that epidermal-glia crosstalk, in this case mediated by a transporter and the FGF receptor, is vital to preserve embryonically-derived circuit architecture during post-developmental growth.
INTRODUCTION
The nervous system is largely established during embryogenesis, but connectivity persists throughout the lifetime of the organism (Benard and Hobert, 2009). For example, in C. elegans, as in other metazoans, neural circuitry is laid out largely during embryogenesis (Sulston et al., 1983). Thereafter the worm grows 100-fold in volume, yet axonal architecture and synaptic contacts are maintained (Benard and Hobert, 2009; Knight et al., 2002). Genetic studies have identified molecules required for the maintenance of axon positions during growth and movement, including L1-CAM, F-spondin, the FGF Receptor among others (Aurelio et al., 2002; Benard and Hobert, 2009; Benard et al., 2012; Benard et al., 2006; Bulow et al., 2004; Johnson and Kramer, 2012; Pocock et al., 2008; Sasakura et al., 2005; Woo et al., 2008). This work indicates two important features regarding the maintenance of nervous system architecture during development. First, the molecules required for maintenance of axon position are distinct from those required for circuit formation. Second, these studies underscore the importance of regulated adhesion in maintenance of axon position.
Circuit architecture also requires the maintenance of synaptic contacts. Synaptic maintenance studies have largely focused on the stability of synapses (Lin and Koleske, 2010; Shi et al., 2012; Wilcox et al., 2011). From these studies we know that neuron-glia interactions can play key roles in the maintenance of synaptic stability (Pfrieger, 2010). Less is known about how synaptic distribution is maintained during post-developmental growth. Synaptic distribution, which is critical for maintenance of the embryonically-derived circuit architecture, requires both maintenance of correct synaptic contact and prevention of formation of inappropriate contacts.
We perform a screen in C. elegans and identify cima-1. In cima-1 mutants, inappropriate contacts between glia and axons promote the formation of ectopic synapses. Glia inappropriately contact axons in these mutants, likely due to increased adhesion with epidermal cells during growth. CIMA-1 is a SLC17 family solute transporter that modulates epidermal-glia interaction via FGFR. We reveal a potential mechanism for the role of SLC17 transporters in maintenance of synaptic distribution. Furthermore, we suggest that reducing adhesion during growth is as important as promoting adhesion to maintain correct synaptic connectivity.
RESULTS
AIY synapses form during embryogenesis and are maintained during growth
The AIY interneurons are a pair of bilaterally symmetric neurons in the nematode nerve ring (Figure 1A). Although these neurons contact many potential synaptic partners, they display remarkable specificity for both synaptic partner and position (White et al., 1986). In adult animals, the observed pattern of synaptic outputs in AIY is reproducible across animals (Colón-Ramos et al., 2007). To determine when AIY synaptic outputs are established, we examined the AIY presynaptic pattern in C. elegans larval stages using GFP::RAB-3 (Nonet et al., 1997). We observed that the presynaptic pattern was already established by the time the animals hatched at larval stage 1 (L1) (Figures 1A, 1B, 1C and (Colón-Ramos et al., 2007)). We also observed that while the length of the neurite and synaptic zones increase as the animal grows, the relative distribution of presynaptic sites is maintained (Figures 1B, 1C, 1J and 1K). Our findings indicate that the presynaptic pattern in the AIY interneuron is established during embryogenesis, and is maintained as the animal grows.
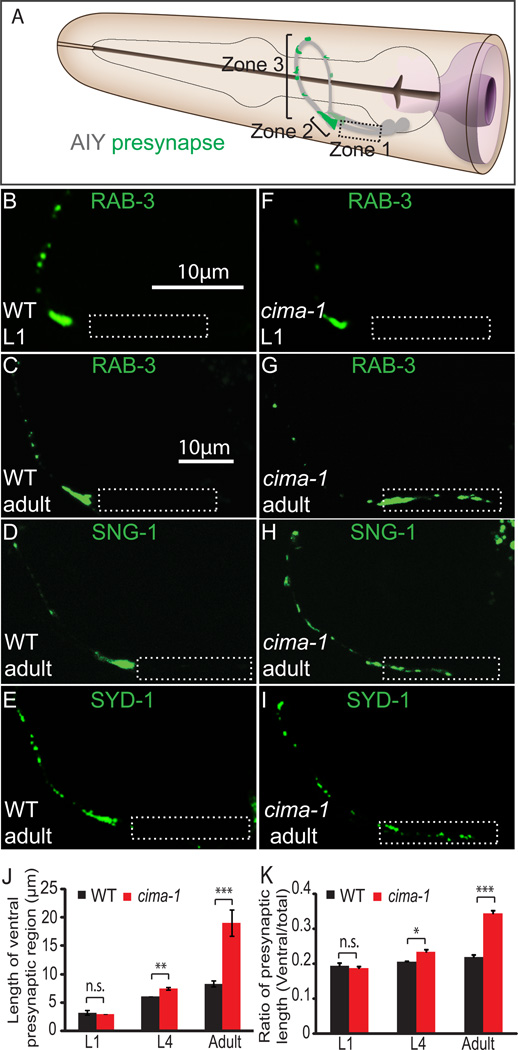
Figure 1. cima-1 is required for maintenance of AIY presynaptic distribution during growth.
(A) Schematic diagram of bilaterally symmetric AIYs (grey) in the C. elegans head (modified from WormAtlas with permission). Green marks presynaptic positions. There are three distinct anatomical regions along the AIY neurite: a segment proximal to AIY cell body that is devoid of synapses (Zone 1, dashed box); a dense presynaptic region at the dorsal turn of the AIY neurite (Zone 2); and a region with discrete presynaptic clusters at the distal part of the neurite (Zone 3) (Colón-Ramos et al., 2007; White et al., 1986).
(B–E) The AIY presynaptic pattern in wild-type animals. Using synaptic vesicle associated GFP::RAB-3, we observe that AIY presynaptic pattern is already established in newly hatched larval L1 stage animals (B) and maintained in adults (C). A similar pattern was observed when we visualized synaptic vesicle protein SNG-1::YFP (D) and active zone protein GFP::SYD-1 (E) in adults and L1 larva (data not shown).
(F–I) cima-1(wy84) mutant animals fail to maintain the AIY presynaptic pattern in adults. cima-1(wy84) mutant L1 animals display a wild-type presynaptic pattern as visualized with GFP::RAB-3 (F). Adult animals display an abnormal presynaptic pattern as visualized with presynaptic proteins GFP::RAB-3 (G), SNG-1::YFP (H) and GFP::SYD-1 (I). The ectopic presynaptic structure was confirmed with fluorescence electron microscopy (fEM) (Figure S1). In all images, dashed box corresponds to normally asynaptic Zone 1, and scale bars are 10µm. (Scale bar in B applies to B and F; scale bar in C applies to C–E and G–I).
(J–K) Quantification of the AIY presynaptic pattern. Note that the length of ventral portion of the presynaptic region (presynaptic region in Zone 1 and Zone 2) is similar in cima-1(wy84) and wild-type animals at L1 stage, but becomes longer at L4 or adult stages (J). The ratio of the presynaptic length (the length of ventral presynaptic region divided by total presynaptic region as shown in K) is a metric that reflects the general pattern of AIY, and persists in wild-type animals during growth (black bars). Note how in cima-1(wy84) mutant animals this ratio becomes abnormally larger, particularly in post-developmental growth after the L4 stage (red bars). n≥34 for each group. Error bars are s.e.m., *: p<0.05, **: p<0.01, ***: p<0.001 by t-test comparison. See also Figure S1.
cima-1 inhibits ectopic synapses during growth
To identify the mechanisms underlying the maintenance of synaptic distribution during growth, we performed a visual forward genetic screen and isolated cima-1(wy84) (for circuit maintenance). GFP::RAB-3 distribution was indistinguishable between cima-1(wy84) and wild-type animals at larval L1 stage (Figures 1B, 1F, 1J and 1K). cima-1(wy84) adult animals, however, displayed a highly penetrant ectopic GFP::RAB-3 localization in the normally asynaptic Zone 1 of AIY (Figure 1G, >90% of animals, n>200 animals). Quantification of the cima-1(wy84) phenotype in adult animals revealed that while the length of Zone 3 remained similar between mutants and wild-type adult animals, the ventral presynaptic region (Zone 2, and ectopic presynaptic structures in Zone 1) was twice as long in cima-1(wy84) mutants (Figures 1G and 1J). The synaptic defect in cima-1(wy84) was confirmed using synaptic vesicle proteins SNB-1 and SNG-1, as well as active zone protein SYD-1 (Figure 1D–E, 1H–I and data not shown). Fluorescence electron microscopy (fEM) also demonstrated the presence of ectopic presynaptic sites in cima-1(wy84) animals (Figure S1) (Watanabe and Jorgensen, 2012; Watanabe et al., 2011). Together, our data indicate that while cima-1(wy84) is not required for establishing presynaptic distribution, but is required for maintaining it.
Ectopic presynaptic sites in cima-1 mutants are not onto postsynaptic partner RIA
The synapses in Zone 2 of wild-type animals are formed primarily onto postsynaptic partner RIA (Figure 2A and (White et al., 1986)). To determine whether the ectopic synapses are targeted to RIA, we simultaneously imaged RIA and the presynaptic sites in AIY. We observed that in wild-type and cima-1(wy84) juvenile animals, RIA projects to Zone 2 correctly (Figures 2B and 2F). However, we noted two differences in the adult mutants. First, in adult cima-1(wy84) animals the RIA neurite is extended posteriorly (Figure 2G). Second, we also observed ectopic presynaptic sites in Zone 1 that extend beyond the area of contact between AIY and RIA (compare Figures 2C with 2G, 2D with 2H).
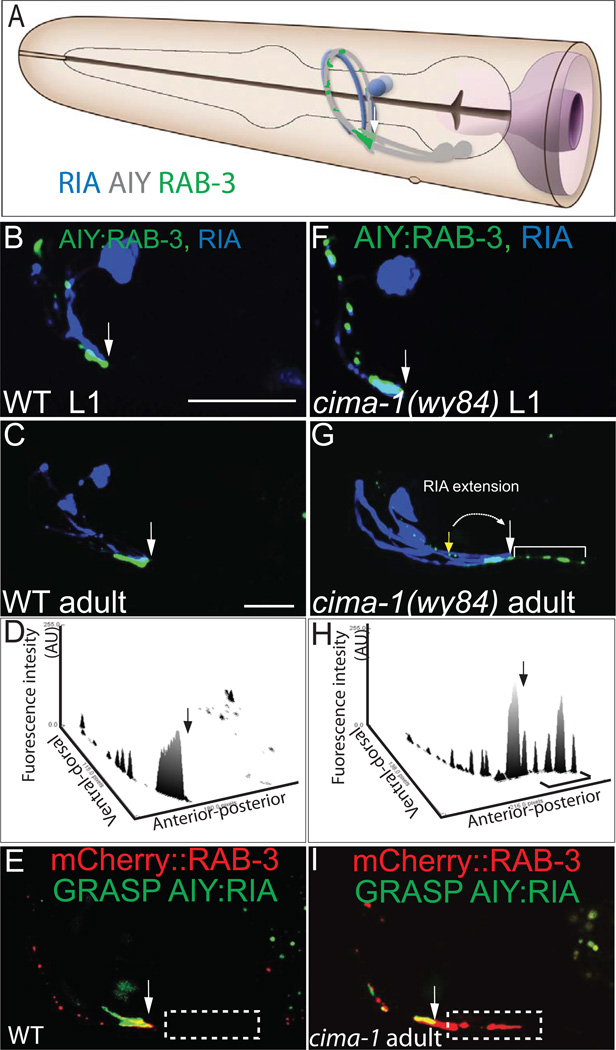
Figure 2. cima-1 (wy84) mutants have a posteriorly extended Zone 2 and ectopic presynaptic structures in Zone 1.
(A) AIY (grey) forms synapses onto postsynaptic partner RIA (blue) in Zone 2 (Colón-Ramos et al., 2007; White et al., 1986); Schematic diagram modified from WormAtlas with permission. (B–C) Simultaneous visualization of synaptic vesicles in AIY (pseudocolor green) and postsynaptic GLR-1 sites in RIA (pseudocolor blue) in a wild-type animal. Note that in both L1 (B) and adults (C), RIA contacts AIY in Zone 2, and not in Zone 1. The arrow indicates the transition between Zone 2 and Zone 1, as determined by the position where RIA contacts AIY.
(D) Three dimensional profile of AIY presynaptic CFP::RAB-3 (pseudocolor green) fluorescence intensity (arbitrary units) of (C). The arrow indicates the transition between Zone 2 and Zone 1.
(E) Simultaneous visualization of GRASP GFP (which indicates contact between presynaptic neuron AIY and postsynaptic partner RIA) and mCherry::RAB-3 in a wild type animal. Note that the AIY:RIA contact indicated by GFP overlaps with presynaptic mCherry::RAB-3 at Zone 2 region.
(F–G) The AIY presynaptic pattern and RIA morphology are wild type in newly hatched (L1 stage) cima-1(wy84) mutant animals (F). However, at the adult stage the RIA neurite is posteriorly extended (from AIY ventral turn indicated by yellow arrow to the posterior site indicated by white arrow) and ectopic presynapses are seen in Zone 1 (bracket) (G). (H) Three dimensional profile of AIY presynaptic GFP::RAB-3 fluorescence intensity (arbitrary units) of the image in (G).
(I) Simultaneous visualization of GRASP GFP and mCherry::RAB-3 in a cima-1(wy84) animal. Note that presynaptic mCherry::RAB-3 extends beyond the AIY:RIA contact region (indicated by GRASP GFP signal).
Ectopic presynaptic sites in AIY are bracketed. All arrows except the yellow in (G) indicate the end of Zone 2 and the beginning of Zone 1. The yellow arrow in (G) indicates where the end of Zone 2 and the beginning of Zone 1 should be. Scale bars are 10µm (scale bar in B applies to B and F; scale bar in C applies to C, G, E and I).
To further examine the relationship between AIY:RIA contact and the ectopic presynaptic sites in Zone 1, we performed GFP-reconstitution across synaptic partners (GRASP) (Feinberg et al 2008). GRASP is based on two GFP fragments (“GFP 1–10” and “GFP 11”) that can reconstitute a functional GFP molecule only when they are in close proximity. A version of GRASP based on the transmembrane protein CD4 allows assessment of cell-cell contact sites (Feinberg et al 2008). We expressed this version of GRASP in AIY (CD4::GFP 11) and RIA (CD4::GFP 1–10) to specifically visualize AIY:RIA contact, and simultaneously labeled AIY presynaptic sites with mcherry::RAB-3. We observed that ectopic presynaptic sites were present beyond the AIY:RIA contact region (Figure 2E and 2I). Our studies indicate that the abnormal distribution of presynaptic structures in cima-1(wy84) adults results from two events: a posterior displacement of synapses between AIY and RIA in Zone 2, and the emergence of ectopic presynaptic sites, which are not apposed to postsynaptic cell RIA, in Zone 1.
cima-1 encodes a membrane transporter in the SLC17 family
Our SNP mapping, genetic rescue and Sanger sequencing data suggest that the cima-1(wy84) allele corresponds to a G to A mutation in the unnamed gene F45E4.11, and that this mutation is predicted to alter a conserved glycine at residue 388 to glutamate (Figures 3A, 3B and 3F). A second allele of F45E4.11(gk902665), with a nonsense mutation at R476, phenocopies the cima-1(wy84) AIY defect (Figure 3C). We also observed a similar AIY presynaptic maintenance defect when knocking down F45E4.11 by RNAi (Figures 3E). Together, our genetic data indicate that cima-1(wy84) is a missense, loss-of-function mutation in F45E4.11 (hereafter called cima-1).
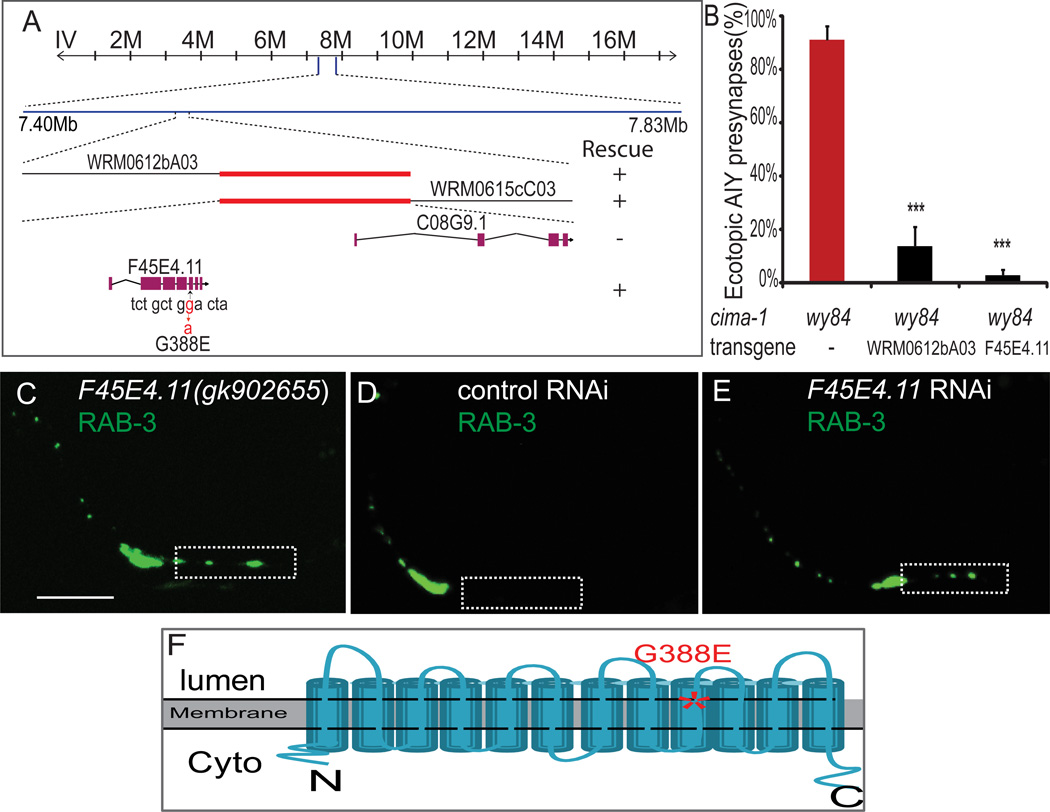
Figure 3. cima-1(wy84) is an allele of F45E4.11, which encodes a conserved SLC17 family transporter.
(A) SNP mapping indicates that the genetic lesion corresponding to the cima-1(wy84) allele is between 7.40Mb and 7.83Mb on chromosome IV. Two overlapping fosmids in this region (WRM0612bA03 and WRM0615cC03) rescue the cima-1(wy84) AIY presynaptic defect. Those two fosmids overlap in a genomic area that includes just two genes: F45E4.11 and C08G9.1. Only F45E4.11 was able to rescue the AIY defect. Sequencing data indicates a missense mutation in the coding region that alters conserved G388 to E.
(B) Quantification of the percentage of animals displaying the AIY presynaptic patterning defect in cima-1(wy84) mutants transformed with fosmid WRM0612bA03 (which includes gene F45E4.11) or with just gene F45E4.11. n≥86 for each category. Error bars are s.e.m., ***: p<0.001 by t-test.
(C) A different F45E4.11 allele, gk902655, contains a nonsense mutation in the coding region (R476 to opal stop codon) and phenocopies the wy84 allele.
(D, E) Knockdown of F45E4.11 by RNAi phenocopies the cima-1(wy84) presynaptic phenotype in AIY. Animals fed with bacteria transfected with control vector L4440 show normal AIY presynaptic distribution (D), while animals fed with bacteria expressing F45E4.11 dsRNA phenocopy the cima-1(wy84) AIY presynaptic phenotype (E). The scale bar is 10µm and applies to (C–E). In (C–E), Zone 1 region is highlighted with a dashed box.
(F) A schematic diagram of the predicted cima-1 topology. The mutated G388 (asterisk) in cima-1(wy84) is located in ninth transmembrane domain. CIMA-1 is a member of SLC17 transporter family (See also Figure S2).
cima-1 encodes a 12-transmembrane domain protein that is homologous to the SLC17 family of solute carrier transporters and similar to Sialin, a lysosomal transporter associated with neurodegenerative disease (Reimer, 2013; Sreedharan et al., 2010; Verheijen et al., 1999) (Figure 3F, and S2).
cima-1 acts in epidermal cells
To understand the mechanism by which cima-1 suppresses ectopic presynapses, we first examined its endogenous expression pattern. We generated a transcriptional GFP reporter and found cima-1 expression starts during embryogenesis and persists in adult animals (Figures 4A–4D and S3A–S3H). In adults, cima-1 is primarily expressed in epidermal cells. Expression was also observed in intestinal cells, but not in neurons (including AIY) or in glia (Figure S3E–S3H).
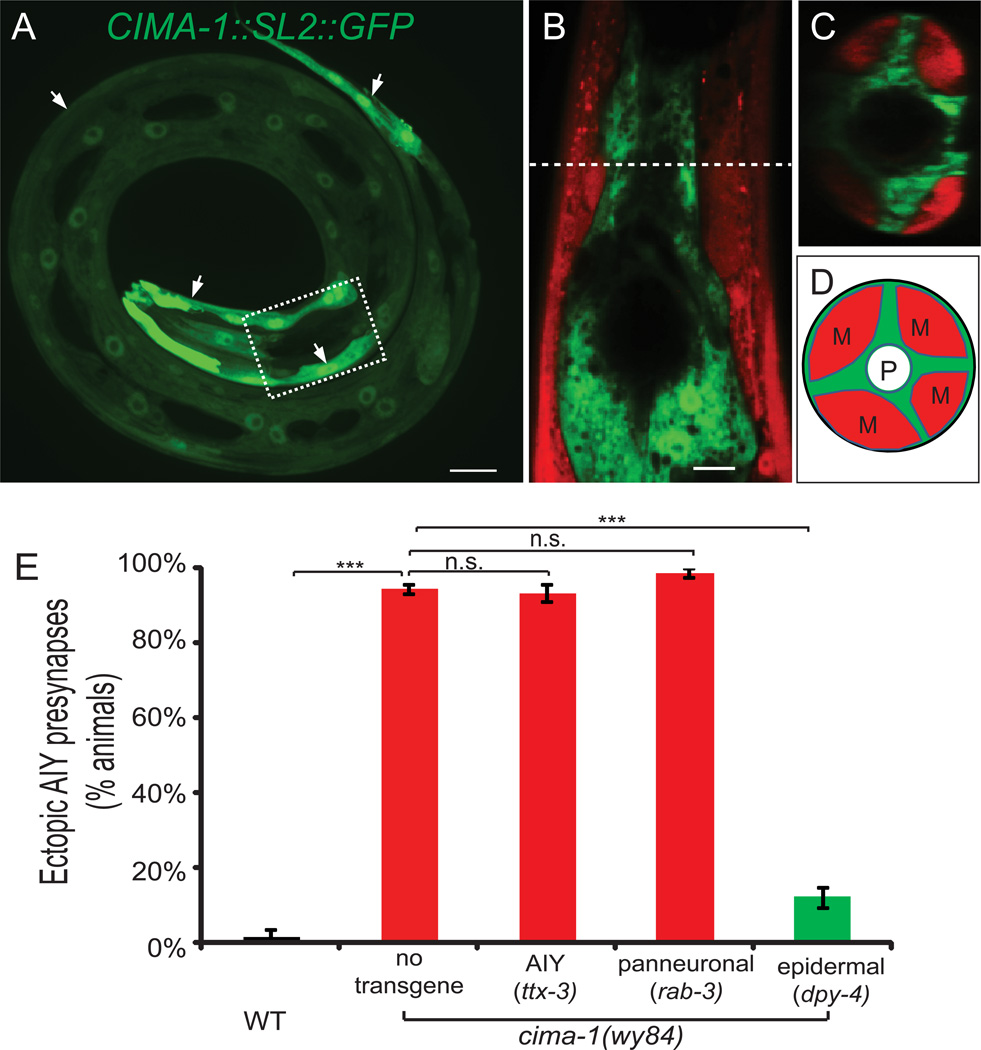
Figure 4. cima-1 is expressed and required in epidermal cells for maintenance of the AIY presynaptic pattern.
(A) A larval animal displaying the endogenous cima-1 expression pattern as determined by rescuing construct CIMA-1(genomic)::SL2::GFP. The dashed box corresponds to the region where AIY is located (AIY not shown; this region is also similar to the region examined in (B and C)). Arrows point at CIMA-1 expressing epidermal cells.
(B–D) Simultaneous visualization of cima-1(genomic)::SL2::GFP and body wall muscle reporter Pmyo-3::mcherry. Note the non-overlapping expressing pattern of cima-1 in epidermal cells and Pmyo-3::mcherry in muscles, both in the sagittal cross-section (B) and in the transverse cross-section (C). Dashed line in (B) indicates site of transverse cross-section image in (C). And (D) is the schematic drawing of (C), with muscle quadrants (“M”), epidermal cells (green) and pharynx (P) labeled.
(E) Quantification of tissue-specific rescue. Expression of cima-1 cDNA in AIY (ttx-3 promoter) or pan-neuronally (rab-3 promoter) does not rescue the AIY presynaptic defect in cima-1(wy84) mutant animals. However, expression of cima-1 cDNA in epidermal cells (dpy-4) robustly rescues the AIY presynaptic defect (see also rescue with epidermal promoters rol-6, dpy-7 and col-19 in Figure S3I). n≥50 for each group. Error bars are s.e.m., n.s.: not significant, ***: p<0.001 by t-test. Scale bars are 10µm. See also Figure S3, S4 and S5.
Consistent with the expression pattern, we observed that expression of CIMA-1 cell-specifically in epidermal cells results in robust rescue of the AIY phenotype (Figure 4E and S3I). Conversely, expression of CIMA-1 cell-specifically in the AIY interneurons (ttx-3 promoter), in all neurons (rab-3 promoter), or in neurons and intestine (aex-3 promoter) did not rescue (Figure 4E and S3I). These data suggest that cima-1 acts in epidermal cells to maintain the AIY presynaptic pattern.
We note that although cima-1 is required in epidermal cells, cima-1(wy84) mutant animals do not exhibit obvious defects in body morphology, epidermal cell morphology, cellular fusion events, epidermal development or general neuroarchitecture in larvae or young adults (Figure S4 and data not shown).
Next, we wanted to determine the temporal requirement of cima-1. As AIY presynaptic defects are only observed in adults, we hypothesized that expression of cima-1 after larval development would be sufficient to rescue the AIY presynaptic defect. To test this we expressed cima-1 using a col-19 promoter that expresses just in epidermal cells and after larval development (Cox and Hirsh, 1985; Liu and Ambros, 1991). We observed significant rescue of the AIY presynaptic defect, consistent with cima-1 playing a post-developmental role in epidermal cells to maintain synaptic distribution (Figure S3I).
The cima-1 phenotype is affected by animal size
Animals increase their size after concluding larval development. To examine if cima-1 is required to maintain synaptic positions during post-developmental growth, we visualized mutants that have abnormally short (dumpy or Dpy) or long (Lon) body length (Figures S5A–S5C; (Page and Johnstone, 2007)). The presynaptic pattern in Dpy animals retained a wild type distribution in AIY (data not shown). Similarly, lon-3(e2175) animals display a longer synaptic region in AIY, but their synaptic distribution does not phenocopy cima-1 (Figure S5C, S5F and S5J). However, cima-1(wy84) lon-3(e2175) double mutants display a significantly enhanced AIY presynaptic maintenance defect (Figures S5I and S5J). Importantly, the enhancement of the presynaptic distribution phenotype in cima-1(wy84) lon-3(e2175) double mutants was observed after late L4 stage (data not shown). Moreover, cima-1(wy84) dpy-4(e1166) double mutants suppressed the cima-1(wy84) AIY presynaptic defect (Figure S5E). This suppression is likely due to the shorter size of the animal, as a number of Dpy alleles with different genetic identities gave us the same result (dpy-7(e88), dpy-9(e12), and dpy-6(e14)) (Figure S5H and data not shown). Together, our findings support a role for cima-1 in maintaining presynaptic distribution during post-developmental growth.
cima-1 is required for maintenance of glial morphology during growth
The ventral cephalic sheath cells (VCSC) are non-neuronal cells (glia) similar to vertebrate astrocytes and are sandwiched between the cima-1-expressing hyp7 epidermal cell and the AIY interneurons (Figure 5A and (Shaham, 2006; White et al., 1986)). To visualize these glial cells, we expressed cytoplasmic mCherry using the glia-specific hlh-17 promoter (McMiller and Johnson, 2005).
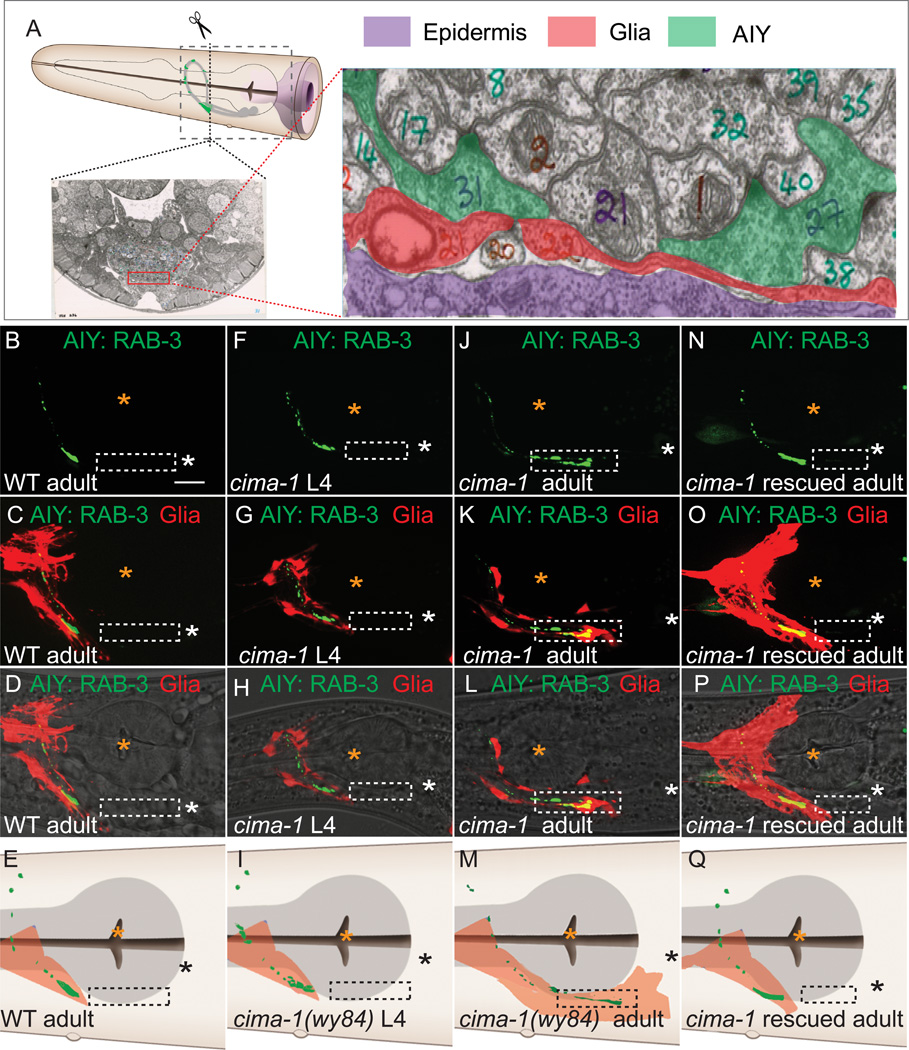
Figure 5. cima-1 is required for maintenance of VCSC glial morphology during growth.
(A) Relative position of epidermal cells, VCSC glia and AIY in C. elegans. A cross section of EM image of a wild-type animal in the Zone 2 region of AIY (from WormAtlas.org and WormImage.org)(White et al., 1986). VCSC glia (pseudocolored red in the micrograph) lie between the epidermal cells (purple) and AIY Zone 2 synapses (green, note vesicles and dense projections in the two AIY-neurite cross sections). The dashed box in the schematic of the worm head represents the region where images (and cartoons) in (B–Q) were obtained.
(B–Q) Simultaneous visualization of AIY presynaptic sites (green) and VCSC glia (red) in wild type adult animal (B–D), cima-1(wy84) L4 animal (F–H), cima-1 (wy84) adult animal (J–L), or cima-1(wy84) adult animal rescued with Pdpy-4::cima-1 (N–P). Images (D, H, L and P) are as (C, G,K and O), but overlaid with DIC. Note that in cima-1(wy84) adult animals, VCSC glia abnormally distend to the Zone 1 region and overlap with AIY ectopic presynapses (dashed box; J–M). Both glia and AIY presynaptic defects are rescued by expressing cima-1 cDNA in epidermal cells (N–Q). In all images, white and black asterisk represent the location of the AIY cell body, and orange asterisks mark pharyngeal grinder. Note that in K and L, synapses are formed in Zone 1, past the orange asterisk. (E, I, M, and Q) are cartoons of (D, H, L and P) with pharynx in grey (see schematic in A). The hlh-17 promoter labels both dorsal and ventral glial cells. The dorsal glia not labeled in (K and L) is due to the mosaic retention of the transgenic marker. Scale bar in (B) corresponds to 10µm and applies to all images. See also Figure S6.
In wild-type animals VCSC glia are in close proximity to AIY Zone 2, but are never observed near the asynaptic Zone 1 (Figures 5B–5E). In larval stages, cima-1(wy84) VCSC morphology is indistinguishable from that of wild-type animals (Figures 5F–5I). However in adult stages, the glial processes (end-feet) were abnormally distended posteriorly onto Zone 1 of AIY (Figures 5J–5M). Cell-specific expression of cima-1 in epidermal cells was sufficient to rescue the glial morphology (Figure 5N–5Q). Thus, cima-1 is required in epidermal cells for maintenance of glial morphology during post-larval growth.
Does position of the glial end-feet correlate with the emergence of ectopic synaptic sites? To address this question, we conducted longitudinal studies and observed a tight temporal and spatial correlation between the position of the glial endfeet and the position of AIY presynaptic sites (Figure S6). Our findings indicate that cima-1 acts in epidermal cells to maintain VCSC glial morphology during post-larval growth, and reveal a correlation between the defective maintenance of glial morphology, and the emergence of ectopic presynaptic sites in AIY.
Glial cell ablation suppresses ectopic synaptic sites in cima-1 mutants
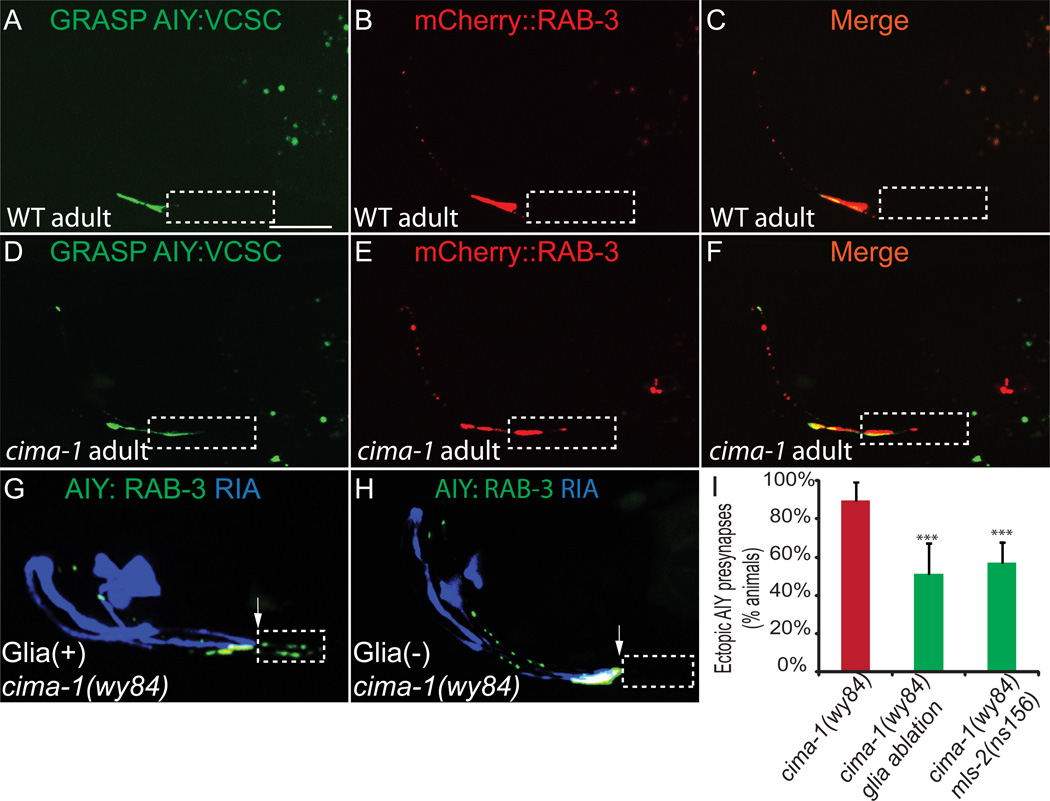
Our findings are consistent with a model in which distended glial end-feet in cima-1(wy84) mutants result in ectopic contact between glia and AIY, which in turn induces the formation of ectopic synaptic sites. To examine this hypothesis, we performed GFP-reconstitution across synaptic partners (GRASP) between glia and AIY (Feinberg et al 2008). We expressed CD4::GFP 11 in AIY and CD4::GFP 1–10 in glia to examine sites of AIY:glia contact. Consistent with published EM data (Figure S1A–S1B and (White et al., 1986)), we observed that in wild-type animals the GRASP signal was restricted to Zone 2 and co-localized with the synaptic marker mCherry::RAB-3 (Figures 6A–6C). cima-1(wy84) mutant animals displayed an ectopic GRASP signal in Zone 1 which co-localized with the ectopic mCherry::RAB-3 (Figures 6D–6F). Our findings suggest that ectopic AIY:glia contact in cima-1(wy84) mutant animals results in ectopic presynaptic specializations (Figures 6E and 6F).
Figure 6. VCSC glia abnormally contact AIY and are required for formation of ectopic presynaptic sites in Zone 1 in cima-1(wy84).
(A–F) Simultaneous visualization of GRASP GFP signal (which indicates contact between AIY and VCSC glia) (A, D) and mCherry::RAB-3 (B, E) in wild-type (A–C) and cima-1 (wy84) (D–F) adult animals. Note that in cima-1 (wy84) mutants VCSC glia abnormally contact AIY in Zone 1 (indicated by GRASP GFP in dashed box), and that these sites correlate with sites of ectopic mCherry::RAB-3 in Zone 1.
(G) In cima-1 (wy84) adult animals, AIY forms ectopic presynaptic sites in Zone 1 (dashed box) beyond Zone 2 (determined by the position of postsynaptic partner RIA in blue). Arrow indicates the end of Zone 2 and the beginning of Zone 1.
(H) As in (G), but with VCSC genetically ablated through the cell-specific expression of caspases. Note suppression of ectopic presynaptic sites in Zone 1 (dashes box).
(I) Quantification of the percentage of animals displaying ectopic presynaptic sites in Zone 1 in cima-1(wy84) adult mutants; in cima-1(wy84) adult mutants expressing caspases cell-specifically in VCSC glia, or in cima-1(wy84) mls-2(ns156) double mutants. The scale bar in (A) is 10µm and applies to (A–H). n≥37 for each genotype. Error bars represent 95% confidence interval. *** : p<0.001 between groups as determined by Fisher’s exact test. See also Figure S1.
To test if glia:AIY contact is required for ectopic synapse formation, we ablated VCSC in two ways. First, we genetically ablated the glia by expressing caspases in these cells (Chelur and Chalfie, 2007). The cell death was confirmed by the absence of VCSC GFP. We observed that cell-specific ablation of the glia in cima-1(wy84) mutants significantly suppressed ectopic presynaptic sites in Zone 1 (Figures 6H and 6I). Second, we examined the AIY presynaptic pattern in cima-1(wy84) mls-2(ns156) double mutants. mls-2 is a transcription factor required for VCSC development, and in mls-2(ns156) mutant animals the glia do not properly develop (Yoshimura et al., 2008). Consistent with caspase ablation results, we also observed that ectopic presynaptic sites in Zone 1 are suppressed in cima-1(wy84) mls-2(ns156) double mutant animals (Figures 6I). It should be noted however that loss of the glia in these experiments did not fully suppress the cima-1 phenotype. Incomplete suppression could result from two possibilities: 1) caspase and genetic ablations do not completely eliminate the glia, or 2) other tissues besides VCSC glia also influence the persistence of ectopic presynaptic sites in AIY.
Together, these data suggest that epidermally-expressed cima-1 regulates glial morphology during post-larval growth, and that maintenance of correct glial morphology during growth is required for preventing the emergence of ectopic presynaptic sites in AIY.
Ectopic synapses do not require embryonic specification genes
During embryogenesis AIY synapse formation requires UNC-6/Netrin, which is transiently expressed by VCSC glia (Colón-Ramos et al., 2007; Wadsworth et al., 1996). UNC-6 instructs presynaptic assembly by signaling through its receptor UNC-40/DCC, expressed in AIY. We used the Netrin receptor, unc-40, to test if Netrin signaling is required for the cima-1 mutant phenotype. To achieve this, we generated cima-1(wy84) unc-40(e271) double mutants and visualized the AIY presynaptic pattern. In juvenile worms, we observed a general reduction of presynaptic vesicle clusters in cima-1(wy84) unc-40(e271) compared to wild-type animals, as expected (Figure S7H and (Colón-Ramos et al., 2007)). In adults, cima-1(wy84) unc-40(e271) animals still displayed ectopic presynaptic sites in Zone 1 similar to cima-1(wy84) single mutants (Figures S7A–S7G). These findings suggest that VCSC glia use different molecular mechanisms for presynaptic assembly during embryonic development and for the maintenance of presynaptic distribution during postdevelopmental growth.
EGL-15(5A)/FGFR is required for ectopic synapse formation
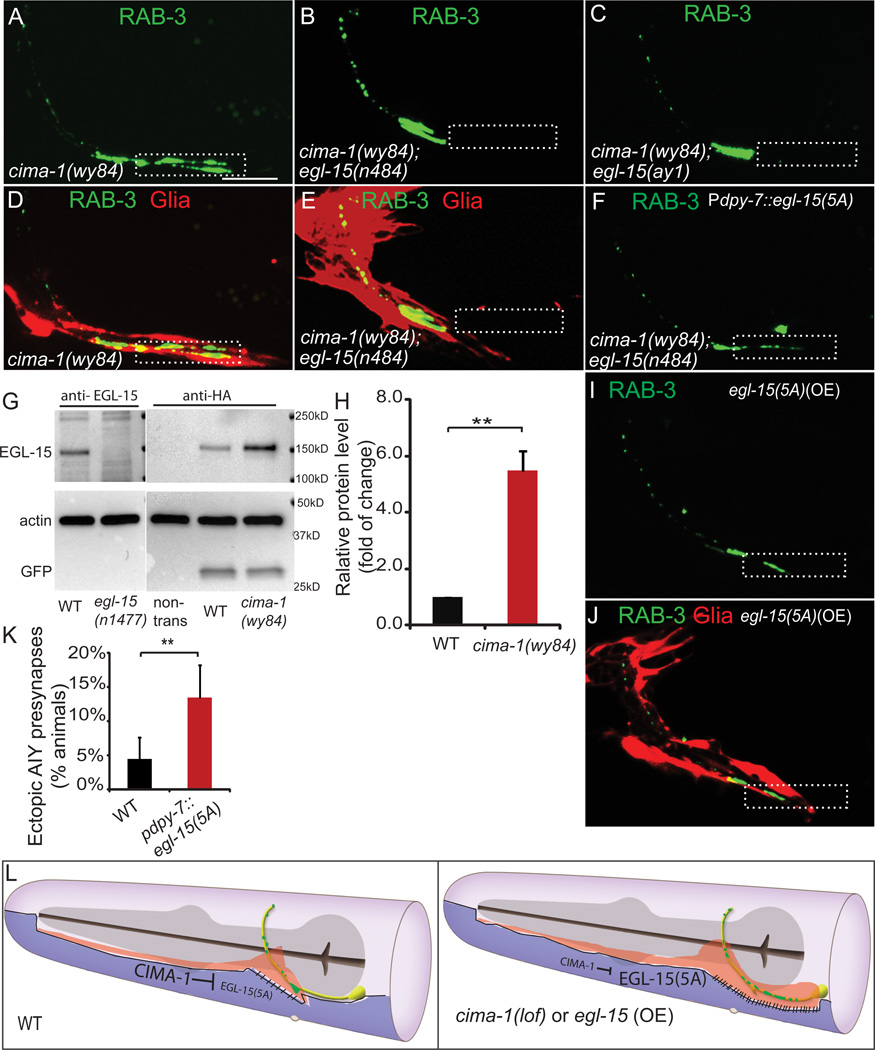
To understand the molecular mechanisms by which cima-1 affects AIY presynaptic maintenance during post-larval growth, we conducted candidate suppressor screens using RNAi and available mutants. These genetic approaches revealed that mutations of egl-15, the only FGFR in C. elegans, suppress the cima-1 AIY presynaptic defect (Figures 7A, 7B, 7C, and S7I; n>300 animals).
Figure 7. egl-15/fgfr is required for ectopic synapse formation in cima-1(wy84) animals.
(A–C) GFP::RAB-3 in cima-1(wy84) adult mutants (A), cima-1(wy84) egl-15(n484) double mutant (B) and cima-1(wy84) egl-15(ay1) double mutant (C). Note that alleles egl-15(n484) and egl-15(ay1), which specifically disrupt egl-15(5A) isoform, suppress cima-1(wy84) presynaptic distribution defect.
(D–E) Simultaneous visualization of presynaptic sites in AIY (green) and glia (red) in cima-1(wy84) (D) and cima-1(wy84) egl-15(n484) double mutant adult animals (E). Note that egl-15(n484) allele suppresses both the AIY presynaptic defect and the glia morphology defect in cima-1(wy84).
(F) Expression of egl-15(5A) cDNA in the epidermal cells (using the dpy-7 promoter) reverts the suppression of the cima-1(wy84) AIY presynaptic phenotype by egl-15(n484).
(G) EGL-15 crackle antibody (a gift from M Stern), detects endogenous EGL-15, the 141kD band in wild type (lane 1), but not in egl-15(n1477) mutants (lane 2), which produced the C-terminus truncated EGL-15 (M Stern, personal communication). HA antibody specifically recognizes HA-tagged EGL-15(5A) expressed in epidermal cells (lane 4 and 5), but not in wild type animals without the transgene (lane 3). For comparison in lanes 4 and 5, the same HA-tagged EGL-15(5A) expressing transgenic line were used. Note that EGL-15(5A) protein levels are higher in cima-1(wy84) mutant animals (lane 5) as compared to wild type animals (lane 4). Actin and coinjection marker Punc-122::GFP were used as loading control.
(H) Quantification of the EGL-15(5A)::HA protein levels from four independent blots. n≥150 for each group. Error bars are s.e.m., **: p<0.01 by student’s t-test.
(I–J) AIY presynaptic GFP::RAB-3 (I) is mislocalized to Zone 1 region abnormally ensheathed by VCSC (J) upon over-expression of egl-15(5A) in epidermal cells by using the dpy-7 promoter (compare to Fig. 5C).
(K) Quantification of the percentage of animals with the phenotype shown in (I). Error bars represent 95% confidence interval. ** : p<0.01 groups as determined by Fisher’s exact test. (L) A model for cima-1 and egl-15(5A) in epidermal cells (purple) regulating VCSC glia (red) morphology and AIY presynaptic distribution (green). In wild-type animals, cima-1 negatively regulates egl-15(5A), thereby reducing epidermal-glia adhesion and preventing glia extension during growth. This interaction contributes to maintaining wild type VCSC morphology, which in turn specifies correct synaptic distribution during growth (left cartoon). In animals with cima-1 loss-of-function, or animals in which egl-15(5A) is overexpressed, the interaction between the epidermal cells and VCSC is misregulated, resulting in VCSC glia distension posteriorly, ectopic contacts between glia and axons and ectopic presynaptic sites. See also Figure S7
egl-15/FGFR is required during C. elegans development for sex myoblast migration, axon outgrowth, and fluid homeostasis, and null alleles of egl-15 are larval lethal (Bulow et al., 2004; Goodman et al., 2003). Splicing isoform egl-15(5A), but not isoform egl-15(5B), is also required post-embryonically to maintain axon position during growth and movement (Bulow et al., 2004). We observed that egl-15(n484), a nonsense allele specific to the egl-15(5A) isoform, suppressed cima-1(wy84) ectopic presynaptic sites (Figures 7A, 7B and S7I). Similarly egl-15(ay1), an egl-15(5A) splicing acceptor mutation (Goodman et al., 2003), also suppressed cima-1(wy84) AIY ectopic synapses (Figures 7C and S7I). These data demonstrate that the formation of ectopic presynaptic sites in cima-1 mutants requires egl-15(5A).
We next examined VCSC glial morphology in cima-1(wy84) egl-15(n484) double mutants. We observed that egl-15(n484) also suppresses the abnormal glial morphology observed in cima-1(wy84) mutants (Figures 7D and 7E). Consistent with this observation, glia ablation did not enhance suppression of the cima-1(wy84) egl-15(n484) double mutants (Figure S7I). Our data suggest that egl-15(n484) suppresses the AIY presynaptic defect by suppressing abnormal VCSC glial morphology in cima-1(wy84) mutants.
EGL-15(5A)/FGFR acts in the epidermis independent of kinase activity
Expression of egl-15(5A) cDNA using a pan-neuronal promoter did not restore ectopic presynapses to egl-15(n484) cima-1(wy84) double mutants. However, expression of egl-15(5A) in epidermal cells restored ectopic presynapses to egl-15(n484) cima-1(wy84) double mutants (Figure 7F and S7I), suggesting that FGFR isoform 5A, like cima-1, acts in the epidermal cells.
EGL-15 is a receptor tyrosine kinase activated by FGF ligands, which activates downstream Ras pathways (Borland et al., 2001; Sundaram, 2006); kinase activity is required by EGL-15(5B) to regulate axon outgrowth and fluid homeostasis (Bulow et al., 2004; Goodman et al., 2003; Huang and Stern, 2004). However, EGL-15(5A) does not require the intracellular kinase domain to maintain axon positions (Bulow et al., 2004). Similarly, we found that neither egl-17(ay6) nor let-756(s2613) suppress the presynaptic phenotype in cima-1(wy84) mutants (data not shown), suggesting that FGF ligands, egl-17 and let-756, are not required for ectopic presynapse formation in cima-1 mutants.
To further test if the kinase domain of EGL-15(5A) is required for the formation of ectopic synapses, we expressed the previous described egl-15(5A) extracellular domain (“egl-15(5A)ecto”; (Bulow et al., 2004)) in the epidermal cells in egl-15(n484) cima-1(wy84) double mutant animals, and found that expression of the ectodomain reverted egl-15(n484) suppression of cima-1(wy84) (Figure S7I). Together, these findings indicate that egl-15(5A) is required in epidermal cells in a kinase-independent manner to distort glial morphology in cima-1 mutants.
CIMA-1 negatively regulates EGL-15(5A)/FGFR protein levels
To determine how cima-1 acts in epidermal cells to maintain presynaptic positions, we first examined its subcellular localization. We observed CIMA-1::RFP is largely colocalized with lysosomal marker GFP::CUP-5 (Figures S3V–S3Y, Pearsons correlation=0.53), suggesting that CIMA-1 localizes primarily to lysosomes. However, alleles that disrupt lysosomal function (such as glo-1(zu391), glo-4(ok623), rab-7(ok511), and cup-5 (ar465)) did not phenocopy cima-1(wy84) (data not shown). Our findings suggest that the cima-1 phenotype does not result from general lysosomal dysfunction.
CIMA-1 localization is reminiscent of that seen for Sialin, a vertebrate homologue of CIMA-1 that also localizes to lysosomes and vesicles, and that in humans is associated with neurodegenerative disease (Verheijen et al., 1999). Sialin regulates transmembrane and extracellular adhesion molecules (Galuska et al., 2010; Hildebrandt et al., 2009; Morin et al., 2004; Myall et al., 2007; Prolo et al., 2009; Verheijen et al., 1999). Based on CIMA-1 localization, and its genetic interaction with EGL-15(5A), we hypothesized that CIMA-1 could be acting at the lysosome to regulate EGL-15(5A) in epidermal cells. To examine this hypothesis we generated transgenic animals expressing C-terminus HA- tagged EGL-15(5A) just in epidermal cells, and probed protein levels by western blots in both cima-1(wy84) and wild type animals. We consistently found that EGL-15(5A) levels are 5-fold higher in cima-1(wy84) animals than in wild-type animals (Figure 7G and 7H, p=0.007). This result suggests that cima-1 is required for the regulation of EGL-15(5A) protein levels in epidermal cells.
To further test if the synaptic defect in cima-1 is due to the increase of EGL-15(5A) in epidermal cells, we generated transgenic lines over-expressing EGL-15(5A) in epidermal cells and observed that over-expression of EGL-15(5A) in wild type animals resulted in abnormal glial morphology and ectopic presynaptic specializations in Zone 1, similar to the phenotypes observed for cima-1(wy84) mutants (Figures 7I–7K). Together, our findings support a model whereby the transporter cima-1 maintains glial morphology and presynaptic distribution by negatively regulating EGL-15(5A)/FGFR during growth. We hypothesize that reduction of EGL-15(5A) levels would result in reduced adhesion between glia and epidermal cells, which would be critical in maintaining glia location, and therefore, synapse location during post-developmental growth.
DISCUSSION
In this study, we identify a cellular and molecular mechanism that maintains presynaptic distribution during C. elegans post-developmental growth. We uncovered the requirement of a SLC17 family transporter CIMA-1. CIMA-1 antagonizes a specific FGFR isoform, EGL-15(5A), in epidermal cells to modulate glial morphology, in turn modulating AIY synaptic distribution.
CIMA-1 acts post-developmentally to regulate synaptic positions during growth. Between the L1 to L4 stage, animals undergo a four-fold change in length. However, during these stages, cima-1 mutants resemble wild type animals. We hypothesize that cima-1-independent mechanisms scale synaptic growth during development and that cima-1 is required to regulate synaptic positions during post-developmental growth. Two lines of evidence support this hypothesis. First, cima-1 expression after larval stages using the col-19 promoter rescues the presynaptic distribution phenotype in AIY. Second, in the cima-1 mutant background, Lon mutants display an enhanced presynaptic maintenance phenotype and Dpy mutants suppress the cima-1 phenotype. These findings demonstrate a relationship between animal size and the post-developmental expressivity of the cima-1 phenotype, and indicate a role for cima-1 in maintenance of the synaptic pattern during postdevelopmental growth.
cima-1 negatively regulates an FGFR isoform, egl-15(5A), to modulate glial morphology during growth. Four lines of evidence support this model. First, like cima-1, egl-15(5A) is also expressed, and required, in epidermal cells to modulate epidermal-glia interaction. Second, in the epidermal cells of cima-1 mutant animals, EGL-15(5A) protein levels are significantly increased. Third, loss of egl-15(5A) in cima-1 mutants mostly restores glial morphology and correct presynaptic patterning in AIY. Fourth, overexpression of egl-15(5A) in wild type animals results in abnormally extended glia and in ectopic presynaptic sites in AIY. EGL-15(5A) was previously implicated in the maintenance of axon position in the ventral nerve cord (Bulow et al., 2004). This function of EGL-15(5A) is also mediated by its ecto-domain, which was hypothesized to provide an adhesive substratum as a part of a larger adhesive complex (Benard and Hobert, 2009; Bulow et al., 2004). Our findings now demonstrate that CIMA-1 can negatively regulate EGL-15(5A) to maintain glial morphology during growth.
CIMA-1 could act as a sugar transporter to regulate EGL-15(5A) protein levels. CIMA-1 is a member of the solute carrier SLC17 family that includes Sialin. While Sialin is capable of transporting a variety of cargos depending on its biological context (Miyaji et al., 2010, 2011; Qin et al., 2012), its role as a lysosomal transporter of acidic monosaccharides has been implicated in neurological diseases (Verheijen et al., 1999; Wreden et al., 2005). Importantly, several studies suggest that Sialin regulates intercellular adhesion via the modulation of glycoconjugate export from the lysosome (Galuska et al., 2010; Hildebrandt et al., 2009; Morin et al., 2004; Myall et al., 2007; Prolo et al., 2009). CIMA-1 also localizes to the lysosome. Although we have not yet identified the specific cargo for CIMA-1, our phenotypic characterization uncovers an in vivo function for this transporter in maintaining presynaptic distribution by regulating specific isoform of the FGFR, EGL-15(5A). EGL-15 is N-glycosylated (Polanska et al., 2009). We hypothesize that CIMA-1 could act like Sialin in transporting acidic monosaccharides that could modify and regulate protein levels of EGL-15(5A). Although our findings are consistent with this hypothesis and demonstrate a genetic interaction between cima-1 and EGL-15(5A) in vivo, it is likely that cima-1 also regulates other molecules, as mutations of egl-15(5A) do not completely suppress the cima-1 phenotype.
The role of glia in synapse formation and function is well-established. In both vertebrates and invertebrates, glia-derived molecules promote synapse formation during development (Allen et al., 2012; Colón-Ramos et al., 2007; Fuentes-Medel et al., 2012; Pfrieger, 2010; Stevens, 2008). Glia are also required in vivo for maintenance of synaptic function in adult animals. For example, ablation of glia in adult Rana pipiens decreases presynaptic function at neuromuscular junctions (Reddy et al., 2003). While glia are known to play roles in maintenance of synaptic function, their role in maintenance of synaptic positions is less clear. A recent study demonstrates that growth of glial processes is coordinated with neuromuscular junction growth in vivo (Brink et al., 2012). We show a post-developmental role for glia in maintaining synaptic distribution and circuit architecture during growth.
The transduction of growth information from epithelial cell, to glia, to neuron may be common. The epidermal epithelium in C. elegans coordinates growth in the organism. For example, genes expressed in epidermal cells regulate molting, body morphogenesis and animal size (Chisholm and Hardin, 2005; Chisholm and Hsiao, 2012; Chisholm and Xu, 2012). Our work shows that the interaction of epidermal cells with glia translates growth information to the neurons to limit synapse distribution. The epithelial-glia interaction we uncovered here is reminiscent of the neurovascular unit in vertebrates. Astrocytes play a fundamental role mediating communication between epithelial cells and neurons in the vasculature of the brain (Abbott et al., 2006; Banerjee and Bhat, 2007; Giaume et al., 2010; Kim et al., 2006; Tam and Watts, 2010; Wolburg et al., 2009). For example, astrocytes indirectly control blood flow to neurons by coupling neuronal activity to the epithelial cells of the vasculature (Allan, 2006; Koehler et al., 2009). Astrocytes also developmentally couple epithelial cells of the vasculature with neurons during embryogenesis (Tam and Watts, 2010). It is not yet known if astrocytes translate growth information from epithelial cells in the vasculature to position synapses as the animal grows. However, given existing roles for astrocytes in coupling functional and developmental information between epithelial cells and neurons, we speculate that analogous, glia-dependent mechanisms like those in C. elegans could maintain synaptic position during animal growth in metazoans.
EXPERIMENTAL PROCEDURES
For further experimental details on strains (Table S1), culture conditions and statistics, please see the extended experimental information.
EMS screen and RNAi
cima-1(wy84) was isolated from a visual forward EMS mutagenesis screen aimed at identifying mutants with defects in GFP::RAB-3 distribution. cima-1 was mapped to an interval between 7.40Mb and 7.83Mb on chromosome IV. The eighteen fosmids that cover this region were injected into cima-1 (wy84) mutants and examined for rescue of AIY presynaptic defects. The F45E4.11 region in cima-1(wy84) was sequenced with Sanger sequencing technique. Bacteria-mediated RNAi was performed as described in (Kamath et al., 2001).
Constructs and nematode transformation
Expression clones were made in the pSM vector, a derivative of pPD49.26 (A. Fire) with extra cloning sites (S. McCarroll and C.I. Bargmann, unpublished data). Constructs are listed in Table S2, and detailed cloning information will be provided upon request. Transgenic strains (1–30ng µl-1) were generated using standard techniques (Mello and Fire, 1995) and listed in Table S2.
Microscopy and imaging
C. elegans animals (cima-1(wy84), Pttx-3::GFP) were prepared for fluorescence electron microscopy as previously described (Watanabe et al., 2011). AIY neurons in two cima-1(wy84) animals were identified based on fluorescence. Fluorescence images and electron micrographs were correlated based on the fiducial markers. Epidermal and VCSC glial cells were identified based on their morphology. A total length of 6 µm of an AIY and a VCSC glial cell were reconstructed from serial electron micrographs as shown in Figure S1F. Reconstruction of AIY and glia in the wild-type animal used EM images (JSH and N2U) prepared by Dr. John White (White et al., 1986), downloaded from http:www.wormimage.org and reconstructed with TrakEM2 EM. For confocal microscopy, images of fluorescently tagged fusion proteins were captured in live C. elegans using an UltraView VoX spinning disc confocal microscope (PerkinElmer) as described and analyzed using Volocity software (Improvision) or ImageJ (Schneider et al., 2012; Stavoe and Colón-Ramos, 2012).
Protein blots
We quantified EGL-15::HA in wild type and cima-1(wy84) mutant animals using olaEx1288 (Pdpy-7::egl-15(5A)::HA::MYC). 40–60 transgenic L4 animals were used for the blots. Reagents and detailed procedures are in the extended experimental procedures section. This experiment was repeated four times with similar results. We also performed an identical experiment with a second, independent transgenic strain (olaEx1411) and three replicated western blots with similar results.
Supplementary Material
HIGHLIGHTS.
Synapse location during post-developmental growth depends on glia location
Epidermal-glia interaction positions glia and limits synapse distribution
Solute transporter, CIMA-1, can modulate epidermal-glia interaction via FGFR
Reducing adhesion is important to maintain correct neuroarchitecture during growth
ACKNOWLEDGEMENTS
Cartoons in Figures 1A, 2A, 5A and 7L are modifications with permission from the Neuron pages of WormAtlas (www.wormatlas.org) by Z.F. Altun and D.H. Hall. The electron micrographs in Fig 5A and Suppl. Fig 1C and 1D are shown with permission from animals “JSH” and “N2U”, each prepared by J. White, E. Southgate, N. Thomson and S. Brenner at the LMB/MRC labs in Cambridge, England (White et al., 1986). With help from John White, the JSH and N2U image archives are now conserved in the Hall lab in NYC, and available online at www.wormimage.org.
We thank Dr. H. Bülow, Dr. M. Stern, Dr. S. Shaham, Dr. T. Kinnunen, Dr. J. Fares and Dr. K. Shen for strains and reagents. Some strains were provided by the CGC, which is funded by NIH (P40 OD010440). We also thank A. Pérez and A. Roque for technical assistance, Dr. X. Song for helping with the protein blots, and D. Hall, S. Margolis, P. De Camilli, S. Strittmatter, M. Hammarlund and members of the Colón-Ramos lab for helpful discussions and sharing of advice. This work was funded by the following grants to D.C.-R. (R00 NS057931, R01 NS076558, a fellowship from the Klingenstein Foundation and the Alfred P. Sloan Foundation and a March of Dimes Research Grant) and to E.M.J (NIH R01 NS034307, NSF 0920069). E.M.J. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution Z.S. and D.C-R. designed experiments. S.W. and E.J. performed the fEM experiments. R. C. performed wild type EM reconstruction and AIY Zone 1 length quantification. Z.S. performed all other experiments. Z.S. and D.C-R. analyzed and interpreted the data. Z.S., S.W., E.J. and D.C.-R. wrote the paper.
REFERENCES
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Allan S. The neurovascular unit and the key role of astrocytes in the regulation of cerebral blood flow. Cerebrovasc Dis. 2006;21:137–138. doi: 10.1159/000090447. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelio O, Hall DH, Hobert O. Immunoglobulin-domain proteins required for maintenance of ventral nerve cord organization. Science. 2002;295:686–690. doi: 10.1126/science.1066642. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard C, Hobert O. Looking beyond development: maintaining nervous system architecture. Curr Top Dev Biol. 2009;87:175–194. doi: 10.1016/S0070-2153(09)01206-X. [DOI] [PubMed] [Google Scholar]
- Benard CY, Blanchette C, Recio J, Hobert O. The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002819. e1002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard CY, Boyanov A, Hall DH, Hobert O. DIG-1, a novel giant protein, non-autonomously mediates maintenance of nervous system architecture. Development. 2006;133:3329–3340. doi: 10.1242/dev.02507. [DOI] [PubMed] [Google Scholar]
- Borland CZ, Schutzman JL, Stern MJ. Fibroblast growth factor signaling in Caenorhabditis elegans. Bioessays. 2001;23:1120–1130. doi: 10.1002/bies.10007. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink DL, Gilbert M, Xie X, Petley-Ragan L, Auld VJ. Glial processes at the Drosophila larval neuromuscular junction match synaptic growth. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037876. e37876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow HE, Boulin T, Hobert O. Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron. 2004;42:367–374. doi: 10.1016/s0896-6273(04)00246-6. [DOI] [PubMed] [Google Scholar]
- Chelur DS, Chalfie M. Targeted cell killing by reconstituted caspases. Proc Natl Acad Sci U S A. 2007;104:2283–2288. doi: 10.1073/pnas.0610877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm AD, Hardin J. Epidermal morphogenesis. WormBook. 2005:1–22. doi: 10.1895/wormbook.1.35.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm AD, Hsiao TI. The Caenorhabditis elegans epidermis as a model skin.I: development, patterning, and growth. WIREs Dev Biol. 2012 doi: 10.1002/wdev.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm AD, Xu S. The Caenorhabditis elegans epidermis as a model skin. II: differentiation and physiological roles. WIREs Dev Biol. 2012 doi: 10.1002/wdev.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GN, Hirsh D. Stage-specific patterns of collagen gene expression during development of Caenorhabditis elegans. Mol Cell Biol. 1985;5:363–372. doi: 10.1128/mcb.5.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Ashley J, Barria R, Maloney R, Freeman M, Budnik V. Integration of a Retrograde Signal during Synapse Formation by Glia-Secreted TGF-beta Ligand. Curr Biol. 2012;22:1831–1838. doi: 10.1016/j.cub.2012.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, Geyer R, et al. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci U S A. 2010;107:10250–10255. doi: 10.1073/pnas.0912103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Goodman SJ, Branda CS, Robinson MK, Burdine RD, Stern MJ. Alternative splicing affecting a novel domain in the C. elegans EGL-15 FGF receptor confers functional specificity. Development. 2003;130:3757–3766. doi: 10.1242/dev.00604. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Muhlenhoff M, Oltmann-Norden I, Rockle I, Burkhardt H, Weinhold B, Gerardy-Schahn R. Imbalance of neural cell adhesion molecule and polysialyltransferase alleles causes defective brain connectivity. Brain. 2009;132:2831–2838. doi: 10.1093/brain/awp117. [DOI] [PubMed] [Google Scholar]
- Huang P, Stern MJ. FGF signaling functions in the hypodermis to regulate fluid balance in C. elegans. Development. 2004;131:2595–2604. doi: 10.1242/dev.01135. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Kramer JM. Neural maintenance roles for the matrix receptor dystroglycan and the nuclear anchorage complex in Caenorhabditis elegans. Genetics. 2012;190:1365–1377. doi: 10.1534/genetics.111.136184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2 doi: 10.1186/gb-2000-2-1-research0002. RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006;39:339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- Knight CG, Patel MN, Azevedo RB, Leroi AM. A novel mode of ecdysozoan growth in Caenorhabditis elegans. Evol Dev. 2002;4:16–27. doi: 10.1046/j.1525-142x.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ambros V. alternative temperal control systems for hypodermal cell differentiation in Carnorhabditis elegans. Nature. 1991;350:162–165. doi: 10.1038/350162a0. [DOI] [PubMed] [Google Scholar]
- McMiller TL, Johnson CM. Molecular characterization of HLH-17, a C. elegans bHLH protein required for normal larval development. Gene. 2005;356:1–10. doi: 10.1016/j.gene.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- Miyaji T, Omote H, Moriyama Y. A vesicular transporter that mediates aspartate and glutamate neurotransmission. Biol Pharm Bull. 2010;33:1783–1785. doi: 10.1248/bpb.33.1783. [DOI] [PubMed] [Google Scholar]
- Miyaji T, Omote H, Moriyama Y. Functional characterization of vesicular excitatory amino acid transport by human sialin. J Neurochem. 2011;119:1–5. doi: 10.1111/j.1471-4159.2011.07388.x. [DOI] [PubMed] [Google Scholar]
- Morin P, Sagne C, Gasnier B. Functional characterization of wild-type and mutant human sialin. EMBO J. 2004;23:4560–4570. doi: 10.1038/sj.emboj.7600464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myall NJ, Wreden CC, Wlizla M, Reimer RJ. G328E and G409E sialin missense mutations similarly impair transport activity, but differentially affect trafficking. Mol Genet Metab. 2007;92:371–374. doi: 10.1016/j.ymgme.2007.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AP, Johnstone IL. The cuticle. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.138.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev. 2010;63:39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Pocock R, Benard CY, Shapiro L, Hobert O. Functional dissection of the C. elegans cell adhesion molecule SAX-7, a homologue of human L1. Mol Cell Neurosci. 2008;37:56–68. doi: 10.1016/j.mcn.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Polanska UM, Duchesne L, Harries JC, Fernig DG, Kinnunen TK. N-Glycosylation regulates fibroblast growth factor receptor/EGL-15 activity in Caenorhabditis elegans in vivo. J Biol Chem. 2009;284:33030–33039. doi: 10.1074/jbc.M109.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prolo LM, Vogel H, Reimer RJ. The lysosomal sialic acid transporter sialin is required for normal CNS myelination. J Neurosci. 2009;29:15355–15365. doi: 10.1523/JNEUROSCI.3005-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci U S A. 2012;109:13434–13439. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 2003;40:563–580. doi: 10.1016/s0896-6273(03)00682-2. [DOI] [PubMed] [Google Scholar]
- Reimer RJ. SLC17: A functionally diverse family of organic anion transporters. Mol Aspects Med. 2013;34:350–359. doi: 10.1016/j.mam.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura H, Inada H, Kuhara A, Fusaoka E, Takemoto D, Takeuchi K, Mori I. Maintenance of neuronal positions in organized ganglia by SAX-7, a Caenorhabditis elegans homologue of L1. EMBO J. 2005;24:1477–1488. doi: 10.1038/sj.emboj.7600621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham S. Glia-neuron interactions in the nervous system of Caenorhabditis elegans. Curr Opin Neurobiol. 2006;16:522–528. doi: 10.1016/j.conb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Shi L, Fu AK, Ip NY. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neurosci. 2012;35:441–453. doi: 10.1016/j.tins.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Sreedharan S, Shaik JH, Olszewski PK, Levine AS, Schioth HB, Fredriksson R. Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics. 2010;11:17. doi: 10.1186/1471-2164-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavoe AK, Colón-Ramos DA. Netrin instructs synaptic vesicle clustering through Rac GTPase, MIG-10, and the actin cytoskeleton. J Cell Biol. 2012;197:75–88. doi: 10.1083/jcb.201110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B. Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals. 2008;16:278–288. doi: 10.1159/000123038. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Sundaram MV. RTK/Ras/MAPK signaling. WormBook. 2006:1–19. doi: 10.1895/wormbook.1.80.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- Verheijen FW, Verbeek E, Aula N, Beerens CE, Havelaar AC, Joosse M, Peltonen L, Aula P, Galjaard H, van der Spek PJ, et al. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat Genet. 1999;23:462–465. doi: 10.1038/70585. [DOI] [PubMed] [Google Scholar]
- Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Jorgensen EM. Visualizing proteins in electron micrographs at nanometer resolution. Methods Cell Biol. 2012;111:283–306. doi: 10.1016/B978-0-12-416026-2.00015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Punge A, Hollopeter G, Willig KI, Hobson RJ, Davis MW, Hell SW, Jorgensen EM. Protein localization in electron micrographs using fluorescence nanoscopy. Nat Methods. 2011;8:80–84. doi: 10.1038/nmeth.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wilcox KC, Lacor PN, Pitt J, Klein WL. Abeta oligomer-induced synapse degeneration in Alzheimer's disease. Cell Mol Neurobiol. 2011;31:939–948. doi: 10.1007/s10571-011-9691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- Woo WM, Berry EC, Hudson ML, Swale RE, Goncharov A, Chisholm AD. The C. elegans F-spondin family protein SPON-1 maintains cell adhesion in neural and non-neural tissues. Development. 2008;135:2747–2756. doi: 10.1242/dev.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreden CC, Wlizla M, Reimer RJ. Varied mechanisms underlie the free sialic acid storage disorders. J Biol Chem. 2005;280:1408–1416. doi: 10.1074/jbc.M411295200. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Murray JI, Lu Y, Waterston RH, Shaham S. mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development. 2008;135:2263–2275. doi: 10.1242/dev.019547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.