Abstract
Recent reports have highlighted greater complexity, plasticity and functional diversity of mononuclear phagocytes (MPCs), including monocytes, macrophages and dendritic cells (DCs), in our organs, than previously understood. The functions and origins of MPCs resident within healthy organs, especially in the kidney, are less well understood, while studies suggest they play roles in disease states distinct from recruited monocytes. We developed an unbiased approach using flow cytometry to analyze MPCs residing in the normal mouse kidney, and identified five discrete subpopulations according to CD11b/CD11c expression as well as F4/80, CD103, CD14, CD16 and CD64 expression. In addition to distinct marker profiles, these subpopulations have different lineages and expression of genes involved in tissue homeostasis, including angiogenesis. Among them, the CD11bint CD11cint F4/80hi subpopulation notably exhibited high capacity to produce a representative anti-inflammatory cytokine, IL-10. Each subpopulation had different degrees of both macrophage (phagocytosis) and DC (antigen presentation) capacities, with a tendency to promote differentiation of regulatory T cells, while two of these showed expression of transcription factors reported to be highly expressed by classical DCs, and proclivity to exit the kidney following stimulation with LPS. In summary, resident kidney MPCs comprise discrete subpopulations, which cannot be simply classified into the conventional entities, and they produce anti-inflammatory and tissue-homeostatic factors to differing degrees.
Introduction
Conventionally mononuclear phagocytes (MPCs) have been shown to comprise monocytes, macrophages and dendritic cells (DCs) (1). Monocytes arise in bone marrow, circulate in the blood and traffic to peripheral organs. In steady state, these monocytes are believed to differentiate into resident macrophages, which function to maintain tissue homeostasis through phagocytosis of apoptotic bodies and production of growth factors. DCs are cells with specialized capacity to present antigen to T lymphocytes, along the capacity to internalize and process antigen. Unlike macrophages, DCs are not believed to derive from monocytes in steady state (2). In inflammatory states, however, monocyte efflux from bone marrow and its recruitment into the affected organs increases, and monocytes become inflammatory MPCs, which often have both macrophage and DC characteristics. All of these MPCs have substantial roles in inflammation. Over the last 10 years, elegant studies have demonstrated that functionally discrete monocyte subpopulations exist with distinct recruitment characteristics and distinct receptor and cytokine repertoires.
Recently, a common precursor in the bone marrow, called monocyte, macrophage, and dendritic cell precursor (MDP), was found to give rise to most of monocytes, macrophages and DCs (3). It can differentiate into monocytes and common DC precursor (CDP) (2). Pre-classical DCs (pre-cDCs) and plasmacytoid DCs (PDC) are believed to derive from CDP, and traffic to lymphoid and non-lymphoid organs, producing resident DCs, whereas their mode of recruitment is less well understood. In addition, pre-cDCs are thought to supply a population of dendritic cells characterized by expression of the integrin αE, also known as CD103, in non-lymphoid tissue (4). In intestine, they are potent at antigen cross-presentation, which can activate or suppress T cell activity according to other environmental cues (5). However, their contribution to homeostasis or disease outside of the intestine is poorly understood. Finally recent studies provide evidence that mononuclear phagocytes arising in the yolk sac during the first stages of embryogenesis traffic to the central nervous system during development to become permanently residing microglia, and that these embryonic mononuclear phagocytes also take up residence in peripheral organs including the kidney (6). Although ablation studies and adoptive transfer studies have dissected unequivocal roles for monocytes in regulating responses to disease in the kidney both favorably and deleteriously (7, 8), the role of mononuclear phagocytes normally residing the kidney in health and disease states is relatively unknown. Several studies, however, indicate that they expand in chronic disease by local proliferation, that they play different roles from inflammatory monocyte-derived cells in disease with different cytokine profiles, and that they may regulate organ growth and repair (7, 9).
MPCs are defined functionally by the name macrophage to describe phagocytic properties, or DC to describe antigen presentation properties, and they are the two main types of resident myeloid cells across tissues. However, it is well known that macrophages can show vast heterogeneity in phenotype and function depending on the context, which includes different tissues and different disease states. They can present antigens, like DCs, in certain conditions. Thus, even this function, which is often regarded as specific for DCs, is common to both.
The purpose of this study is to define resident MPC subpopulations in normal kidney to determine their potential functional contributions.
Materials and Methods
Animals
C57BL/6 (B6) and BALB/c mice were from Charles River Laboratories. FVB/N CX3CR1-GFP (CX3CR1gfp/+) (10), B6 CCR2-GFPTr (11), B6 CSF1R-GFPTr (12), B6 GM-CSF−/− (13), CSF1R−/− on mixed background strain (14) and B6 IL-10–β–lactamase reporter (ITIB) (15) mice have been previously described. B6 LysMCre/Cre and B6 Gt(ROSA)26Sortm14(CAG-tdTomato)Hze (R26RtdTomato) mice were from the Jackson Laboratory. They were bred to obtain LysmCre/+;R26RtdTomato/+ mice. Mice with a targeted deletion of Recombination Activation Gene 2 (RAG2−/−) on BALB/c background were from Taconic Labs. Ovalbumin-specific DO11.10 TCR-transgenic mice were generously provided by Dr. Abul Abbas (University of California, San Francisco). ITIB mice with homozygous reporters were used for the experiments. 5 μg per gram body weight of Lipopolysaccharide E. coli 0111:B4 (Sigma) suspended in phosphate buffer saline (PBS) was injected intraperitoneally, and samples were taken after 48 hours. All animal studies were performed under protocols approved by Department of Comparative Medicine, University of Washington.
Preparation of single cells from kidney or lymph nodes
After systemic perfusion with ice-cold PBS to remove blood, kidney was resected, de-capsulated, diced, then incubated at 37 °C for 30 min in DMEM/F12 media (Mediatech) including 0.2 mg/mL of Liberase TL (Roche Applied Science) and 100 U/mL of DNase (Roche Applied Science) as described (7). Addition of DMEM/F12 media including 10% FBS was followed by filtration of the suspension through cell strainer with 40 μm mesh (Fisher Scientific). The filtrate was then centrifuged at 600 g for 5 minutes, and the obtained pellet was resuspended as single cells. Kidney draining lymph nodes were identified by injection of 10μl of Evan’s blue dye into the kidney and identification of nodes, which had taken up the blue dye 5–15mins later. LNs were dissociated by standard trituration methods (16), prior to washing and resuspending in FACS buffer.
Processing of blood for flow cytometry
The whole blood was drawn into a syringe including EDTA dissolved in PBS for anticoagulation. The blood, further diluted in PBS, was centrifuged at 300 g for 3 minutes. The pellet was treated twice with ACK lysis buffer (150 mM ammonium chloride, 10 mM potassium bicarbonate, 0.1 mM EDTA) for red blood cell lysis and washed with PBS. The obtained pellet was resuspended as blood leukocytes for flow cytometry.
Flow cytometry
Cell pellets were resuspended in ice-cold FACS buffer (PBS including 1% bovine serum albumin, 2 mM EDTA, and 0.01% sodium azide) including 2% mouse serum, plated in round-bottom 96-well plate, and incubated with antibody mixture on ice for 30 minutes. Directly conjugated anti-CD45 (clone 30-F11), anti-CD3e (clone 145-2C11), anti-CD19 (clone 1D3), anti-CD49b (clone DX5), anti-Ly6G (clone 1A8), anti-CD11b (clone M1/70), anti-CD11c (clone N418), anti-F4/80 (clone BM8), anti-MHC class II (clone M5/114.15.2), anti-CD103 (clone 2E7), anti-CD86 (clone GL1), anti-CD14 (clone Sa2-8), anti-CD16/32 (clone 93), anti-CD64 a and b (clone X54-5/7.1), anti-Ly6C (AL-21), and anti-CD135 (A2F10) antibodies were from BioCentury, eBioscience or BD Biosciences and used at 1:200 dilution. After incubation, cells were washed with FACS buffer twice, followed by analysis using BD FACSCanto II (BD biosciences) or by sorting with BD FACSAria II (BD biosciences). To obtain total cell numbers, in some experiments PE-conjugated counting beads (Spherotech, Illinois) were added to the single cell suspension for cell counting according to manufacturer’s guidelines. In experiments evaluating CD135 expression cells were incubated for 3h after purification prior to application of antibodies. Blood monocytes were defined as leukocytes which express CD45 and CD11b but do not express Ly6G, CD19, CD3e or CD49b. Kidney MPCs were defined as cells from kidneys in which all blood had been flushed, which express CD45 but did not express Ly6G, CD19, CD3e or CD49b.
Immunofluoresence microscopy
Normal C57BL/6 kidneys were prepared and labeled as previously described and images were captured by confocal microscopy (8).
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Total RNA was extracted using RNeasy Mini Kit (QIAGEN). Complementary DNA (cDNA) was synthesized using iScript cDNA Synthesis Kit (Bio-Rad). qRT-PCR assay was performed using an ABI7900HT (Applied Biosystems) and iTaq SYBR Green Supermix with ROX (Bio-Rad). The cycling conditions were initial denaturation at 95°C for 2.5 minutes, followed by PCR cycles of 95°C for 15 seconds and 58°C for 30 seconds. Specificity of each primer pair was confirmed by verification of single PCR product with expected size in agarose gel electrophoresis. The sequences of primers are shown in Table SI.
T cell differentiation assay
RAG2-deficient DO11.10 (DR) mice were generated as a source of homogenous naïve antigen-specific CD4+ T cells containing no effector or regulatory T cell populations (17). Purified CD4+ T cells from spleen and peripheral lymph nodes of donor DR mice were obtained by no-touch magnetic bead isolation (Miltenyi Biotec) followed by positive cell sorting for CD4+ T cells. Bone marrow-derived dendritic cells and macrophages were prepared as described (18, 19). Briefly, single-cell suspensions of bone marrow were prepared and cultured with 20 ng/ml GM-CSF for dendritic cells, or 20% L929-conditioned media for macrophages for 4–6 days. All APC cultures were aliquoted and then incubated with 5 μg/ml ovalbumin (323–339) peptide for one hour at 37°C, then washed three times with plain media. 3 × 104 purified CFSE-labeled DR T cells were mixed with APC populations at indicated ratios in complete RPMI media supplemented with 5% FBS, and cultured for 5 days. On day 5, the cultures were re-stimulated with plate-bound anti-CD3e (10 μg/mL, clone 145-2C11) and anti-CD28 (1 μg/ml, clone PV-1) for 5 hours in the presence of Golgi inhibitor (BD Biosciences). Cells were harvested and stained for viability (eBioscience, Fixable Viability Dye), surface CD4 and DO11.10 TCR (KJ1-26 antibody), and intracellular Foxp3 (clone FJK-16s), IFNγ (clone MG1.2), IL-4 (clone BVD6-24G2), IL-10 (clone JES6-16E3) and IL17a (clone TC11-18H10.1) (antibodies from eBioscience). Negative control samples were stained with isotype control antibodies. T cells were analyzed by flow cytometry as described above.
Phagocytosis assay
Sorted mononuclear phagocytes were incubated in DMEM/F12 medium including 10% FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin, with latex beads-FITC complex in Phagocytosis Assay Kit (Cayman Chemical Company) for 2 hours, following analysis by FACSCanto II.
LPS stimulation of renal mononuclear phagocytes
Sorted cell subpopulations were incubated in DMEM/F12 medium including 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 100 ng/mL LPS (Sigma) for 16 hours. The stimulated cells were used for qRT-PCR analysis as mentioned above.
IL-10 reporter mouse experiments
Single cells from ITIB mouse kidney were incubated in PBS including 1.8 μM CCF4-AM (Invitrogen) and 7.2 mM probenecid (Sigma) at room temperature for 90 minutes with gentle shaking. Following one wash with PBS, cells were stained with antibodies as described above prior to flow cytometry analysis.
Statistics
All data are given as mean ± SEM. Significant differences were analyzed using Student t-test between two groups and ANOVA among groups more than three. P values <0.05 were regarded as significant.
Results
The integrins CD11b and CD11c in conjunction with F4/80, CD103, CD16, CD14 and CD64 discriminate five discrete mononuclear phagocyte populations
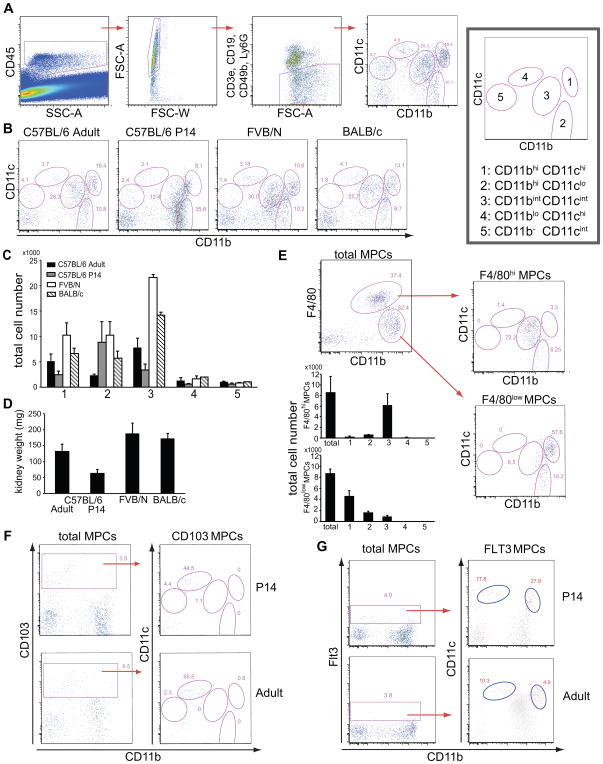
Although it has been thought that CD11b is expressed by macrophages whereas CD11c is expressed by DCs, no clear functional correlate has been demonstrated for these receptors, and recent studies have challenged the specificity, particularly in non-lymphoid organs. We analyzed all tissue leukocytes from normal adult kidneys by flow cytometry from C57BL/6, FVB/N and BALB/c strains of mice. After excluding all lymphocytes and granulocytes, the remaining cells were identified as MPCs (Fig. 1A). Kidney MPCs were assessed for cell surface expression of the markers CD11b and CD11c (Fig. 1 B). In each strain, five populations of MPCs were discriminated according to expression levels of CD11b and CD11c: (1) CD11bhi CD11chi; (2) CD11bhi CD11clo; (3) CD11bint CD11cint; (4) CD11blo CD11chi; (5) CD11b− CD11cint. However the ratio of each subpopulation was distinct in the different strains and young mice (P14) also showed distinct distribution of subpopulations. To evaluate this further, total leukocyte numbers were evaluated (Fig. 1 C) and these showed substantially higher levels of MPC1 and MPC3 that could not be explained simply by increased kidney size (Fig 1 D). Moreover MPC5 was the smallest population and was lower in BALB/c and FVB/N mice.
Figure 1.
Five distinct subpopulations of resident mononuclear phagocytes (MPCs) in normal kidney show both “macrophage” and “dendritic cell” markers by flow cytometry. (A) Gating strategy for identification of MPCs in normal mouse kidney concluded by a representative CD11b/CD11c flow cytometry plot of resident MPCs in normal kidney with right-hand plot showing key and nomenclature for each population. (B) Representative CD11b/CD11c MPC plots for 3 different strains of adult mice and 14 day old C57BL/6 mice with representative percentages. (C) Total number of each MPC subpopulation per kidney. (D) Average Kidney weight by mouse strain. (E) Representative CD11b, F4/80 plot of total MPCs (left) and CD11b/CD11c plots for F4/80hi MPCs (right, upper) and F4/80lo MPCs (right, lower). Most F4/80hi MPCs reside in CD11bint CD11cint subpopulation. Graphs show total numbers of F480hi and F4/80lo MPCs in each MPC population. (F) Plots showing CD103+ cells in young and adult kidneys and their distribution in CD11b/CD11c plots. (G) Plots (%) showing rare Flt3+ MPCs in young and adult normal kidney and their distribution (red events) in CD11b/CD11c plots. For (A) and (B), experiments were independently performed more than 30 times, using totally more than 80 mice. For others, experiments were independently performed twice or more, using three or more mice each time.
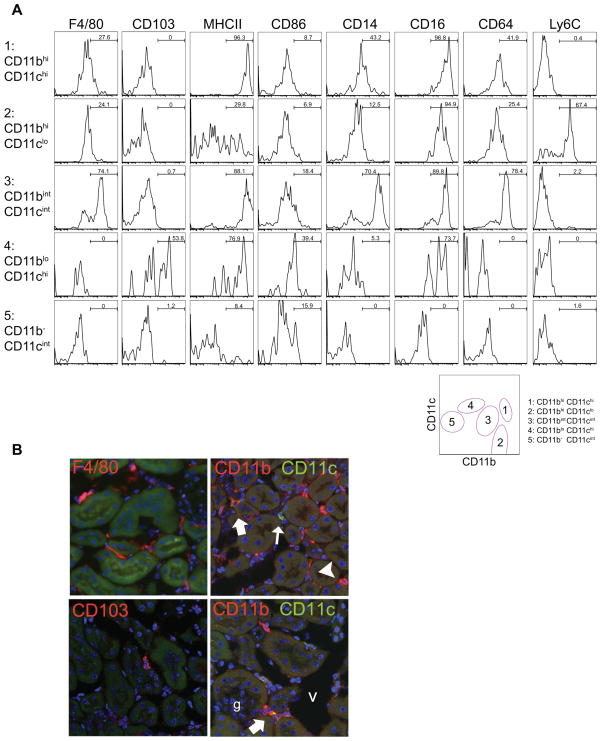
The separation of these MPC populations was readily apparent with concurrent use of F4/80 expression, which has been used as a classical cell surface marker of kidney macrophages, and more recently has been found to be expressed by CD11c+ MPCs residing in the kidney (20, 21). We found that F4/80hi MPCs were predominantly in the population of CD11bint CD11cint MPC3 (Fig 1 E), whereas lower expression of F4/80 was observed in CD11bhi CD11clo MPC2 and CD11bhi CD11chi MPC1 populations (Fig. 1 E, Fig. 2, Table I). The remaining populations did not express F4/80.
Figure 2.
Heterogeneous expression of typical dendritic cell and macrophage markers by kidney MPCs. (A) Histogram plots showing expression of cell surface markers in resident MPC subpopulations in normal C57BL/6 mouse kidney. Representative plots from 3 independent experiments are shown. Bars show % in gated area (B) Fluorescence confocal micrographs (X400) showing CD11b, CD11c, F4/80 and CD103 expression in kidney cortex. Within the cortex F4/80hi and F4/80lo cells can be seen. Rare CD103+ CD3- cells can be seen and co-labeling with CD11b and CD11c identifies cells which express CD11b alone (arrowheads), CD11b and CD11c at high levels (broad arrows), CD11c alone (upper panel small closed arrow) and CD11c at high levels with CD11b at low levels (lower panel small closed arrow)
Table I.
Mean fluorescence intensity of surface markers in MPC subpopulations
| 1: CD11bhi CD11chi | 2: CD11bhi CD11clo | 3: CD11bint CD11cint | 4: CD11blo CD11chi | 5: CD11b− CD11cint | ANOVA | |
|---|---|---|---|---|---|---|
| F4/80 | 2933 ± 224 | 4115 ± 1336 | 8462 ± 2681 | 576 ± 35 | 573 ± 38 | 0.011 |
| CD103 | 523 ± 52 | 360 ± 91 | 764 ± 218 | 6180 ± 1013 | 487 ± 26 | <0.0001 |
| MHCII | 90043 ± 6707 | 26134 ± 11707 | 60825 ± 15701 | 18026 ± 11080 | 2619 ± 427 | <0.001 |
| CD86 | 1633 ± 157 | 2171 ± 902 | 3257 ± 1200 | 3647 ± 168 | 1368 ± 99 | 0.15 |
| CD14 | 6229 ± 484 | 8297 ± 5539 | 21402 ± 7608 | 714 ± 40 | 222 ± 17 | 0.031 |
| CD16 | 32893 ± 5721 | 13460 ± 4040 | 8087 ± 3922 | 15727 ± 6625 | 904 ± 36 | 0.0075 |
| CD64 | 3929 ± 1194 | 3292 ± 1223 | 4381 ± 1846 | 427 ± 303 | 378 ± 44 | 0.077 |
| Ly6C | 92 ± 9 | 2473 ± 68 | 212 ± 35 | 118 ± 20 | 135 ± 10 | < 1 × 10−16 |
Mean ± SEM is shown (n =3).
CD11blo, CD11chi MPC4 population was more readily discriminated by concurrent detection of the integrin CD103 (Fig 1 F). CD103+ MPCs represent 4–6% of kidney resident MPCs, but the vast majority are found in the MPC4 population. Nevertheless in mice at postnatal day 14 (P14), and in adults CD103 was also expressed in a minority of CD11b− CD11cint cells (Fig. 1 F). CD103+ MPCs have been reported elsewhere to be derived from a distinct precursor which expresses the receptor Flt3 (CD135) (4). At P14, Flt3 expressing MPCs represented <5% of all MPCs and there were detected predominantly with the MPC4 population, but a second population of Flt3+ MPCs was detected as a minor subpopulation within MPC1 (Fig 1G). In adults these minor Flt3+ populations persisted but represented fewer cells within MPC4 and MPC1 (Fig. 1 G). A minority of Flt3+ MPCs were CD11b− CD11cint MPC5. Together with the findings that some CD103+ MPCs are also in MPC5 suggest that MPC5 cells may be in a state of flux with MPC4, depending on circumstances.
Expression of MHC class II (MHCII) has been used as a conventional marker of DCs. All subpopulations except CD11b− CD11cint MPC5 expressed MHCII but highest expression was restricted to CD11bhi CD11chi (MPC1) and CD11bint CD11cint (MPC3) cells (Fig. 2 A, Table I). Interestingly, expression of the co-stimulatory molecule CD86 did not correlate well with MHCII, but it was expressed at highest level by the minor population of CD11blo CD11chi cells (MPC4) (Fig. 2 A). All these subsets lacked the plasmacytoid DC (PDC) marker B220 (data not shown), suggesting that PDCs do not populate the normal kidney.
The markers CD14, CD16 and, to some extent, CD64 have been used to discriminate macrophages and monocyte subpopulations. The five subpopulations of MPCs expressed distinct patterns of these receptors. Importantly, CD14 and CD64 were expressed most highly in the CD11bint CD11cint MPC3 population, while CD11bhi CD11chi MPC1 population had the highest expression of CD16 (Fig. 2 A and Table I). While DCs are often defined as CD11c+ MHCII+ cells, MPC3 cells expressed these receptors but concurrently expressed these macrophage markers at high levels (Table II). CD11b− CD11c− MPCs were negative in all the assessed surface makers, and excluded in the subsequent studies (discussed below).
Table II.
Summary of markers in MPC subpopulations
| F4/80 | Ly6c | CD14 | CD16 | CX3CR1 | CCR2 | CSF1R | MHCII | CD103 | Zbtb46 | Batf3 | Irf8 | CCR7 | IL-10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1: CD11bhi CD11chi | + | − | + | ++ | + | +++ | + | ++ | − | ++ | ± | − | + | ± |
| 2: CD11bhi CD11clo | + | + | + | + | + | ++ | ++ | − ~ + | − | − | ± | ± | − | + |
| 3: CD11bint CD11cint | ++ | − | ++ | + | ++ | + | +++ | ++ | − | − | ± | ± | − | ++ |
| 4: CD11blo CD11chi | − | − | − | + | ± | ± | + | + | + | ++ | ++ | ++ | ++ | − |
| 5: CD11b− CD11cint | − | − | − | − | N.A. | ± | − | − | − | − | − | + | ± | − |
N.A.: not applicable.
As would be predicted by the flow cytometric analysis, these distinct subpopulations can be discriminated within the interstitium of the kidney cortex by fluorescence microscopy and are not distributed to any particular location by subpopulation (Fig 2 B).
Kidney mononuclear phagocyte subpopulations can be discriminated by chemokine receptor expression, ontogeny and growth factor dependence
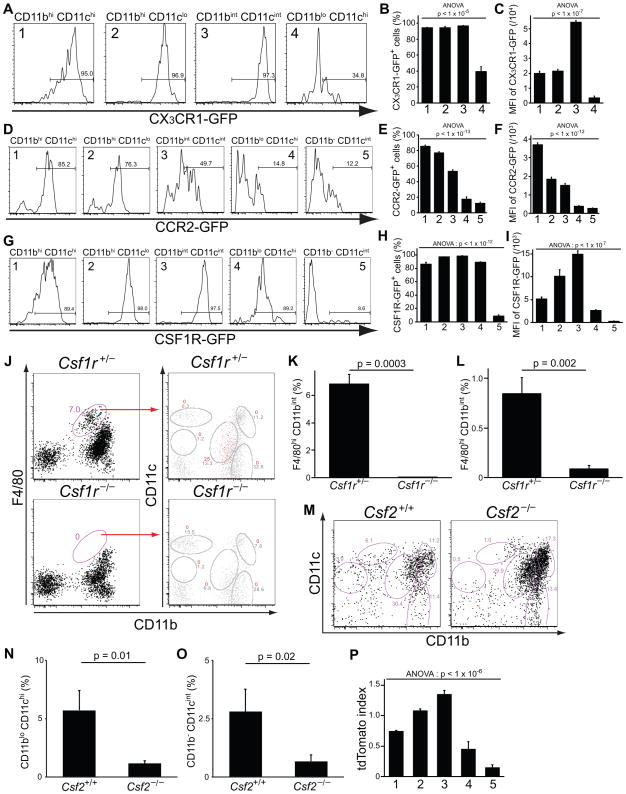
Chemokine receptors play an important role in monocyte, macrophage and DC trafficking. In order to explore chemokine receptor expression in the MPC subpopulations, we assessed expression of CX3CR1, a receptor thought to affect steady state trafficking of monocytes, in Cx3cr1+/GFP mice of FVB/N strain. As noted above, kidney from FVB/N wild type mice have reduced numbers of CD11b− CD11cint MPC5 (Fig. 1 B) and this minor population did not express CX3CR1. The remaining kidney MPCs expressed different levels of CX3CR1, with CD11bint CD11cint, F4/80hi, MPC3 expressing the highest levels (Fig. 3 A–C), and CD11blo CD11chi MPC4 expressing these receptors at the lowest levels.
Figure 3.
MPC subpopulations show differential expression of chemokine receptors, colony-stimulating factor-dependency and distinct ontogeny. Histograms (A, D, G), and graphs of percent positive (B, E, H), or mean fluorescence intensity (MFI) (C, F, I) of GFP in MPC subpopulations from CX3CR1GFP/+ kidneys (n = 3), CCR2-GFPTr mice (n = 4), and CSF1R-GFPTr mice (n = 3), respectively. (J) Representative plots of P14 juvenile kidney MPCs in control Csf1r+/− and Csf1r−/− mice at day 14. In right plots gated cells (%) are shown as red events whereas total events (%) are shown in grey. Note in juvenile kidneys MPC4 expresses CD11b at lower levels compared to adults. (K–L) Graphs showing the proportion of MPCs that are F4/80hi CD11bint MPCs (corresponding to CD11bint CD11cint MPC3) in control Csf1r+/− and Csf1r−/− mice at day 14 (K) (n = 3) or at day 0 (L) after birth. (M–O) Representative plots (%) of kidney MPCs (M) and a graph showing the proportion (N–O) of MPCs that are the CD11blo CD11chi subpopulation (MPC4) and CD11b− CD11cint (MPC5) in adult control and adult Csf2−/− knockout mice (n = 3). (P) A graph showing the ratio of tdTomato-positive kidney MPCs normalized to monocytes in LysmCre/+;R26RtdTomato/+ monocyte fate reporter mice. 1: CD11bhi CD11chi, 2: CD11bhi CD11clo, 3: CD11bint CD11cint, 4: CD11blo CD11chi, 5: CD11b− CD11cint. Data are represented as mean ± SEM.
Similarly, we assessed expression of CCR2 (Fig. 3 D–F), another receptor implicated in monocyte trafficking, using CCR2-GFPTr mice on C57BL/6 background. In most CD11blo CD11chi and CD11b− CD11cint MPCs, receptor expression was not detected. All of the remaining three populations of MPCs expressed CCR2, but CD11bint CD11cint, F4/80hi, MPCs had lower levels. The fact that at steady state key chemokine receptor levels are distinct lends weight to the hypothesis that these cells exist as separate populations.
The receptor for the growth factor colony stimulating factor (CSF)-1, which is also called macrophage colony stimulating factor (M-CSF), is thought to be critical in MPC differentiation and functions (14). In CSF1R-GFPTr mice that report CSF-1 receptor expression by GFP fluorescence, the distribution of CSF-1R expression was also discriminatory (Fig. 3 G–I). Whereas the CD11bhi CD11clo MPC2 and CD11bint CD11cint MPC3 expressed high levels of the receptor, CD11bhi CD11chi MPC1 and CD11blo CD11chi, CD103+ MPC4 expressed intermediate levels, and the CD11b− CD11cint MPC5 did not express it. The absence of the receptor on this latter population suggests that it may have silenced expression or that it functions independently of CSF-1 receptor signaling. To test whether any of the MPCs had absolute dependence on the receptor expression for differentiation and survival, we evaluated MPCs in kidneys from P14 Csf1r−/− mice, since these mice rarely survive beyond weaning and since P14 is the time point when kidney development is complete. In P14 Csf1r−/− mice, CD11bint CD11cint, F4/80hi, subpopulation was selectively absent, suggesting that such cells may require CSF1R for differentiation (Fig. 3 J and K). The absence of F4/80hi cells was also observed in Csf1r−/− mice at day 0 after birth (Fig. 3 L). Importantly, all other subpopulations were preserved. MPC4 was increased in these juvenile mice although the difference was not significant.
CSF-2, granulocyte-macrophage colony stimulating factor (GM-CSF), is another growth factor that plays important roles in myeloid cell differentiation and activation. To test its role in the appearance/maintenance of kidney MPCs, we evaluated kidney MPCs in Csf2−/− mice of C57BL/6 background compared with strain-matched controls. In these adult mice CD11blo CD11chi MPC4 were markedly reduced (Fig. 3 M and N), as were CD11b− CD11cint MPC5 (Fig. 3 M and O) suggesting they are dependent on the expression of GM-CSF for differentiation and/or survival. In addition the proportion of CD103+ MPCs was reduced to < 1% from 4–6% in controls. Recent studies have suggested that CD103+ MPC may derive from a circulating pre-cDC rather than monocyte (4). Consistent with that, some CD11blo CD11chi, CD103+, MPCs express Flt3 (Fig. 1 G), but these observation in Csf2−/− kidney suggest that the majority of MPC4 are not Flt3 dependent rather they are GM-CSF dependent. In addition in Csf2−/− mice there was a trend toward an increase in MPC1, although this was not significant.
When MDPs differentiate into monocytes, they activate expression of the lysosomal protein, lysozyme M (2). To test the origins of the five MPC subpopulations, we assessed whether they had ever been monocytes by fate mapping monocytes by their expression of LysM during bone marrow monocyte differentiation using LysMCre/+ ; R26RtdTomato/+ reporter mice (Fig. 3 P). Compared with monocytes, the MPC2, showed similar levels of tdTomato reporter and MPC3 showed increased levels. These suggest that MPC2 bears a close relationship to monocytes, and consistent with that many express low levels of Ly6C (Fig 2 A). MPC1 population showed somewhat lower proportion with tdTomato reporter and MPC4 (CD11blo CD11chi, CD103+), and MPC5 (CD11b− CD11cint) comprised fewer that have previously expressed the monocyte marker LysM. These findings indicate that MPC4 and MPC5 populations appear in the kidney without having trafficked as monocytes, suggesting they either arise in development from pre-cDCs or arise from another tissue resident progenitor. In addition MPC1 may comprise two subsets one of which may arise from pre-DCs or another tissue progenitor. Consistent with this, a subpopulation within MPC1 expresses Flt3 (Fig 1 G).
CD11blo CD11chi and CD11bhi CD11chi subpopulations have higher expression of transcription factors characteristic of classical dendritic cells
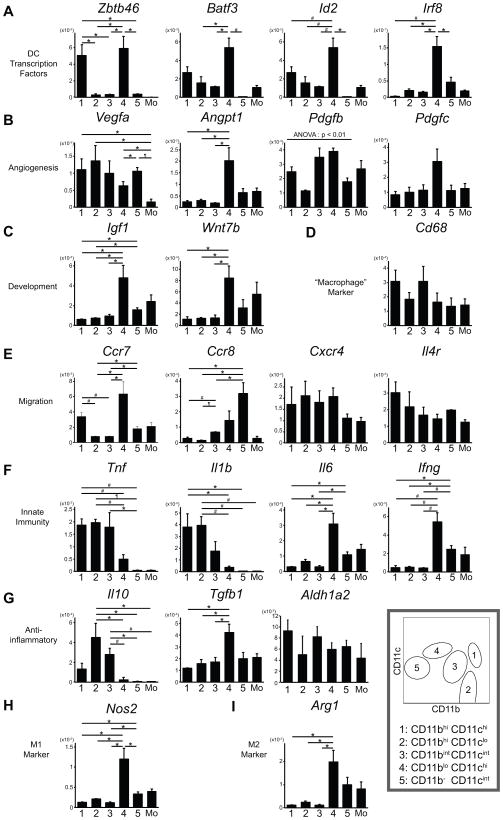
A single study has reported that two kinds of classical dendritic cells (cDCs) exist in non-lymphoid tissues; CD103+ DCs and CD11b+ DCs (4). Several transcription factors have been found to be involved in their development. We analyzed expression of these transcription factors in purified kidney MPC subpopulations. Zbtb46 is a transcription factor that has been identified as a marker specifically expressed by both CD103+ DCs and CD11b+ DCs, but not by other MPCs (22, 23). It was expressed in CD11blo CD11chi, CD103+, MPC4 and CD11bhi CD11chi MPC1 at significantly higher levels (Fig. 4 A), suggesting that the former subpopulation is equivalent to the reported CD103+ DCs and the latter is equivalent to CD11b+ DCs. Other transcription factors, BATF3 and Id2, are reported as indispensable for development of CD103+ DCs, but not CD11b+ DCs, although they are expressed in both types of DCs in peripheral tissues (4, 24). This is consistent with our findings that MPC4 had significantly higher expression of these two transcription factors and MPC1 had intermediate expression (Fig. 4 A). However, only MPC4 expressed Irf8 (Fig. 4 A), a transcription factor necessary for development of both CD103+ and CD11b+ cDCs but reported to be expressed only in CD103+ DCs in peripheral tissues (4, 25). These results are consistent with CD11blo CD11chi MPC4 corresponding to CD103+ DCs and CD11bhi CD11chi MPC1 corresponding to CD11b+ DCs.
Figure 4.
Quantitative RT-PCR analysis of gene expression in normal kidney MPC subpopulations. The expression levels of the gene relative to GAPDH are shown (n = 3). 1: CD11bhi CD11chi, 2: CD11bhi CD11clo, 3: CD11bint CD11cint, 4: CD11blo CD11chi, 5: CD11b− CD11cint, Mo: blood monocytes. Data are represented as mean ± SEM. * p < 0.05, # p <0.01, ¶ p < 1×10−3.
Kidney mononuclear phagocytes differentially express angiogenic, developmental and migratory genes
Macrophages are well known for their roles in angiogenesis and organ growth, as well as roles in innate immunity and phagocytosis. In order to explore whether kidney MPCs play roles in these processes, we assessed gene transcript levels for functionally related genes in each purified subpopulations, together with blood monocytes as control.
The angiogenic factor, vascular endothelial growth factor (VEGF)-A (Vegfa) was expressed in all MPCs at higher levels than monocytes (Fig. 4 B), indicating the resident MPC potential for angiogenesis. Although blood monocytes are similar to CD11bhi CD11clo MPCs in CD11b and CD11c expression, CD11bhi CD11clo MPC2 express significantly higher levels of Vegfa. In addition, MPC4 population had significantly higher expression of another angiogenic factor, Angiopoietin-1 (Angpt1) (Fig. 4 B). These cells also had a tendency to have higher expression of platelet-derived growth factor (PDGF) B and C (Pdgfb and Pdgfc) (Fig. 4 B).
As well as roles in angiogenesis, macrophages play important roles in organogenesis, and determining organ size (9). They also can promote repair (8). Two developmental pathways implicated in these processes are the insulin-like growth factor (IGF) pathway and the WNT pathway. As in angiogenic factors, CD11blo CD11chi MPC4 expressed significantly more Igf1 and Wnt7b than other subpopulations (Fig. 4 C).
Because CD68, a lysosome-associated phagocytic receptor, is often utilized as “macrophage” marker, but not DC marker, we assessed its expression level in each MPC subset. Strikingly, all MPC populations express similar levels of CD68 (Fig. 4 D).
Next we assessed in more detail chemokine receptors, CCR7 and CCR8, implicated in emigration of MPCs to lymph nodes where they can function as APCs (26, 27). CD11blo CD11chi MPC4 and CD11b− CD11cint MPC5 had higher expression of Ccr7 and Ccr8 (Fig. 4 E). Ccr7 is also highly expressed in CD11bhi CD11chi MPC1. These results suggest that these three subpopulations might present antigens to T cells in vivo.
CXCR4 plays a role in angiogenic myeloid cell recruitment (28). All MPC populations express similar levels of this receptor (Fig. 4 E), implicating potential functions in angiogenesis. The interleukin (IL)-4 receptor has been implicated in reactive proliferation of resident macrophages (29) and dendritic cell maturation (30), and it is also expressed in all MPC populations (Fig. 4 E).
Macrophages and dendritic cells are sentinels in mounting innate immune responses to tissue injury. In addition, macrophages can play an anti-inflammatory role, depending on the context. However, little is known about the pro- and anti-inflammatory proclivity of resident kidney MPCs. Thus, we assessed the transcriptional expression of representative cytokines in MPC subpopulations. Tnf and Il1b, pro-inflammatory cytokine genes, were significantly highly expressed in CD11bhi CD11chi MPC1 and CD11bhi CD11clo MPC2 (Fig. 4 F). In contrast, CD11blo CD11chi MPC4 and CD11b− CD11cint MPC5 subsets had higher expression of other pro-inflammatory cytokines, Il6 and Ifng. Anti-inflammatory cytokines were also produced at differing levels. CD11bhi CD11clo MPC2 and CD11bint CD11cint MPC3 had significantly more transcripts of Il10, whereas Tgfb1 was highly expressed in CD11blo CD11chi cells (Fig. 4 G). Interestingly RALDH2, an enzyme that regulates retinoic acid synthesis and has been implicated in stimulating regulatory T cells, and macrophage polarization was expressed at high levels by all MPC subsets (Fig 4 G). Overall these results suggest that MPC subpopulations have distinct functions in innate immunity.
It is well known that macrophages have much heterogeneity, and its typical examples are M1 and M2 macrophages, which promote and suppress inflammation, respectively. Surprisingly, CD11blo CD11chi subpopulation had significantly higher expression in both a representative M1 marker Nos2 and M2 marker Arg1 (Fig. 4 H and I).
Kidney mononuclear phagocyte populations show differential capacity for phagocytosis
The detailed expression profiles of these kidney MPC populations suggest they all may have both phagocytic and APC functions. We therefore assessed function in ex vivo assays. Phagocytosis of latex beads has been used as a ‘gold standard’ to experimentally uncover phagocytic function typical of macrophages, since only professional phagocytes have the capacity to phagocytose inert beads (31). MPCs were collected together and incubated with fluorescent latex beads for 2 hours at 37°C. Internalization of beads in respective subpopulations was assessed by flow cytometry. All the MPC subpopulations showed phagocytic function, but to differing degrees (Fig. 5 A). CD11bhi CD11clo MPC2 exhibited the highest capacity to phagocytose whether assessed as proportion of phagocytosing cells or the mean fluorescence intensity (Fig. 5 B and C). The rare population of CD11blo CD11chi, CD103+ MPC4 also showed significant phagocytic capacity whereas CD11b− CD11cint MPC5 showed a much lower capacity. Collectively, all MPC subpopulations can be considered to be phagocytic cells.
Figure 5.
The capacity of kidney MPCs to phagocytose latex beads ex vivo. (A) Representative histograms of kidney MPC subpopulations after incubation with FITC-conjugated latex beads. (B–C) Graphs showing the proportion of MPCs with phagocytosed beads (B) and of mean fluorescence intensity (C) in each subpopulation (n =4). Data are represented as mean ± SEM.
Kidney mononuclear phagocyte populations show differential capacity for stimulation of naïve T-cells and driving T-cell polarization
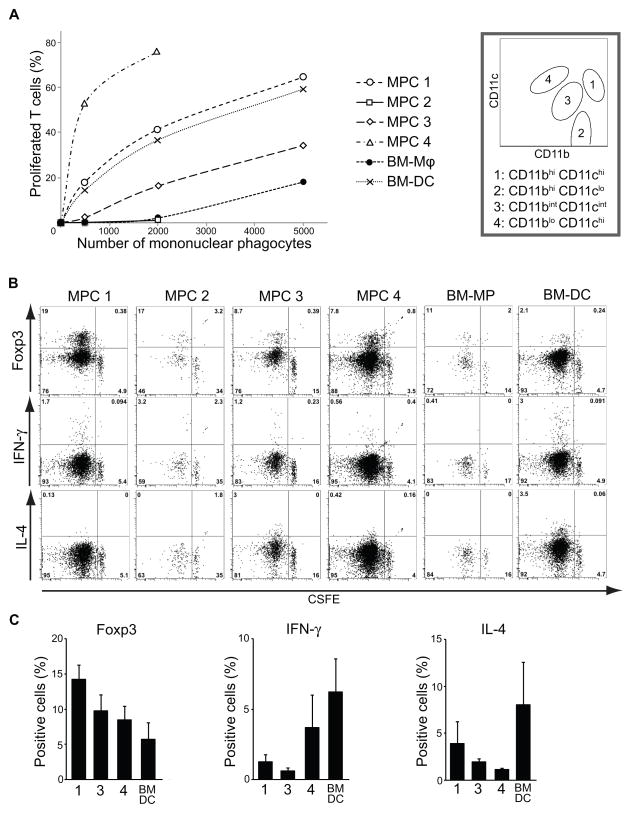
The capacity to successfully present antigen to naïve T cells is the ‘gold standard’ of antigen-presenting cell (APC) or DC function. Since all MPC populations expressed some level of MHC class II and co-stimulatory molecule CD86, we assessed the capacity of kidney MPC subpopulations to present antigen, in steady state, successfully to naïve T cells from the DO11.10 mice with the Rag2−/− mutation on the BALB/c background. These T cells can only respond to I-Ad MHC class II-restricted ovalbumin peptide 323–339 epitope because they have only the ovalbumin-specific T cell receptor expressed by the transgene and lack other T cell receptors due to deficiency of its recombination. Cultured bone marrow derived DC (BM-DC) and macrophage (BM-Mφ) were used as controls. Purified kidney MPC populations or aliquots of bone marrow derived cells, previously pulsed with ovalbumin peptide, were incubated with CFSE labeled naïve T cells for 5 days in non-polarizing conditions, and re-stimulated with anti-CD3e and anti-CD28 antibodies, followed by assessment of T cell proliferation (Fig. 6 A–C). As expected, BM-DC showed a much higher capacity to stimulate antigen-specific T-cell proliferation compared with BM-Mφ. Intriguingly, however, there were marked differences in the capacity of kidney MPC populations to stimulate T-cell proliferation. CD11bhi CD11clo MPCs (MPC2) had a low capacity to stimulate T-cells, similar to BM-Mφ, but CD11bhi CD11chi MPCs (MPC1) had a much higher capacity, similar to BM-DC, whereas CD11bint CD11cint, F4/80hi, MPCs (MPC3) had an intermediate capacity to stimulate T cells. Even more surprising was the finding that CD11blo CD11chi, CD103+, cells (MPC4) showed pronounced APC capacity, much greater than CD11bhi CD11chi, implicating these non-monocyte derived MPCs as the most potent APCs in the kidney. CD11b− CD11cint MPCs were too few in number to collect from BALB/c mice.
Figure 6.
Kidney MPC populations exhibit unique differential capacities for stimulating antigen-specific CD4+ T cell proliferation and polarization. (A) Graph showing the proportion of viable DO11.10/RAG2−/−; T cells that have proliferated as measured by CFSE dilution after incubation with aliquots of ovalbumin peptide-pulsed MPCs from each purified kidney MPC subpopulation (MPC1: CD11bhi CD11chi, MPC2: CD11bhi CD11clo, MPC3: CD11bint CD11cint, MPC4: CD11blo CD11chi), or from bone marrow-derived macrophages (BM-Mφ) or bone marrow-derived DCs (BM-DC). (B) Representative flow cytometric plots showing T cell survival and proliferative responses (CSFE dilution) and expression of markers of differentiation (FoxP3, IFNγ, IL4) in response to incubation of 30,000 naïve T cells with 2000 ovalbumin peptide-pulsed APCs. Gates were set using isotype control and no APC experimental control. (C) Graphs showing the proportion of viable proliferated T cells that expressed intracellular Foxp3 (left panel) or produced intracellular IFNγ (middle) or IL-4 (right) after re-stimulation following initial incubation with 2000 APCs. BM-MP and BM-DC were used as controls. MPC2 and BM-MP were excluded due to failure to stimulate >75% of T cells to proliferate. Data are represented as mean ± SEM. The experimental conditions were reproduced in duplicates or triplicates and the experiment performed twice.
Next we studied the polarization of naïve T cells induced by MPC subpopulations, by assessing TH1 (IFN-γ), TH2 (IL-4), TH17 (IL-17) and Treg (Foxp3) differentiation (Fig. 6 B–C). No TH17 differentiation was observed (data not shown), but CD11bhi CD11chi MPCs induced a high proportion of Treg and did not stimulate TH2 differentiation. By contrast, CD11blo CD11chi, CD103+, MPC4 induced a low proportion of Treg and a higher proportion of TH1 cells. In addition, CD11bint CD11cint, F4/80hi, MPCs, the most abundant subpopulation in steady-state kidney, induced a high proportion of Treg differentiation. By contrast BM-DC stimulated higher proportions of TH1 and TH2 and lower proportions of Treg. Collectively these studies implicate MPC1, 3 and 4 in maintenance of immunological tolerance.
In response to the pathogen-associated molecule, Lipopolysaccharide, kidney mononuclear phagocyte populations show distinct cytokine repertoires
We were interested to understand how resident kidney MPCs respond to a pro-inflammatory agent. To test this, MPC subpopulations and autologous blood monocytes were purified, and gene expression was assessed after 16-hour exposure, ex vivo, to the innate activator Lipopolysaccharide (LPS) which activates cells via TLR4 and CD14 (32) (Fig. 7). CD11bhi CD11chi and CD11bint CD11cint subpopulations had higher expression of pro-inflammatory Tnf and Il1b, especially compared to monocytes, whereas another pro-inflammatory cytokine Il6 was more highly expressed in CD11bhi CD11clo and CD11blo CD11chi (Fig. 7 A). The expression of an anti-inflammatory cytokine, Il10, showed a similar pattern as in Il6 (Fig. 7 B). The expression of another anti-inflammatory cytokine Tgfb1 was extremely low or could not be detected (data not shown).
Figure 7.
Quantitative RT-PCR analysis of gene expression in MPC subpopulations stimulated ex vivo with Lipopolysaccharide. The expression levels of the gene relative to GAPDH are shown (n =3). 1: CD11bhi CD11chi, 2: CD11bhi CD11clo, 3: CD11bint CD11cint, 4: CD11blo CD11chi, 5: CD11b− CD11cint, Mo: blood monocytes. Data are represented as mean ± SEM. * p < 0.05, # p <0.01, ¶ p < 1×10−3, § p < 1×10−4.
LPS is a typical inducer of M1 activation in macrophages. M1 markers, Cxcl10 and Il23a, were highly expressed in CD11blo CD11chi and CD11bhi CD11clo MPCs (Fig. 7 C). These two MPC subsets also had higher expression of another M1 inducer, Ifng. Intriguingly, a typical M2 activation marker, Arg1, was also expressed in these two subpopulations with significantly higher levels (Fig. 7 D). We also assessed other M1 markers, Nos2, Il12a, and Il12b, but their expression levels were extremely low (data not shown).
Next we evaluated expression of inflammatory chemokines, including Cxcl2, Ccl2, and Ccl3, which showed differential expression in MPC subpopulations (Fig. 7 E). Ccr7, a chemokine receptor related to MPC migration to lymph nodes, was highest in CD11blo CD11chi, CD103+, cells (Fig. 7 F). This population as well as CD11bhi CD11clo cells had higher expression of genes related to tissue repair, Vegfa, Pdgfb, and Igf1 (Fig. 7 G).
CD11bint CD11cint, F4/80hi mononuclear phagocytes are the main IL-10 producing MPCs in vivo
Since the resident MPCs predominantly stimulated Treg-cell proliferation in APC assays, we reasoned that some of the MPCs may play an anti-inflammatory role. To study this, we analyzed IL-10 production in vivo using a novel, highly sensitive IL-10 reporter mouse. These mice have the knock-in transgene β-lactamase under the IL-10 promoter, endogenous IL-10 gene, and IRES, and β-lactamase expression in respective cells can be detected by flow cytometry due to its enzymatic ability to convert green fluorescence to blue (15). In steady state, most MPCs do not synthesize IL-10, but a proportion of CD11bint CD11cint, F4/80hi, MPCs generate IL-10 in healthy kidneys (Fig. 8 A), suggesting they may have a unique role in preventing inflammatory responses. To investigate IL-10 up-regulation in kidney MPCs in the context of systemic inflammation, we utilized a model of systemic administration of LPS to mice. It is known that IL-10 is elicited as a counter-balance against systemic inflammatory response. Following systemic LPS administration, approximately 40% of MPCs activated IL-10 production (Fig. 8 B). Analysis of the MPC subpopulations that expressed IL-10 indicated that the CD11bint CD11cint, F4/80hi, subpopulation showed the highest ratio of IL-10 producing cells (Fig. 8 C). These findings show that this subpopulation has a greater capacity to IL-10 production in vivo.
Figure 8.
Interleukin-10 expression and quantification of kidney MPC subpopulations in vivo in steady state or following systemic lipopolysaccharide exposure. (A) Representative flow cytometry plots of kidney MPCs in highly sensitive IL-10 reporter (Il10IB/IB) and control (Il10+/+) mice. The right panel shows the distribution of IL-10-positive cells by F4/80 and CD11b (n=4). (B) Representative plots and a graph of IL-10 positive cells showing a remarkable increase in IL-10 expression of kidney MPCs with systemic LPS treatment, with the right panel showing the distrubtion of IL10+ cells by F4/80 and CD11b. (C) IL-10 histograms in CD11bhi CD11clo (2), CD11bint CD11cint (3), and CD11blo CD11cint (5) cells and a graph showing the proportion of IL-10 positive cells within each subpopulation (n =3) indicating that the majority of IL10+ cells are MPC3. (D) Representative flow cytometry panels showing the distribution of MPCs with or without LPS stimulation. (E) The graph showing the changes in the absolute number of MPCs within each subpopulation normalized to kidney weight. The filled bars correspond to those treated with vehicle, while the blank bars correspond to those treated with LPS (n =3). (F–G) Representative plots showing non-lymphocyte cells in lymph nodes in steady state or 48h after LPS injection. Note marked increase in F4/80+ CD11b+ cells as well as CD11c+ cells. Data are represented as mean ± SEM. * p < 0.05, # p <0.01, ¶ p < 1×10−3.
Mononuclear phagocyte populations show differential evanescence from the kidney following systemic LPS administration in vivo
To study the in vivo response of MPCs to systemic administration of LPS, we compared resident kidney MPC populations with those 48 h after LPS treatment. Over a 48-hour time period, two MPC populations showed almost complete disappearance from the kidney in response to LPS (Fig. 8 D–E). These populations are the CD11bhi CD11chi MPC1 and CD11blo CD11chi, CD103+, MPC4. CD11b− CD11cint MPC5 also declined in number although this was not complete (Fig. 8 E). In addition there was a significant reduction in the number of CD11bint CD11cint F4/80hi MPC3 population (Fig 8 E). Although this population did not disappear, numerically, the greatest number of MPCs disappeared from the kidney from this population of cells. To evaluate the meaning of this evanescence we assessed kidney draining lymph nodes (LNs) for the migration of MPCs. Forty-eight hours after LPS administration, there was a substantial increase in CD11bhi, CD11chi, F4/80hi MPCs in the LNs, but no clear population of CD103+ MPCs (Fig 8 F–G). Although this is not a fate map, these studies are consistent with substantial number of MPC1 and MPC3 cells migrating to LNs to present antigen. The fate of MPC4 cells is unclear.
Discussion
We used an unbiased approach to identify five distinct subpopulations of resident MPCs in the normal kidney, based on the different CD11b/CD11c integrin expression patterns in conjunction with F4/80, CD103 and CD14. By using this strategy we were able to discriminate 5 distinct subpopulations. To our knowledge, this is the first study that comprehensively analyzed and classified kidney MPCs, although other studies have previously discriminated MPCs in other organs (16, 33). These kidney subpopulations also show different patterns in MPC-related surface markers, monokine receptor expression, and gene expression profiles at steady state, and respond to LPS stimulation with distinct cytokine expressions. However, these resident subpopulations all express a range of growth factors that may regulate vasculature and tissue homeostasis. A potent anti-inflammatory cytokine, IL-10, is expressed by these resident MPCs, and it is remarkably elevated with LPS stimulation. They also share the capacity of phagocytosis and antigen presentation to naïve T cells, but to different degrees. The presentation to naïve T cells predominantly promotes differentiation of regulatory T cells. These results therefore identify discrete resident MPC subpopulations that share both macrophage and DC functions as well as macrophage and DC markers even within each subpopulation. A major conclusion of these findings is that conventional dichotomous macrophage and DC functions, phagocytosis and antigen presentation, respectively, are context-specific functions of resident MPCs rather than functions restricted to distinct cells; “macrophages” or “DCs”.
Among the five MPC subpopulations, CD11bint CD11cint, F4/80hi, MPC3 are the most abundant in normal kidney. Their most striking feature is high proclivity for IL-10 production both at steady state and in response to pathogenic stimulus. This subpopulation also has a tendency to stimulate differentiation of regulatory T cells. Furthermore, these MPCs appear to be the same MPCs described by Schulz et al. as arriving from yolk sac progenitors (6) (34), adding further weight to their distinct function in kidney. Lower CCR2 expression in this population than in CD11bhi F4/80lo populations (CD11bhi CD11chi and CD11bhi CD11clo) might reflect the difference of their ontogeny. In addition, only this CD11bint CD11cint MPC3 subpopulation requires CSF1R for their existence in the kidney, although other subpopulations also express this receptor at a lower level than CD11bint CD11cint population. A recent study reported that CSF1R signaling promotes repair from kidney injury through skewing MPC functions (35). Collectively, these finding have several important implications. Firstly, CD11bint CD11cint, F4/80hi, MPC3 may serve as endogenous defenders against inflammation. Secondly, since resident MPCs may expand in chronic diseases (7), it is quite possible that they represent a significant population of reparative macrophages (also known as regulatory or M2 macrophages). In addition, since the number of MPC3 cells different significantly between mouse strains it is possible that some of the immunological differences noted between these strains relates the differences in this cell population. Finally, although MPC3 have many characteristics of macrophages, nevertheless, numerically they leave the kidney in greatest numbers after systemic LPS injection and have significant APC capacity.
A second major finding from this study is that there exists a relatively rare resident population of CD103+ (CD11blo CD11chi) MPCs that have extremely higher capacity for antigen presentation with a potentially greater tendency to stimulate TH1 cells. These CD103+ MPCs also express the migratory chemokine receptor CCR7 and have an inclination to disappear from the kidney in response to LPS exposure, although at this time their fate is unclear. These findings suggest that they may have distinct APC roles in the kidney, potentially in local antigen presentation. In addition, relatively few of these MPCs appear to derive from monocytes. Consistent with this, some of MPC4 population also express Flt3, a receptor central for DC development, and had the highest expression of all the cDC development-related transcription factors that we assessed, including Zbtb46, Batf3, Id2, and Irf8 (36). Nevertheless this population was selectively dependent on GM-CSF for their existence. These results suggest that CD11blo CD11chi MPC4 correspond to CD103+ cDCs, although the latter cells have been conventionally limited to populations of CD11c+ MHCII+ cells. Furthermore, CD11blo CD11chi MPCs had higher expression of angiogenic factors, especially the ones related to pericyte recruitment, including Angpt1, Pdgfb, and Pdgfc, and in response to LPS activate genes consistent with M2 macrophage phenotype. Therefore, the may also have an important role in angiogenesis and/or maintaining the homeostasis of vasculature as well as regulating inflammation.
In addition to this relatively rare but functionally important population, there is a small population of CD11b− CD11cint MPC5, that expresses CD11c but most do not express CD103 or Flt3. These cells also do not derive from a monocyte precursor. The function of this subpopulation is currently unclear since they are rare and this has rendered functional studies incomplete. They are also dependent on CSF2 (GM-CSF), which has a role in development of DCs in vivo (37). One possibility is that they are a quiescent precursor of CD11blo CD11chi, CD103+ MPCs, which is also supported by the finding that they have intermediate expression of Irf8, a transcription factor known to be expressed in earlier stages of DC development (25). However, further studies will be required to validate this hypothesis. The alternative is that they represent a novel population of MPCs that have higher Ccr8 expression and capacity to emigrate from the kidney.
The CD11bhi CD11chi MPC1 subpopulation exhibits complicated characteristics. A minority of these cells express Flt3 and MPC1 have high expression of Zbtb46 suggesting that they are equivalent to CD11b+ DCs. Indeed, they have high capacity for antigen presentation, stimulating differentiation of Treg cells, they express the migratory chemokine receptor Ccr7 highly, and appear to migrate avidly to kidney LNs in response to LPS (Table II). On the other hand, this subpopulation has monokine receptor CX3CR1 and CCR2 expression at high levels, whereas the CD11blo CD11chi, CD103+, subpopulation does not. These two subsets also differ in inflammatory cytokine expression, suggesting the possibility that they have distinct roles in inflammatory states, although further studies are needed to clarify their lineage and features in detail. One possibility is that the Flt3+ subpopulation within MPC1 are CD11b+ DCs derived from a pre-DC as described elsewhere (4) but that the remaining Flt3− cells within MPC1 can also function as CD11b+ DCs. Other studies support the notion that circulating monocytes can give rise to non-conventional DCs in non lymphoid tissues (16, 33, 38), and MPC1 bears similarities to CD64+ monocyte-derived tissue DCs described by others (39, 40). Our studies indicate that MPCs that are not derived from pre-DCs have all the capacity to function as DCs in the normal kidney. Further studies are required to dissect the origins of these MPC populations in the normal kidney and their differential responses to homeostatic and inflammatory stimuli.
The CD11bhi CD11clo subpopulation is similar to blood monocytes in terms of CD11b and CD11c expression. However, we found clear and significant differences between these two types of cells. The former resident cells have significantly higher expression of Vegfa, Tnf, Il1b, and Il10 at steady state than the latter circulating cells, suggesting that CD11bhi CD11clo subpopulation plays a role as sentinels in tissues relevant to maintaining homeostasis.
We did not analyze CD11b− CD11c− MPC subpopulation because they lack all the MPC-related markers that we assessed. According to the gating strategy that we used, they also lack lymphocyte (T cell, B cell, and NK cell) and granulocyte maker. One possibility is that they are atypical lymphocytes, such as innate lymphoid cells, although further studies are needed for their characterization.
Another major observation is that all of the MPCs likely play concurrent roles in vascular homeostasis. All the subpopulations express typical angio-regulatory factors Vegf, Angpt1 and Pdgf genes in steady state. Recent studies have implicated macrophages and CSF-1 in tissue growth including kidney during development (9). A major factor in organ size is angiogenesis, but it will be interesting to understand whether the MPC subpopulations play similar or distinct roles in vascular regulation.
Inflammatory cytokines are expressed with different levels among MPC subpopulations in normal kidney. For example, Tnf and Il1b are more highly expressed in CD11bhi CD11chi, CD11bhi CD11clo, and CD11bint CD11cint populations, while Il6 and Ifng are more highly expressed in CD11blo CD11chi and CD11b− CD11cint populations. However, when they are stimulated with LPS ex vivo, pro-inflammatory cytokines Tnf and Il1b are generally down-regulated compared to steady state. Furthermore, an M1 marker Nos2 is remarkably decreased (undetectable by qRT-PCR), while an M2 marker Arg1 is increased by LPS stimulation. Taken together, these findings may further support the idea that resident MPCs have anti-inflammatory and reparative roles in tissue injury.
We conclude that the normal kidney has five discrete MPC subpopulations which exhibit distinct characteristics, functions and ontogeny. These functions are likely to be important in homeostasis and immunological tolerance. Overall, the subpopulations share both macrophage and DC characteristics, suggesting that these functions are more related to context than separate lineage. Finally, in response to systemic pathogenic exposure, kidney MPCs may serve as critical sentinels for the adaptive immune response and play important anti-inflammatory and tissue-reparative roles that are distinct from monocytes.
Supplementary Material
Acknowledgments
This work was supported by Institute for Stem Cell and Regenerative Medicine at University of Washington, NIH grants DK94768, DK93493, DK84077, TR000504 and AHA grant 12040023 (J.S.D.), NIH grant DK83375 (P.J.N.), an Overseas Research Grant from Mochida Memorial Foundation for Medical and Pharmaceutical Research (T.K.) and by the Deutsche Forschungsgemeinschaft DFG (J.L., Li 2074/1-1).
We thank Dr. E. Richard Stanley (Albert Einstein College of Medicine, New York) for providing transgenic mice, and Jen-Feng Lai (BRI) and Deby Kumasaka (FHCRC) for technical assistance with experiments.
References
- 1.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 4.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234:268–281. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 6.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 7.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 8.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, Sakkal S, Samuel CS, Ramsay RG, Deane JA, Wells CA, Little MH, Hume DA, Ricardo SD. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol. 2011;179:1243–1256. doi: 10.1016/j.ajpath.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 13.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 15.Bouabe H, Liu Y, Moser M, Bosl MR, Heesemann J. Novel highly sensitive IL-10-beta-lactamase reporter mouse reveals cells of the innate immune system as a substantial source of IL-10 in vivo. J Immunol. 2011;187:3165–3176. doi: 10.4049/jimmunol.1101477. [DOI] [PubMed] [Google Scholar]
- 16.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Thompson LJ, Valladao AC, Ziegler SF. Cutting edge: De novo induction of functional Foxp3+ regulatory CD4 T cells in response to tissue-restricted self antigen. J Immunol. 2011;186:4551–4555. doi: 10.4049/jimmunol.1003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weischenfeldt J, Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc. 2008 doi: 10.1101/pdb.prot5080. pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 22.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo IF, Jung S, Lira SA, Randolph GJ. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutz MB, Schnare M, Menges M, Rossner S, Rollinghoff M, Schuler G, Gessner A. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574–3580. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 31.Desjardins M, Griffiths G. Phagocytosis: latex leads the way. Curr Opin Cell Biol. 2003;15:498–503. doi: 10.1016/s0955-0674(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 32.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jojic V, Shay T, Sylvia K, Zuk O, Sun X, Kang J, Regev A, Koller D, Best AJ, Knell J, Goldrath A, Jojic V, Koller D, Shay T, Regev A, Cohen N, Brennan P, Brenner M, Kim F, Rao TN, Wagers A, Heng T, Ericson J, Rothamel K, Ortiz-Lopez A, Mathis D, Benoist C, Bezman NA, Sun JC, Min-Oo G, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Monach P, Blair DA, Dustin ML, Shinton SA, Hardy RR, Laidlaw D, Collins J, Gazit R, Rossi DJ, Malhotra N, Sylvia K, Kang J, Kreslavsky T, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S C Immunological Genome Project. Identification of transcriptional regulators in the mouse immune system. Nature immunology. 2013;14:633–643. doi: 10.1038/ni.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114:835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- 38.Khandelwal P, Blanco-Mezquita T, Emami P, Lee HS, Reyes NJ, Mathew R, Huang R, Saban DR. Ocular Mucosal CD11b+ and CD103+ Mouse Dendritic Cells under Normal Conditions and in Allergic Immune Responses. PloS one. 2013;8:e64193. doi: 10.1371/journal.pone.0064193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Gregoire C, Malissen B, Guilliams M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188:1751–1760. doi: 10.4049/jimmunol.1102744. [DOI] [PubMed] [Google Scholar]
- 40.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. European journal of immunology. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.