Abstract
Mitochondrial dynamics, the fusion and fission of individual mitochondrial units, is critical to the exchange of the metabolic, genetic and proteomic contents of individual mitochondria. In this regard, fusion and fission events have been shown to modulate mitochondrial bioenergetics, as well as several cellular processes including fuel sensing, ATP production, autophagy, apoptosis, and the cell cycle. Regulation of the dynamic events of fusion and fission occur at two redundant and interactive levels. Locally, the microenvironment of the individual mitochondrion can alter its ability to fuse, divide or move through the cell. Globally, nuclear-encoded processes and cellular ionic and second messenger systems can alter or activate mitochondrial proteins, regulate mitochondrial dynamics and concomitantly change the condition of the mitochondrial population. In this review we investigate the different global and local signals that control mitochondrial biology. This discussion is carried out to clarify the different signals that impact the status of the mitochondrial population.
Keywords: Mitochondrial Dynamics, Cell Cycle, Autophagy, Fusion, Fission, mtDNA, Mitochondrial Movement, Bioenergetics, Mitophagy
Introduction
Mitochondria exist as a dynamic network within living cells, undergoing fusion and fission events that facilitate inner and outer membrane fusion and the exchange of organelle contents such as solutes, metabolites, proteins, and mitochondrial DNA (mtDNA)[1-6]. Processes of fusion and fission are necessary for many cellular functions including mitosis, fuel sensing, ATP production, autophagy, and apoptosis [6-11]. Mitochondria are evolutionarily derived from the endosymbiotic relationship of prokaryotic bacteria within eukaryotic cells. Although the coexistence of these two organisms have become genetically integrated through natural selection, the individual mitochondrion is still in many ways its own organism [12-14]. In this review we explore the dynamic relationship between “global” cellular and “local” organelle specific control of individual mitochondria, and the implications of these controls on the mitochondrial population.
Mitochondrial proteins are encoded both by nuclear and mtDNA, however all mitochondrial proteins can be post-translationally modified, degraded, oxidized, or otherwise altered by conditions specific to the individual organelle. This process can be described as “local” control which refers to the effect of micro-environmental changes at the level of the individual mitochondrion that affect fission and fusion events. Local control can affect the capacity of the individual mitochondrion to function in energy production, sequester apoptotic stimuli, or propagate mtDNA. For example, loss of mitochondrial membrane potential (Δψm) can lead to the isolation of the mitochondrial unit from the mitochondrial network, and increased probability of autophagic degradation [6;15].
In contrast to “Local” controls, “Global” controls are mechanisms that can either override or monopolize mechanisms of local control to alter the dynamic status of mitochondria in the entire cell. The cell cycle is a prime example of global control. A key phase of the cell cycle is G1-S mitochondrial biogenesis which is controlled by nuclear transcription factors PGClα, PPARα, NRF1/2, and ERRα[16] [17]. Activation of these transcription factors leads to increased mitochondrial mass, respiratory capacity and energy production required during the etropic S phase of cell division [17]. Observational findings during the cell cycle have demonstrated that individual mitochondria act in a concerted manner at different stages of the cell cycle [17-20]. Specifically, network hyperfusion was found to occur at G1-S phase and be required for progression to S phase [16]. Additionally, hyper fragmentation of the mitochondrial network begins in S Phase [20]. Lastly, fragmented mitochondria localize to opposite telomeres of daughter mitochondria in M phase suggesting concerted movement and dispersion between daughter cells. A depiction of this process as well as the roles of global and local control can be found in Figure 1.
Figure 1. Mitochondrial Life in the Cell Cycle.
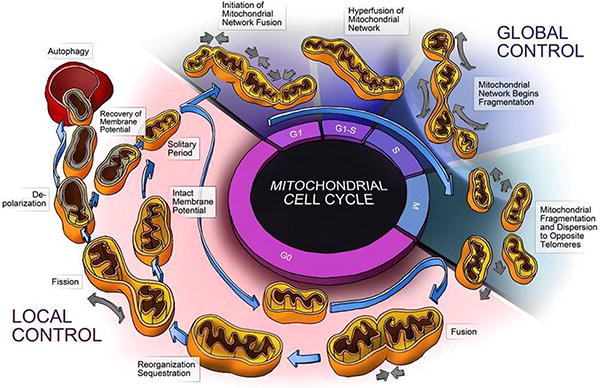
This diagram depicts the normal life cycle of an individual mitochondria during the G0 phase of the cell cycle. The mitochondrion undergoes fusion, fission, depolarization, and degradation by autophagy. This process is depicted as one of local control where by mitochondrial events are largely dictated by the local energetic status and associated local signals. During the cell cycle Global signals cause concerted changes in the mitochondrial population, as noted by Hyperfusion in G1-S and fragmentation during M phase. These global population effects are governed by the cells demand for energy required by cell division and the need for homogenization and sequestration of cellular components during met-phase. The cell cycle serves as an elegant example of the parities of local and global control.
Regulation and Monitoring of Dynamic Events
Recent findings have begun to elucidate the life cycle of a mitochondrion. It has been shown that mitochondria exist in networked and solitary phases within the cell, undergoing cycles of fusion and fission in response to a variety of stimuli [6;10;16;21-24]. Additionally, mitochondria can be subjugated from the dynamic life cycle and targeted for degradation by the autophagosome. [6;25;26]. Biophysical and morphologic parameters can be monitored for extended periods of time using mitochondrially targeted photo activatable GFP (mtPA-GFP) [27-29]. Real time tracking of fluorescently-labeled individual mitochondria enables quantification of size, motility, shape, Δψm as well as temporal properties of fusing organelles. Gerenscer and Nicholls have developed the Optical Flow Method (OF) by which mitochondrial movement, redox status, and Δψm can be correlated using imaging techniques [30]. These experiments confirm that mitochondria exist in two states, networked and solitary, and that fusion and fission events are correlated to the bioenergetic state change of individual mitochondria.
In several cell types prolonged tracking (up to 2 hrs) of individual mitochondria demonstrated the organelle's ability to maintain a stable Δψm during the solitary period [6;15;27]. During most of that time (95% of the recording period) the Δψm of the individual mitochondrion was within ±2.7 mV of its average baseline. This observation indicates that continuous deterioration in Δψm is an unlikely (or infrequent) route for the generation of depolarized mitochondria under normal conditions. The majority of fission events yield heterogeneous daughter mitochondria with opposite Δψm deflections, usually greater than 5 mV. Probability analysis has found that the depolarized daughter mitochondria are 6 times less likely to rejoin the mitochondrial network through fusion [6]. Non-fusing mitochondria can be identified using a procedure in which a small group of mitochondria are tagged by laser photoconversion of matrix-targeted mitochondrial photoactivatable green fluorescent protein (mtPA-GFP) and observed over time. These mitochondria fail to dilute their activated mtPA-GFP through fusion events. Co-staining with the membrane potential dependent dye TMRE revealed that non-fusing mitochondria are depolarized by ∼7 mV compared to average Δψm, while co-staining with an OPA1 antibody, that distinguishes the various isoforms, shows that such mitochondria contain approximately 50% less total OPA1 levels compared to the typical mitochondria [6]. This identifies inhibition of fusion as a selective process or initial checkpoint that generates depolarized mitochondria. Characteristics of these depolarized mitochondria make them significantly less likely to fuse with the mitochondrial network and subsequently more likely to be degraded by autophagy [6]. The importance of these events to organelle functionality make the control by local and global signals of particular interest. A summary of the regulation steps discussed below and the appropriate references can be found in Table 1.
Table 1. Regulation of Mitochondrial Dynamics.
| Control Mechanism | Outcome | Reference | |
|---|---|---|---|
| Fission | |||
| Global Cellular Control | |||
| Ubiquitination of DRP1 (MARCH V) | Inhibition of Fission | [84] | |
| cAMP Dependent Phosphorylation of DRP1 | Inhibition of Fission | [85] | |
| Calcium dependent recruitment of DRP1 from Cytosol | Fission induced by global calcium | [34] [84]; [36] | |
| Dephosphorylation of DRP1 by Calcineurin regulates translocation to mitochondria | Fission induced elevated cytosolic calcium | [85] [86] |
|
| Increase of Bax/Bak induces Fission. Bax and Bak in healthy cell controls fusion through Mfn2 | Interacts at mitochondrial fission sites to promote fission | [37;38] | |
| Sumoylation of DRP1 at fission sites | Sumoylation protects DRP1 from degradation and allows fission to proceed | [40] [41] |
|
| Local Organelle Control | |||
| Loss of Membrane potential leads to OPA1 processing by metalloproteases | Increased OPA1 processing inhibits fusion of individual mitochondrion | [46] [50] [51] [45] |
|
| Increased OPA1 processing in response to local membrane potential and ATP levels | OPA1 processing inhibits fusion and induces mitophagy | [6;44;50] | |
| Fusion | |||
| Global Cellular Control | |||
| Activation of PGC1a/PGC-1b/ERRa induces MFN2 mRNA. PGC-1b induces mitochondrial fusion through Mfn2. | Increased Fusion/Mitochondrial Biogenesis | [87] [10] |
|
| BID disrupts the OPA1 cristae junction complex | The size of cristae junction is regulated independently of OPA1 mitochondrial fusion activity | [88] | |
| Mito tubularization and network fusion at G1-S of cell cycle. | G1-S stimulates global mitochondrial fusion, mitosis stimulates fission | [16;20] [19] | |
| Local Organelle Control | |||
| Functional interaction of OPA1 with MFN1. | Functional interaction of Mfn1 and Opa1 but not Mfn2. | [43;89] | |
| OPA1 processing by Metalloproteases blocks fusion | Fusion inhibited at the local level by protease activity | [50], [51], [45] [89] [44] |
|
| Low Local GTP level induces outer membrane tethering | Initial fusion promoted in energy deficient environments | [71] | |
| High Intra-mitochondrial GTP levels required for inner membrane fusion | Complete fusion regulated by energetic status | [71] | |
| Mitochondrial movement on microtubules is essential for fusion | Inhibition of movement arrests fusion | [24] | |
| Solitary Period | |||
| Global Cellular Control | |||
| Increase of cellular calcium increases mitochondrial motility and fusion (Miro) | Release of cellular calcium stores increases mitochondrial movement | [75] [90] | |
| Global ADP levels increase Mitochondrial movement to synapses | ADP signals mitochondrial motility | [81] | |
| GPCR Ga12 is expressed in mitochondria and regulates motility | GPCR sensitive to GDP/GTP levels regulate mitochondrial motility | [82] | |
| Local Organelle Control | |||
| Local ATP level/membrane potential regulates motility | Increased energetic capacity increases movement | [75] | |
| Mito movement along Microtubules occurs in an energy dependent manner | Individual mitochondria move at different rates along microtubules based on ATP levels | [78] [79]; [75] | |
| Local redox status of Mitochondria impacts MMP and Velocity of movement | Elevated Oxidation leads to Loss of MMP. Loss of MMP leads to increased motility | Gerenscer and Nicholls 22 |
Factors Affecting Mitochondrial Fragmentation
In eukaryotic cells, mitochondrial fission requires local organization of FIS1 and recruitment of GTPase DRP1 for assembly of the fission machinery that subsequently leads to membrane scission [10;31-35]. Global cellular signals related to energetic status, mitosis, and cell death have the capability to affect fission. However, the recruitment, organization and assembly of these, proteins ultimately can be regulated by the individual mitochondrion. Complete understanding of the regulation of this process has yet to be determined; however several levels of global and local control have been elucidated.
Secondary modifications of proteins and global ion signals have been shown to be strong global regulators of the fission machinery. DRP1 recruitment to the mitochondrial membrane has been found to be a calcium dependent process [34;36]. Dephosphorylation of cytosolic DRP1 by calcineurin has been shown to cause mitochondrial localization of DRP1 [21]. Both DRP1 and FIS1 have been found to interact with BCL-2 family members and thus globally regulated by apoptotic and anti-apoptotic machinery [18;36-39]. Additionally, DRP1 levels have been found to be regulated by sumoylation and other posttranslational modifications affecting mitochondrial fission [40;41]. The cell cycle has been found to cause uniform changes in the fission state of mitochondria, and in particular hyperfragmentation has been noted at the stage of M phase [17-19].
Local control of fission at the level of the individual mitochondrion appears to be largely regulated by the energetic status of the mitochondrion itself. Loss of membrane potential and ATP production can lead to OPA1 processing and mitochondrial fragmentation due to inhibition of its fusion activity [42-44]. Several studies have investigated OPA1 processing as a key regulator of mitochondrial fragmentation[18;31;44-46] [44]. OPA1 processing is dependent on membrane potential, metal ion levels, and ATP, and proteases PARL and Yme1 identified as key players, although the precise steps of this process still need to be elucidated[42;43;46-51].
Current understanding suggests three key steps to mitochondrial fragmentation: a) organization of FIS1 proteins around a fission site [52]; b) recruitment of DRP1 from the cytosol to the FIS1 sites [34]; c) inhibition of fusion in response to local processing of OPA1 in individual mitochondria. It should be noted however that inhibition of fusion has not been found to induce fission directly; however it does induce membrane fragmentation suggesting the importance of fusion/fission balance [32;33;53]. Of particular interest to the theory of local control of fission is that the energetic status of the individual mitochondria as dictated by Ca2+, membrane potential, and ATP levels are key regulators of mitochondrial fragmentation [44;46;49;50;54]. These findings suggest that in most cases generation of a depolarized mitochondrion, a process regulated by fission, is therefore impacted by energetic status [55-57]. In this model fission events represent a checkpoint for mitochondrial depolarization and degradation by mitophagy. This suggests that through fission the cell has evolved a mechanism for “programmed organelle death” on a daughter mitochondrion, a concept previously discussed in the literature[58;59]. Therefore, despite cellular control of the fission stimuli, the individual mitochondrial fission event is a locally controlled checkpoint for the generation of polarized and depolarized mitochondria. However, since fission events can also produce daughter mitochondria with equivalent membrane potential that interact normally with the mitochondrial network the fission event itself is not the key regulation [27;32;33]. Rather the process that allows fission to selectively generate a depolarized daughter mitochondrion and subjugate damaged or ineffective components will be the key regulation step. This area of research is of great interest and should increase our understanding of the regulation of mitochondrial dynamics
Local and Global Control of Mitochondrial Fusion
While fission can generate depolarized daughter mitochondria, it is fusion or rather lack of fusion of depolarized mitochondria back to the network that relegates it for degradation. The precise signal that initiates mitochondrial fusion has not been elucidated, although it is likely that it is initiated by both local and global signals. Fusion events allow rapid diffusion of matrix proteins and ions [8;32;33;60-62], with slower migration of inner and outer membrane components and mtDNA nucleoids [3;5;27;63]. Fusion events can be separated into two categories transient (outer membrane only) and complete (inner and outer membrane) fusion. Recent evidence suggests that these two types of fusion have alternate stimuli and purpose, however both play an important role in mitochondrial function [2;22].
In mammals, mitochondrial dynamic proteins MFN1, MFN2, and OPA1 have been shown to be critical for mitochondrial membrane fusion [18;64-68]. Further processing of these proteins, in particular OPA1, by proteolytic processing and interactions with BCL-2 family members has been found and speculated to affect fusion events and alter mitochondrial morphology [37;42;46;50]. Several examples of global control of this function exist. The proapoptotic protein BID was found to interact with OPA1 at the cristae junction and impair fusion [42;69]. Transcriptional stimulation of mitochondrial fusion by PGC1β is performed through selective induction of Mfn2 mRNA transcripts [10]. The cell cycle has been found to cause uniform changes in the fusion state of mitochondria, and in particular hyper-fusion has been noted at the stage of G1-S [18-20;37;70]. The local environment may regulate not only fusion itself but the type of fusion, transient or complete, as OPA1 processing could disrupt its interaction with MFN1 and block complete fusion [42;43;46]. Similarly GTP levels and processing of OPA1 by energy dependent proteases has been found to prevent transient fusion, complete fusion, or both depending on the conditions [42;44;45;49-51;71]. This suggests, as with fission, that fusion events have multiple regulation steps and it is a process regulated by energetic status. For a detailed review of the mitochondrial fusion proteins and their regulation see the following references [21;31-33;35;72].
It has been suggested that fusion provides a restorative function to depolarized mitochondria[32]. Fusion equilibrates matrix components as well as membrane potential of fused mitochondria in a range of several seconds [7;27;28;60]. Tracking of individual mitochondria demonstrated high probability for fission events briefly after fusion events, suggesting that fusion events are key regulator of the rate of dynamics [15;22;27]. Furthermore, tracking of fission and Δψm shows that depolarized mitochondria generated during fission events are 6 times less likely to become involved in a consecutive fusion event within the next 10 minutes as compared to their sisters generated during the same events (“fission-mate”)[6]. This finding suggested that depolarized mitochondria may remain in the cell as non-fusing mitochondrion. Importantly, once the fission event has generated a depolarized mitochondrion, this depolarized mitochondrion meets a second checkpoint at the level of fusion. The ability of a mitochondrion to re-fuse to the network is a key determinant of its fate. At this checkpoint local responsiveness of the individual mitochondrion to regulation of its fusion machinery can determine whether the mitochondrion rejoins the network or is relegated for autophagic processing. Thus, the fusion event establishes a secondary checkpoint for the fate of the individual mitochondrion.
Control of the Solitary Mitochondrion
Mitochondria spend the majority (Over 90%) of its life cycle as a solitary organelle [73]. In the solitary phase of the mitochondrial life cycle, the mitochondria does not directly participate in dynamic events. Of particular interest is what affects the length of this period, and how that impacts the rate of mitochondrial dynamics.
There is evidence to suggest that association of mitochondria with cytoskeleto and mitochondrial motility has a stron influence on the period of time spent between fusion events. Since fusion is initiated by tethering of MFN1/2 proteins on the outer membrane [31;47;48;66;67;74], movement may facilitate the chance for proper alignment between the engaging mitochondria. The work of Yi et al suggest that mitochondrial motility can be regulated by both calcium and ATP levels [75]. These results again point to energy status (ATP levels) as well cellular signals (Ca2+) as regulators of individual mitochondrial motility and thus perhaps dynamic rate [22]. In H2c9 cardiomyoblasts motile mitochondria have higher chance to fuse compared to static organelles. Mitochondria were shown to travel along microtubules by association with dyneins and kinesins [76;77]. Specifically, mitochondria in neurons have been found to travel both anterograde and retrograde along axonal projections in a mitochondrial membrane and ATP production dependent manner [75;78;79]. Mitochondria with high membrane potential and elevated ATP production are directed to sites of vesicular release and ATP demand while depolarizing mitochondria are returned to the soma [76;80]. Additionally an increase in global ADP levels has been found to drive mitochondrial movement toward synapses [81]. Furthermore, the Ga12 receptor found on mitochondria regulates mitochondrial movement in a GTP/GDP dependent manner [82]. Recent work by Gerenscer and Nichols using OF method of tracking mitochondrial movement suggests that mitochondrial movement can be directly impacted by energetic and redox status. In neurons, mitochondria with low Δψm travel more rapidly then those with high Δψm, and are more likely to travel retrograde away from sites of high ATP demand [30]. Additionally high oxidative potential in individual mitochondria exhibit a loss of Δψm. This suggests that oxidative damage may affect MMP and therefore movement of mitochondria. These results suggest that individual mitochondria have selective movement based on energy levels. This regulation of the solitary phase of the mitochondrial life cycle is likely to directly affect the rate of mitochondrial dynamics. Thus, stimuli that alter the energetic status of individual mitochondrion exert selective pressure not only during the networked state and during fission but also during the solitary phase.
Mitochondrial Dynamics and Mitochondrial DNA
Fusion and fission allow for diffusion and redistribution of mtDNA and reduces the heterogeneity in mtDNA content between mitochondria within the cell [3;4;31;32;35;62;83]. Increased heterogeneity in mtDNA content has been correlated with several aging related diseases [35]. Our recent findings as well as the findings of others suggest that fission is an initial step in quality maintenance that ends up with the removal of bioenergetically compromised mitochondria by autophagy. [6]. This suggests that mtDNA mutations that lead to respiratory dysfunction and loss of Δψm would be selectively excluded from the mitochondrial network by fission and subjugated for autophagy. This process results in the removal of bioenergetically compromising mutations and offers a mechanism of natural selection. In support of this idea, it has been shown that mtDNA nucleiods tightly regulate their genetic material and do not freely exchange between nucleiod units [5]. This infers that mtDNA nucleiods constitute the basic mitochondrial unit, and that mutations within nucleiods can be propagated or eliminated based of degradation or proliferation of the mitochondrial unit. As we discussed mitochondrial dynamics are linked to the degradation and production of mitochondria within the cell and thus the global and local control of this process has the potential to impact mtDNA mutation and overall cellular mtDNA heteroplasmy. However, the duration between fusion events (5-30 minutes) is significantly shorter than the half life of most of the respiratory chain subunits. Therefore, it is likely that inhibition of mitochondrial fusion for durations that exceed the lifespan of respiratory chain components (range of days) may be essential for producing a bioenergetic phenotype from mtDNA mutations and deletions; thereby allowing their sequestration and elimination. It is therefore possible that adaptation to a new environment could benefit from an extended period where fusion is inhibited and the mitochondrial network is fragmented.
Future Topics
Based on the quantifications of fusion and fission events, time spent in networked and solitary phases, specific rates of fusion/fission the total number of events in the cellular life cycle can be predicted. This could imply that the cell evolved a required amount of fusion/fission events for each life cycle. This number may be a function of the need for complementation and homogenization of mitochondrial components in preparation for cell division or the need for proper dispersion of mtDNA copies. The matrix PA-GFP diffusion assay is a method for quantification of this, as it measures the time it takes for matrix contents to be equilibrated across the cell through mitochondrial dynamics and the number of dynamic events required for this process. Additionally, the OF method developed by Gerenscer and Nicholls can be used to quantify organelle velocity offering an accurate method for quantification of the solitary period and mitochondrial movement [30]. Interestingly, primary cells, which are less proliferative then their differentiated counterparts, display a slower rate of matrix diffusion [38]. This finding may implicate the rate of mitochondrial dynamics and thus diffusion as intrinsic parts of the cellular life cycle. As the mechanisms that regulate mitochondrial dynamics, both at a local and global level, become elucidated, it will be important to determine the impact of modulation of these factors on the ability of the mitochondria to network and properly diffuse components. The cell cycle exerts global control which may up or down regulation fusion/fission, yet ultimately individual events are responsive to the local mitochondrial status. Therefore in future work it will be critical to examine diffusion and dynamic event rates in response to alterations in local and global control mechanisms.
Abbreviations
- Δψ
Mitochondrial membrane potential
- Bcl-2
B-cell Lymphoma -2
- Bid
BH3 interacting domain
- Drp1
Dynamin related protein 1
- ERRα
Estrogen related receptor alpha
- Ga12
GPCR a 12
- mtDNA
Mitochondrial DNA
- mtPAGFP
Mitochondrial photo activatable green fluorescent protein
- MFN1/2
Mitofusin 1 and 2
- NRF1/2
Nuclear respiratory factor 1/2
- OF
Optical flow method
- OPA1
Optic atrophy protein 1
- PARL
presenilin-associated rhomboid-like
- PGC1α
PPAR gamma coactivator 1 alpha
- PPARα
Peroxisome proliferation activated receptor alpha
- TMRE
tetramethylrhodamine ethyl ester
- Yme1L
yme1-like protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
Reference List
- 1.Malka F, Lombes A, Rojo M. Organization, dynamics and transmission of mitochondrial DNA: focus on vertebrate nucleoids. Biochim Biophys Acta. 2006;1763:463–472. doi: 10.1016/j.bbamcr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, Rojo M. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6:853–859. doi: 10.1038/sj.embor.7400488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legros F, Malka F, Frachon P, Lombes A, Rojo M. Organization and dynamics of human mitochondrial DNA. J Cell Sci. 2004 Jun 1;117:2653–2662. doi: 10.1242/jcs.01134. [DOI] [PubMed] [Google Scholar]
- 4.Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilkerson RW, Schon EA, Hernandez E, Davidson MM. Mitochondrial nucleoids maintain genetic autonomy but allow for functional complementation. J Cell Biol. 2008 Jun 30;181:1117–1128. doi: 10.1083/jcb.200712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008 Jan 23;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakada K, Inoue K, Ono T, Isobe K, Ogura A, Goto YI, Nonaka I, Hayashi JI. Inter-mitochondrial complementation: Mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med. 2001;7:934–940. doi: 10.1038/90976. [DOI] [PubMed] [Google Scholar]
- 8.Arimura S, Yamamoto J, Aida GP, Nakazono M, Tsutsumi N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc Natl Acad Sci U S A. 2004 May 18;101:7805–7808. doi: 10.1073/pnas.0401077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skulachev VP. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci. 2001;26:23–29. doi: 10.1016/s0968-0004(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 10.Liesa M, Borda-d'Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, Palacin M, Vidal-Puig A, Zorzano A. Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS One. 2008;3:e3613. doi: 10.1371/journal.pone.0003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial Networking Protects Beta Cells from Nutrient Induced Apoptosis. Diabetes. 2009 Jul 29; doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margulis L. Symbiosis and evolution. Sci Am. 1971;225:48–57. doi: 10.1038/scientificamerican0871-48. [DOI] [PubMed] [Google Scholar]
- 13.Gray MW, Burger G, Lang BF. The origin and early evolution of mitochondria. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-6-reviews1018. REVIEWS1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999 Mar 5;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 15.Wikstrom JD, Katzman SM, Mohamed H, Twig G, Graf SA, Heart E, Molina AJ, Corkey BE, de Vargas LM, Danial NN, Collins S, Shirihai OS. beta-Cell mitochondria exhibit membrane potential heterogeneity that can be altered by stimulatory or toxic fuel levels. Diabetes. 2007;56:2569–2578. doi: 10.2337/db06-0757. [DOI] [PubMed] [Google Scholar]
- 16.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009 Jul 21;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarpulla RC. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene. 2002 Mar 6;286:81–89. doi: 10.1016/s0378-1119(01)00809-5. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arakaki N, Nishihama T, Owaki H, Kuramoto Y, Suenaga M, Miyoshi E, Emoto Y, Shibata H, Shono M, Higuti T. Dynamics of mitochondria during the cell cycle. Biol Pharm Bull. 2006;29:1962–1965. doi: 10.1248/bpb.29.1962. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007 Apr 13;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 21.Scorrano L. Proteins that fuse and fragment mitochondria in apoptosis: con-fissing a deadly con-fusion? J Bioenerg Biomembr. 2005;37:165–170. doi: 10.1007/s10863-005-6572-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Hajnoczky G. Ca2+-dependent regulation of mitochondrial dynamics by the Miro-Milton complex. Int J Biochem Cell Biol. 2009;41:1972–1976. doi: 10.1016/j.biocel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da CS, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009 Jun 3;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X. Mitochondrial 'kiss-and-run': interplay between mitochondrial motility and fusion-fission dynamics. EMBO J. 2009 doi: 10.1038/emboj.2009.255. Mitochondrial 'kiss-and-run': interplay between mitochondrial motility and fusion–fission dynamics. Liu X., Weaver D. Shirihai OS and Hajnoczky G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007 Jun 15;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008 Dec 1;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twig G, Graf SA, Wikstrom JD, Mohamed H, Haigh SE, Elorza A, Deutsch M, Zurgil N, Reynolds N, Shirihai OS. Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. Am J Physiol Cell Physiol. 2006;291:C176–C184. doi: 10.1152/ajpcell.00348.2005. [DOI] [PubMed] [Google Scholar]
- 28.Jakobs S, Schauss AC, Hell SW. Photoconversion of matrix targeted GFP enables analysis of continuity and intermixing of the mitochondrial lumen. FEBS Lett. 2003 Nov 6;554:194–200. doi: 10.1016/s0014-5793(03)01170-0. [DOI] [PubMed] [Google Scholar]
- 29.Molina AJ, Shirihai OS. Monitoring mitochondrial dynamics with photoactivatable [corrected] green fluorescent protein. Methods Enzymol. 2009;457:289–304. doi: 10.1016/S0076-6879(09)05016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerencser AA, Nicholls DG. Measurement of instantaneous velocity vectors of organelle transport: mitochondrial transport and bioenergetics in hippocampal neurons. Biophys J. 2008 Sep 15;95:3079–3099. doi: 10.1529/biophysj.108.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005 Oct 15;14 Spec No. 2:R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005 Jul 15;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003 Jan 20;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006 Jun 30;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Kong D, Xu L, Yu Y, Zhu W, Andrews DW, Yoon Y, Kuo TH. Regulation of Ca2+-induced permeability transition by Bcl-2 is antagonized by Drpl and hFis1. Mol Cell Biochem. 2005;272:187–199. doi: 10.1007/s11010-005-7323-3. [DOI] [PubMed] [Google Scholar]
- 37.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002 Dec 23;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol. 2004 Feb 16;164:493–499. doi: 10.1083/jcb.200309082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003 Sep 19;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 40.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004 Feb 17;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Dimmer KS, Scorrano L. (De)constructing mitochondria: what for? Physiology (Bethesda) 2006;21:233–241. doi: 10.1152/physiol.00010.2006. [DOI] [PubMed] [Google Scholar]
- 42.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, Derks C, Dejaegere T, Pellegrini L, D'Hooge R, Scorrano L, De SB. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006 Jul 14;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Cipolat S, Martins de BO, Dal ZB, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004 Nov 9;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baricault L, Segui B, Guegand L, Olichon A, Valette A, Larminat F, Lenaers G. OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res. 2007 Oct 15;313:3800–3808. doi: 10.1016/j.yexcr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006 Dec 8;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 46.Guillery O, Malka F, Frachon P, Milea D, Rojo M, Lombes A. Modulation of mitochondrial morphology by bioenergetics defects in primary human fibroblasts. Neuromuscul Disord. 2008;18:319–330. doi: 10.1016/j.nmd.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004 Dec 15;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara N, Jofuku A, Eura Y, Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun. 2003 Feb 21;301:891–898. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 49.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007 Aug 27;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007 Aug 27;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jofuku A, Ishihara N, Mihara K. Analysis of functional domains of rat mitochondrial Fis1, the mitochondrial fission-stimulating protein. Biochem Biophys Res Commun. 2005 Jul 29;333:650–659. doi: 10.1016/j.bbrc.2005.05.154. [DOI] [PubMed] [Google Scholar]
- 53.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005 Apr 12;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 54.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003 Mar 31;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 56.Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12:1613–1621. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 57.Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 58.Mijaljica D, Prescott M, Klionsky DJ, Devenish RJ. Autophagy and vacuole homeostasis: a case for self-degradation? Autophagy. 2007;3:417–421. doi: 10.4161/auto.4441. [DOI] [PubMed] [Google Scholar]
- 59.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008 Jan 23;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Partikian A, Olveczky B, Swaminathan R, Li Y, Verkman AS. Rapid diffusion of green fluorescent protein in the mitochondrial matrix. J Cell Biol. 1998 Feb 23;140:821–829. doi: 10.1083/jcb.140.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 2002;12:178–184. doi: 10.1016/s0962-8924(01)02246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffin EE, Detmer SA, Chan DC. Molecular mechanism of mitochondrial membrane fusion. Biochim Biophys Acta. 2006;1763:482–489. doi: 10.1016/j.bbamcr.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Wikstrom JD, Twig G, Shirihai OS. What can mitochondrial heterogeneity tell us about mitochondrial dynamics and autophagy? Int J Biochem Cell Biol. 2009;41:1914–1927. doi: 10.1016/j.biocel.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003 Jul 1;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- 65.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 66.Eura Y, Ishihara N, Oka T, Mihara K. Identification of a novel protein that regulates mitochondrial fusion by modulating mitofusin (Mfn) protein function. J Cell Sci. 2006 Dec 1;119:4913–4925. doi: 10.1242/jcs.03253. [DOI] [PubMed] [Google Scholar]
- 67.Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134:333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- 68.Rojo M, Legros F, Chateau D, Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002 Apr 15;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 69.Frezza C, Cipolat S, Scorrano L. Measuring mitochondrial shape changes and their consequences on mitochondrial involvement during apoptosis. Methods Mol Biol. 2007;372:405–420. doi: 10.1007/978-1-59745-365-3_29. [DOI] [PubMed] [Google Scholar]
- 70.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009 Jul 21;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meeusen S, Devay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006 Oct 20;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 72.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004 Aug 6;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 75.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004 Nov 22;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 77.Fehrenbacher KL, Yang HC, Gay AC, Huckaba TM, Pon LA. Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr Biol. 2004 Nov 23;14:1996–2004. doi: 10.1016/j.cub.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004 Jun 1;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 79.Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005 Aug 4;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 80.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005 Dec 1;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mironov SL. Complexity of mitochondrial dynamics in neurons and its control by ADP produced during synaptic activity. Int J Biochem Cell Biol. 2009;41:2005–2014. doi: 10.1016/j.biocel.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 82.Voyno-Yasenetskaya T, Conklin BR, Gilbert RL, Hooley R, Bourne HR, Barber DL. G alpha 13 stimulates Na-H exchange. J Biol Chem. 1994 Feb 18;269:4721–4724. [PubMed] [Google Scholar]
- 83.Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–275. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]
- 84.Nakamura H, Tanimoto K, Hiyama K, Yunokawa M, Kawamoto T, Kato Y, Yoshiga K, Poellinger L, Hiyama E, Nishiyama M. Human mismatch repair gene, MLH1, is transcriptionally repressed by the hypoxia-inducible transcription factors, DEC1 and DEC2. Oncogene. 2008 Jul 10;27:4200–4209. doi: 10.1038/onc.2008.58. [DOI] [PubMed] [Google Scholar]
- 85.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cereghetti GM, Stangherlin A, Martins de BO, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008 Oct 14;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes. 2006;55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 88.Frezza C, Cipolat S, Martins de BO, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De SB, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006 Jul 14;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 89.Guillery O, Malka F, Landes T, Guillou E, Blackstone C, Lombes A, Belenguer P, Arnoult D, Rojo M. Metalloprotease-mediated OPA1 processing is modulated by the mitochondrial membrane potential. Biol Cell. 2008;100:315–325. doi: 10.1042/BC20070110. [DOI] [PubMed] [Google Scholar]
- 90.Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008 Dec 30;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]


