Abstract
The intestinal epithelium and underlying lamina propria contain T cells that play important roles in maintaining colonic homeostasis. These T cells mediate substantial and specific regulation to ensure that pathogenic microorganisms are eliminated while commensal bacteria are tolerated. There is considerable evidence supporting the notion that the altered ratio between Foxp3+CD4+ T regulatory cells and T effector cells in the colonic microenvironment might contribute to the initiation and progression of inflammation and eventually development of colon cancer. Recent findings on the heterogeneity and plasticity of T regulatory cells, such as the identification of IL-17+Foxp3+CD4+ and the RORγt+Foxp3+CD4+ subsets, in patients with colorectal inflammation and cancer have provided a new twist in our understanding of the pathogenesis of colonic diseases. Phenotypic and functional properties of IL-17-producing Foxp3+CD4+ T cells as well as the significant implications of these cells in the initiation and progression of colorectal diseases are discussed in this review.
Keywords: T regulatory cells, Th17, IL-17, RORγt, Inflammatory bowel disease, Colon cancer
1. Introduction
Regulatory T (TReg) cells expressing the transcription factor forkhead box P3 (Foxp3) are naturally present in the immune system. TReg cells suppress the activation, proliferation and effector functions of a wide range of immune cells, including CD4+ and CD8+ T cells, natural killer (NK) and NKT cells, B cells and antigen presenting cells in vitro and in vivo [1]. TReg cells have key roles in the prevention of autoimmune responses and the development of immunopathology as well as in the maintenance of homeostasis. As a double-edged sword, TReg cells can also suppress antitumor immune responses and can favor tumor progression. The unique functional properties of TReg cells make these cells a primary target in the search for new cell-based immunotherapeutic approaches. However, recent studies showed that TReg cells are not a homogeneous and terminally differentiated cell population. Instead, these cells are heterogeneous in gene expression, phenotype and function. In addition, TReg cells are strongly affected by other immune components including effector cells and innate immune cells during initiation and progression of disease. Thus, to achieve the translational goal of applying TReg cells in the treatment of diseases, understanding of the TReg cell immunobiology in the context of various tissues and pathogenesis of various diseases is required. Lymphocytes of mucosal tissues form a relatively autonomous immune subsystem, with specialized adaptations appropriate for this particular microenvironment [2-4]. The intestinal mucosa is normally maintained in a state of controlled inflammation in which equilibrium exists between protective immunity and tolerance to self-antigen and commensal bacteria [5]. Protective immunity against different classes of pathogens depends on the generation of distinct types of immune responses mediated and coordinated by effector cells specifically TH1, TH2 and TH17. On the other hand, TReg cells are involved in the maintenance of tolerance. A series of observations suggest that TReg cells correlate with poor prognosis in many cancer types, including breast, lung, melanoma, and ovarian carcinoma [6] due to their suppressive effects on anti-tumor immunity. However, several studies have shown that TReg cells are protective in cancer by virtue of their ability to control inflammation in an IL-10 dependent manner [7]. In fact, Foxp3 expression has been indicative of better prognosis in gastric cancer, head and neck, and breast cancer [8-10]. Recent identification of IL-17-producing Foxp3+CD4+ T cells in IBD, colon cancer and polyposis might provide a potential explanation for the conflicting observations regarding the role of TReg cells in cancer. Furthermore, the advancements in understanding TReg immunobiology locally and systemically and the influence of inflammatory microenvironments on the differentiation of effector cells and on the stability of TReg cells would improve the use of TReg as cell-based immunotherapy.
2. Heterogeneity of TReg cells
Foxp3+CD4+ TReg cells are not homogeneous in gene expression, phenotype and suppressive function. The transcription factor Foxp3, a master control gene for the development and function of both mouse and human TReg cells [11-14], is currently the definitive marker of TReg cells. However, Foxp3 can be transiently expressed in activated human T cells. This transient Foxp3 expression in T cells does not enable suppression but, instead, makes separation of TReg cells from activated T effector cells difficult to perform. Therefore, studies to determine the frequency of various T cell subsets in diseases require further scrutiny and mandate the use of multiple parameters instead of Foxp3 alone to define TReg cells. In spite of multiple attempts for the identification of the proper marker combination including CD25, CD127 and CD62L to delineate human TReg cells, the identification and purification of human TReg remains a challenge. Recent studies have shown that CD45RA or CD45RO, which are mutually exclusive, are particularly useful markers when combined with CD25 and/or Foxp3 [15]. CD45RA+Foxp3+ naïve TReg cells, are present in peripheral blood and prevalent in cord blood [16, 17] and are considered the human counterparts of mouse natural TReg cells that develop in the thymus. Naïve TReg cells proliferate after in vitro stimulation via their TCR and are highly resistant to apoptosis [18], in contrast to CD45RO+Foxp3+ TReg cells, which tend to be hyporesponsive and apoptotic after activation in vitro. Effector CD45RO+Foxp3+ TReg cells, which derive mainly from naïve TReg cells, as well as CD45RA+Foxp3+ naïve TReg cells have potent in vitro suppressive activity [15]. In contrast to the human system, naïve mouse CD4+ T cells lack transient and promiscuous expression of Foxp3 after activation. But naive mouse T cells readily convert to Foxp3+ TReg (iTReg) cells following in vitro stimulation with TGF-β or retinoic acid [19]. These induced TReg cells share many phenotypic markers and regulatory functions natural TReg cells, but do not exhibit the signature gene transcription profile of natural TReg cells [20]. Instead, the gene profile of induced TReg cells is Foxp3 independent [15].
3 Characterization of in vitro induced IL-17-producing Foxp3+CD4+ T cells
TReg cells are well known to produce IL-10 and TGF-β during activation and these cytokines are considered to be part of the mechanisms via which TReg cells mediate their suppressive function [21]. Recent findings reveal that TReg cells are capable of producing IL-17. There is an intimate link between TH17 cells and TReg cells. Exposure of antigen-activated naïve T cells to TGF-β in vitro results in transcriptional up-regulation of both Foxp3 and RORγt [22]. Furthermore, T cells co-expressing Foxp3 and RORγt have been identified in vivo in both mice and humans [23]. Foxp3 is able to physically bind to RORγt and to inhibit the transcriptional activity of RORγt thereby blocking IL-17 production. Therefore, in steady state conditions, TReg cells are unable to produce IL-17 [22]. A number of research groups have reported that during activation in the presence of appropriate inflammatory stimuli in vitro, both human and murine TReg cells display a IL17+Foxp3+CD4+ phenotype and can produce IL-17 [24-27]. Recent studies including our own work have identified that IL-1β is a critical mediator in the conversion of TReg cells into IL-17-producing cells in vitro. In the presence of IL-1β, TReg cells express increased levels of RORγt and are able to secrete IL-17 in response to T cell receptor (TCR)-mediated stimulation. The activation of MAPK pathways via IL-1β/IL-1R might have an active role in this differentiation process [24]. We have determined that Runx1, a transcription factor required for the expression of Foxp3 and RORγt, is highly expressed in IL-17-prducing Foxp3+CD4+ cells compared to Foxp3+CD4+ T cells, and silencing of Runx1 with small interfering RNA significantly reduced the numbers of IL-17-producing cells induced by IL-1β [28]. Thus, sustained expression of Runx1 in TReg cells by IL-1β might be responsible for the conversion of TReg cells into IL-17-producing RORyt+Foxp3+CD4+ cells. Although these in vitro generated IL-17-producing Foxp3+CD4+ T cells retain phenotypic properties of TReg cells, including expression of CD25, CTLA-4 and GITR, these cells represent a distinct subset of TReg cells. IL-17-producing Foxp3+CD4+ T cells express increased levels of ICOS compared to TReg cells, and more importantly, display potent suppressive activity and are capable of inducing apoptosis of responder T cells [28].
As mentioned above, Foxp3+Treg cells represent a heterogeneous population consisting of committed TReg cells and of a minor subpopulation that retains developmental plasticity [29]. It is tempting to speculate that this plasticity-retaining subset gives rise to TReg cells capable of producing IL-17. Several attempts have been made to identify the origin of IL-17-producing Foxp3+CD4+ T cells. Most of the studies have demonstrated that effector CD45RO+ TReg cells but not CD45RA+ naïve TReg cells are capable of producing IL-17 upon TCR stimulation in the presence of the combination of IL-1β, IL-2, IL-23 and IL-21 [23]. CD45RA+ naïve TReg cells retain stable CpG methylation across the RORC locus even upon prolonged ex vivo expansion and as a consequence, they display only a marginal tendency to express RORγτ and to develop into IL-17-producing cells. In contrast, stimulation-induced DNA demethylation of RORC occurs selectively in effector CD45RA- TReg cells [30]. IL-17-producing Foxp3+CD4+ T cells like TH17 cells express CCR6, a trait of tissue-homing effector T cells [31, 32], suggesting that CCR6 combined with other markers such as CD45RA, CD45RO and Foxp3 could be used to identify IL-17-producing TReg cells in vitro and in vivo. In addition, studies by Raffin et al. have determined that IL-1 receptor type 1 (IL-1R1) positive effector TReg cells can become IL-17-secreting effectors in response to TCR-mediated stimulation in the presence of IL- 1β, suggesting that ex vivo expression of IL-1R1 in human TReg cells identifies an early intermediate in the conversion of IL-17-producing cells from TReg cells [33].
4. Roles of TReg cells and TH17 cells in inflammation and colon cancer
The intestinal tract contains large numbers of immune cells involved in the encounter with microbial antigens. Under normal conditions, the lumen of the intestine is covered by a layer of intestinal epithelial cells (IECs), which are joined together by tight junctions forming a protective layer impeding bacterial invasion [34]. Immune responses in the colon take place in three distinct compartments: the lamina propria, the isolated lymphoid follicles (ILFs) scattered throughout the colon, and the mesenteric lymph nodes. The latter two sites may play important roles in initiating immune responses and in dictating quantitative and qualitative features of the responses. Cells in the lamina propria are involved in antigen sampling, and this is the site in which effector cells accumulate during inflammation [35].
TReg cells actively suppress enteroantigen-reactive cells and contribute to the maintenance of intestinal immune homeostasis. Distinct TReg cell subsets coexist in the intestinal mucosa and mesenteric lymph nodes. Studies have shown that commensal bacteria can direct the development of Foxp3+ TReg cells with a unique ‘inducible” genetic signature, suggesting the existence of a mechanism via which microbiota can actively promote mucosal tolerance by inducing the differentiation of conventional T cells into Foxp3+ TReg cells [36]. In fact, it is widely accepted that ‘inducible’ rather than ‘natural’ TReg cells are particularly effective in maintaining immune homeostasis of the intestine (Figure 1). The use of murine models of inflammatory bowel disease (IBD) has indicated that these IL-10-producing inducible TReg cells play an important role in preventing or limiting colitis [37]. The precise origin of these inducible TReg cells remains unclear.
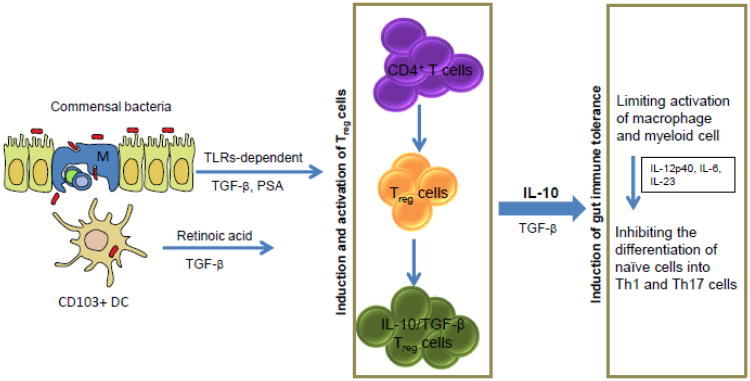
Figure 1. Induction of TReg cell mediated gut immune tolerance.
In the steady state, gut immune tolerance is induced and maintained by a variety of mechanisms, which regulate the differentiation and activation of TReg cells. Some commensal bacteria can induce the differentiation of CD4+ T cells into TReg cells in a TLRs dependent manner. Clostridium spp. can induce generation of IL-10 producing TReg cells via epithelial cell-derived TGF-β, whereas Bacteroides fraglilis can induce IL-10 producing TReg cells via polysaccharide A (PSA). CD103+DC can also induce TReg cells through their ability to produce retinoic acid and TGF-β. TReg cells act on macrophage and myeloid cells to inhibit their ability to produce colitogenic cytokines including IL-12p40, Il-6 and IL-23, which are required for the differentiation of CD4+ T cells into Th1 and Th17 cells. Both Th1 and Th17 cells are associated with initiation and progression of IBD and colon cancer. TReg cells suppress inflammatory responses mainly through production of anti-inflammatory cytokines TGF-β and more importantly IL-10.
In mucosal surfaces, TH17 cells are thought to protect the host from infections, whereas several types of TReg cells keep effector T cell populations in check and prevent T cell-mediated destruction of intestinal tissue [38-41]. There is compelling evidence that Crohn's disease (CD) is driven by TH17 cells [42]. CD, one type of IBD, is believed to be the result of an aberrant response of the gut-associated lymphoid tissue to bacterial and/or dietary antigens. Increased serum IL-17 levels have been observed in patients with CD compared to healthy individual. In addition, IL-17 mRNA expression and the number of IL-17+ T cells were also increased in the inflamed mucosa from patients with active CD [43]. However, blocking IL-17 is ineffective for the treatment of CD, suggesting that although IL-17 is involved in the pathogenesis of CD, IL-17 itself is unlikely to serve as a therapeutic target for CD [44]. Increased numbers of TH17 cells were also observed in the intestinal mucosa in patients with ulcerative colitis (UC), another well-characterized type of IBD [45]. It is unclear whether TH17 cells play the same role in the pathogenesis of these two types of IBD or whether these two forms of IBD share a common mechanism underlying the accumulation of TH17 cells as a consequence of the inflammation process in the intestinal mucosa. Of note, concomitantly with the increased frequency of TH17 cells, there is also an increased number of TReg cells in the lamina propria, mesenteric lymph nodes, and inflamed intestinal mucosa of IBD patients [46]. Although the presence of IL-17, TH17 cells and TReg cells in the inflamed intestine is well established, their dynamics and contribution to the IBD disease remain elusive.
TReg cells are an obstacle for immune surveillance and immune therapy of cancer due to suppressing anti-tumor immune responses. However, studies using ApcMin/+ mice revealed a protective role of IL-10-producing TReg cells in bacterial-induced chronic inflammation and cancer [47] and even hereditary colon cancer [48], suggesting that TReg cells might play a protective role in cancer by suppressing inflammation (Figure 2). Mast cells are essential hematopoietic components promoting the development of adenomatous polyps [49]. Adoptive transfer of TReg cells protect against colon cancer by suppressing focal mastocytosis in adenomatous polyps due to IL-10 production [7]. Conversely, in polyp-ridden mice, TReg cells are aberrant, fail to produce IL-10 and instead produce IL-17 thereby promoting rather than suppressing mastocytosis [7, 50]. These observations suggest that the role of TReg cells in the development of cancer depends on their unique functional properties as identified by their ability to produce specific cytokines.
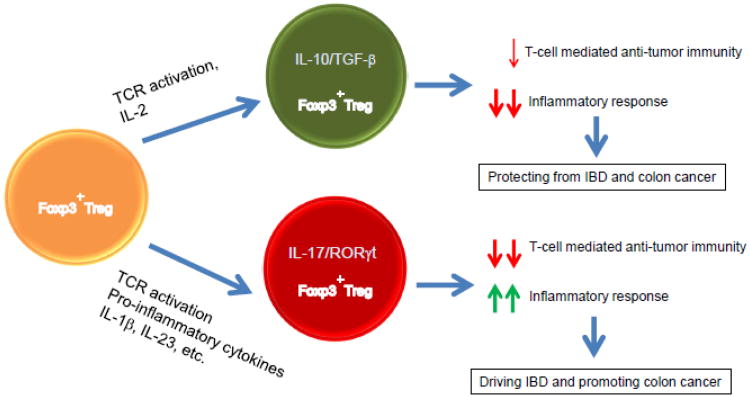
Figure 2. Opposing effects of Foxp3+TReg and RORγt+Foxp3+TReg cells in the development of IBD and colon cancer.
Foxp3+ TReg cells suppress both T-cell mediated anti-tumor immune response and inflammatory response at least in part by secretion of IL-10 and TGF-β in response to TCR-mediated stimulation. Given their ability to suppress inflammatory processes, Foxp3+ TReg cells play an important role in controlling and suppressing the pathogenesis of IBD and subsequently the development of colon cancer. However, in the context of an inflammatory milieu, Foxp3+ TReg cells are able to express RORγt and have the potential to express and secrete IL-17. The resultant RORγt+Foxp3+TReg cells display potent suppressive activity on T-cell mediated immune responses, whereas their antiinflammatory function is compromised. As a consequence, RORγt+Foxp3+ TReg cells are unable to control inflammation and rather promote instead of suppressing the development of colon cancer.
IL-17, produced locally in the tumor microenvironment, plays important roles in both angiogenesis and tumor immunity. Inhibition of IL-17A at tumor sites by intratumoral injection of an adenovirus vector expressing siRNA against the mouse IL-17A gene (Ad-si-IL-17) significantly inhibited tumor growth. It was found that inhibition of IL-17 at tumor sites significantly suppressed CD31, MMP9 and VEGF expression in tumor tissue. Furthermore, the cytotoxic activity of tumor infiltrating CD8+ lymphocytes in mice treated with ad-si-IL17A was significantly higher than in control mice [51]. Further experimental evidence supports the idea that IL-17 promotes tumor growth. Specifically, ablation of IL-17 significantly reduced tumor development in mice bearing a heterozygote mutation in the adenomatous polyposis coli (APC) gene (ApcMin/+ mice). There was also a decrease in inflammatory cytokines and proinflammatory mediators and reduced lymphocytic infiltration, suggesting that IL-17 promotes spontaneous intestinal tumorigenesis [52]. Consistently with a positive role of IL-17 in promoting tumor development, tumor tissues have a higher frequency of IL-17+ cells T cells compared with untransformed bowel tissues [53]. The role of IL-17 in promoting tumor growth provides additional support for the already well-established connection between inflammation and tumorigenesis. However, this study did not identify whether Ad-si-IL-17 induced IL-17 ablation only in T effector cells or whether its effects were also extended to TReg cells, a population that is also capable of producing IL-17 and is intimately linked to the development of inflammation and cancer in the bowel.
TReg cells can affect tumor progression by mediating suppression of the effector T-cell responses and by inducing production of angiogenetic factors by TH17 cells. Tumor infiltrating TH17 cells themselves have also been postulated to offset the anti-tumor response of IFN-γ+ effector cells [54]. Thus, both TReg cells and TH17 cells can affect cancer progression through independent and interconnected effects although the precise mechanisms of such counter-regulation remain poorly understood. Understanding the regulation of the TReg/TH17 axis may result in novel approaches for the control of tumor progression. Studies by Baba et al. suggested that human CD45RA+ TReg cells promote the differentiation of human TH17 cells, implying that CD45RA+ TReg cells positively govern TH17 development. These findings might provide an explanation why TH17 cells are often detected alongside TReg cells within tumor tissues or systemically in cancer patients [7, 55, 56] and further suggest that the TReg population might be an appropriate target for cancer immunotherapy.
5. Paradoxical roles of IL-17-producing Foxp3+CD4+ cells in anti-inflammatory response and anti-tumor immunity
Inflammatory and tumor tissues often have lower frequency of CD69+ T effector cells but a higher frequency of TH17 cells and TReg cells compared to healthy or untransformed tissues [53]. The mechanisms by which TH17 cells and TReg cells accumulate in such inflammatory and tumor tissues and their roles in the pathogenesis of these diseases remain unsolved. The identification of IL-17+Foxp3+ T cells in inflamed intestinal mucosa of patients with IBD and cancer but not in healthy control might provide some clues to the above questions.
IL-17+Foxp3+ cells accumulated in colorectal cancer (CRC) tissue express CCR6, TGF-β and IL-6, and significantly suppress CD8+ cell-mediated immune responses during in vitro culture [57, 58]. Interestingly, the suppressive activity of IL-17+Foxp3+ cells is attenuated by pretreatment with an anti-IL-17 antibody, suggesting that this subset of T cells may be a novel therapeutic target in the treatment of CRC [58]. In a similar study, abundant IL-17+Foxp3+ cells expressing high levels of TGF-β, CXCR3, CCR6, and RORγt were also detected in CRC. In addition, co-culture of IL-17+Foxp3+ cells isolated from transformed tissues with sphere cells generated from bone marrow derived mononuclear cells under hypoxia, could induce sphere cells to express CRC markers such as CD133, CD44s, CD166, EpCAM, and ALDH1 [59, 60]. Addition of neutralizing anti-IL-17 antibody in the co-culture abolished the expression of CRC markers in sphere cells, suggesting that IL-17+Foxp3+ TReg cells drive cancer-initiating mechanisms and that IL-17 itself plays a critical role in this process [60].
A different study showed that TReg cells become pathogenic in the context of CRC when they acquire expression of RORγt [61]. In this model, expression of RORγt marks a distinct subset of activated TReg cells, which have the potential to express IL-17 and have been identified in human CRC tumor. These RORγt-expressing TReg cells expand in CRC in a manner dependent on cancer-stage. Moreover, enriched RORγt+ TReg cells obtained from CRC display potent T-cell suppressive function. Conversely, RORγt deficiency restored the anti-inflammatory properties of TReg cells, protected against polyposis and improved anti-tumor immunity as determined by increased IFN-γ production [61]. These findings suggest that RORγt expressed in Foxp3+ TReg cells might serve as a primary therapeutic target for improvement of the anti-inflammatory effects of TReg cells, thereby inhibiting tumor development. Furthermore, these findings suggest that RORγt+ Treg rather than IL-17-producung cells might have a causative role in the pathogenesis of CRC development in the context of bowel inflammation (Figure 1).
6. Conclusions
The immunobiology of TReg cells has been intensively investigated since the discovery of TReg cells and their master transcription factor Foxp3, more than a decade ago. Such efforts have led to significant advances in our understanding of the development of TReg cells and the molecular mechanisms underlying their suppression function. Recent studies have focused on understanding the reciprocal relationship between TReg and other immune components surrounding TReg cells. Through these studies it has been recognized that TReg cells are subjected to regulation mediated by other immune cell populations, and as a result, the functional and phenotypic properties of TReg cells can be altered. Currently developing evidence supports the idea that the role of TReg cells in various disease settings varies considerably depending on disease stage, inflammatory conditions and activation of effector cells. Identification of the IL-17+Foxp3+TReg and RORγt+Foxp3+ TReg subsets in human IBD and CRC confirm that Foxp3+ TReg cells are capable of expressing transcription factors that control the differentiation of effector cells and can potentially produce proinflammatory cytokines. Although the exact mechanisms underlying the generation of IL-17+ TReg cells remain unclear, soluble mediators such as IL-1β, TGF-β and IL-23 might be involved in this process. The precise role of these TReg subsets in inflammation and tumor immunity is still debatable but most studies agree that IL-17+TReg and RORγt+TReg suppress anti-tumor immunity and promote tumor growth. Thus, targeting RORγt and IL-17 in TReg cells might be required in order to improve the properties of TReg cells employed for cell-based therapies in the context of inflammation and cancer.
Highlights.
Foxp3+ TReg cells are able to express RORγt and produce IL-17
IL-17+ TReg cells are accumulated in the inflamed intestinal mucosa.
IL-17+ TReg cells have potent T-cell suppressive activity.
RORγt + TReg cells are unable to suppress inflammation.
IL-17+/RORγt+ TReg cells are pathogenic TReg cells with proinflammatory properties.
Acknowledgments
Supported by NIH grants HL107997-01, R56AI43552 and by the Leukemia and Lymphoma Society Translational Research Program TRP 6222-11
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Bienenstock J, McDermott M, Befus D, O'Neill M. A common mucosal immunologic system involving the bronchus, breast and bowel. Adv Exp Med Biol. 1978;107:53–59. doi: 10.1007/978-1-4684-3369-2_7. [DOI] [PubMed] [Google Scholar]
- 3.Jorde R, Burhol PG. Diurnal profiles of gastrointestinal regulatory peptides. Scand J Gastroenterol. 1985;20:1–4. doi: 10.3109/00365528509089624. [DOI] [PubMed] [Google Scholar]
- 4.Smart CJ, Trejdosiewicz LK, Badrel-Din S, Heatley RV. T lymphocytes of the human colonic mucosa: functional and phenotypic analysis. Clin Exp Immunol. 1988;73:63–69. [PMC free article] [PubMed] [Google Scholar]
- 5.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos PD, Rudensky AY. Treg cells in cancer: a case of multiple personality disorder. Sci Transl Med. 2012;4:164fs144. doi: 10.1126/scitranslmed.3005283. [DOI] [PubMed] [Google Scholar]
- 7.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Re. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, Brasnu DF, Tartour E. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 9.Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladoire S, Arnould L, Mignot G, Coudert B, Rebe C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, Fumoleau P, Ghiringhelli F. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;125:65–72. doi: 10.1007/s10549-010-0831-1. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 13.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 14.Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 16.Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, Fazekas de Saint Groth B. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 17.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Lopes JE, Chong MM, Ivanov, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Kim J, Boussiotis VA. IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J Immunol. 2010;185:4148–4153. doi: 10.4049/jimmunol.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008;181:3137–3147. doi: 10.4049/jimmunol.181.5.3137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Patsoukis N, Petkova V, Boussiotis VA. Runx1 and Runx3 are involved in the generation and function of highly suppressive IL-17-producing T regulatory cells. PLoS One. 2012;7:e45115. doi: 10.1371/journal.pone.0045115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidl C, Hansmann L, Andreesen R, Edinger M, Hoffmann P, Rehli M. Epigenetic reprogramming of the RORC locus during in vitro expansion is a distinctive feature of human memory but not naive Treg. Eur J Immunol. 2011;41:1491–1498. doi: 10.1002/eji.201041067. [DOI] [PubMed] [Google Scholar]
- 31.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 32.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 33.Raffin C, Raimbaud I, Valmori D, Ayyoub M. Ex vivo IL-1 receptor type I expression in human CD4+ T cells identifies an early intermediate in the differentiation of Th17 from FOXP3+ naive regulatory T cells. J Immunol. 2011;187:5196–5202. doi: 10.4049/jimmunol.1101742. [DOI] [PubMed] [Google Scholar]
- 34.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 35.Newberry RD, Lorenz RG. Organizing a mucosal defense. Immunol Rev. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 36.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitt EG, Haribhai D, Williams JB, Aggarwal P, Jia S, Charbonnier LM, Yan K, Lorier R, Turner A, Ziegelbauer J, Georgiev P, Simpson P, Salzman NH, Hessner MJ, Broeckel U, Chatila TA, Williams CB. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. J Immunol. 2012;189:5638–5648. doi: 10.4049/jimmunol.1200936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan YY, Flavell RA. TGF-beta and regulatory T cell in immunity and autoimmunity. J Clin Immunol. 2008;28:647–659. doi: 10.1007/s10875-008-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanai T, Nemoto Y, Kamada N, Totsuka T, Hisamatsu T, Watanabe M, Hibi T. Homeostatic (IL-7) and effector (IL-17) cytokines as distinct but complementary target for an optimal therapeutic strategy in inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:306–313. doi: 10.1097/MOG.0b013e32832bc627. [DOI] [PubMed] [Google Scholar]
- 43.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza A, Shata MT. Letter: pathogenicity of Th17 cells may differ in ulcerative colitis compared with Crohn's disease. Aliment Pharmacol Ther. 2012;36:204. doi: 10.1111/j.1365-2036.2012.05124.x. author reply 205. [DOI] [PubMed] [Google Scholar]
- 45.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 46.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 47.Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- 48.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 49.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, Weissleder R, McNagny KM, Khazaie K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayata K, Iwahashi M, Ojima T, Katsuda M, Iida T, Nakamori M, Ueda K, Nakamura M, Miyazawa M, Tsuji T, Yamaue H. Inhibition of IL-17A in tumor microenvironment augments cytotoxicity of tumor-infiltrating lymphocytes in tumor-bearing mice. PLoS One. 2013;8:e53131. doi: 10.1371/journal.pone.0053131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girardin A, McCall J, Black MA, Edwards F, Phillips V, Taylor ES, Reeve AE, Kemp RA. Inflammatory and regulatory T cells contribute to a unique immune microenvironment in tumor tissue of colorectal cancer patients. Int J Cancer. 2013;132:1842–1850. doi: 10.1002/ijc.27855. [DOI] [PubMed] [Google Scholar]
- 54.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 55.Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette MT, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, Farcet JP, Sobhani I. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57:772–779. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 56.Maruyama T, Kono K, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Izawa S, Fujii H. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010;101:1947–1954. doi: 10.1111/j.1349-7006.2010.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, Zou W. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 58.Ma C, Dong X. Colorectal cancer-derived Foxp3(+) IL-17(+) T cells suppress tumour-specific CD8+ T cells. Scand J Immunol. 2011;74:47–51. doi: 10.1111/j.1365-3083.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 59.Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382–390. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, Zhang B, Liu T, Yang P. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 2011;89:85–91. doi: 10.1189/jlb.0910506. [DOI] [PubMed] [Google Scholar]
- 61.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]