Summary
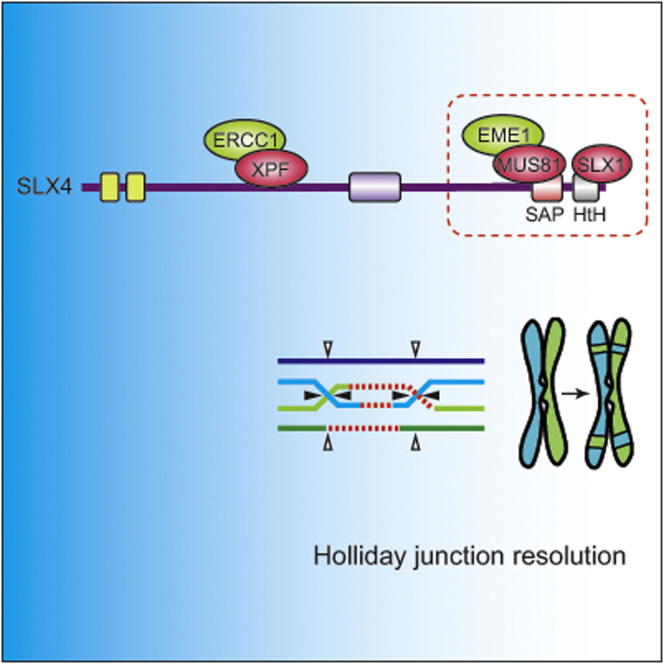
Holliday junctions (HJs) are X-shaped DNA structures that arise during homologous recombination, which must be removed to enable chromosome segregation. The SLX1 and MUS81-EME1 nucleases can both process HJs in vitro, and they bind in close proximity on the SLX4 scaffold, hinting at possible cooperation. However, the cellular roles of mammalian SLX1 are not yet known. Here, we use mouse genetics and structure function analysis to investigate SLX1 function. Disrupting the murine Slx1 and Slx4 genes revealed that they are essential for HJ resolution in mitotic cells. Moreover, SLX1 and MUS81-EME1 act together to resolve HJs in a manner that requires tethering to SLX4. We also show that SLX1, like MUS81-EME1, is required for repair of DNA interstrand crosslinks, but this role appears to be independent of HJ cleavage, at least in mouse cells. These findings shed light on HJ resolution in mammals and on maintenance of genome stability.
Graphical Abstract
Highlights
-
•
Resolution of Holliday junctions in mouse cells requires the SLX1 nuclease
-
•
SLX1 acts cooperatively with MUS81-EME1 in HJ resolution and ICL repair
-
•
Mutations in SLX4 that prevent it binding to SLX1 and MUS81-EME1 abolish HJ resolution
-
•
DNA substrates of SLX1 and MUS81-EME1 in ICL repair appear to be different from HJs
Introduction
SLX4 coordinates a multiprotein complex that is important for DNA repair. In metazoans, this complex includes three structure-selective nucleases: XPF-ERCC1, MUS81-EME1, and SLX1 (Andersen et al., 2009; Fekairi et al., 2009; Muñoz et al., 2009; Saito et al., 2009; Svendsen et al., 2009). Together these nucleases confer the complex with the ability to cleave a wide range of branched DNA structures in vitro, which mimic DNA intermediates that occur during the repair of damaged DNA and broken DNA replication forks. Both MUS81-EME1 and XPF-ERCC1 are required for the repair of DNA interstrand crosslinks (ICLs) in mammalian cells, and the latter is also required for repair of UV-induced lesions (Ciccia et al., 2008). Depletion of SLX4 from human cells using siRNA duplexes does not affect UV repair but causes pronounced hypersensitivity to agents that induce DNA ICLs (Fekairi et al., 2009; Muñoz et al., 2009; Svendsen et al., 2009). The importance of SLX4 in ICL repair in humans is underscored by the observation that biallelic mutations in SLX4/FANCP cause Fanconi anemia (FA) (Kim et al., 2011; Stoepker et al., 2011), a cancer predisposition syndrome accompanied by developmental, skeletal, and hematological defects (Auerbach, 2009).
Despite the clear importance of the SLX4 complex in DNA repair, little is known about the underlying molecular mechanisms. For example, it is not yet known if SLX1 is involved in ICL repair, partly because depletion of SLX1 from human cells destabilized SLX4, preventing the functional analysis of SLX1 (Muñoz et al., 2009). Furthermore, it is not yet known how SLX4 affects the associated nucleases, and there has been some debate about whether the exquisite hypersensitivity of SLX4-defective cells reflects the loss of regulation of one or more of these nucleases. In this light, a recent study concluded that the role of SLX4 in ICL repair involves XPF-ERCC1 only, because a fragment of SLX4 lacking amino acids 1–499, that did not interact with XPF-ERCC1, did not rescue the mitomycin-C (MMC) sensitivity of Slx4 hypomorphic MEFs (Crossan et al., 2011). However, the first 499 amino acids of SLX4 also contain two ubiquitin-binding domains that are vital for ICL repair but that are not required for SLX4 to interact with XPF (Kim et al., 2011; Stoepker et al., 2011). Two studies used SLX4 deletion mutants lacking the C-terminal helix-turn-helix (HtH) domain to investigate the importance of SLX1 binding for ICL repair. One study found that the HtH deletion mutant fully rescues the sensitivity of Slx4 hypomorphic MEFs (Crossan et al., 2011), whereas the other showed that this mutant only partly rescued the MMC sensitivity of SLX4-defective FA cells (Kim et al., 2013). Therefore, the functional relevance of the binding of nucleases to SLX4 in ICL repair remains unclear.
The SLX4 complex is capable of processing Holliday junctions (HJs) in vitro (Fekairi et al., 2009; Muñoz et al., 2009; Svendsen et al., 2009). HJs are four-way DNA junctions at which two chromatids are topologically intertwined. These structures arise during homologous recombination (HR), a process required for repairing unscheduled double-strand breaks (DSBs) or damaged replication forks in mitotic cells. HJs are also key intermediates during meiotic recombination (Schwartz and Heyer, 2011; West, 2009). Ultimately HJs must be removed to enable chromosome segregation, and two distinct modes of HJ removal have been identified in mammalian cells (Schwartz and Heyer, 2011). The first pathway involves the dissolution of double HJs (dHJs) by the BTR complex (BLM-TOPIII-RMI1-RMI2). The coupled helicase and topoisomerase activities of BLM and TOPIII, respectively, disassemble HJs, resulting exclusively in noncrossover products (Chaganti et al., 1974; Wu and Hickson, 2003, 2006). This pathway dominates in mitotic cells, possibly because minimizing crossovers lowers the incidence of loss of heterozygosity (LOH) that would increase disease risk and impair organism fitness (LaRocque et al., 2011).
Alternatively, HJs can be resolved by nucleases (Schwartz and Heyer, 2011; West, 2009). Depending on the symmetry of the cleavage, crossover or noncrossover products may occur. Cells from Bloom syndrome (BS) patients lacking BLM show a large increase in the frequency of sister chromatid exchanges (SCEs), which are thought to result from the crossovers generated by nucleolytic resolution of dHJs that escape dissolution (Chaganti et al., 1974; Wechsler et al., 2011). To date, three nuclear HJ resolving activities have been identified in mammalian cells: MUS81-EME1, SLX1, and GEN1 (Bailly et al., 2010; Fekairi et al., 2009; Ip et al., 2008; Muñoz et al., 2009; Svendsen et al., 2009; Taylor and McGowan, 2008; Wechsler et al., 2011; West, 2009). GEN1 cleaves HJs symmetrically to produce nicked linear duplex products (Ip et al., 2008; Rass et al., 2010), whereas SLX1 introduces a mixture of symmetric and asymmetric cuts across the junction (Fekairi et al., 2009; Muñoz et al., 2009; Svendsen et al., 2009). In contrast to GEN1 and SLX1, MUS81-EME1 does not cleave intact HJs efficiently, but prefers nicked junctions and recombination intermediates such as extended D-loop structures (Boddy et al., 2001; Ciccia et al., 2003; Doe et al., 2002; Gaillard et al., 2003; Whitby et al., 2003).
The elevated SCE frequency in BS cells requires GEN1 and MUS81-EME1 (Wechsler et al., 2011). However, although GEN1 cleaves intact HJs efficiently in vitro, MUS81-EME1 does not. Instead MUS81-EME1 shows a strong preference for nicked HJs, suggesting that it might act on junctions that are subjected to prior nicking by a different nuclease. One possible candidate is SLX1 because it can process intact HJs efficiently in vitro. Furthermore, SLX1 and MUS81-EME1 bind to the HtH motif and the SAP domain of SLX4, respectively (Fekairi et al., 2009; Kim et al., 2013; Svendsen et al., 2009). The close proximity of the SLX4 SAP and HtH domains places SLX1 close enough to MUS81-EME1 on SLX4 to suggest the possibility of cooperation between the two nucleases. In this sense, one function of SLX4 in its capacity as a scaffold would be to facilitate serial processing of HJs, a possibility raised previously (Svendsen et al., 2009). In this study, we investigated whether SLX1 is involved in HJ resolution and ICL repair, and we tested potential cooperation with MUS81-EME1 and the functional significance of binding of these nucleases to SLX4.
Results
Disruption of the Murine Giyd2/Slx1 and Btbd12/Slx4 Genes
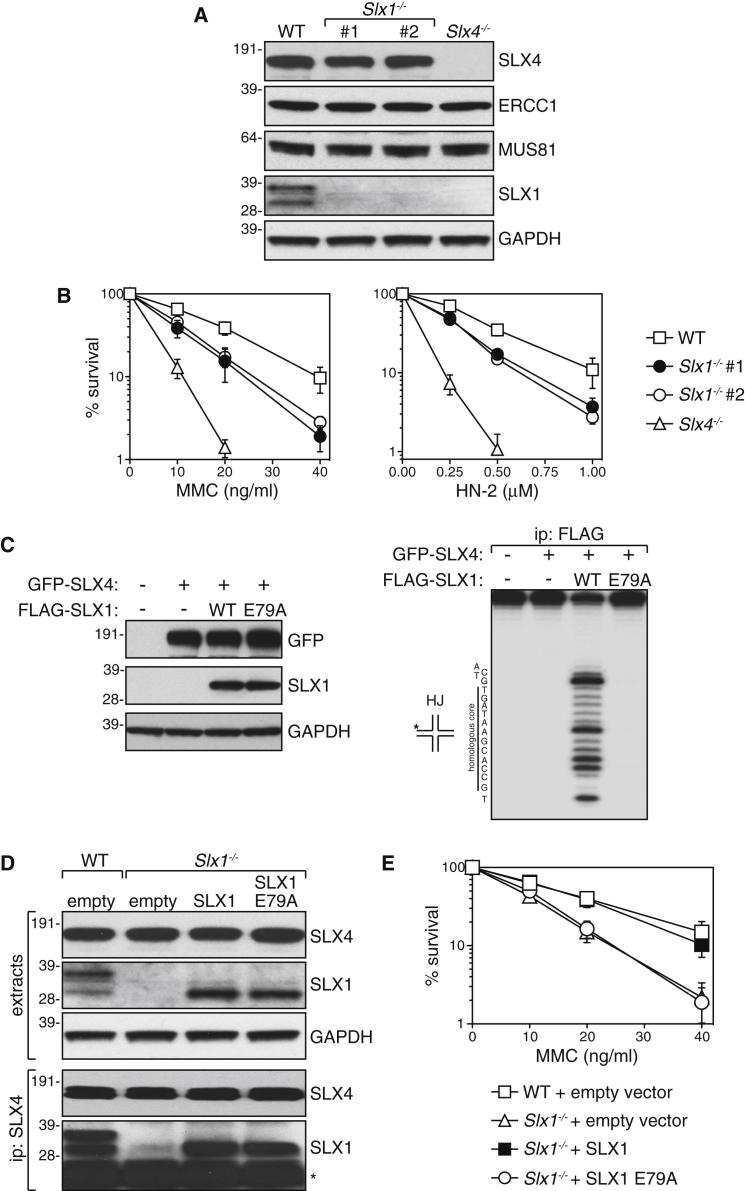
There is currently no information on SLX1 function in mammals. To study the in vivo roles of SLX1, we disrupted the murine Giyd2 (Slx1) gene by eliminating the transcription start site and the remainder of the first exon (Figure S1A). Gene disruption was confirmed by Southern blotting and PCR (Figures S1B and S1C). Both Slx1+/− and Slx1−/− mice were born at Mendelian frequencies (Table S1) without overt morphological, developmental, or hematological defects. Adult mice were fertile (data not shown). SLX1 protein was undetectable in extracts of Slx1−/− mouse embryonic fibroblast extracts (MEFs) (Figure 1A) or testis extracts (Figure S1D) by western blotting. The expression levels of SLX4, ERCC1, and MUS81 proteins, however, were normal in Slx1−/− MEFs (Figure 1A).
Figure 1.
SLX1 Is Involved in the Repair of DNA ICLs
(A) Western blot analysis of MEF extracts from wild-type (WT), Slx1−/−, and Slx4−/− mice. Two separate Slx1−/− MEF clones were tested. (See also Figures S1 and S2.)
(B) Clonogenic survival analysis of MEFs exposed to genotoxins. For each genotype, cell viability of untreated cells is defined as 100%. Data are represented as mean ± SEM, n = 3. (See also Figure S3.)
(C) HEK293 cells stably expressing tetracycline-inducible (“+”) GFP-tagged mouse SLX4 were transfected with mouse SLX1 (WT), SLX1 active site mutant E79A, or left untransfected (“−”). Cell extracts were subjected to western blotting (left panel). Anti-FLAG immunoprecipitates were incubated with a synthetic radiolabeled four-way junction containing a 12 bp homologous core. Reaction products were subjected to denaturing PAGE (right panel).
(D) Western blot analysis of Slx1−/− MEFs complemented with untagged versions of wild-type SLX1, SLX1 E79A, or empty vector (top panels). Extracts were subjected to immunoprecipitation with anti-SLX4 antibodies, and precipitates were probed with the antibodies indicated (bottom panels). ∗, IgG light chain.
(E) Clonogenic survival analysis of complemented Slx1−/− MEFs from (D) exposed to increasing doses of MMC. For each genotype, cell viability of untreated cells was defined as 100%. Data are represented as mean ± SEM, n = 3.
We also disrupted the murine Btbd12 (Slx4) gene (Figure S2A); gene disruption was confirmed by Southern blotting, PCR (Figures S2B and S2C), and western blotting (Figure S2D). The expression levels of SLX4-associated proteins ERCC1 and MUS81 were normal in MEFs from the Slx4−/− mice but SLX1 protein was undetectable by western blotting (Figure 1A). This suggests that SLX4 regulates SLX1 protein stability, and therefore SLX4 null mice lack both SLX1 and SLX4 proteins. Initially, no viable Slx4−/− offspring were obtained from crossing Slx4 heterozygotes. However, after backcrossing the heterozygotes five times, we obtained viable Slx4−/− mice, albeit at sub-Mendelian frequencies (Table S2). Slx4−/− mice were on average around 10%–15% smaller than heterozygotes or wild-type mice at the age of 6 weeks (data not shown). No overt developmental or morphological defects were observed. Although mating Slx4−/− males with Slx4−/− females resulted in viable progeny, testes in males were on average 47% smaller than in wild-type mice at 10 weeks of age (data not shown). Smaller testis size is in line with two previous reports describing a hypomorphic Slx4 mouse strain made by the European Conditional Mouse Mutagenesis Program (EUCOMM) (Crossan et al., 2011; Holloway et al., 2011). It is worth noting that the EUCOMM mice were reported to exhibit phenotypes reminiscent of those seen in FA patients such as aplastic anemia (Crossan et al., 2011), even though mouse knockouts of other FA genes did not recapitulate most of these phenotypes (Bakker et al., 2013). However, we saw no evidence of an FA-like syndrome in the Slx4−/− or Slx1−/− mice we generated in this study (data not shown).
SLX1 Nuclease Activity Is Required for Repair of DNA ICLs
We next investigated if SLX1 is involved in ICL repair assessed by hypersensitivity to genotoxins that induce ICLs. As shown in Figure 1B, Slx1−/− MEFs are hypersensitive to ICL-inducing agents such as nitrogen mustard (HN-2) and MMC, and embryonic stem cells (ESCs) from Slx1−/− mice were also hypersensitive to MMC (Figure S3A). The sensitivity of Slx1−/− MEFs to agents that induce ICLs was much less pronounced than Slx4−/− MEFs, probably because SLX4 binds to several nucleases involved in ICL repair in addition to SLX1. Slx1−/− MEFs and ESCs were not more sensitive to camptothecin (CPT), ionizing radiation (IR), hydroxyurea (HU), or UV light than wild-type cells (Figures S3A and S3B). Defects in the repair of ICLs often result in chromosome abnormalities (Auerbach, 2009). In this light, we observed a slight increase in the number of chromosome abnormalities such as chromatid breaks and radial structures in Slx1−/− MEFs exposed to MMC compared with wild-type cells, and a much larger increase in Slx4−/− MEFs (Figure S3C).
We next tested if the nuclease activity of SLX1 is required for cellular resistance to ICL-inducing agents. To this end we mutated a highly conserved residue, Glu79, in the SLX1 URI-type nuclease domain to alanine. This mutation abolished the activity of FLAG-tagged mouse SLX1 immunoprecipitated from HEK293 cells in the cleavage of a radioactively [5′-32P]-labeled HJ with a core that could undergo a number of steps of branch migration, thereby presenting all possible dinucleotides at the point of exchange (Figure 1C) (Muñoz et al., 2009). Next, Slx1−/− MEFs were infected with viruses expressing untagged SLX1 wild-type or E79A, or with empty virus; mutation of E79 in SLX1 did not affect interaction with SLX4 (Figure 1D). As shown in Figure 1E, wild-type SLX1, but not the E79A mutant, rescued the MMC hypersensitivity of Slx1−/− MEFs. Together, these data provide evidence that SLX1 is involved in repair of DNA ICLs in mammals.
SLX1 Is Required for the Nucleolytic Processing of HJs
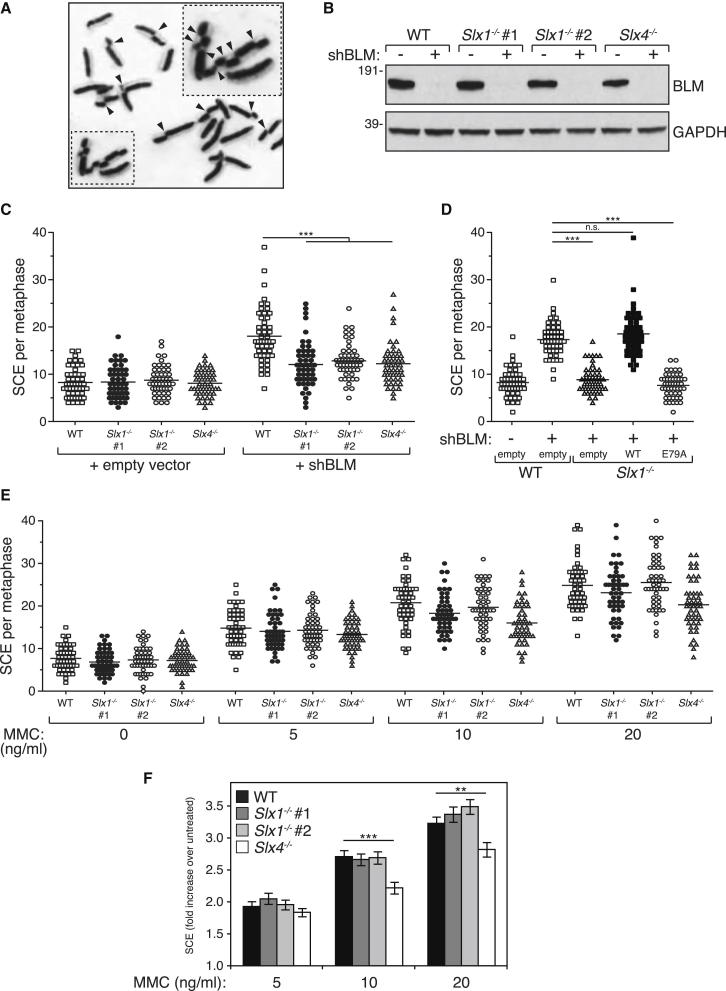
SLX1 is capable of processing HJs in vitro (Fekairi et al., 2009; Muñoz et al., 2009; Svendsen et al., 2009). We next investigated whether SLX1 is required for HJ resolution in vivo using the elevated SCE frequency observed in BLM-depleted cells as readout (Figure 2A). To this end, we used shRNA-expressing retroviruses to deplete BLM from Slx1−/− MEFs (Figure S4A) (Sfeir et al., 2009). As shown in Figure 2B, BLM protein was undetectable in MEF extracts when cells were infected with retrovirus expressing the BLM shRNA, but not when empty virus was used. Depletion of BLM from wild-type MEFs caused an increase in SCE frequency from 8.3 SCE per metaphase to 18 SCE per metaphase (Figure 2C), but in two separate clones of Slx1−/− MEFs, the SCE frequency after BLM depletion was diminished to around 12 SCE per metaphase. A similar defect in SCE was observed in Slx4−/− MEFs (Figure 2C), in agreement with a previous analysis of BS cells depleted of SLX4 (Wechsler et al., 2011). The SCE defect in Slx1−/− MEFs depleted of BLM was rescued by expression of wild-type SLX1 but not by the nuclease-inactive SLX1 E79A mutant (Figure 2D). Therefore, SLX1 is required for nucleolytic resolution of HJs in vivo.
Figure 2.
Defective HJ Resolution in Slx1−/− Cells
(A) Representative image of a metaphase spread for SCE analysis in MEFs after depletion of BLM with shRNA-expressing retroviruses. SCE events are indicated by arrowheads.
(B) Western blot analysis of MEFs, of the genotypes indicated, infected with retroviruses expressing a BLM-specific shRNA (+) or with virus prepared with empty vector as control (−).
(C) Scatterplot of SCE frequencies in MEFs depleted of BLM as described in (B). Fifty metaphases were analyzed for each condition, and significance was calculated using one-way ANOVA (∗∗∗p < 0.0001) followed by Bonferroni’s Multiple Comparison Test. Each point represents the total number of SCEs in a single mitotic spread. The horizontal line in each data set represents mean SCE frequency.
(D) Same as (C) except that Slx1−/− MEFs infected with viruses expressing SLX1, SLX1 E79A, or empty virus were analyzed. Significance was calculated as above; ∗∗∗p < 0.0001; n.s., nonsignificant.
(E) Scatterplot of SCE frequencies in wild-type, Slx1−/−, and Slx4−/− MEFs after exposure to increasing concentrations of MMC. Fifty metaphases were counted for each condition. Each point represents the total number of SCEs in a single mitotic spread. The horizontal line in each data set represents mean SCE frequency.
(F) Same as (E) except that the data were plotted as fold increase over untreated cells. Data are represented as mean ± SEM. Experimental significance was calculated using an unpaired t test; ∗∗p < 0.01; ∗∗∗p < 0.001.
We also analyzed SCEs formed during the repair of ICLs. Exposure of wild-type MEFs to MMC caused a dose-dependent increase in SCE frequency, but Slx1−/− MEFs were indistinguishable from wild-type cells in this regard (Figures 2E and 2F). In contrast, the MMC-induced increase in SCE frequency was reduced by more than 30% in Slx4−/− MEFs (Figures 2E and 2F). Thus, although SLX4 contributes to the generation of SCE during ICL repair, SLX1 does not appear to be involved. This could be due, in principle, to redundancy between SLX1 and nucleases such as MUS81-EME1, as discussed below.
SLX1 Is Epistatic with MUS81-EME1 in ICL Repair and HJ Resolution
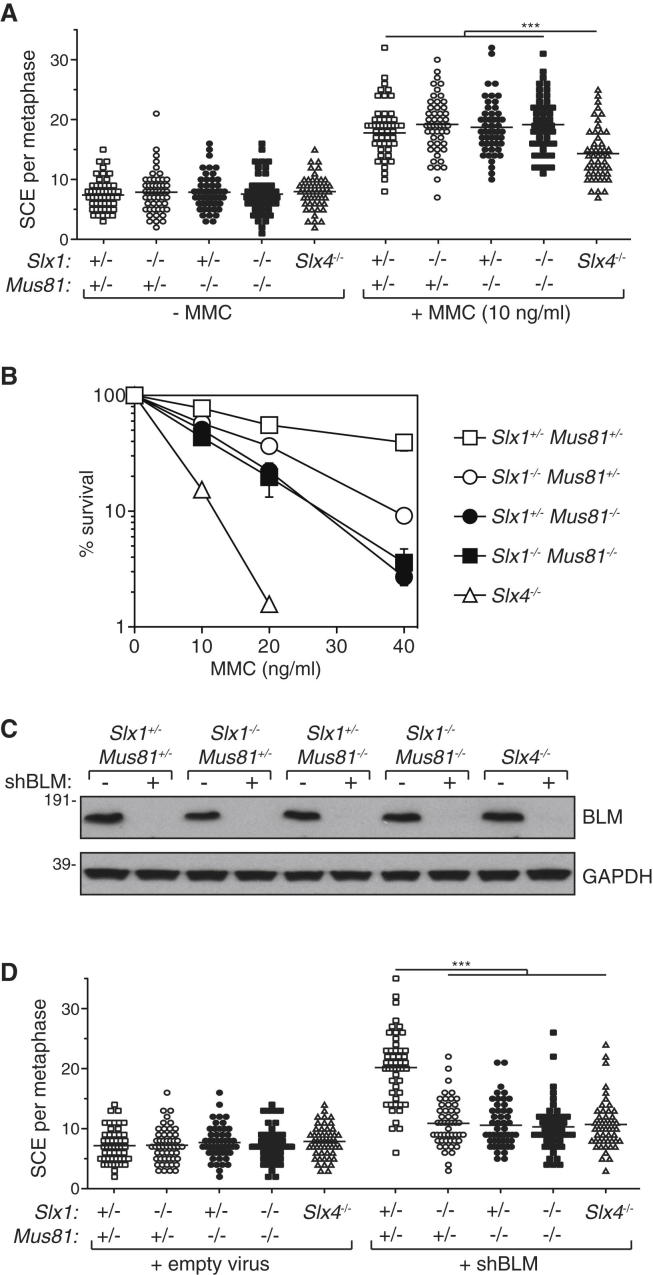
The phenotypes associated with disruption of Slx1 in mice described above are somewhat reminiscent of those reported for Mus81−/− mice (Dendouga et al., 2005). In both cases, the mice are viable and fertile with no gross abnormalities, but MEFs are hypersensitive to ICL-inducing agents. Cells from both mice also show a higher than normal level of chromosome abnormalities after exposure to MMC. These observations, together with the physical proximity of SLX1 and MUS81-EME1 bound to SLX4, suggest that these two nucleases might act in the same pathway, perhaps cooperatively. In order to test this possibility, we generated Slx1−/− Mus81−/− double knockout mice to enable epistasis analysis. These mice were born at Mendelian frequencies (Table S3), showing no developmental, morphological, or hematological abnormalities. Moreover, the fertility of Slx1−/−, Mus81−/−, or Slx1−/− Mus81−/− littermates, or testis size in males, is not significantly different from wild-type mice (data not shown). To study HJ resolution, we isolated MEFs from Slx1−/− Mus81−/− embryos, and as control we used MEFs from single knockout embryos (Slx1−/− Mus81+/− and Slx1+/− Mus81−/−). Double heterozygous (Slx1+/− Mus81+/−) embryos were used as negative control (Figure S4B).
As mentioned earlier, SLX1 had no effect on SCEs generated in MEFs exposed to MMC (Figures 2E and 2F), and similar data were reported for Mus81−/− cells (McPherson et al., 2004). However, it was possible this is because SLX1 acts redundantly with MUS81-EME1. However, as shown in Figure 3A, the increase in SCE frequency induced by MMC in cells lacking both MUS81 and SLX1 was indistinguishable from control cells (Slx1+/− Mus81+/−) and the Slx1−/− or Mus81−/− single knockouts. We also compared the MMC sensitivity of Slx1−/− Mus81−/− MEFs with the respective single knockouts in clonogenic survival assays. As shown in Figures 3B, the MMC hypersensitivity of Mus81−/− cells was slightly more pronounced than Slx1−/− cells, and Slx1−/− Mus81−/− cells were not more sensitive than the most sensitive of the single knockouts (Mus81−/−). Similar results were obtained using alternative clones of MEFs (Figure S4C). Taken together, these observations suggest that SLX1 and MUS81-EME1 are epistatic in terms of ICL repair, at least judged by hypersensitivity to MMC, but they are not required for SCE induced by ICLs in MEFs.
Figure 3.
SLX1 and MUS81 Act Epistatically in HJ Resolution
(A) Scatterplot of SCE frequencies in MEFs of the genotypes indicated after exposure to 10 ng/ml MMC. Fifty metaphases were counted for each condition and significance was calculated using a one-way ANOVA (∗∗∗p < 0.0001) followed by Bonferroni’s Multiple Comparison Test. Each point represents the total number of SCEs in a single mitotic spread. The horizontal line in each data set represents the mean SCE frequency.
(B) Clonogenic survival analysis of MEFs of the genotypes indicated exposed to MMC. For each genotype, cell viability of untreated cells was defined as 100%. Data are represented as mean ± SEM, n = 3. (See also Figure S4.)
(C) Western blot analysis of MEFs, of the genotypes indicated, infected with retroviruses expressing a BLM-specific shRNA (+). Viruses prepared with empty vector served as control (−).
(D) Cells from (C) were analyzed for SCE frequencies. Fifty metaphases were counted for each condition and significance was calculated as in (A). Each point represents the total number of SCEs in a single mitotic spread. The horizontal line in each data set represents mean SCE frequency.
We went on to explore the possibility that SLX1 and MUS81-EME1 are also epistatic in the resolution of HJs. To this end, we depleted BLM from MEFs of various genotypes using shRNA-expressing retroviruses (Figure 3C). Depletion of BLM from control MEFs caused an increase in SCE frequency from around 7 SCE per metaphase to around 20 SCE per metaphase. In Slx1−/− or Mus81−/− single knockout MEFs, the SCE frequency after BLM depletion was diminished to around 10 SCE per metaphase, and no further decrease was observed in Slx1−/− Mus81−/− double knockout cells leaving the residual levels of SCE unaffected (Figure 3D). Therefore, SLX1 and MUS81-EME1 are epistatic with regard to the resolution of HJs that escape dissolution, suggesting that they function in the same pathway.
SLX4 Mutations that Prevent Interaction with SLX1 and/or MUS81-EME1
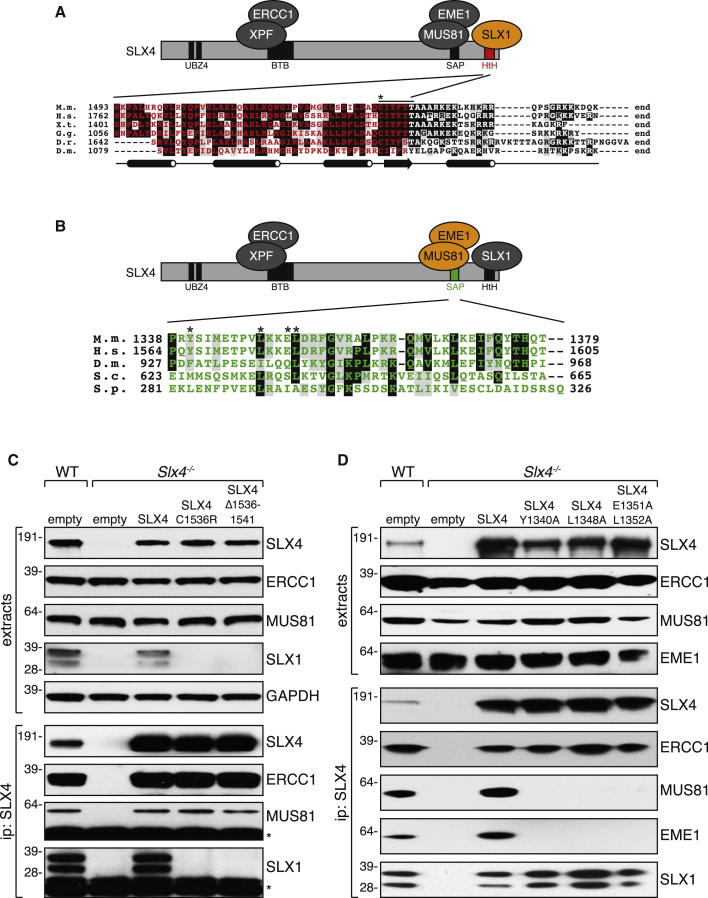
The data above suggest that SLX1 and MUS81-EME1 act together in HJ resolution, and we next set out to test if this requires the tethering of the two nucleases to SLX4 where they bind close together. The SLX1-interacting region of SLX4 has been localized to a small C-terminal fragment containing the HtH motif (Figure 4A) (Fekairi et al., 2009; Svendsen et al., 2009). A 200 amino acid fragment containing the HtH domain was subjected to saturated mutagenesis, followed by reverse yeast two-hybrid screening in order to find SLX4 mutations that abolish interaction with SLX1 (data not shown). Two such mutations were identified: a single point mutation in a highly conserved Cys residue in the SLX4 HtH domain (C1536R), and a small deletion mutant lacking six amino acids (Cys1536-Thr1541; Figure 4A). Full-length SLX4 bearing these mutations, or wild-type SLX4, was stably expressed in Slx4−/− MEFs. As shown in Figure 4C, SLX1 is undetectable in extracts of Slx4−/− MEFs, suggesting the stability of SLX1 requires that it binds to SLX4. Stable expression of wild-type SLX4 restored normal levels of SLX1 in Slx4−/− MEFs. However, the SLX4 mutants C1536R or Δ1536-1541, expressed at levels similar to wild-type SLX4, did not restore SLX1 expression (Figure 4C). Furthermore, no SLX1 could be detected in SLX4 immunoprecipitates from cells expressing these mutant forms of SLX4, but MUS81 and ERCC1 were present at normal levels (Figure 4C). These data indicate that the interaction of SLX4 with SLX1 is required for SLX1 stability, and therefore cells expressing SLX4 mutants that cannot interact with SLX1 are essentially null for SLX1 expression. Nonetheless, these mutants can be used to investigate if SLX1 contributes to the roles of SLX4 in DNA repair.
Figure 4.
SLX4 Mutations that Prevent Interaction with SLX1 and MUS81
(A) Alignment of the C terminus of SLX4 from different species. The HtH domain is highlighted in red. The black line above the alignment refers to the six amino acid deletion in SLX4 that abolished interaction with SLX1, and the asterisk indicates Cys1536. Jalpred3-based secondary structure prediction is indicated below the alignment with barrels representing α helices and the arrow a β sheet. M.m., Mus musculus; H.s., Homo sapiens; X.t., Xenopus tropicalis; G.g., Gallus gallus; D.r., Danio rerio; D.m., Drosophila melanogaster.
(B) Alignment of the SAP domain of SLX4 from different species (highlighted in green). Tyr1340, Leu1348, Glu1351, and Leu1352 are indicated with asterisks. S.c., Saccharomyces cerevisiae; S.p., Saccharomyces pombe.
(C) Slx4−/− MEFs were infected with retroviruses expressing SLX4 wild-type (SLX4), SLX4 C1536R, or SLX4 Δ1536-1541. Extracts were subjected to western blot analysis (upper panels) or immunoprecipitation with anti-SLX4 antibodies (lower panels).
(D) Same as (C) except that Slx4−/− MEFs were infected with retroviruses expressing SLX4 wild-type (SLX4), SLX4 bearing alanine mutations at Y1340, L1348, or E1351+L1352.
We also set out to generate SLX4 mutants that cannot interact with MUS81-EME1. A previous study deleted the entire SAP domain of SLX4 to abolish interaction with MUS81-EME1, and the resulting SLX4 deletion fragment could only partly rescue the MMC sensitivity of FANCP cells (Kim et al., 2013). However, even though in budding yeast Slx4 does not interact with Mus81-Mms4Eme1 (Schwartz et al., 2012), we found that deleting the entire SAP domain of yeast Slx4 perturbs its function (data not shown). These data indicate that the SAP domain of yeast Slx4 is functionally important independent of MUS81 interaction, and it is likely that the same is true of human SLX4. On this basis we sought to engineer more subtle changes in mouse SLX4 that would abolish the interaction with MUS81-EME1. A fragment containing the SAP domain of SLX4 was subjected to saturated mutagenesis, reverse yeast two-hybrid and alanine scanning (data not shown). These experiments revealed several mutations in SLX4 that abolished interaction with MUS81-EME1: Y1340A, L1348A, and a combination of E1351A and L1352A (Figure 4B). Full-length SLX4 bearing these mutations, or wild-type SLX4, were stably expressed in Slx4−/− MEFs. As shown in Figure 4D, the SLX4 Y1340A, L1348A, and E1351A L1352A mutants were unable to interact with MUS81-EME1 in coimmunoprecipitation experiments, but their ability to interact with SLX1 and ERCC1 was unaffected.
Binding of SLX1 and MUS81-EME1 to SLX4 Is Essential for HJ Resolution
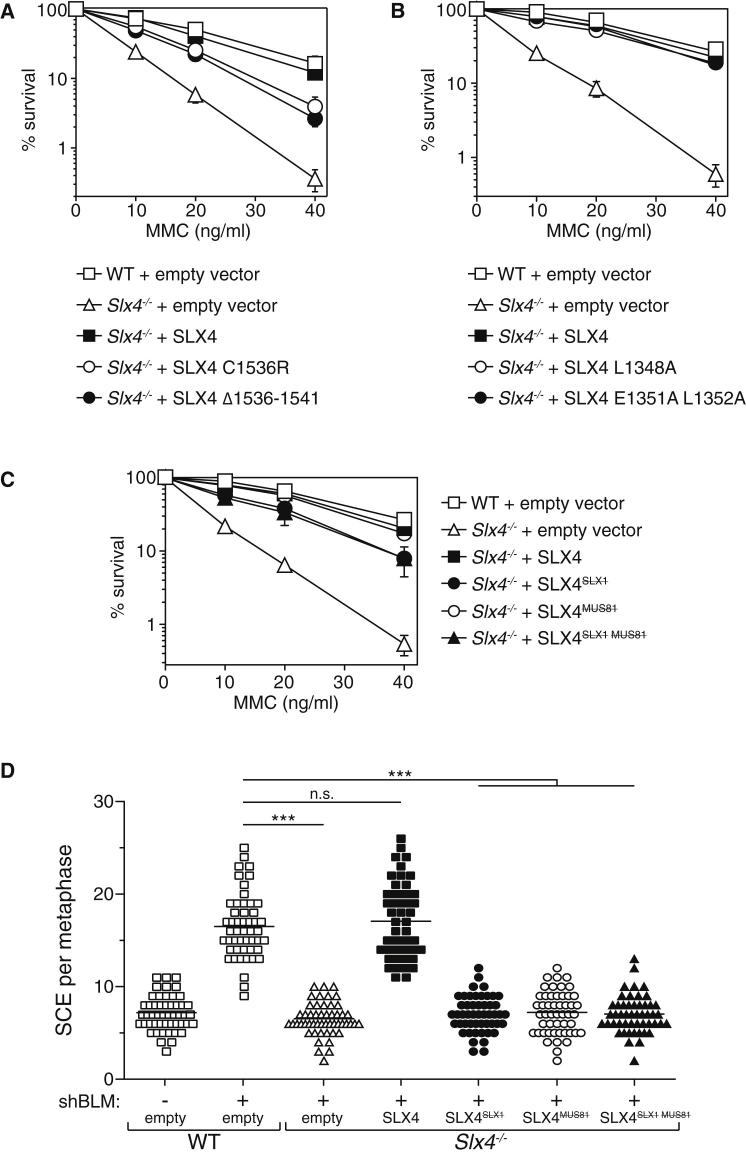
We next examined whether the SLX4 mutants that are unable to interact with SLX1 or MUS81-EME1 are able to rescue the defects observed in Slx4−/− cells, starting with analysis of ICL repair. As shown in Figure 5A, the SLX4 C1536R and Δ1536-1541 mutants, that are incapable of interacting with SLX1, only partly rescued the MMC hypersensitivity of Slx4−/− MEFs. The residual sensitivity of these cells was similar to that of Slx1−/− cells (Figure 1B), consistent with a failure of C1536R and Δ1536-1541 SLX4 mutants to restore SLX1 expression in Slx4−/− cells (Figure 4C). On the other hand, the SLX4 L1348A and E1351A L1352A mutants, both of which fail to interact with MUS81-EME1, were able to fully rescue the MMC hypersensitivity of Slx4−/− MEFs (Figure 5B). Referring to the SLX4 C1536R mutant hereafter as SLX4SLX1 and the SLX4 E1351A L1352A as SLX4MUS81, we combined these mutations to generate SLX4SLX1 MUS81 (Figure S5), and found that this compound mutant restored MMC hypersensitivity of Slx4−/−cells to the same degree as the SLX4SLX1 mutant (Figure 5C). These data suggest that MMC hypersensitivity of cells lacking SLX4 is at least partly due to lack of SLX1 and that the interaction of SLX4 with MUS81-EME1 is not required for ICL repair in MEFs.
Figure 5.
HJ Resolution Requires Binding of SLX1 and MUS81-EME1 to SLX4
(A) Clonogenic survival analysis of Slx4−/− MEFs stably expressing SLX4 wild-type (SLX4), SLX4 C1536R, or SLX4 Δ1536-1541, exposed to MMC. For each genotype, cell viability of untreated cells was defined as 100%. Wild-type MEFs and Slx4−/− MEFs infected with empty virus were used as controls. Data are represented as mean ± SEM, n = 3.
(B) Same as (A) except that SLX4 bearing alanine mutations at L1348 or E1351+L1352 were examined.
(C) Clonogenic analysis as in (A) and (B) except that SLX4 mutations C1536R (SLX4SLX1) and E1351A+L1352A (SLX4MUS81) were combined to prevent interaction with both SLX1 and MUS81 (SLX4 SLX1MUS81). (See also Figure S5.)
(D) Scatterplot of SCE frequencies in MEFs infected with retroviruses expressing a BLM-specific shRNA or control virus (−). The following MEFs were examined: Slx4−/− MEFs infected with SLX4 wild-type or mutants of SLX4, i.e., SLX4SLX1, SLX4MUS81, or SLX4SLX1MUS81. Wild-type MEFs and Slx4−/− MEFs infected with viruses prepared from empty vector were used as control. Fifty metaphases were counted for each condition and significance was calculated using one-way ANOVA (∗∗∗p < 0.0001) followed by Bonferroni’s Multiple Comparison Test. Each point represents the total number of SCE in a single mitotic spread. The horizontal line in each data set represents mean SCE frequency.
We went on to test if the SLX4SLX1 and SLX4MUS81 mutants are defective in HJ resolution. As shown in Figure 5D, the elevation in SCE frequency triggered by BLM depletion is diminished in Slx4−/− MEFs compared with wild-type MEFs. This defect is rescued by expression of wild-type SLX4, but not by the SLX4SLX1, SLX4MUS81 mutants or the SLX4SLX1MUS81 compound mutant. Therefore, it is likely that the binding of both SLX1 and MUS81-EME1 to SLX4 is essential for HJ resolution. In contrast, the binding of SLX1, but not MUS81-EME1, is important for ICL repair in MEFs (Figure 5C). These data suggested strongly that the role of SLX1 and MUS81-EME1 in ICL repair is independent of HJ resolution at least in cells from mice, and we set out to investigate this possibility.
An Hje-SLX1 Fusion Protein with a Preference for HJs
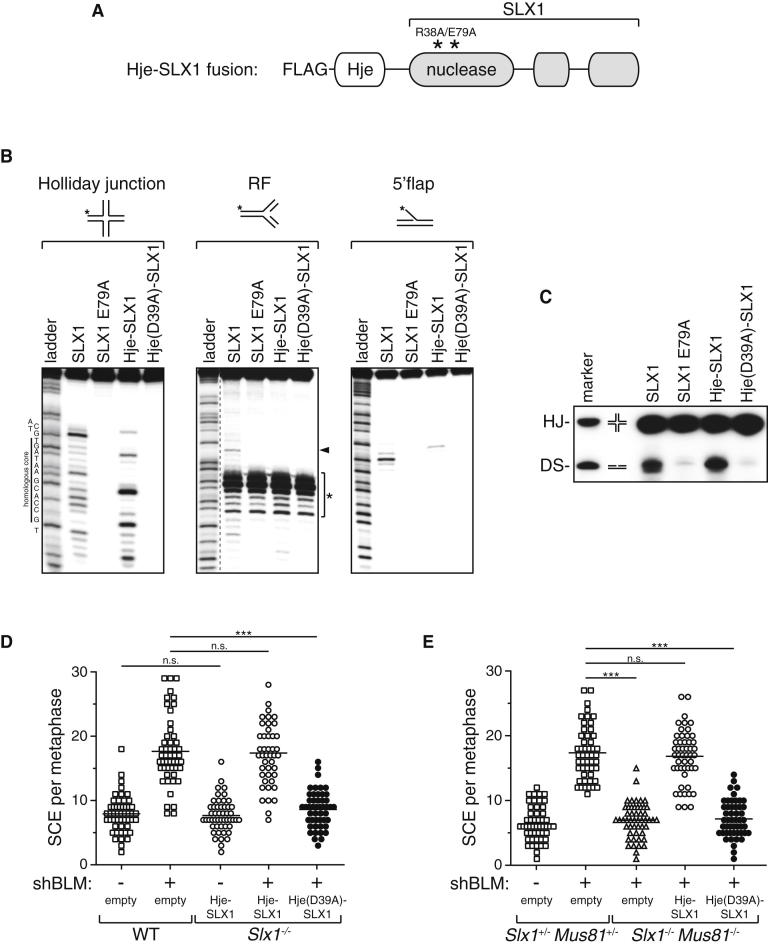
The data above show that SLX1 and MUS81-EME1 are both involved in HJ resolution, judged by measuring the increased SCE frequency caused by BLM depletion. However, SLX1 and MUS81-EME1 are both capable of cleaving a range of branched DNA structures besides HJs in vitro. This consideration, together with the observation that BLM can act on a similar range of branched substrates (Mohaghegh et al., 2001; van Brabant et al., 2000), raised the possibility that the SCEs in BLM-depleted cells arise from nucleolytic processing of structures other than four-way junctions. In an attempt to investigate this possibility, we set out to see if the cleavage of HJs by SLX1 and MUS81-EME1 is physiologically relevant. With this in mind, we mutated the SLX1 nuclease domain (R38A E79A) to inactivate it and fused this SLX1 derivative, which should maintain the ability to bind SLX4, to an archaeal resolving enzyme Hje. This enzyme was reported to have a marked preference for HJs compared with other resolvases (Kvaratskhelia and White, 2000). We next asked if the resulting fusion protein, referred to as Hje-SLX1 (Figure 6A), can rescue defects associated with loss of SLX1. We used an analogous fusion construct containing a catalytically inactive D39A Hje mutant as a negative control. We confirmed that FLAG-tagged versions of the Hje-SLX1 fusion protein, and the catalytically inactive derivative, bind to SLX4 in coimmunoprecipitation experiments in HEK293 cells (Figure S6B).
Figure 6.
HJs Are Physiological Substrates of SLX1 and MUS81
(A) Schematic diagram of a Hje-SLX1 fusion protein. The SLX1 nuclease domain was mutated (R38A E79A) to inactivate it and fused to an archaeal resolving enzyme Hje known to cleave Holliday junctions preferentially.
(B) HEK293 cells stably expressing GFP-tagged SLX4 were transfected with SLX1 wild-type or SLX1 E79A, Hje-SLX1, or Hje (D39A)-SLX1. Anti-FLAG immunoprecipitates were incubated with synthetic DNA structures: mobile Holliday junction (b strand-labeled Jbm5), replication fork (RF)-like structure (b strand-labeled), or 5′ flap (a3 strand-labeled). Reaction products were subjected to denaturing PAGE. Sequencing ladders were obtained by subjecting the corresponding substrates to Maxam and Gilbert‘s purine-specific reaction. Lower exposure is shown for the RF sequencing ladder. (See also Figure S6.)
(C) Same as (B) except the products from the HJ reaction were subjected to nondenaturing PAGE. HJ and duplex DNA (DS) of the same size as the predicted products of symmetrical cleavage of the HJ were run in parallel as markers.
(D) Scatterplot of SCE frequencies in MEFs infected with retroviruses expressing a BLM-specific shRNA (+) or with control virus (−). The following MEFs were examined: Slx1−/− MEFs infected with Hje-SLX1, Hje (D39A)-SLX1, or empty virus. Wild-type MEFs infected with empty virus were used as control. Fifty metaphases were counted for each condition and significance was calculated using one-way ANOVA (∗∗∗p < 0.0001; n.s., nonsignificant) followed by Bonferroni’s Multiple Comparison Test. Each point represents the total number of SCEs in a single mitotic spread. The horizontal line in each data set represents mean SCE frequency.
(E) Same as (D) except that Slx1−/−Mus81−/− MEFs expressing Hje-SLX1, Hje (D39A)-SLX1, or empty virus were examined.
Next, anti-FLAG immunoprecipitates were incubated with radiolabeled DNA substrates. We used a four-way junction with a 12 bp homologous core (HJ), a replication fork (RF) analog (a three-way junction where one strand is discontinuous at the junction), and a 5′ flap. Reaction products were separated by gel electrophoresis under denaturing conditions. As shown in Figure 6B, both SLX1 and the Hje-SLX1 fusion exhibited strong cleavage activity on the four-way junction substrate. Consistent with previous reports, SLX1 cleaved the four-way junction at multiple positions (Fekairi et al., 2009; Muñoz et al., 2009; Svendsen et al., 2009); the Hje-SLX1 fusion protein also cleaved at several points, but these were different from SLX1 (Figure 6B; Figure S6A). Nonetheless, nondenaturing gel electrophoresis showed that Hje-SLX1 introduced symmetric cleavages within the homologous core of the HJ, resulting in the production of linear duplex DNA as required for the productive resolution of the junction (Figure 6C). The Hje-SLX1 derivative, which is inactivated by the D39A mutation, showed no detectable activity toward the HJ substrate (Figure 6B). Although all of the FLAG immunoprecipitates exhibited some nonspecific activity toward the RF substrate (Figure 6B, asterisk), a weak SLX1-specific cleavage product was observed in FLAG-SLX1 immunoprecipitates that was not present in SLX1 E79A precipitates (Figure 6B, arrowhead). This product was not observed in Hje-SLX1 precipitates, indicating that this fusion protein cannot cleave replication forks. The 5′ flap substrate was cleaved by FLAG-SLX1 one nucleotide before the junction, and by contrast, the Hje-SLX1 fusion exhibited only very weak activity three nucleotides from junction (Figure 6B; Figure S6A). These experiments demonstrated that Hje-SLX1 binds to SLX4 and acts as a potent HJ resolving enzyme with restricted substrate specificity compared with SLX1.
HJs Are Physiological Substrates of SLX1 and MUS81-EME1
We next examined whether the Hje-SLX1 fusion could restore the cellular functions perturbed in cells lacking SLX1. Untagged versions of Hje-SLX1 and the inactive D39A derivative were stably expressed in Slx1−/− MEFs (Figure S6C, left panels). Cells were then depleted of BLM. Strikingly, the Hje-SLX1 fusion protein, but not the D39A version, restored SCE frequency in BLM-depleted Slx1−/− MEFs similar to wild-type SLX1 (Figure 6D). Expression of Hje-SLX1 in cells that were not depleted of BLM had no apparent effect on SCE levels (Figure 6D). The Hje-SLX1 fusion and the inactive D39A derivative were also stably expressed in Slx1−/− Mus81−/− MEFs (Figure S6C, right panels). This experiment revealed that Hje-SLX1 but not the D39A version restored SCE frequency after BLM depletion back to wild-type levels (Figure 6E). Taken together, these data strongly suggest that the formation of SCE in BLM-depleted cells, which was shown to require binding of SLX1 and MUS81-EME1 to SLX4, results from the resolution of HJs. Finally, we tested the ability of the Hje-SLX1 fusion protein to reverse the ICL repair defects in cells lacking SLX1. Intriguingly, the Hje-SLX1 fusion protein was incapable of rescuing the MMC hypersensitivity of Slx1−/− MEFs in contrast to wild-type SLX1 (Figure S6D). These data strengthen the notion that the role of SLX1 in ICL repair in MEFs involves cleavage of DNA structures other than HJs.
Discussion
In this study, we demonstrate that SLX1 is involved in cellular responses to DNA damage in mammals. Consistent with its reported ability to process HJs in vitro (Fekairi et al., 2009; Muñoz et al., 2009; Svendsen et al., 2009), we found that SLX1, like MUS81-EME1, plays a major role in the nucleolytic resolution of HJs in vivo. SLX1 and MUS81-EME1 are both required for resolving the majority of HJs that escape dissolution by BLM, and they act epistatically in this regard. We also found that the tethering of SLX1 and MUS81-EME1 to SLX4, where they bind in close proximity, is essential for the ability of these nucleases to promote HJ resolution. The simplest interpretation of these findings is that SLX1 and MUS81-EME1 act cooperatively in the processing of HJs, as previously postulated by others (Svendsen et al., 2009). In this model SLX1 acts on intact HJs to create nicked junctions, which are then cleaved by MUS81-EME1 (Boddy et al., 2001; Ciccia et al., 2003; Doe et al., 2002; Svendsen et al., 2009; Whitby et al., 2003). Close juxtaposition of SLX1 and MUS81-EME1 on SLX4 might position the respective active sites to favor coordinated first-strand cleavage by SLX1 and second-strand cleavage by MUS81-EME1. This idea might help to solve the longstanding dilemma of how MUS81-EME1 participates in HJ resolution in vivo when the recombinant enzyme only weakly cleaves intact HJs in vitro compared with nicked HJs (Boddy et al., 2001; Ciccia et al., 2003; Doe et al., 2002; Whitby et al., 2003).
The model whereby SLX1 and MUS81-EME1 act together in HJ resolution is based on measuring SCE frequency upon BLM depletion. But do the SCEs observed in BLM-depleted cells result from the cleavage of HJs? It is possible that the SCEs resulting from depletion of BLM reflect instead the cleavage of branched DNA structures other than HJs. This is a particular concern because BLM, like MUS81-EME1 and SLX1, is active toward a range of branched DNA structures in vitro (Mohaghegh et al., 2001; van Brabant et al., 2000). Furthermore, the cleavage of the precursors to HJs such as extended D-loop structures can produce crossovers that would manifest as SCEs (Osman et al., 2003). Thus, it is possible that the SCEs in BLM-depleted cells occur as a result of the cleavage of recombination structures other than HJs. However, the demonstration that the Hje-SLX1 fusion protein with increased specificity for HJs can reverse the SCE defect in Slx1−/− and Slx1−/− Mus81−/− cells strongly suggests that SCE formation in BLM-depleted cells reflects HJ cleavage by SLX1 and MUS81-EME1.
In this study we demonstrate that SLX1 is involved in ICL repair in mammalian cells, and similarly to HJ resolution, SLX1 appears to act epistatically with MUS81-EME1 in ICL repair. However, several observations suggest that the epistatic interaction between SLX1 and MUS81-EME1 in ICL repair does not involve the cleavage of HJs, at least in mice. First, the SCEs formed in MMC-treated MEFs do not require SLX1 or MUS81-EME1, unlike the SCEs observed in BLM-depleted cells. Second, whereas HJ resolution in BLM-depleted MEFs requires binding of both SLX1 and MUS81-EME1 to SLX4, cellular resistance to MMC requires binding of SLX1 but not MUS81-EME1. Third, the Hje-SLX1 fusion protein, which is much more specific for HJs than SLX1, can rescue the SCE defect in Slx1−/− and Slx1−/− Mus81−/− MEFs depleted of BLM, but it cannot rescue the MMC hypersensitivity of these cells. These observations suggest that the role of SLX1 and MUS81-EME1 in the repair of ICLs involves the processing of structures other than HJs. How might SLX1 and MUS81-EME1 cooperate in ICL repair? MUS81-EME1 is known to act at the “unhooking” step of ICL repair, a process that involves incisions on either side of the ICL that produce DSBs (Hanada et al., 2006, 2007). However, DSB induction in cells exposed to ICL-inducing agents does not require SLX1 (data not shown). Therefore, the epistatic interaction of SLX1 and MUS81-EME1 in ICL repair is independent of DSB induction. One possible explanation is that SLX1-SLX4 processes recombination intermediates during the repair of the DSBs generated by the pool of MUS81-EME1 that is not bound to SLX4.
It is clear that the dominant mode of HJ removal in mitotic cells—at least those arising spontaneously—involves BLM, and HJ resolution by nucleases acts to cleave junctions that escape dissolution. The BLM pathway has been supposed to minimize the risk of loss of heterozygosity, since this pathway generates noncrossover outcomes exclusively (LaRocque et al., 2011). However, in meiotic cells crossover formation is necessary and should therefore involve nucleolytic HJ resolution. Since SLX1 and MUS81-EME1 resolve the majority of HJs that escape dissolution in mitotic mammalian cells, one might predict that they might dominate in meiosis. However, although Mus81−/− mice show meiotic defects, they are fertile, suggesting that other nucleases can mediate HJ processing in the absence of MUS81-EME1 (Dendouga et al., 2005; Holloway et al., 2008). This is unlikely to be SLX1, since the fertility and gonad size of Slx1−/−, Mus81−/−, or Slx1−/− Mus81−/− mice generated in this study were not significantly different from wild-type mice (data not shown). Since Slx4−/− mice exhibit subfertility and hypogonadism, but Slx1−/− Mus81−/− do not, the third SLX4-associated nuclease, XPF-ERCC1, might be involved in meiotic HJ resolution. In this light, recent studies in nematodes found that XPF/BLM and SLX1/MUS81-EME1 act on parallel pathways in worm meiosis (Agostinho et al., 2013; O’Neil et al., 2013; Saito et al., 2013). Moreover, SLX4 and XPF-ERCC1 are important for crossover generation in Drosophila (Yildiz et al., 2002). It will be interesting to test if XPF-ERCC1 is involved in HJ resolution in mammals. However, since Slx4−/− mice can generate offspring, it must be that nucleases that do not associate with SLX4 can resolve HJs in the absence of SLX4 complex components. This might be GEN1. It will be interesting to make the relevant combinations of gene knockouts in mice to investigate this. Given the importance of meiotic HJ resolution in heredity, and in preventing defects in chromosome segregation, it will be vitally important to decipher the precise mechanisms underlying the resolution of meiotic HJs.
Experimental Procedures
Depletion of BLM from MEFs Using shRNA
The protocol was adapted from a previous report (Sfeir et al., 2009). Briefly, viruses expressing a BLM-specific shRNA were produced by cotransfecting 293T cells with pSUPER.retro.puro (Open Biosystems) expressing the BLM target sequence 5′-gga cct gct gga aga ttt a-3′ and with pCMV-VSV-G and pCMV-Gag-Pol. MEFs were infected four times in 6 hr intervals with virus-containing supernatant supplemented with 8 μg/ml polybrene. Twenty-four hours after the last infection, MEFs were selected with 3 μg/ml puromycin. Knockdown efficiency was confirmed by western blot.
SCE Assay
Cells were treated for 24 hr with 10 μM BrdU before replacing the medium with fresh BrdU and the indicated dose of MMC for an additional 24 hr. When SCE analysis was performed after BLM knockdown, cells were selected for the uptake of virus in the presence of 3 μg/ml puromycin 24 hr after the last infection. MEFs were treated with two cycles of BrdU as described above (Figure S4A). At 6 hr before the end of the second BrdU cycle, nocodazole was added at a final concentration of 0.15 μg/ml. Cells were harvested and prepared for metaphase spreads. Spreads were stained with Hoechst 33258, followed by UV irradiation and staining with Giemsa according to standard protocols. Fifty spreads were analyzed for each condition on a DeltaVision wide-field deconvolution microscope (Olympus).
Full details of all other experimental procedures are given in Supplemental Information.
Acknowledgments
We are grateful to James Hastie, Hilary MacLauchlan, and the Antibody Production Team at Division of Signal Transduction Therapy, University of Dundee and to DNA Sequencing Service and Oligonucleotide Synthesis Service at CLS, University of Dundee. We thank CIRRU staff, especially Lorraine Malone and Carol Clacher, for help with animal husbandry and ESC derivation, and we are thankful to Gail Fraser and Elaine Forsyth for help with genotyping. We thank Victoria McGuire for help with generating knockout mice and Catherine Lamm for histological analyses. We are grateful to Clare McGowan for the kind gift of Mus81−/− mice and to Simon Boulton for sending the mice. We are grateful to members of the Rouse lab for critical reading of this manuscript. We thank Anton Gartner for communicating data prior to publication, useful discussions, and critical reading of the manuscript. We are also grateful to Steve West for communicating data prior to publication. This study was also supported by the Medical Research Council (MRC), the pharmaceutical companies supporting the Division of Signal Transduction Therapy Unit (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck KgaA, Janssen Pharmaceutica, and Pfizer) associated with the MRC PPU (D.C., N.N., R.T., T.J.M., J.S.C.A., J.R.), and Cancer Research UK (A.-C.D. and D.M.J.L.).
Published: September 26, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes six figures, four tables, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2013.08.036.
Supplemental Information
References
- Agostinho A., Meier B., Sonneville R., Jagut M., Woglar A., Blow J., Jantsch V., Gartner A. Combinatorial regulation of meiotic holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet. 2013;9:e1003591. doi: 10.1371/journal.pgen.1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L., Bergstralh D.T., Kohl K.P., LaRocque J.R., Moore C.B., Sekelsky J. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Mol. Cell. 2009;35:128–135. doi: 10.1016/j.molcel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A.D. Fanconi anemia and its diagnosis. Mutat. Res. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly A.P., Freeman A., Hall J., Déclais A.C., Alpi A., Lilley D.M., Ahmed S., Gartner A. The Caenorhabditis elegans homolog of Gen1/Yen1 resolvases links DNA damage signaling to DNA double-strand break repair. PLoS Genet. 2010;6:e1001025. doi: 10.1371/journal.pgen.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker S.T., de Winter J.P., te Riele H. Learning from a paradox: recent insights into Fanconi anaemia through studying mouse models. Dis. Model. Mech. 2013;6:40–47. doi: 10.1242/dmm.009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy M.N., Gaillard P.H., McDonald W.H., Shanahan P., Yates J.R., 3rd, Russell P. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell. 2001;107:537–548. doi: 10.1016/s0092-8674(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Chaganti R.S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom’s syndrome lymphocytes. Proc. Natl. Acad. Sci. USA. 1974;71:4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A., Constantinou A., West S.C. Identification and characterization of the human mus81-eme1 endonuclease. J. Biol. Chem. 2003;278:25172–25178. doi: 10.1074/jbc.M302882200. [DOI] [PubMed] [Google Scholar]
- Ciccia A., McDonald N., West S.C. Structural and functional relationships of the XPF/MUS81 family of proteins. Annu. Rev. Biochem. 2008;77:259–287. doi: 10.1146/annurev.biochem.77.070306.102408. [DOI] [PubMed] [Google Scholar]
- Crossan G.P., van der Weyden L., Rosado I.V., Langevin F., Gaillard P.H., McIntyre R.E., Gallagher F., Kettunen M.I., Lewis D.Y., Brindle K., Sanger Mouse Genetics Project Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat. Genet. 2011;43:147–152. doi: 10.1038/ng.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendouga N., Gao H., Moechars D., Janicot M., Vialard J., McGowan C.H. Disruption of murine Mus81 increases genomic instability and DNA damage sensitivity but does not promote tumorigenesis. Mol. Cell. Biol. 2005;25:7569–7579. doi: 10.1128/MCB.25.17.7569-7579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe C.L., Ahn J.S., Dixon J., Whitby M.C. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J. Biol. Chem. 2002;277:32753–32759. doi: 10.1074/jbc.M202120200. [DOI] [PubMed] [Google Scholar]
- Fekairi S., Scaglione S., Chahwan C., Taylor E.R., Tissier A., Coulon S., Dong M.Q., Ruse C., Yates J.R., 3rd, Russell P. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138:78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard P.H.L., Noguchi E., Shanahan P., Russell P. The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol. Cell. 2003;12:747–759. doi: 10.1016/s1097-2765(03)00342-3. [DOI] [PubMed] [Google Scholar]
- Hanada K., Budzowska M., Modesti M., Maas A., Wyman C., Essers J., Kanaar R. The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. EMBO J. 2006;25:4921–4932. doi: 10.1038/sj.emboj.7601344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Budzowska M., Davies S.L., van Drunen E., Onizawa H., Beverloo H.B., Maas A., Essers J., Hickson I.D., Kanaar R. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat. Struct. Mol. Biol. 2007;14:1096–1104. doi: 10.1038/nsmb1313. [DOI] [PubMed] [Google Scholar]
- Holloway J.K., Booth J., Edelmann W., McGowan C.H., Cohen P.E. MUS81 generates a subset of MLH1-MLH3-independent crossovers in mammalian meiosis. PLoS Genet. 2008;4:e1000186. doi: 10.1371/journal.pgen.1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway J.K., Mohan S., Balmus G., Sun X., Modzelewski A., Borst P.L., Freire R., Weiss R.S., Cohen P.E. Mammalian BTBD12 (SLX4) protects against genomic instability during mammalian spermatogenesis. PLoS Genet. 2011;7:e1002094. doi: 10.1371/journal.pgen.1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S.C.Y., Rass U., Blanco M.G., Flynn H.R., Skehel J.M., West S.C. Identification of Holliday junction resolvases from humans and yeast. Nature. 2008;456:357–361. doi: 10.1038/nature07470. [DOI] [PubMed] [Google Scholar]
- Kim Y., Lach F.P., Desetty R., Hanenberg H., Auerbach A.D., Smogorzewska A. Mutations of the SLX4 gene in Fanconi anemia. Nat. Genet. 2011;43:142–146. doi: 10.1038/ng.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Spitz G.S., Veturi U., Lach F.P., Auerbach A.D., Smogorzewska A. Regulation of multiple DNA repair pathways by the Fanconi anemia protein SLX4. Blood. 2013;121:54–63. doi: 10.1182/blood-2012-07-441212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaratskhelia M., White M.F. An archaeal Holliday junction resolving enzyme from Sulfolobus solfataricus exhibits unique properties. J. Mol. Biol. 2000;295:193–202. doi: 10.1006/jmbi.1999.3363. [DOI] [PubMed] [Google Scholar]
- LaRocque J.R., Stark J.M., Oh J., Bojilova E., Yusa K., Horie K., Takeda J., Jasin M. Interhomolog recombination and loss of heterozygosity in wild-type and Bloom syndrome helicase (BLM)-deficient mammalian cells. Proc. Natl. Acad. Sci. USA. 2011;108:11971–11976. doi: 10.1073/pnas.1104421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J.P., Lemmers B., Chahwan R., Pamidi A., Migon E., Matysiak-Zablocki E., Moynahan M.E., Essers J., Hanada K., Poonepalli A. Involvement of mammalian Mus81 in genome integrity and tumor suppression. Science. 2004;304:1822–1826. doi: 10.1126/science.1094557. [DOI] [PubMed] [Google Scholar]
- Mohaghegh P., Karow J.K., Brosh R.M., Jr., Bohr V.A., Hickson I.D. The Bloom’s and Werner’s syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001;29:2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz I.M., Hain K., Déclais A.C., Gardiner M., Toh G.W., Sanchez-Pulido L., Heuckmann J.M., Toth R., Macartney T., Eppink B. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol. Cell. 2009;35:116–127. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- O’Neil N.J., Martin J.S., Youds J.L., Ward J.D., Petalcorin M.I., Rose A.M., Boulton S.J. Joint molecule resolution requires the redundant activities of MUS-81 and XPF-1 during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9:e1003582. doi: 10.1371/journal.pgen.1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman F., Dixon J., Doe C.L., Whitby M.C. Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol. Cell. 2003;12:761–774. doi: 10.1016/s1097-2765(03)00343-5. [DOI] [PubMed] [Google Scholar]
- Rass U., Compton S.A., Matos J., Singleton M.R., Ip S.C., Blanco M.G., Griffith J.D., West S.C. Mechanism of Holliday junction resolution by the human GEN1 protein. Genes Dev. 2010;24:1559–1569. doi: 10.1101/gad.585310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T.T., Youds J.L., Boulton S.J., Colaiácovo M.P. Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genet. 2009;5:e1000735. doi: 10.1371/journal.pgen.1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T.T., Lui D.Y., Kim H.M., Meyer K., Colaiácovo M.P. Interplay between structure-specific endonucleases for crossover control during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9:e1003586. doi: 10.1371/journal.pgen.1003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E.K., Heyer W.D. Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma. 2011;120:109–127. doi: 10.1007/s00412-010-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E.K., Wright W.D., Ehmsen K.T., Evans J.E., Stahlberg H., Heyer W.D. Mus81-Mms4 functions as a single heterodimer to cleave nicked intermediates in recombinational DNA repair. Mol. Cell. Biol. 2012;32:3065–3080. doi: 10.1128/MCB.00547-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A., Kosiyatrakul S.T., Hockemeyer D., MacRae S.L., Karlseder J., Schildkraut C.L., de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoepker C., Hain K., Schuster B., Hilhorst-Hofstee Y., Rooimans M.A., Steltenpool J., Oostra A.B., Eirich K., Korthof E.T., Nieuwint A.W. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat. Genet. 2011;43:138–141. doi: 10.1038/ng.751. [DOI] [PubMed] [Google Scholar]
- Svendsen J.M., Smogorzewska A., Sowa M.E., O’Connell B.C., Gygi S.P., Elledge S.J., Harper J.W. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138:63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E.R., McGowan C.H. Cleavage mechanism of human Mus81-Eme1 acting on Holliday-junction structures. Proc. Natl. Acad. Sci. USA. 2008;105:3757–3762. doi: 10.1073/pnas.0710291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brabant A.J., Ye T., Sanz M., German J.L., III, Ellis N.A., Holloman W.K. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- Wechsler T., Newman S., West S.C. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature. 2011;471:642–646. doi: 10.1038/nature09790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.C. The search for a human Holliday junction resolvase. Biochem. Soc. Trans. 2009;37:519–526. doi: 10.1042/BST0370519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby M.C., Osman F., Dixon J. Cleavage of model replication forks by fission yeast Mus81-Eme1 and budding yeast Mus81-Mms4. J. Biol. Chem. 2003;278:6928–6935. doi: 10.1074/jbc.M210006200. [DOI] [PubMed] [Google Scholar]
- Wu L., Hickson I.D. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- Wu L., Hickson I.D. DNA helicases required for homologous recombination and repair of damaged replication forks. Annu. Rev. Genet. 2006;40:279–306. doi: 10.1146/annurev.genet.40.110405.090636. [DOI] [PubMed] [Google Scholar]
- Yildiz O., Majumder S., Kramer B., Sekelsky J.J. Drosophila MUS312 interacts with the nucleotide excision repair endonuclease MEI-9 to generate meiotic crossovers. Mol. Cell. 2002;10:1503–1509. doi: 10.1016/s1097-2765(02)00782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.