Abstract
The “Malaria Evolution in South Asia” (MESA) program project is an International Center of Excellence for Malaria Research (ICEMR) sponsored by the US National Institutes of Health. This US–India collaborative program will study the origin of genetic diversity of malaria parasites and their selection on the Indian subcontinent. This knowledge should contribute to a better understanding of unexpected disease outbreaks and unpredictable disease presentations from Plasmodium falciparum and Plasmodium vivax infections. In this first of two reviews, we highlight malaria prevalence in India. In particular, we draw attention to variations in distribution of different human-parasites and different vectors, variation in drug resistance traits, and multiple forms of clinical presentations. Uneven malaria severity in India is often attributed to large discrepancies in health care accessibility as well as human migrations within the country and across neighboring borders. Poor access to health care goes hand in hand with poor reporting from some of the same areas, combining to possibly distort disease prevalence and death from malaria in some parts of India. Corrections are underway in the form of increased resources for disease control, greater engagement of village-level health workers for early diagnosis and treatment, and possibly new public–private partnerships activities accompanying traditional national malaria control programs in the most severely affected areas. A second accompanying review raises the possibility that, beyond uneven health care, evolutionary pressures may alter malaria parasites in ways that contribute to severe disease in India, particularly in the NE corridor of India bordering Myanmar Narayanasamy et al., 2012.
Keywords: Malaria, Plasmodium falciparum, Plasmodium vivax, India, South Asia, Epidemiology, Drug resistance, ICEMR
1. Introduction
The US National Institute of Health is supporting ten International Centers of Excellence for Malaria Research (ICEMR) world-wide. Over a period of 7 years, the goal is to capture a broader and more detailed picture of malaria incidence in different parts of the world and to identify questions and study sites that will help national and international programs understand and control malaria more efficiently, with the long term of goal of global malaria eradication (Rao, 2012).
In South Asia, the largest country, India, offers vast variations in geography and regional ties to surrounding communities and countries. This makes for many possible ways in which malaria parasite populations can mix to affect drug responses, disease severity, and transmission.
The present review addresses the broad theme of diversity in South Asia and how it affects malaria presentations throughout the subcontinent. An accompanying second review discusses the study plan for the “Malaria Evolution in South Asia” ICEMR, including selection and characteristics of the study sites, the research tools being deployed, potential new findings and training plans for long-term continuity of the program and improved human health.
2. South Asia, a diverse home for human malaria parasites
2.1. Weather, size, geography, and people
The countries of Afghanistan, Bangladesh, India, Nepal, Pakistan, Sri Lanka, and countries to the west vary in size, political structures and fluidity, human genetics, and ecological diversity (Fig. 1). There are also variations in public health resources, and disease burdens from other infectious diseases. Despite these differences, the countries of South Asia are interdependent with respect to life style, geographic proximity, and approach to health care. India, because of its size and diversity, captures many, if not all, aspects of the culture and disease patterns seen within the region.
Fig. 1.
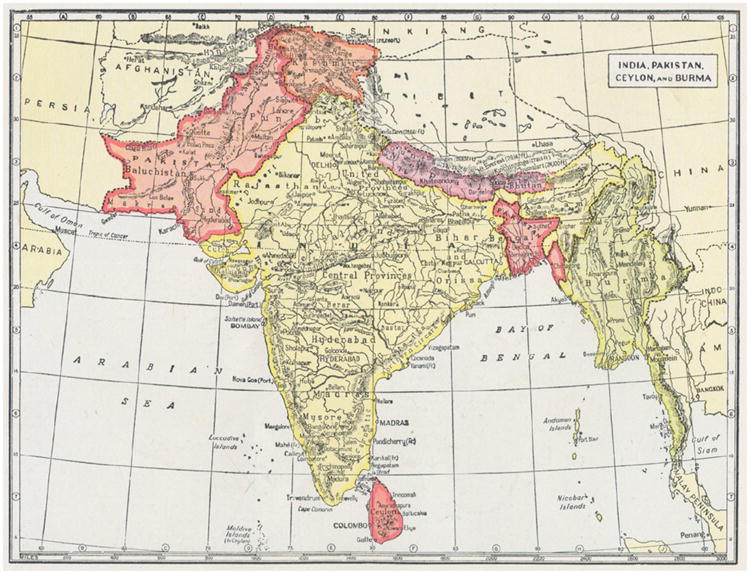
An old map showing one view of South Asia countries, with pre-independence names: India (without Indian Kashmir), West Pakistan (now Pakistan), Afghanistan, East Pakistan (now Bangladesh), Ceylon (now Sri Lanka), Nepal, Bhutan, and Burma (now Myanmar). http://www.probertencyclopaedia.com/photolib/maps/.
In South Asia, India has more than 3 million square kilometers of land (http://www.worldfactsandfigures.com/countries/india.php), vast amounts of which are well suited for the breeding of mosquitoes and propagation of malaria parasites. Its climate is strongly influenced by the Himalayas and the Thar Desert, both of which drive the monsoons. The Himalayas prevent cold Central Asian winds from blowing in, keeping the bulk of the Indian subcontinent warmer than most locations at similar latitudes. The Thar Desert plays a crucial role in attracting the moisture-laden southwest summer monsoon winds that, between June and October, provide the majority of India's rainfall. Four major climatic groupings predominate in India: tropical wet, tropical dry, subtropical humid, and mountainous.
India is the seventh-largest country in the world by geographical area, is the second-most populous country and has a coastline of 7517 km (4700 miles). India is the world's most culturally, linguistically and genetically diverse geographical entity, after the African continent. It has an estimated population of 1.2 billion. India's urban population increased 11-fold during the twentieth century and is increasingly concentrated in large cities. By 2001, there were more than 350 million people in Indian cities, with more than 15 million in Mumbai, Delhi and Kolkata each. Yet, at this time, more than 70% of India's population continues to reside in rural areas. The interplay between city-based and forest-based concentration of human reservoirs for parasites and easy movement of workers from one part of India to another, mixed with varying degrees of innate and acquired protection in different human communities, raises many opportunities for dissecting important traits that contribute to malaria protection or vulnerability.
India's nominal per capita income was US $1371 in 2011, ranked 138th in the world. Yet, economic reforms since 1991 have transformed India into one of the fastest growing economies. It has the world's ninth largest economy and the fourth largest purchasing power. With an annual GDP growth rate of 8.5%, the economy is among the most rapidly growing in the world. It has the world's second largest labor force with 478 million working people.
2.2. Health changes and challenges
From 1947 to 2007, Indian life expectancy has more than doubled (32 years to 68.6 years), the infant mortality rate has decreased 76.3 percent (146 per 1000 live births to 35) and the crude death rate has fallen 74 percent (25.1 per 1000 population to 6.6).
India still faces considerable challenges. The country accounts for a large share of the world's disease burden. Although India makes up 16.5 percent of the world's population, it accounts for “a third of diarrheal diseases, tuberculosis, respiratory and other infections; a third of parasitic infestations and pre-natal conditions; a quarter of maternal conditions; a fifth of nutritional deficiencies, diabetes and cardiovascular diseases; and the second largest number of HIV/AIDS cases in the world”. In India, annually, 2.2 million infants and children die from preventable illnesses, 100,000 mothers die during childbirth, 500,000 people die of tuberculosis and 5 million people suffer from HIV/AIDS. Diarrhea and malaria continue to be major killers (World Health Organization, Commission on Macroeconomic and Health, 2001).
Providing healthcare and disease prevention services to India's growing population of more than one billion people is challenging in the face of increased competition for resources. India spends only about 5% of GDP on health care and of this, only one fifth is public spending. Health policy in India has shifted its focus from being a comprehensive, universal healthcare system to a selective and targeted program-based healthcare policy with the public domain being confined to family planning, immunization, selected disease surveillance and medical education and research.
3. Malaria prevalence
3.1. Fever data
India has a robust administrative structure to capture raw data on fevers and parasite slide positivity on a national scale. The National Vector Borne Disease Control Program (NVBDCP) prescribes a fortnightly national active surveillance for fever, knowing that about 10% of the population will have fever at some point in a year. It is assumed that if all or most of the fever cases are slide-examined for malaria, most of the incidence of malaria can be captured through Annual Blood Examination Rate (ABER).
In 2008, ABER showed 8.7% coverage in India (Fig. 2a). However, a closer look at the district level numbers reveals wide variations in compliance with Program guidelines (Fig. 2b). Some 314 districts, with 57% of the total 1.1 billion Indian population, had less than the desired 10% coverage for malaria surveillance. The remaining 316 (43%) districts had adequate malaria surveillance (data not shown). Some 49 districts (population: 40.6 million) had ABER inspections for greater than 20% of the population.
Fig. 2.
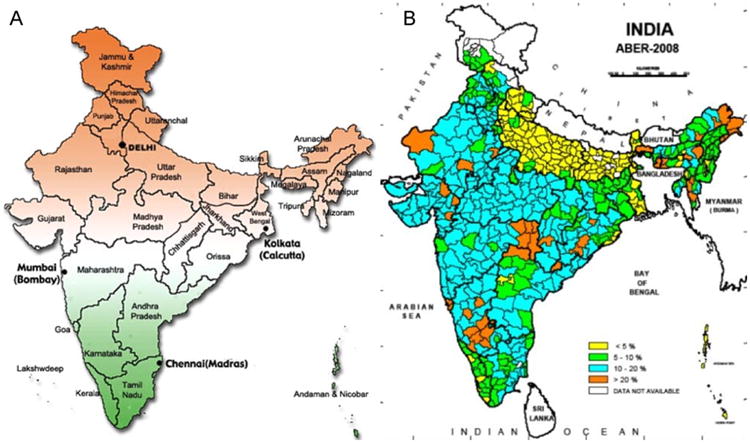
(A) Present state map of India (http://blog.lookindia.in). This is to help readers identify the different states mentioned in the text in the context of malaria epidemiology in India. (B) Districts of India vary widely in ABER compliance (2008 data). Some malaria prone N and NE districts (see Fig. 3) are among the poorest in malaria surveillance compliance (Map prepared by NIMR, Goa, using data from the National Vector Borne Disease Control Program, New Delhi).
3.2. Parasitemia
The risk of malaria infection varies greatly in different regions. The Annual Parasite Incidence (API) is an index to express malaria cases per thousand individuals. Based on the NVBDCP data from 2008, the API was <2 in most of India, particularly in districts in the Northern, Western and Southern parts of the country (Fig. 3). Districts with API of 2–5 were scattered across central India. The most malaria prone districts, with >5 API, were clustered in the eastern states of Chhattisgarh, Jharkhand and Orissa as well as in the northeastern states found east of Bangladesh near the Myanmar border. The districts with the highest incidence, API >20, were in Orissa (7 districts), Arunachal (5), Meghalaya (2), Chhattisgarh (2), Assam (1), Mizoram (1), Jharkhand (1), and Andaman and Car Nicobar Islands (1). In 2008, these 20 districts with the highest malaria burden had API ranging from 21 to 74 in a population of 10.7 million (Fig. 3).
Fig. 3.
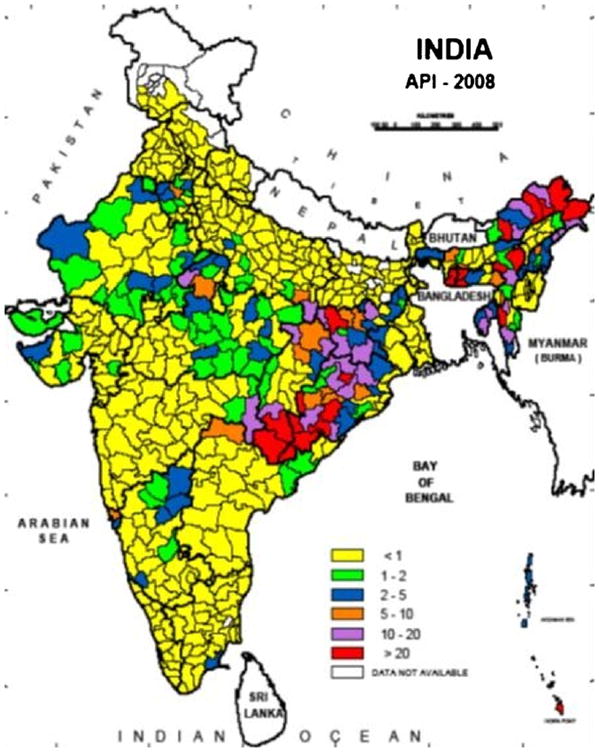
Annual Parasite Incidence (Slide positive malaria cases/1000 population) in India for the year 2008 (Map prepared by NIMR, Goa, using data from the National Vector Borne Disease Control Program, New Delhi).
3.3. Plasmodium falciparum versus Plasmodium vivax prevalence
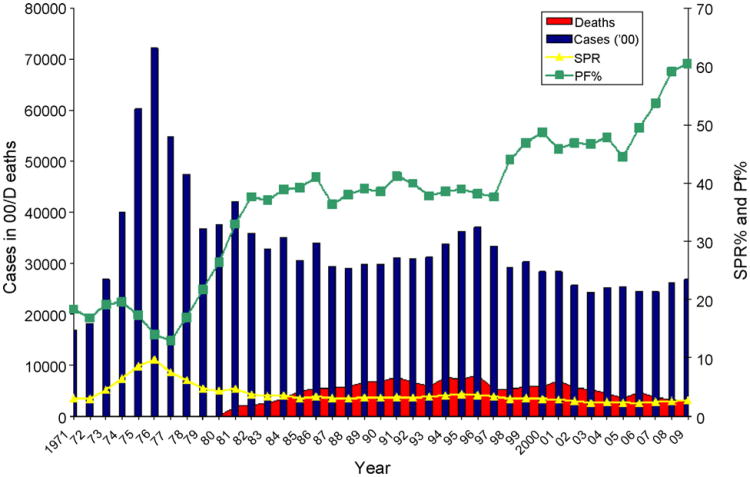
The relative ratio of P. vivax and P. falciparum varies greatly in different parts of India and often within states. Most of the Indo-Gangetic plains, northern hilly states, northwestern India and the southern Tamil Nadu state have <10% P. falciparum infections with the rest being P. vivax. The states sustaining hyperendemic malaria and the highest ratio of P. falciparum tend to be inhabited by ancient ethnic tribes mainly in the forest ecosystems (30–90% P. falciparum). Nearby fringe areas can be meso to hyper endemic, but still with a preponderance of P. falciparum (90% or even more). Other localized areas in the same state can have as low as 10 to 30% P. falciparum (Kumar et al., 2007). The representation of P. falciparum over P. vivax has been increasing in India over the last 30 years to about 60% (Fig. 4, green symbols).
Fig. 4.
Changes in malaria prevalence in the South East Asia Region as reported by the member countries to WHO from 1971 to 2009. In this region, after resurgence of malaria in the mid seventies in the post eradication era, reported incidence of malaria has stabilized as seen in the trends of API and SPR. SPR represents Slide Positivity Rates (malaria incidences per 1000slides read). In contrast, P. falciparum proportion has consistently increased to reach 60% in recent years. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Data source: www.who.searo.int; WHO (2001a,b).
3.4. Absolute numbers
Today, of 1.2 billion people in India, 80.5% live in malaria risk areas. Of these, 4, 32 and 44% live in areas of high, moderate and low risk to malaria respectively (http://www.searo.who.int/). There are an estimated 1.5–2 million reported malaria cases every year in India and they are divided evenly between P. falciparum (Pf) and P. vivax (Pv) (Fig. 4) (Singh et al, 2009). This fact is important. In the global malaria research community, India is often thought to be a P. vivax dominated country. Overall, there may be over 1,000,000 cases of P. falciparum in India to match over 700,000 P. vivax cases. Studies place malaria-related economic losses at about US $0.5–1.0 billion annually.
The question of mortality from malaria has significant uncertainties. There has been recent high profile debate and discussion about malaria mortality in India (Dhingra et al, 2010; Basnyat, 2011; Deonarine, 2011; Kumar et al, 2011; Shah et al., 2011a,b; Sharma et al, 2011; Valecha et al., 2011). Verbal autopsy interviews suggest that malaria deaths in India around 2001–2003 may have been as high as 200,000 per year (Dhingra et al., 2010; Sharma et al., 2011). These numbers are an order of magnitude higher than recent WHO estimates, which are higher than Government estimates. Techniques underlying verbal autopsy evoke understandable skepticism (Basnyat, 2011; Deonarine, 2011; Shah et al., 2011a,b; Valecha et al., 2011). However, estimates based on certified death reports to the Government of India for roughly the same period (1997–1998) also suggest about 140,000 malaria deaths per year in India after factoring in very different reporting rates from different parts of India (Kumar et al, 2011). A special commission was convened in 2011 by the Government of India with the goal of understanding true malaria death rates in India.
According to the 2010 WHO report (released in 2011), the overall pattern of malaria cases may have decreased in recent years, but the death rates remain largely unchanged. In 2000, there were only 892 malaria deaths in India by WHO criteria, and they are now estimated at 1133 deaths in 2009 in India from 1.5 million reported cases for that year (WHO, 2011a,b).
3.5. Comparisons to neighboring countries
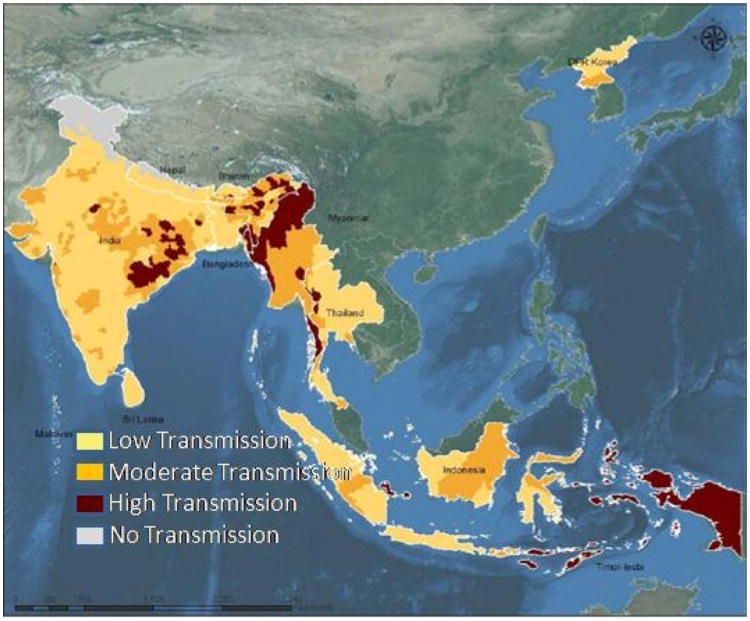
There are 107 countries and territories endemic to malaria in the world. The South East Asian Region of WHO is comprised of 11 countries, including Bangladesh, Bhutan, Democratic People's Republic of Korea, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, Thailand and Timor-Leste. Malaria intensity within most of these countries is highly variable (Fig. 5).
Fig. 5.
Malaria endemic zones of South Asia. Highest transmission is shown in rust, and low transmission in yellow. These WHO estimates are likely to undergo continual reevaluation as more internal research activities take place in these countries, as well as cross checks from global NIH ICEMR activities. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Source: www.who.searo.int; WHO (2001a,b).
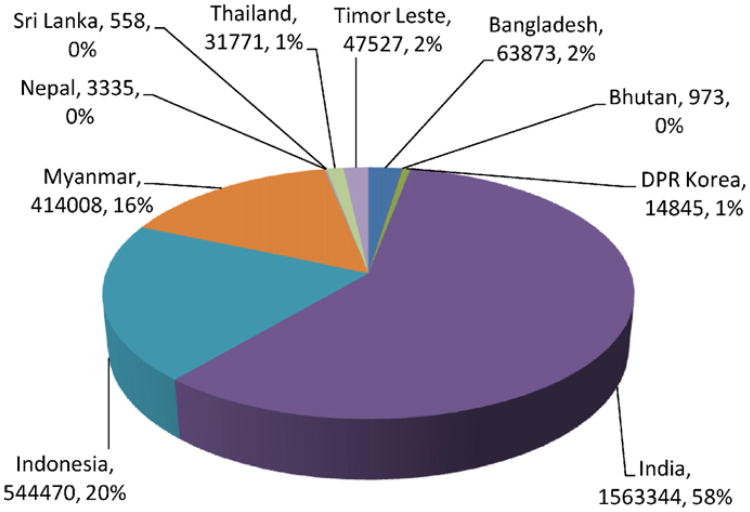
In the past, the South Asia/Southeast Asia region annually was estimated to contribute about 30% to the estimated 300–500 million global cases of malaria and about 8% of the global 1 million malaria deaths (Breman, 2001). By these WHO calculations, the estimated number of malaria cases in South Asian region are on the order of 90–167 million cases and estimated deaths are on the order of 125,000 per year. The majority of the malaria incidence in South East Asia is contributed by India followed by Indonesia, Myanmar, and Thailand (Fig. 6).
Fig. 6.
India was estimated to be the largest contributor of malaria to South and South East in 2009.
After the national malaria eradication campaigns of 1957–1959, malaria was negligible in the region with only 1037 reported cases and no deaths. During the resurgence phase in the 1960s and 1970s, malaria made a spectacular come-back in the region. At its peak, there were 7.2 million cases with API of 8.8, SPR of 9.7% and P. falciparum representing 14% of the cases. During the 1990s, the reported incidence stabilized to about 2–3 million malaria cases and the reported deaths attributable to malaria increased to 8061 in 1996. This increase in mortality corresponded to the gradual rise in P. falciparum to about 60% of malaria infections (Fig. 4). However, as discussed, the true burden of mortality may be higher than reported by the National Malaria Control Programs of the member countries.
In 2009, a total of 2.7 million cases of malaria were reported in South East Asia (source WHO). Of these, India alone contributed 1.6 million (58%) cases (Fig. 6). This was followed by Indonesia with 0.5 million cases (20%). Among the rest of the countries, Myanmar contributed 0.4 million (16%). The other countries together contributed to the remaining 6% of malaria.
4. Antimalarials in India and drug resistance
The Government of India regularly monitors antimalarial drug effectiveness and adjusts National Guidelines accordingly (National Institute of Malaria Research Publication, 2011). The following is an overview with respect to each recently used antimalarial approach.
4.1. Chloroquine
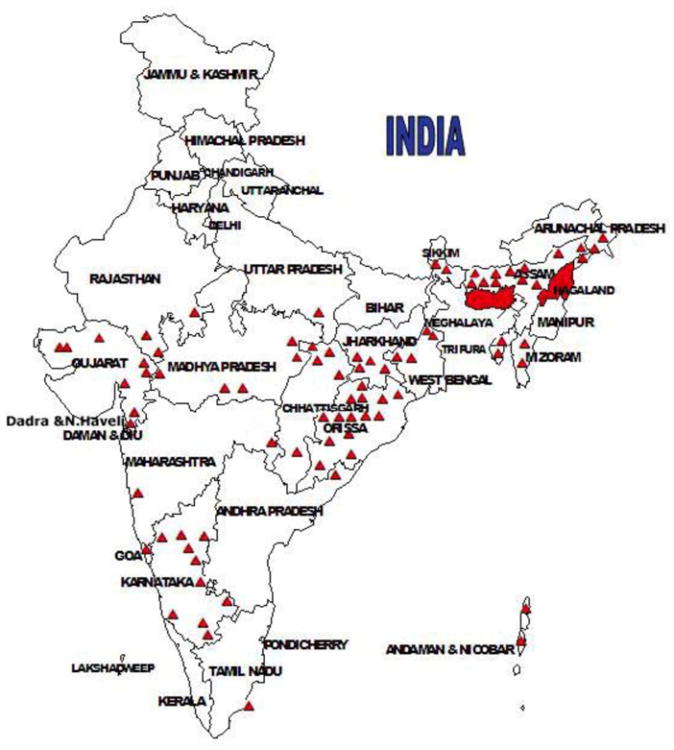
For decades chloroquine was the drug of choice in India, as it was in many parts of the world. However, chloroquine resistance against P. falciparum was reported early in Assam in 1973 (Sehgal et al., 1973; Pattanayak et al., 1979; Arora et al., 2008). There are country-wide reports of chloroquine resistance, especially from Orissa, the Northeastern states, Madhya Pradesh, and the Western states of Rajasthan and Gujarat (Misra et al, 1995; Sharma, 1996; Kumar et al., 2007; Valecha et al, 2009; Shah et al., 2011a,b). Today chloroquine-resistance against P. falciparum is common throughout India (Fig. 7) and chloroquine is not to be prescribed for treating P. falciparum.
Fig. 7.
Distribution of confirmed chloroquine resistance sites (red triangles) in India based on data from the National Vector Borne Diseases Control Program, India; Shah et al. (2011a,b). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Source: http://nvbdcp.gov.in/DRUG.html.
Of course, chloroquine is also less desirable for other reasons. It is not gametocytocidal for P. falciparum and thus cannot block transmission. It is also ineffective as an antirelapse drug for P. vivax infections. Another major disturbing development is the possible reduced sensitivity of P. vivax to chloroquine, which still is the drug of choice for treating P. vivax in most parts of India (Garg et al., 1995; Dua et al., 1996; Singh, 2000; Kshirsagar et al., 2000). In spite of case reports from some parts of the country, systematic clinical studies close to some of these sites have not shown chloroquine resistance in P. vivax (Shah et al., 2011a,b). In the absence of good culture systems for P. vivax and due to limited well-controlled clinical trials, the jury is still out on whether there is common, widespread resistance to chloroquine in P. vivax populations in India
4.2. Sulphadoxine–pyrimethamine
This second-line drug combination of sulphadoxine– pyrimethamine is still surprisingly effective against P. falciparum, even though development of resistance is a constant worry. A series of single-step mutations in the codons 108, 51, 59 and 164 in the dihydrofolate reductase (DHFR) gene and in the codons 436, 437, 540, 581 and 623 in the dihydropteroate synthase (DPHS) can confer resistance in P. falciparum. In India, although DHFR mutations have been reported, there is limited population-adjusted clinical data on sulphadoxine-pyrimethamine (SP) resistance. Results from 26 studies carried out between 1978 and 2007 indicate resistance in North Eastern region especially at the Indo–Myanmar border in Arunachal Pradesh (Shah et al., 2011a,b).
4.3. Quinine
In India, this old standby drug continues to be effective, but is reserved for treating complicated malaria. It produces many side effects with oral or parenteral use. Quinine use requires close patient monitoring over a long duration of therapy, if used alone. Mefloquine, although effective to treat multidrug resistant falciparum malaria in India, has a long duration of exposure in the blood, which some believe makes it predisposed to development of resistance.
4.4. Endoperoxides
The artemisinin group of drugs are highly effective. However, with possible weakened effectiveness, recrudescence is now a common phenomenon (WHO, 2007a,b). Dose schedules with the endoperoxides are determined empirical, safety in pregnancy is still being debated, and there is also concern about neurological side effects. Based on WHO recommendation, most of the countries have banned monotherapy with artemisinin. The national standards recommend ACT (Artemisinin-based Combination Therapy) with SP as a common partner-drug to treat P. falciparum infection (National Institute of Malaria Research Publication, 2011). ACTs present new prospects for avoiding drug resistance and are recommended for the treatment of P. falciparum malaria (WHO, 2007a,b).
4.5. Primaquine
This is the only 8-aminoquinoline available for gametocytocidal action in P. falciparum and for antirelapse activity in P. vivax. It is contra indicated in pregnancy and infants and there is at present no alternative therapy available. As per the current drug policy of India, primaquine is prescribed for 14 days in P. vivax for antirelapse treatment. However, patient compliance to this long regimen cannot be assured especially in the field where it is dispensed by health workers.
4.6. Antimicrobials
The malaria parasite has subcellular organelles like mitochondria and apicoplast, which are of protist origin and are sensitive to some classic antimicrobials. A combination of parenteral quinine and doxycycline is used for severe malaria, but not in pregnant women or children under 8. Instead, clindamycin is recommended.
5. Disease severity
5.1. Disease classifications
Severe malaria is not a single syndrome. In one approach, the disease has been divided into three major syndromes: cerebral malaria, severe malaria anemia, and respiratory distress (Miller et al., 2002). A number of factors, host and parasite related, influence malaria severity including host age and acquired immunity (Marsh, 1992). In regions of high malaria transmission, children bear the brunt of malaria morbidity and mortality. In Africa, the mean age of children with severe malaria anemia is always lower than those with cerebral malaria (Miller et al., 2002). By comparison, non-immune adults can suffer any of the three major syndromes, are more susceptible to severe respiratory complications than children and frequently experience multi-organ failure, which is not observed in children.
5.2. P. falciparum complications and increasing mortality
In India, the incidence of severe disease may differ across the country. For example, between 1995 and 2001, mortality from complicated P. falciparum malaria in Vellore in the southern state of Tamil Nadu was 7.9%, while in Jabalpur (Madhya Pradesh, Central India) and Rourkela (Orissa, Eastern India) it was 25.6 and 30% respectively (Shukla et al., 1995; Harris et al., 2001). In Central India, 8.5% (152/1783) admitted in a tertiary care hospital with complicated P. falciparum infections had cerebral malaria (CM) (Shukla et al., 1995). Of the 152 CM patients, 39 (25.6%) eventually died. A majority of them were in the 16–40 years age group and had hyper-parasitaemia and hypoglycemia. Delayed diagnosis and comatose condition appeared to contribute to the high mortality rate in the patients.
Disease severity patterns even within particular parts of India may be shifting, possibly related to the introduction of drug-resistant parasite strains or increases in P. falciparum infections in historic P. vivax regions. For instance, in a tertiary care industrial hospital at Rourkela, the total number of patients admitted with complicated malaria significantly increased from 14% (62/431) in 1995–1997 to 24% (236/996) admitted with complicated malaria in 2000-2002. Similarly, cases of acute renal failures doubled from 22% (47/369) to 44% (117/265) and deaths in patients without renal involvement also increased from 13% (47/369) to 17% (119/731) among these complicated cases (Kumar et al, 2007). A general shift in the clinical profile in patients with complicated malaria has been suggested and multiple organ dysfunction/failure is reportedly becoming a common feature. For example, in a tertiary care hospital in Cuttack, only 11% (96/879) of cases admitted were without complications, while 382 (43%) had either cerebral or renal or hepatic involvement, 298 (34%) had cerebral malaria with either renal or hepatic involvement and 103 (12%) had multi organ failure. About 18% (138/783) died due to malaria (Dr. B.K.Das: Personal communication). Such percentages are not uncommon at many tertiary care centers across India.
5.3. P. vivax severity
Complications due to the “benign species” P. vivax have been reported from Bikaner, India and also from other sites in recent years (Valecha et al., 1992; Patial et al., 1998; Beg et al, 2002; Kochar et al., 2009). In Bikaner, Rajasthan a study was carried out from September 2003 to December 2005, in 1091 patients admitted with malaria (Kochar et al., 2009). Of these, 635 (58%) were P. falciparum and 456 (42%) were P. vivax mono infection confirmed with peripheral blood smear, rapid test and PCR. Out of the P. vivax patients, there were 40 (8.7%) with severe manifestations in the age range of 18–60 years (mean ± SD = 29 ± 11 years). The severe manifestations were present in patients either alone or in different combinations. Hepatic dysfunction and jaundice was found in 23 (57.5%), renal dysfunction in 18 (45%), severe anemia in 13 (32.5%), cerebral malaria in 5 (12.5%), acute respiratory distress syndrome (ARDS) in 4 (10%), shock in 3 (7.5%), hypoglycaemia in 1 (2.5%), thrombocytopaenia in 5 (12.5%) and multiorgan dysfunction in 19 (47.5%).
A case of severe P. vivax in Goa for the first time showed histopathological evidence for parasite presence in newly effected organs (Valecha et al., 2009). With increasing careful documentation of severe P. vivax cases not only from India but also from Papua in Indonesia, Papua New Guinea and Brazil, severe P. vivax is being increasingly accepted as a manifestation of human malaria (Basat and Alonso, 2011)
6. Mosquito vectors in India
6.1. Mosquito species
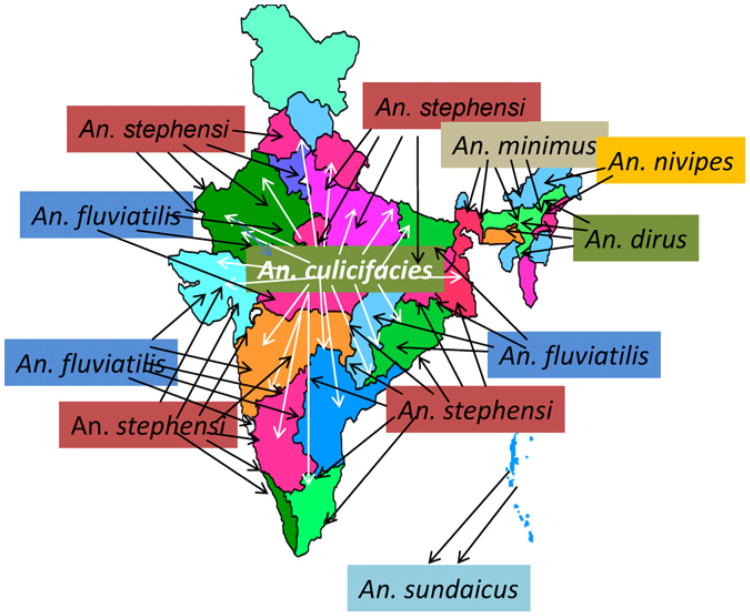
India reports a wide distribution of 9 anopheline vectors transmitting 3 Plasmodial species, P. falciparum, P. vivax and Plasmodium malariae (Kumar et al, 2007). Anopheles culicifacies is the principal vector of rural malaria and is widely distributed. Anopheles stephensi, on the other hand, is primarily a vector in urban areas. Anopheles fluviatilis is a vector in the hills and foothills while Anopheles minimus, Anopheles nivipes and Anopheles dirus are vectors of the northeastern states (Fig. 8). Anopheles sundaicus is restricted to the Andaman and Car Nicobar islands. Besides these, Anopheles annularis and Anopheles varuna are considered secondary vectors with wide distribution (Nagpal and Sharma, 1994). It is suspected that An. annularis plays a key role in malaria transmission in Jharkhand, Orissa, West Bengal and Chhattisgarh states (Rao, 1983).
Fig. 8.
General distribution of primary vectors of malaria in India. An. cuclicifacies has wide distribution in rural plains, An. stephensi is an urban vector, An. fluviatilis is a vector in hills and foothills in the mainland, while An. minimus, An. nivipes and An. dirus are important vectors in the North Eastern India. An. sundaicus is restricted to the Andaman and Car Nicobar Islands. An. annularis (not shown in the figure) is suspected to play an important role in malaria transmission in the Eastern Indian states of Jharkhand and Orissa.
6.2. Transmission rates
A careful epidemiologic survey of malaria transmission was carried out in a hyperendemic area of Sundargarh District, Orissa, India. It involved 13 tribal villages within a short distance, some in forests and some in the plains (Sharma et al., 2004, 2006). Malaria transmission was perennial in both areas, dominated by P. falciparum (85%). However, the number of P. falciparum cases per 1000 individuals per year was 284 in forests and 31 in the plains. The parasite rate was 14% and 1.7% respectively. The entomological inoculation rate (EIR) was 0.311 in the forest and 0.014 in the plains. The intense transmission in forest areas was attributed to the highly anthropophagic vector An. fluviatilis sibling species S, complemented by An. culicifacies sibling species C. In plains, malaria transmission was by An. culicifacies sibling species C. In forest villages, as seen in hyperendemic parts of Africa, clinical malaria occurred more frequently in children between the ages of 0–5 years and declined gradually with age, presumably as immunity built up.
In Goa, Korgaonkar et al. (in press) carried out all night mosquito landing collections in rural and urban parts of the state, and out of the 4191 mosquitoes, 5 genera and 23 species collected: 10 species belonged to Anopheles, 8 to Culex, 3 to Aedes and 1 each to Mansonia and Armigeres. A total of 11 vector species pursued human hosts, including 55 (1.3%) An. stephensi mosquitoes in urban areas. Two of these 55 An. stephensi were evaluated for Circumsporozoite Protein (CSP) by ELISA and were found positive for P. falciparum sporozoites.
6.3. Mosquito control
Not surprising, many vectors have become resistant to insecticides like DDT, HCH/Dieldrin, Malathion, and Fenitrothion. Some vectors even show low sensitivity to the latest class of synthetic pyrethroid compounds. Some of these insecticides have been banned in the last three decades and the replacement insecticides are expensive. Some vector species like An. dirus, An. fluviatilis, and An. minimus are exophilic and exophagic and avoid sprayed surfaces. In urban situations, Indoor Residual Spraying (IRS) is not practical and in rural situations, there are operational constraints and resource limitations, which restrict coverage of target populations with IRS. Thus, IRS cannot be relied upon as a principal vector control tool, although it still has an important role to play as a part of a larger public health campaign. There is a growing argument in favor of re-introduction of DDT in areas where it was withdrawn many years ago and where, as in South Africa and Solomon island, vectors may still be controlled with DDT (Breman et al., 2004; Dash et al., 2007). The low cost of DDT is attractive, if food chain contamination can be avoided in a public health setting (Curtis and Townson, 1998). In the recent years, there has been increasing reliance on LLINs especially in stable malaria zones with the assistance of the World Bank or Global Funds for Aids Tuberculosis and Malaria (GFATM). Additionally, some high malaria burden states have also procured 1 million LLINs with internal resource mobilization.
7. Migrant workers in South Asia
In India, one potentially important factor in the spread of P. falciparum is migrant workers. The common press (TNN, 2011; Sharma, 2011), as well as the scientific community and scientific publications (Dash et al., 2008), refer to human migrations in South Asia as the cause of emerging virulence in South Asian malaria. Specifically, the booming economies of certain cities in South Asia attract migrant workers from poorer states. While it may be that the majority of cases of severe malaria in government hospitals are from migrant laborers, the causes of this representation and its consequences on malaria public health requires careful examination.
Malaria linked with migration of population is a complex phenomenon. Human populations move for (i) economic activity e.g. labor work in projects, gem/met al mining, agriculture, (ii) under distress due to famines, floods, earthquakes, conflicts, or (iii) for tourism/recreation (Prothero, 1977). Such migration may lead to permanent change of residence or there may be temporary change of residence after which the individuals return to their location of origin (termed, circulation). All these patterns can influence local malaria epidemiology, including transmission and its seasonality. In South Asia, movements on forest fringes are responsible for ‘forest malaria’ (Sharma and Konrachine, 1991). Refugee movements have played a significant role in the spread of drug resistant P. falciparum malaria in South East Asia since 1970s, particularly during war in Vietnam and Cambodia and recent unstable political conditions on the Thai–Myanmar border (Verdrager, 1986).
South Asia is potentially one of the most dynamic regions in terms of population movement that promotes malaria transmission. Migrant malaria is virtually invisible to organized disease control measures. The inter-country boundaries are porous and traditional ethnic people cross national boundaries freely to interact with fellow tribes across borders. Many of these areas are forested and roads are poorly maintained so official authorities have little control over local events including provision of health care. Of particular interest is the vast “corridor” running through Myanmar, Thailand, Cambodia, Laos, and Vietnam to the Yunnan province of China. This is notorious for trading of legal as well as illegal commodities. In these areas, An. dirus, An. minimus and An. maculatus transmit malaria outdoors as these species are both exophilic and exophagic and not amenable to classic vector control measures. This makes for a difficult situation with respect to malaria control.
There are three identified risk factors related to population movements which exacerbate malaria in South and South East Asia: (1) those which increase malaria transmission, (2) those which predispose communities to severe and complicated malaria and (3) those which promote transmission of drug-resistant parasite strains. From a control perspective, migration malaria is particularly challenging because migrants generally are thought to remain out of the ambit of organized health services, they are hard to track and monitor, and they generally do not have the resources or access to comply with control strategy of the country (e.g. use of ITNs, choice of antimalarial drugs or drug regimens). The availability of spurious/fake drugs further complicates the scenario. The social causes of why some isolated communities do not access or are not served by mainstream public health efforts remains to be fully studied.
8. Malaria management in South Asia
8.1. Control infrastructure in India
There are a number of well-structured National Disease Control and Elimination programs implemented by local governments under the umbrella of the recently created National Rural Health Mission (NRHM). They follow technical and operational guidelines of the National Vector Borne Disease Control Program (NVBDCP) of the Government of India for malaria and for other vector borne diseases.
In the government-funded health care system of India, there are three tiers:
A primary health care system having a network of primary health centers and sub-centers in rural areas, where roughly 70% of Indians live. In cities, the equivalent are urban health centers and urban health posts or dispensaries, which function under municipal councils and corporations.
District hospitals for secondary care.
Medical colleges and government hospitals for tertiary care.
Among private health care providers, there are general practitioners, hospitals and polyclinics that are professionally managed. Many individuals go to traditional healers in their communities, especially before malaria symptoms become too serious. Tertiary care hospitals are also operated by large public sector industrial units and through support from large private sector industry who maintain hospitals for their employees and local communities (Kumar et al., 2007).
NVBDCP goals are implemented, in part, through the primary health care system with the assistance of “multi-purpose workers” who operate at the grassroots level. In areas with poor access, fever treatment depots (FTDs) provide care and treatment at the community level. Early detection and complete treatment, selective vector control, and behavioral-change communication are the key components of the current malaria control strategy of the NVBDCP.
8.2. Strategic approaches to control malaria
The South Asia region is initiating efforts to help meet Millennium Development Goals (MDGs; http://www.un.org/millenniumgoals/aids.shtml) for halting malaria morbidity and mortality by the year 2015. Such an approach must address many significant technical, economic and administrative realities.
In this paragraph, we highlight some important issues and initiatives. In India, as in other countries where malaria continues to pose a major challenge, there is a need to estimate the true burden of malaria. This should help with thoughtful establishment of priorities for health planning, allocation of existing resources for malaria control, and advocacy for additional resources. Disease control programs have massive data gathering and dissemination challenges. Spatial and temporal maps to track populations at risk for malaria transmission at the country, sub-country and local levels would be of great value. Selective vector control approaches involving ITNs/LLINs coverage, focal IRS and environmental measures (such as biological Larvicides), and even selective use of DDT, may potentiate gains from other control efforts. P. falciparum dominant regions that are generally inaccessible need better ITN/LLINs and rapid spot diagnosis, followed by complete treatment to contain transmission. Advocacy at the country level must press for strict regulatory measures and pharmaco-vigilance to check counterfeit drug trade. The WHO has done well by leading campaigns to ban the use of artesunate monotherapy to thwart development of widespread resistance to artemisinin-based antimalarials. Now similar international efforts may be needed to decrease the spread of fake drugs. Finally, globally and in South Asia in particular, there is a need to balance attention between P. falciparum and P. vivax malaria. The latter accounted for 40% of the total malaria cases in 2009. This could become a large crisis, given the potential for chloroquine resistance in P. vivax and recent reports of complicated malaria and mortality from this species (Basat and Alonso, 2011; Valecha et al., 2009).
Malaria elimination
Global eradication of malaria is once again under active discussion, thanks to policy goals from Melinda and Bill Gates in October 2007, followed by WHO Director-General Margaret Chan's quick and positive response to this possibility and challenge. WHO published an updated field manual on elimination of malaria for low and moderately endemic countries (WHO, 2007a,b). There are 99 such endemic countries in the world. Of these, 32 are currently pursuing an elimination strategy and the remaining 67 are controlling malaria (Das and Horton, 2010). A malaria elimination group has been constituted with 55 active researchers from all over the world. A guide on malaria elimination for policy makers and a prospectus of malaria elimination have been published (Feachem, 2009; Feachem et al., 2009). In the Asia Pacific region, 11 countries (Sri Lanka, Bhutan, Indonesia, Thailand, the Democratic People's Republic of Korea, China, Republic of Korea, Vanuatu, Malaysia, The Solomon Islands and Philippines) are members of the Asia Pacific Malaria Elimination Network (APMEN) and are currently pursuing accelerated malaria control with a final aim to eliminate malaria from these countries. ‘The mission of this diverse but cohesive Network is to collaboratively address the unique challenges of malaria elimination in the region through leadership, advocacy, capacity building, knowledge exchange, and building the evidence base’ (www.apmen.org). The National Vector Borne Disease Control Program in India is also contemplating formulation of a nationwide elimination program during the 12th five year plan period from 2012 to 2017. For a country as diverse as India, with complex malaria epidemiology and massive challenges for management, the elimination agenda is expected to be extremely demanding and an extended battle is to be expected to achieve true elimination.
9. Summary
Overall, while much is known about malaria in South Asia, increased epidemiological, clinical, and basic science studies will be necessary to achieve and sustain global reduction of malaria morbidity and mortality. These are mandated in Millennium Development Goals.
Acknowledgments
The Program Project on “Malaria Evolution in South Asia” is an International Center of Excellence for Malaria Research (ICEMR) supported by the US National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant U19 AI089688. The authors are thankful to our NIMR and WHO colleagues for sharing policy publications, which have been mentioned in this review on malaria.
Abbreviations
- ABER
Annual Blood Examination Rate
- API
Annual Parasite Incidence
- ICEMR
International Center of Excellence for Malaria Research
- IRS
Indoor Residual Spraying
- LLINs
Long Lasting Insecticide Treated Nets
- NVBDCP
National Vector Borne Disease Control Program
References
- Basat Q, Alonso PL. Fathoming severe Plasmodium vivax disease. Nat Med. 2011;17:48–49. doi: 10.1038/nm0111-48. [DOI] [PubMed] [Google Scholar]
- Basnyat B. Malaria-attributed death rates in India. The Lancet. 2011;377:993. doi: 10.1016/S0140-6736(11)60381-4. [DOI] [PubMed] [Google Scholar]
- Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA., Jr Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002;67:230–232. doi: 10.4269/ajtmh.2002.67.230. [DOI] [PubMed] [Google Scholar]
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- Curtis CF, Townson H. Malaria: existing methods of vector control and molecular entomology. Br Med Bull. 1998;54:311–325. doi: 10.1093/oxfordjournals.bmb.a011690. [DOI] [PubMed] [Google Scholar]
- Das P, Horton R. Malaria elimination: worthy, challenging, and just possible. The Lancet. 2010 doi: 10.1016/S0140-6736(10)61551-6. Published online 29, 2010. [DOI] [PubMed] [Google Scholar]
- Dash AP, Adak T, Raghavendra K, Singh OP. The biology and control of malaria vectors in India. Curr Sci. 2007;92:1571–1578. [Google Scholar]
- Dash AP, Valecha N, Anvikar AR, Kumar A. Malaria in India: challenges and opportunities. J Biosci. 2008;33:583–592. doi: 10.1007/s12038-008-0076-x. [DOI] [PubMed] [Google Scholar]
- Deonarine A. Malaria-attributed death rates in India. The Lancet. 2011;377:993–994. doi: 10.1016/S0140-6736(11)60382-6. [DOI] [PubMed] [Google Scholar]
- Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, Bassani DG, Suraweera W, Laxminarayan R, Peto R. Adult and child malaria mortality in India: a nationally representative mortality survey. The Lancet. 2010;376:1768–1774. doi: 10.1016/S0140-6736(10)60831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua VK, Kar PK, Sharma VP. Chloroquine resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996;1:816–819. doi: 10.1111/j.1365-3156.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Feachem RGA, Phillips AA, Targett GA. Shrinking the Malaria Map: A Prospectus on Malaria Elimination. The Global Health Group, UCSF; San Francisco, CA: 2009. pp. 1–185. [Google Scholar]
- Feachem RGA. Shrinking the Malaria Map: A Guide on Malaria Elimination for Policy Makers. 2009:1–66. [Google Scholar]
- Garg M, Gopinathan N, Bodhe P, Kshirsagar NA. P. Vivax malaria resistant to chloroquine: case reports from Bombay. Trans R Soc Trop Med Hyg. 1995;89:656–657. doi: 10.1016/0035-9203(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Harris VK, Richard VS, Mathai E, Sitaram U, Kumar KV, Cherian AM, Amelia SM, Anand G. A study on clinical profile of falciparum malaria in a tertiary care hospital in South India. Indian J Malariol. 2001;38:19–24. [PubMed] [Google Scholar]
- Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in Northwestern India. Am J Trop Med Hyg. 2009;80(2):194–198. [PubMed] [Google Scholar]
- Korgaonkar NS, Kumar A, Yadav RS, Kabadi D, Dash AP. Studies on mosquito biting activity on humans and detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Indian J Med Res. doi: 10.4103/0971-5916.93434. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshirsagar NA, Gogtay NJ, Rajgor D, Dalvi SS, Wakde M. An unusual case of multidrug-resistant Plasmodium vivax malaria in Mumbai (Bombay), India. Ann Trop Med Parasitol. 2000;94(2):189–190. doi: 10.1080/00034980057536. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dua VK, Rathod PK. Malaria-attributed death rates in India. The Lancet. 2011;377:991–992. doi: 10.1016/S0140-6736(11)60379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77:69–78. [PubMed] [Google Scholar]
- Marsh K. Malaria—a neglected disease? Parasitology. 1992;104(Suppl):S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Misra SP, Nandi J, Lal S. Chloroquine versus amodiaquine in the treatment of Plasmodium falciparum malaria in Northeast India. Indian J Med Res. 1995;102:119–123. [PubMed] [Google Scholar]
- Nagpal BN, Sharma VP. Indian Anophelines. Oxford & IBH. Co; 1994. [Google Scholar]
- Narayanasamy K, Chery L, Basu A, Duraisingh MT, Escalante A, Fowble J, Guler JL, Herricks T, Kumar A, Majumder PP, Maki J, Mascarenhas A, Rodrigues J, Roy B, Sen S, Shastri J, Smith J, Valecha N, White J, Rathod PK. Malaria evolution in south asia: knowledge for control and elimination. Acta Tropica. 2012;121:256–266. doi: 10.1016/j.actatropica.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Malaria Research (NIMR), National Vector Borne Disease Control Program (NVBDCP) Guidelines for Diagnosis and Treatment of Malaria in India. Government of India; 2011. [Google Scholar]
- Patial RK, Kapoor D, Mokta JK. Cerebral dysfunction in P. Vivax malaria: a case report. Indian J Med Sci. 1998;52:159–160. [PubMed] [Google Scholar]
- Pattanayak S, Roy RG, Phukan D, Barkakuty BN. Chloroquine resistance in P. falciparum in Assam State. Indian J Med Res. 1979;70(December (Suppl.)):14–19. [PubMed] [Google Scholar]
- Prothero R. Population movements and problems of malaria eradicationin Africa. Bull World Health Organ. 1977;24:405–425. [PMC free article] [PubMed] [Google Scholar]
- Rao M. The International Centres of Excellence for Malaria research. Acta Tropica. 2012;121:157. doi: 10.1016/j.actatropica.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Rao TR. The Anophelines of India. Malaria Research Centre, Indian Council of Medical Research; 1983. [Google Scholar]
- Sehgal PN, Sharma MID, Gogai S. Resistance to chloroquine in falciparum malaria in Assam state, India. J Comm Dis. 1973;5:175–180. [Google Scholar]
- Shah NK, Dhillon GP, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011a;11(1):57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NK, Dhariwal AC, Sonal GS, Gunasekar A, Dye C, Cibulskis R. Malaria-attributed death rates in India. The Lancet. 2011b;377:991. doi: 10.1016/S0140-6736(11)60378-4. [DOI] [PubMed] [Google Scholar]
- Sharma P. 2011. Malaria cases rise in Mumbai, BMC blames migrants. DNA. 2011 May 24; [Google Scholar]
- Sharma SK, Chattopadhyay R, Chakrabarti K, Pati SS, Srivastava VK, Tyagi PK, Mahanty S, Misra SK, Adak T, Das BS, Chitnis CE. Epidemiology of malaria transmission and development of natural immunity in a malaria-endemic village, San Dulakudar, in Orissa state, India. Am J Trop Med Hyg. 2004;71:457–465. [PubMed] [Google Scholar]
- Sharma SK, Tyagi PK, Padhan K, Upadhyay AK, Haque MA, Nanda N, Joshi H, Biswas S, Adak T, Das BS, Chauhan VS, Chitnis CE, Subbarao SK. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh District, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100:917–925. doi: 10.1016/j.trstmh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- Sharma VP, Konrachine AV. Forest malaria in South East Asia. Malaria Research Centre; New Delhi: 1991. [Google Scholar]
- Sharma VP, Jha P, Dhingra N, Jotkar RM, Peto R. Malaria-attributed death rates in India. The Lancet. 2011;377:994–995. [Google Scholar]
- Shukla MM, Singh N, Singh MP, Tejwani BM, Srivastava DK, Sharma VP. Cerebral malaria in Jabalpur, India. Indian J Malariol. 1995;32:70–75. [PubMed] [Google Scholar]
- Singh RK. Emergence of chloroquine-resistant vivax malaria in south Bihar (India) Trans R Soc Trop Med Hyg. 2000;94:327. [PubMed] [Google Scholar]
- Singh V, Mishra N, Awasthi G, Dash AP, Das A. Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009;25:452–457. doi: 10.1016/j.pt.2009.06.004. [DOI] [PubMed] [Google Scholar]
- TNN. 2011. Migrant workers under AMC's scanner for malaria prevention. Times of India. 2011 Jul 27; [Google Scholar]
- Valecha N, Bagga A, Chandra J, Sharma D. Cerebral symptoms with P. vivax malaria. Indian Pediatr. 1992;29:1176–1178. [PubMed] [Google Scholar]
- Valecha N, Pinto RG, Turner GD, Kumar A, Rodrigues S, Dubhashi NG, Rodrigues E, Banaulikar SS, Singh R, Dash AP, Baird JK. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg. 2009;81:758–762. doi: 10.4269/ajtmh.2009.09-0348. [DOI] [PubMed] [Google Scholar]
- Valecha N, Staedke S, Filler S, Mpimbaza A, Greenwood B, Chandramohan D. Malaria-attributed death rates in India. The Lancet. 2011;377:992–993. doi: 10.1016/S0140-6736(11)60380-2. [DOI] [PubMed] [Google Scholar]
- Verdrager J. Epidemiology of the emergence and spread of drug-resistant falciparum malaria in South-East Asia and Australia. J Trop Med Hyg. 1986;89:277–289. [PubMed] [Google Scholar]
- World Health Organization. Commission on Macroeconomics and Health (2001) 2001a http://www.whoindia.org/LinkFiles/Commision_on_Macroeconomic_and_Health_Executive_Summary.pdf.
- World Health Organization. The World Health Report 2001. Geneva, Switzerland: 2001b. [Google Scholar]
- World Health Organization. Malaria Elimination: A Field Manual for Low and Moderate Endemic Countries. Geneva, Switzerland: 2007a. [Google Scholar]
- World Health Organization. Revised Malaria Control Strategy: Focusing on a New Paradigm. Regional Committee Document No SEA/RC60/9 (Rev) 2007b [Google Scholar]
- World Health Organization. World Malaria Report 2010. Geneva, Switzerland: 2011a. [Google Scholar]
- World Health Organization. Eliminating Malaria: Learning from the Past, Looking Ahead. Geneva, Switzerland: 2011b. [Google Scholar]