Abstract
Mitochondria are one of the major sources of reactive oxygen species (ROS) in the cell. When exceeding the capacity of antioxidant mechanisms, ROS production may lead to different pathologies, such as ischemia-reperfusion injury, neurodegeneration, anemia and ageing. As a consequence of the endosymbiotic origin of mitochondria, eukaryotic cells have developed different transport mechanisms that coordinate mitochondrial function with other cellular compartments. Four mitochondrial ATP-binding cassette (ABC) transporters have been described to date in mammals: ABCB6, ABCB8, ABCB7 and ABCB10. ABCB10 is located in the inner mitochondrial membrane forming homodimers, with the ATP binding domain facing the mitochondrial matrix. ABCB10 expression is highly induced during erythroid differentiation and its overexpression increases hemoglobin synthesis in erythroid cells. However, ABCB10 is also expressed in nonerythroid tissues, suggesting a role not directly related to hemoglobin synthesis. Recent evidence points toward ABCB10 as an important player in the protection from oxidative stress in mammals. In this regard, ABCB10 is required for normal erythropoiesis and cardiac recovery after ischemia-reperfusion, processes intimately related to mitochondrial ROS generation. Here, we review the current knowledge on mitochondrial ABC transporters and ABCB10 and discuss the potential mechanisms by which ABCB10 and its transport activity may regulate oxidative stress. We discuss ABCB10 as a potential therapeutic target for diseases in which increased mitochondrial ROS production and oxidative stress play a major role.
Keywords: mitochondria, oxidative stress, ABCB10, ABC-me, erythropoiesis, ischemia-reperfusion
1. INTRODUCTION
Mitochondrial function is associated with reactive oxygen species (ROS) generation. Different antioxidant enzymes (i.e. superoxide dismutase 2, glutathione peroxidases, peroxiredoxin, thioredoxin reductase, etc.) are located in the mitochondria where they maintain ROS below toxic levels. In addition, ROS generation inside the mitochondria in the form of hydrogen peroxide can diffuse to the cytosol and damage other subcellular compartments. Consistent with this, overexpression of catalase targeted inside the mitochondrial matrix was demonstrated to have protective effects in the entire cell [20]. In contrast, less is known about the molecular mechanisms allowing an integrated and coordinated antioxidant response of the mitochondria with the rest of the cell under conditions of increased oxidative stress.
As a consequence of the endosymbiotic origin of mitochondria, eukaryotic cells have developed different transport mechanisms in order to coordinate mitochondrial function with other cellular compartments. Therefore, it is expected that mitochondrial importers and exporters will be essential to coordinate, integrate and maintain a general antioxidant response. Recent data unravel novel mechanisms involved in protection from oxidative stress, as they demonstrate that the mitochondrial ATP binding cassette transporter ABCB10 is required for an efficient general antioxidant response in vivo [38,53]. ABCB10 was originally identified as ABC-me (ATP binding cassettemitochondrial erythroid) in mouse and M-ABC2 in humans [76,88]. ABCB10 is the closest mammalian orthologue to yeast MDL1 and to C. elegans haf-3. ABCB10 is located in the inner mitochondrial membrane, forming homodimers, and its overexpression increases hemoglobin synthesis [29, 76]. ABCB10 expression in nonerythroid tissues suggested a role not directly related to hemoglobin synthesis [53, 76]. Recent ABCB10 and MDL1 studies suggest that mitochondrial export (of yet unidentified molecules) can constitute a novel pathway to protect from oxidative stress in different cell types. In this review, we will discuss the current knowledge on ABCB10 in the context of mitochondrial ABC transporters of its subfamily.
2. STRUCTURE-FUNCTION OF ABC PROTEINS AND ABCB10
a) General features of ABC transporters
Although their functions are versatile, ABC transporters share quite conserved structures. Most of the findings discussed in this section have been described in other, nonmitochondrial, ABC proteins. Given that ABC proteins have highly conserved domains, we expect some similarities in the structure-function with ABCB10. ABC transporters can be formed by diverse numbers of polypeptide chains (from 1 to 5). However, these polypeptide chains always fold to form 2 trans-membrane domains (TMD), which span the biological membrane multiple times via α-helices and recognize the substrate/s, and 2 nucleotide binding domains (NBD), which bind and hydrolyze ATP to provide energy for the substrate transport [33]. ABCB10, as in the case of different ABC transporters, is formed by 2 polypeptide chains (2 hemitransporters or monomers), each of them harboring a TMD and a NBD (Figure 1A and C) [29]. While no reports on the crystal structure of human mitochondrial ABC proteins have been published so far, different mutagenesis studies, the characterization of their domains and crystallographic studies of other ABC proteins provide important insights on how ABCB10 might be functioning:
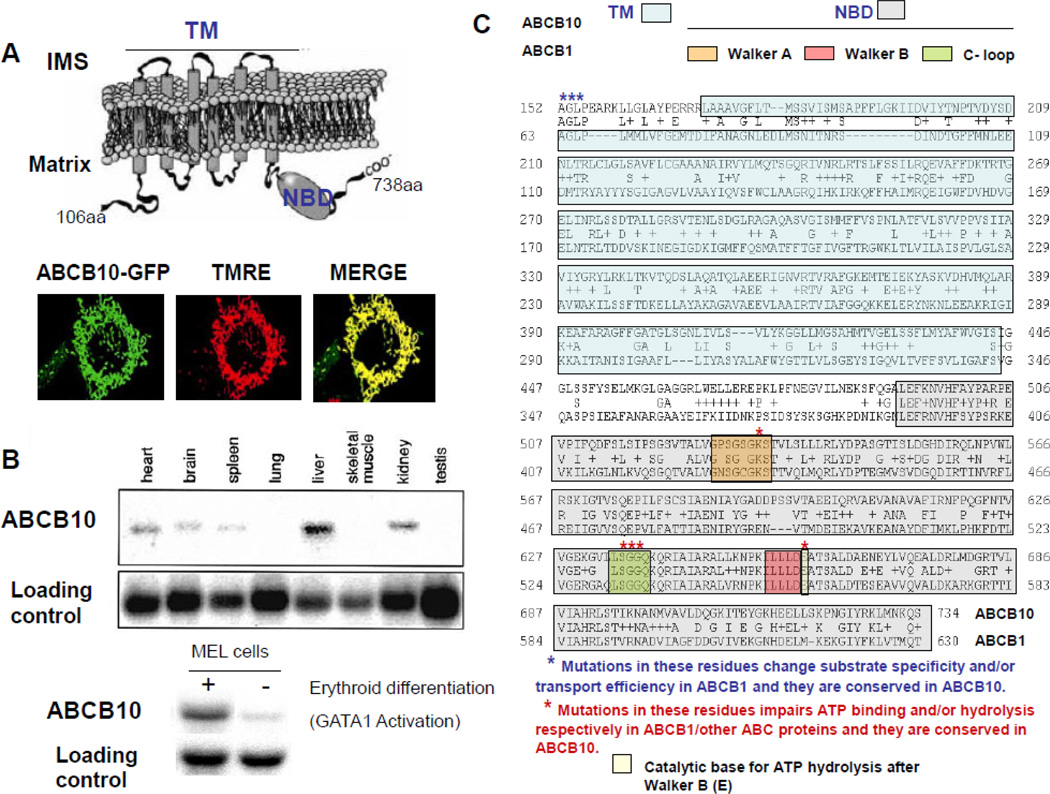
Figure 1. ABCB10 topology in the inner mitochondrial membrane and its functional domains.
A) Cartoon representing ABCB10 topology in the inner mitochondrial membrane. ABCB10 nucleotide binding domain NBD is facing the mitochondrial matrix and 6 transmembrane domains span the inner mitochondrial membrane. ABCB10 forms homodimers and not heterodimers. The location of the NBD, in the C-terminal portion of the protein, suggests that ABCB10 is exporting its substrate/s to the inner membrane space (IMS) (see arrow). The N-terminal part of the protein contains a long mitochondrial pretargeting sequence cleaved after insertion. Fluorescence microscope images of a mouse NIH-3T3 fibroblast expressing ABCB10-GFP (green) and stained with the mitochondrial membrane potential sensitive probe TMRE (red). Merged images show perfect colocalization of ABCB10-GFP with TMRE, demonstrating that ABCB10-GFP targets to mitochondria. B) ABCB10 expression in different mouse tissues measured by Northern blot. Liver, heart, brain and kidney show ABCB10 expression (highest levels in liver, kidney and heart). Differentiation of Mouse erythroleukemia cells (MEL) is associated with GATA-1 activation (master transcription factor regulating terminal eythroid differentiation and hemoglobin synthesis) and a large increase in ABCB10 expression measured by Northern blot. This figure was adapted from reference 76, Shirihai et al. (2000) EMBO Journal. C) Alignment of human ABCB10 (NP_036221) and human ABCB1/MDR1 (NP_000918.2) amino-acidic sequences using Blast P (37% identity covering a 78% of ABCB10 sequence). The transmembrane-TM- and nucleotide binding-NBD- domains are in light green and grey respectively. The Walker A, B and C-loop motifs are highlighted within the NBD in different colors. Residues conserved in ABCB10 that were mutated in functional studies of ABCB1 or other ABC proteins are marked with asterisks.
a.1) Transmembrane domain (TMD)
It is a relatively variable domain and this variability determines ABC transporters specificity to their substrates. For example, the multidrug resistance ABC transporter (MDR1/ABCB1) exports chemotherapeutic drugs through the plasma membrane [11]. The transporter associated with antigen presentation (TAP or ABCB2/ABCB3 heterodimer) transports antigenic peptides across the ER membrane to assemble the MHC complex for the immune response [62], and the cystic fibrosis transmembrane conductance regulator (CFTR or ABCC7) channels chloride and other anions out of epithelial cells [71]. In any specific ABC transporter, the variability in single amino-acids of the TMD determines the molecules transported and the throughput for each one of them. For example, previous studies have shown that single amino acid mutations in TMD alter CFTR/ABCC7 anion selectivity, changing its preference for the different anions (Br−, Cl−, I−, F−) [3]. In addition, similar alterations in the TMD of ABCB1 change substrate specificity [79] and inhibit drug resistance activity [81]. It will be of interest to test whether specific mutations in the TMD of ABCB10 can also change their substrate specificity and lead to different physiological consequences.
a.2) NBD (nucleotide binding domain)
In contrast to TMD, the NBD contains highly conserved residues in different ABC transporters, which form 3 characteristic motifs (in both mitochondrial and non-mitochondrial): the Walker A, the Walker B and the C-loop motifs (Figure 1C). This is mainly due to the fact that these residues bind and catalyze ATP hydrolysis to provide energy for transport. Most of the functional characterizations of the NBD were performed in non-mitochondrial ABC proteins and are summarized in this section. Given this high degree of conservation, we expect some similarities in the structure-function relation in ABCB10. The most conserved residues in the 3 motifs are the following: the Walker A motif is constituted by GXXGXGKS/T (where X is any amino acid), the Walker B motif by HHHHD (H is any hydrophobic amino acid) and the C-loop motif by LSGGQ. These 3 motifs were also identified in ABCB10 (Figure 1C) [76]. During a typical ABC-mediated transport process (as described in the crystal structure of a non-mitochondrial ABC protein, MalK, the ATPase subunit of the maltose ABC transporter from Escherichia coli), two ATP molecules are buried between the two monomers of the ABC transporter. Each ATP is positioned between the Walker A motif of one monomer and the C-loop motif of the other one. In this way, two NBDs form a dimeric sandwich with a head to tail orientation [13]. The lysine residue in the Walker A motif (Figure 1C) is conserved for hydrogen bond interaction with the β and γ phosphates of ATP and stabilization of the hydrolysis transition state during ATP hydrolysis [21]. A mutation of this residue results in almost complete loss of ATPase activity [61], which is due to impaired ATP binding by the NBD [48]. The conserved glutamate residue immediately following the Walker B motif is believed to be the catalytic base for ATP hydrolysis (Figure 1C). This negatively-charged residue polarizes a nearby water molecule to attack the γ phosphate, which releases free energy. This is confirmed by the finding that the mutation in the glutamate residue does not affect the binding of ATP by NBD but significantly reduces ATP hydrolysis [60,83]. The C-loop motif binds to ATP via the interaction of the serine and glycine residues with the γ phosphate. Mutation of these residues of the C-loop decreased ATP hydrolysis in CFTR (ABCC7), MDR1 (ABCB1) and TAP (ABCB2/ABCB3 heterodimer) [14,57,78]. With the stable association of ATP with the NBD maintained by the above conserved residues and domains, one cycle of ATP hydrolysis proceeds, producing the free energy required for substrate transport. One cycle is constituted by ATP hydrolysis, release of Pi and replacement of ADP with new ATP [34]. Although it is still not clear how ATP hydrolysis is related to substrate transport, a model suggests that the ATP hydrolysis at the catalytic site of NBD leads to alteration in the backbone conformation of the LSGGQ sequence constituting the C-loop motif. This alteration is propagated upstream to change the conformation of the substrate-bound TMD, by rotating the variable NBD helical domain that is close to the ATP binding pocket [42]. In this way, the substrate is released from TMD and is transported across the biological membranes. One could expect similar mechanisms in ABCB10. In addition, mutations in any of the conserved NBD residues in ABCB10 are also expected to abrogate its function. However, this needs to be confirmed by crystallographic and mutagenesis studies on ABCB10.
b) Structure-function of mitochondrial ABC transporters and ABCB10
ABCB10 is expected to show a similar structure-function relationship as described above for ABC proteins. As an example, ABCB10 (with an open reading frame of 715 amino acids) has an ~400 amino acid hydrophobic sequence that is believed to be the TMD and spans the mitochondrial inner-membrane six times (Figure 1A and C). After the TMD, there are ~240 amino acids that constitute the NBD, which contains the Walker A motif (GPSGSGKST), the C-loop motif (LSGGQ), and the Walker B motif (LLLDE) (Figure 1C). The NBD of ABCB10 is located at the mitochondrial matrix, suggesting that ABCB10 might transport substrates out of mitochondria through the mitochondrial inner-membrane [76]. In contrast to other ABC non-mitochondrial transporters, ABCB10 has a long mitochondrial targeting sequence (105 amino acids) that localizes ABCB10 into the mitochondrial inner-membrane (Figure 1A). Removal of these 105 amino acids targets ABCB10 to the endoplasmic reticulum [29]. On the other hand, the first 135 aa of ABCB10 can target any membrane protein to the mitochondria [59]. Of the first 105 amino-acids, the hydrophobic middle third (aa 36–70) is essential for the mitochondrial targeting, whereas 1–35 and 71–105aa are required for proper mitochondrial import and insertion of ABCB10 in the inner membrane (but not for mitochondrial targeting) [29]. After trafficking into the mitochondrial innermembrane, the leading sequence is then cleaved by enzymes inside the mitochondrial matrix [29]. This long pre-sequence might have been incorporated during endosymbiosis of the α-proteobacteria Rickettsia prowazekii (which is the probable ancestor of mitochondria [4]), in order to avoid mistargeting of mitochondrial ABC transporters to other cellular compartments or membranes (i.e. endoplasmic reticulum). Of note, there is no conservation of ABCB10 mitochondrial targeting sequence in the closest Rickettsia prowazekii orthologue, demonstrating that this long sequence is an acquired feature during endosymbiosis (ABCB10 identity starts at mouse amino acid 181, which corresponds to amino acid 75 of Rickettsia prowazekii Multidrug resistance protein). This feature was most likely acquired when Rickettsia ABCB10 orthologue was transferred to the host cell nuclear DNA to become ABCB10.
ABCB10 forms a homodimer by combining two hemi-transporters [29], as those shown in other ABC transporters [84]. The residues responsible for ABCB10 dimerization have not been indentified [29]. How this homodimerization contributes to ABCB10 transport activity is unknown. No ABCB10 structure-function studies (apart from the mitochondrial targeting studies discussed) have been performed yet. In addition, no ABCB10 mutants have been described yet in humans and it is unknown whether ABCB10 mutations are associated with pathological states in humans. However, studies performed on other mitochondrial ABC transporters and the study of their structure-function relationship may give some hints about ABCB10 transport activity. The yeast protein ATM1 (ATP binding cassette Transporter Mitochondria 1; see section 4b for a detailed description) has an N-terminal mitochondrial matrix-targeting signal and is localized in the mitochondrial inner-membrane, with a C-terminal NBD facing the matrix [51]. In a similar manner to other non-mitochondrial ABC transporters, the conserved lysine residue in Walker A motif of ATM1 is responsible for the ATP binding by NBD and the homodimerization of the two hemi-transporters. The mutation of the NBD lysine to methionine causes complete loss of ATM1 function [18]. ABCB7 is a mammalian orthologue of yeast ATM1 (see section 4b). Mutations in ABCB7 are related to a disease called X-linked sideroblastic anemia with ataxia (XLSA/A) in humans. The heritage family study shows that the amino acid change of E433K at the C-terminal of the sixth TMD [9,70] or V411L at the beginning of the sixth TMD in ABCB7 is sufficient to cause XLSA/A [56]. These mutations in the TMD would suggest an alteration in the substrate binding, specificity or transport efficiency of ABCB7 as a cause for this disease. On the other hand, there are no reports describing any XLSA/A patients harboring mutations in the conserved residues of the Walker A motif, the Walker B motif or the C-loop motif of the NBD. This lack of mutants could be related to a vital role of this protein in humans and to the likely possibility that mutations in these domains would completely abrogate ABCB7 function. One could expect similar patterns for ABCB10.
The potential substrates described so far for ABCB6 (another mitochondrial ABC transporter related to ABCB7, see section 4) are porphyrins, including heme, coproporphyrinogen III, and protoporphoryrinogen IX [47]. These molecules harbor anionic carboxylate side chains and tetrapyrrole moieties, which could be specifically recognized and bound by TMD of ABCB6. It would be of high interest to find out whether ABCB10 has a similar domain in order to know if it can transport similar molecules, given that both ABCB6 and ABCB10 are involved in heme synthesis (see sections 2 and 4) [47,76]. However, it is possible that ABCB6 might transport other molecules into the mitochondria (or other compartments), as ABCB6 deletion does not cause any defects in erythropoeisis in humans (see section 4) [32].
MDL1 is another mitochondrial ABC transporter in yeast [23] and has a relatively high degree of homology to ABCB10 compared to the rest of mitochondrial ABC transporters (43% identity, covering 78% of the aminoacidic sequence, see sections 2 and 4) (Figure 4). Similarly, its TMD spans the mitochondrial inner-membrane sixth times and its NBD is at the C-terminal residing in the mitochondrial matrix. In Saccharomyces cerevisiae, peptides harboring 6 to 20 amino acids (as a result of protein digestion products by m-AAA protease in the mitochondrial matrix) are exported by MDL1 [87]. Given that MDL1 has a high degree of homology to ABCB10, one may hypothesize that small peptides may also be the substrates of ABCB10. Thus, it would be interesting to study whether these peptides could bind by the uncharacterized substrate binding site in the TMD of ABCB10. In addition, the glutamate immediately after the Walker B motif in MDL1 NBD is the catalytic site for ATP hydrolysis, but this residue is dispensable for ATP binding (consistent with other ABC proteins) [35]. Moreover, mutations of other highly conserved residues, such as glycine in the Walker A motif, aspartate in the Walker B motif and serine in the C-loop motif decreases the release of peptides by MDL1 into the cytoplasm [87]. These data confirm that other mitochondrial ABC proteins have conserved structure-function characteristics present in non-mitochondrial ABC proteins, despite being in membranes with different phospholipid composition.
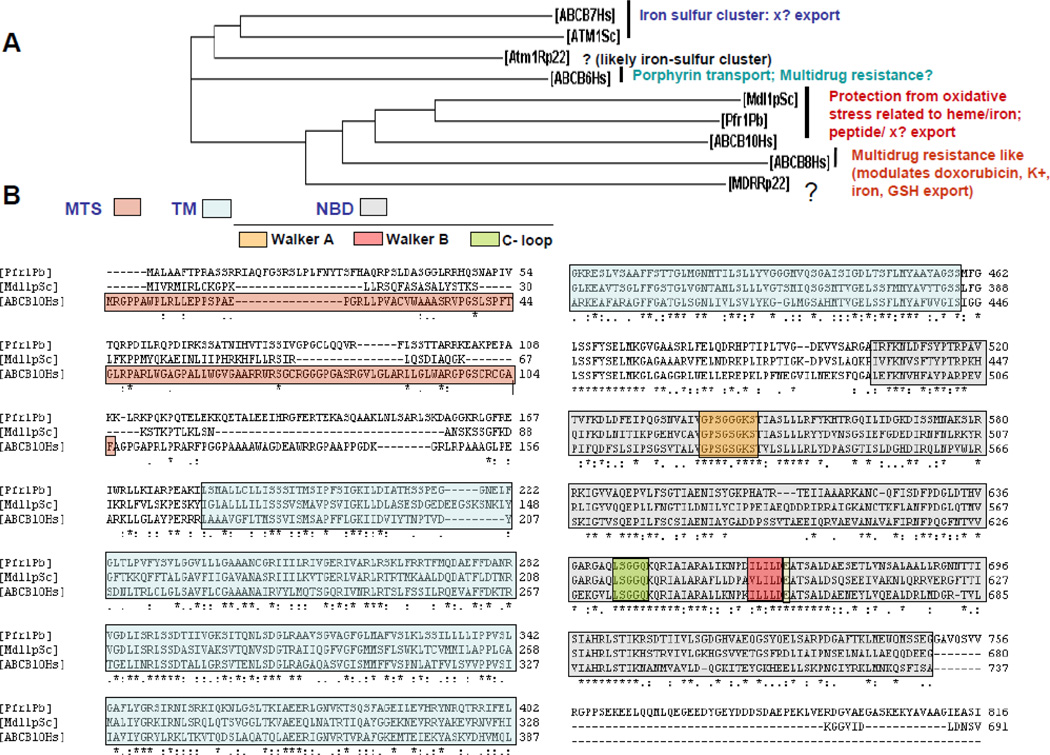
Figure 4. Mitochondrial ABC transporters cluster according to their different physiological roles reported.
A) Phylogenic analysis: dendogram obtained using ClustalW2 software from the EMBL website and using the aminoacid sequences of ABC proteins. Hs: Homo sapiens; Sc: Saccharomyces cerevisae (fungi); Rp22: Rickettsia prowazekii strain 22 (prokaryote, ancestor of mitochondria); Pb: Paracoccidioides brasiliensis (fungi). Note that human ABCB7 clusters together with ATM1 from Saccharomyces cerevisae and from Rickettsia prowazekii, suggesting that its function permitting iron/sulfur cluster synthesis is likely to be conserved. ABCB6, despite its high levels of aminoacid identity by BLAST protein analysis to ABCB7 and ATM1Sc, clusters separately. This separation fits well with ABCB6 heterogeneous localization inside the cell and the likely possibility that it transports multiple substrates. ABCB10, Pfr1 and MDL1 have all been shown to be required for protection under conditions of increased oxidative stress related to heme and iron metabolism. Consistent with this, they cluster together. ABCB8, on the other hand, has been reported to be involved in different processes, such as multidrug resistance in cancer cell lines (in doxorubicin export), mitochondrial K+ ATP channel, iron and GSH export in the heart. These heterogeneous functions suggest a less conserved and more specialized function of ABCB8. In addition, MDR (multidrug resistance protein) of Rickettsia Prowazekii clusters separately from ABCB10 and ABCB8. Rickettsia Prowazekii MDR function is unknown, but we think that it will be closer to ABCB10, as the latter clusters together with lower eukaryotes orthologues, suggesting a more conserved function. B) Sequence alignment of human ABCB10 (ABCB10Hs) with Saccharomyces cerevisae MDL1 (Mdl1pSc) and Paracoccidioides brasiliensis Pfr-1 (Pfr1Pb) using Clustal W2 software. Identical residues are marked with asterisks. The transmembrane-TM- and nucleotide binding-NBD- domains, according to ABCB10 sequence, are in light green and grey respectively. The Walker A, B and C-loop motifs are highlighted within the NBD in different colors. ABCB10 mitochondrial target sequence (MTS) is also highlighted in orange. Note that MTS is the less conserved region of the protein.
3. THE ROLE OF ABCB10 (ABC-me/M-ABC2/mABC2) IN PROTECTING FROM OXIDATIVE STRESS
ABCB10 was identified as ABC-me in mouse and M-ABC2 in humans [76,88]. These different nomenclatures can generate some confusion. Therefore, it is important to clarify that ABCB10, ABC-me, M-ABC2, MTABC2, m-ABC2 and mABC2 are different names for the same protein. This protein is different from ABCB8/mABC1/M-ABC1. ABCB10 is the closest mammalian orthologue to yeast MDL1 (multidrug resistance like 1 protein, 43% identity, covering 78% of the sequence) and to C. elegans haf-3 (44% identity, covering 79% of the sequence). Several studies have shown the importance of MDL1 function in yeast physiology. On the other hand, to our knowledge, no studies have shown in detail the precise function of haf-3 in C. elegans. However, one study describes that repression of haf-3 by siRNA or homozygous deletion, does not activate the mitochondrial unfolded protein response [31]. Determining haf-3 function and its substrates in C. elegans will provide valuable information for the understanding of ABCB10 function in mammals. On the other hand, the precise function of haf-3 homologue, haf-1, was described in the same study and shares important similarities with ABCB10 yeast orthologue MDL1 [31]. Therefore, haf-1 function will be discussed in this review, as its description might provide valuable information in order to understand ABCB10 function, despite not being the closest ABCB10 C. elegans orthologue (ABCB10 shares a 40% of identity with haf-1, covering 77% of its sequence). This similarity is lower when compared to haf-3 but still significant (see above).
a) Function of ABCB10 orthologues in yeast, fungus and C. elegans
MDL1 and MDL2 were cloned using a gene mapping strategy in yeast [23]. To our knowledge, the precise function of MDL2 is still unknown; but its deletion impaired respiratory cell growth in yeast, suggesting that it is required for basal mitochondrial function [87]. In contrast, MDL1 deletion did not impair growth under non-fermentable carbon sources, showing that it is not required to maintain mitochondrial function in the resting state [87]. MDL1 (but not MDL2) exports peptides in isolated mitochondria preparations (cell-free systems) from the matrix to the inter-membrane space. More specifically, these peptides were digestion products of the matrix AAA protease (m-AAA mitochondrial protease), which is involved in protein quality control [87]. This study provided the first in vitro evidence of the physiological role of MDL1.
One can hypothesize that the observed peptide export activity may help preventing mitochondrial dysfunction induced by an abnormal and sudden accumulation of peptides caused by increased damage and turnover (i.e. increased ROS generation or mitochondrial oxidative stress). This accumulation could potentially generate higher order structures (protein aggregates) and irreversibly damage mitochondria, abrogating their ability to cope with the stress (i.e such as β-amyloid peptide accumulation in Alzheimer disease). Furthermore, the formation of these aggregates could be stimulated and/or catalyzed by reactive oxygen species, generating a vicious cycle (aggregates cause dysfunction; mitochondrial dysfunction increases ROS, which promote the formation of more aggregates). Indeed, deletion of Lon protease located in the mitochondrial matrix causes the likely accumulation of protein aggregates, which are associated with mitochondrial dysfunction and ROS generation [10]. Thus, accumulation of certain peptides increased by stress conditions and exacerbated by MDL1 deletion (i.e high ROS) could cause a similar phenotype to Lon activity abrogation (despite the difference that lack of Lon activity would cause the accumulation of misfolded proteins, and lack of MDL-1 accumulation of peptides).
Another possibility is that these peptides exported by MDL1 were not causing or facilitating the damage per se, but that they would harbor a signal in order to communicate the mitochondria status to the rest of the cell. This signal could be essential to activate cellular antioxidant responses or mitochondrial quality control mechanisms [7]. This would impede the recruitment of quality control mechanisms when required and cause the accumulation of dysfunctional mitochondria. Indeed, studies performed in C. elegans support this hypothesis. Haf-1 deletion (MDL1 orthologue in C. elegans) caused an accumulation of peptides inside the mitochondrial matrix, which hampered the activation of the transcription factor ZC376.7 and abrogated a proper mitochondrial unfolded protein response [31]. As a result, Haf-1 deletion caused mild mitochondrial dysfunction (measured as decreased oxygen consumption in worms) under basal conditions, which was exacerbated by exposing Haf-1-null worms to high temperature (30° C, which hampers protein folding in mitochondria) [31]. However, it is unknown whether potential protein aggregates constituted by Haf-1 substrates also contributed to mitochondrial dysfunction or whether decreased mitochondrial respiration was only caused by the lack of activation of quality control mechanisms.
These findings in yeast and C. elegans strongly suggest that MDL1/haf-1 export activity might play a role in mitochondrial stress response, but that it is not essential for viability under basal conditions. In this regard, MDL1 over-expression was shown to prevent the abnormally increased mitochondrial oxidative stress caused by ATM1 deletion through an unknown mechanism [17]. Consistent with this, a study characterizing the pathogenic fungus Paracoccidioides brasiliensis also supports the hypothesis that MDL1 is important to cope with situations of increased stress. This study shows that ABCB10 orthologue Pfr1 is markedly increased under stress conditions. Pfr1 expression was increased by treatment with fluconazole (used to combat fungal infections), which damages the fungus and their mitochondria by inhibiting the cytochrome P450 enzyme involved in the synthesis of ergosterol [30].
b) ABCB10 (ABC-me)
As discussed in previous sections, one would expect that ABCB10 could play a role in peptide export and active protection from increased oxidative stress, as it is the mitochondrial ABC transporter closer to MDL1 in mammals [30,76,88,89]. In contrast, ABCB7, ABCB6 and ATM1 would be the mitochondrial ABC proteins involved in heme and iron metabolism, as they are in a separated phylogenic cluster than MDL1 and ABCB10 (see section 4) [30,88,89].
Despite these phylogenic features, different studies demonstrate that ABCB10, like ATM1 and ABCB7, also plays an important role in heme and iron metabolism [16,76]. A hypothesis that could reconcile both predictions would state that ABCB10 activity confers a specific antioxidant function (not present in lower eukaryotes or prokaryotes) required to protect from oxidative damage specifically associated with mammalian heme metabolism.
This hypothesis suggests that ABCB10 would be a version of MDL1 tuned up to protect mitochondria from the oxidative challenges specifically occurring in multi-cellular eukaryotic organisms (i.e. hemoglobin synthesis). The mechanism for this protection could involve peptide export and/or other molecules specifically regulating antioxidant function and/or avoiding mitochondrial oxidative damage. In this regard, tissues with higher susceptibility to oxidative damage show ABCB10 expression [76,88]. Heart and hematopoetic tissues show high levels of ABCB10 expression in mice (hematopoetic tissues with the highest levels) [76]. These tissues handle two of the most challenging (physiological and pathophysiological) processes dealing with increased mitochondrial oxidative stress: erythropoiesis (hemoglobin synthesis) and cardiac ischemia reperfusion. Furthermore, liver and brain also show considerable levels of ABCB10 expression. In both tissues, mitochondrial oxidative stress plays a major role in the pathogenic consequences of ischemia-reperfusion (as in the heart). In this regard, we expect that ABCB10 will play a protective role from oxidative stress in these tissues too.
b.1) ABCB10 in erythropoeisis and heme metabolism
ABCB10 (ABC-me) was identified in mRNA subtractive analysis of transcripts that are induced during erythroid differentiation by the activation of the transcription factor GATA1 (a master regulator of terminal erythroid differentiation) [76]. ABCB10 was identified as a mitochondrial transporter and was localized in the inner membrane (Figure 1A). ABCB10 nucleotide binding domain (NBD) is facing the mitochondrial matrix, suggesting that ABCB10 could export molecules out of the mitochondria (Figure 1A). However, some eukaryotic ABC proteins showing the same topology (NBD oriented to the matrix) have been shown to act as importers. Thus, ABCB10 location and the possibility of harboring export activity made it an attractive candidate for the missing mitochondrial exporters of amino-levulinic acid (ALA) or heme, involved in heme/hemoglobin synthesis [76]. This hypothesis was further supported by 3 different results:
The increase in hemoglobin levels and synthesis rates triggered by ABCB10 overexpression in erythroid cells, without affecting differentiation (Figure 2B). 2) The increase in ABCB10 expression induced by addition of exogenous amino-levulinic acid (ALA), which increases heme synthesis (by-passing mitochondrial amino-levulinic acid synthesis and export). 3) The decrease in ABCB10 expression by exogenously added physiological heme levels (the final product exported by the mitochondria) [76]. These results (2 and 3) suggested that ABCB10 could be involved in heme export rather than in ALA export.
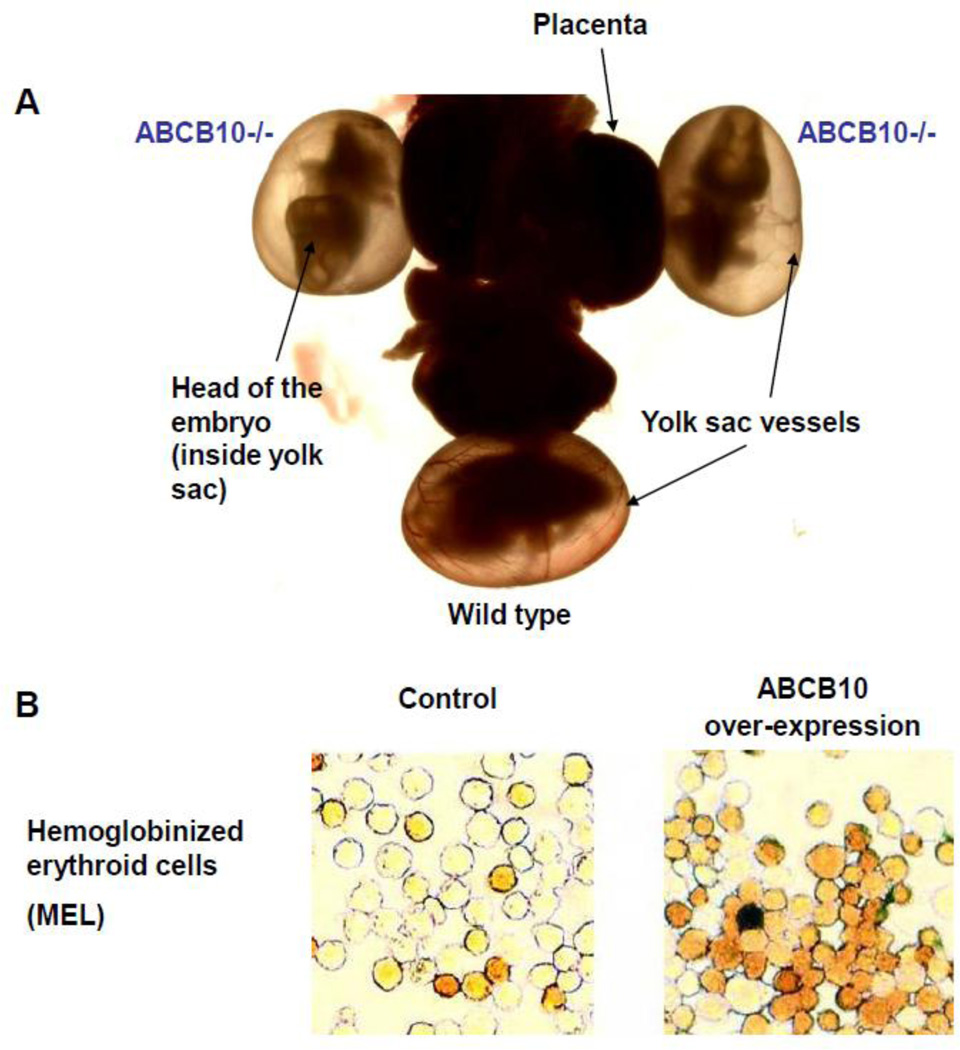
Figure 2. ABCB10 is essential for erythropoiesisin vivo.
A) Light microscope image of day 10.5 embryos still attached to the placenta and inside the yolk sac. The 2 embryos in the upper part of the image harbor inactivated ABCB10 alleles (−/−) and showed beating hearts. The embryo in the bottom part of the image was a wild type. Note that the wild type embryo has hemoglobinized cells in the vasculature of the yolk sac (give red color to vessels), whereas the ABCB10 −/− embryos show vessels with no red color (lack of hemoglobinized cells). The yolk sac is the main site of primitive erythropoiesis at day 9.5– 10.5. See reference 38 for a more detailed description. B) Representative images of differentiated MEL cells (murine erythroleukemia cells) stably transfected with an empty vector (control) or containing ABCB10 cDNA (ABCB10 over-expression) and stained with benzidine for hemoglobin detection (orange / brown color). This figure panel was adapted from reference 76, Shirihai et al. (2000) EMBO Journal.
This in vitro data was corroborated in vivo by our recent studies showing that ABCB10 expression is essential for erythropoesis in vivo [38] (Figure 2A). ABCB10 KO mice die at embryonic day 12.5 and are severely anemic at day 10.5, when primitive erythropoiesis takes place and heme synthesis rates are maximal in erythroid precursors (Figure 2A) [38]. This phenotype closely resembles GATA-1 KO mice. GATA-1 is the master transcription factor regulating terminal erythropoiesis, as it controls the expression of multiple transcripts essential for red blood development (and also ABCB10 expression). Thus, GATA-1 mice are severely anemic at embryonic day 10 and die at day 11, which is very similar to ABCB10 KO embryos phenotype [38,66,67]. In addition, GATA-1 KO erythroid differentiation is stalled beyond the pro-erythroblast stage (when heme synthesis rates are maximal), as in ABCB10 KO [38,66,67]. Together, these data demonstrate that ABCB10, a downstream target of GATA-1, is essential for red blood cell differentiation.
This model challenged a major prediction in regard to ABCB10 function: If ABCB10 was to export a heme synthesis intermediate, one would predict that hemoglobin synthesis would not be possible in the absence of ABCB10, and that erythroid cells would die with no hemoglobin in them. This was not the case. Histology of ABCB10 KO embryonic yolk sac and cytology of embryonic blood (day 10.5) showed the presence of a minor population of hemoglobinized erythroid precursors (10% of erythroid precursors had hemoglobin and some of them were apoptotic) [38]. Therefore, there should be mitochondrial export of heme and amino-levulinic acid in the absence of ABCB10, as hemoglobin synthesis is still possible.
How could ABCB10 regulate heme synthesis while not directly transporting an intemediate? Our initial hypothesis was by protecting mitochondria and/or the cell from increased oxidative stress associated with heme/iron metabolism. Indeed, this function protecting from mitochondrial damage or oxidative stress would be conserved to a certain extent with the yeast and C. elegans orthologues of ABCB10, as described in the previous section (2a) [17,31].
Furthermore, multiple lines of evidence demonstrate the intimate relation between erythropoesis, heme metabolism and oxidative stress in mammals. One example is the mice deficient in one of the main antioxidant enzymes located in the mitochondrial matrix, Superoxide Dismutase 2 (sod2) [25,26]. Lethally irradiated mice were rescued by implanting hematopoietic stem cells (HSC) with deleted sod2 expression (sod2 −/−) [25,26]. Despite the rescue from lethality, these mice had a decreased number of circulating red blood cells, showing features of sideroblastic anemia; that is, granules with iron, inside the red blood cells [25,26]. Interestingly, reconstitution of T, and B, myeloid cells from HSC sod2 −/− was not affected [25,26]. These data strongly demonstrated that mitochondrial antioxidant activity was particularly important for erythrocyte differentiation and did not affect HSC differentiation to other lineages. This was somehow expected, as the high levels of heme synthesis required for hemoglobinization involve a large increase in free iron import inside the mitochondria and formation of different heme intermediates, which can cause severe oxidative damage.
Therefore, in order to test whether mitochondrial oxidative damage was contributing to defective erythropoiesis caused by ABCB10 deletion, we differentiated ABCB10−/− erythroid progenitors to red blood cells ex vivo (from ABCB10 KO day 10.5 embryonic blood) and in vitro (from ABCB10 KO embryonic stem cells) in the presence of a SOD2 mimetic (MnTBAP) [38]. Treatment with the catalytic antioxidant MnTBAP partially restored hemoglobin synthesis and partially prevented apoptosis after differentiation of ABCB10 −/− cells to erythrocytes. Furthermore, a mainly cytosolic ROS scavenger (less efficient in the mitochondria), N-acetyl-cysteine, was completely unable to prevent these defects [38]. These results demonstrated that ABCB10 activity is required to avoid oxidative damage, particularly associated with mitochondrial superoxide production during heme synthesis and erythrocyte differentiation. However, this rescue by MnTBAP was partial, which could be explained by three different scenarios:
Efficiency of the antioxidant treatment: possibly the maximal capacity of the MnTBAP as an antioxidant was not sufficient to completely prevent the increase in oxidative stress associated with the lack of ABCB10 and erythrocyte differentiation.
ABCB10 was playing an additional role in erythrocyte differentiation not directly associated to protection from oxidative damage. Indeed, it is still possible that ABCB10 transported essential heme synthesis intermediates (such as ALA or heme per se) when hemoglobin synthesis rates are high, whereas other transporters would be responsible for ALA or Heme export at lower or basal synthesis rates. These additional transporters could explain hemoglobin synthesis in erythroid cells without ABCB10 expression.
MnTBAP is not a good catalase mimetic and the increase in H2O2 from dismutation of superoxide could be still leading to cell death. It will be of interest to increase both superoxide dismutase and catalase activities and study whether this treatment could completely rescue defective erythropoeisis caused by ABCB10 deletion. In this regard, if an antioxidant treatment completely rescuing hemoglobinization in ABCB10 −/− cells was identified, it would be a strong proof that ABCB10 would not be involved in the export of essential heme intermediates (ALA) or heme per se when synthesis rates increase.
Different studies show that ABCB10 plays additional roles in regulating heme synthesis. In vitro data demonstrated that ABCB10 is required to stabilize mitoferrin 1 (Mfrn1) protein levels specifically during erythroid differentiation of murine erythroleukemia cell line (MEL cells) [16]. Overexpression of ABCB10 in COS7 (non-erythroid cells) and undifferentiated MEL cells also stabilized Mfrn1 expression [16]. Therefore, ABCB10 has an additional indirect function regulating heme synthesis, by allowing maximal iron import to the mitochondria through Mfrn1 stabilization. Indeed, Ferrochelatase was also found in the same complex with over-expressed ABCB10 and over-expressed Mfrn1 [15]. Whether the formation of this complex is required to stabilize Ferrochelatase or for Ferrochelatase function is unknown [15]. Given that Ferrochelatase was also found to interact with ABCB7, it would be interesting to determine the exact composition of these ABCB10 complexes in vivo and whether they are physiologically relevant [15,80]. Regarding the interaction between ABCB10/Mfrn1, we confirmed the likely existence of this regulatory pathway in vivo, as day 10.5 embryonic blood from ABCB10 KO show a specific reduction in Mfrn1 protein levels (larger than in other mitochondrial proteins) [38].
On the other hand, different observations suggest that the interaction between ABCB10 and Mfrn1 is not essential for hemoglobinization or for the independent function of both proteins. The first evidence is that hemoglobinization can occur in the absence of ABCB10, demonstrating that Mfrn1 is still active [38]. Furthermore, treatment with antioxidant molecules increased hemoglobinization in ABCB10 KO cells, demonstrating that Mfrn1 activity can be increased in the absence of ABCB10 [38]. Given that iron can cause oxidative stress, it is likely that ABCB10-mediated modulation of the mitochondrial oxidative status could be an important signal determining Mfrn1 protein levels. Thus, it is possible that ABCB10 could regulate Mfrn1 levels independently of their physical presence in the same complex. Reduction in iron import under conditions of increased ROS (associated with ABCB10 reduction) may function to maintain cell viability and mitochondrial function during erythroid differentiation. In this regard, it would make physiological sense that if ABCB10 protective activity was impaired, the mitochondria would not maintain a high iron import activity through Mfrn1, thereby avoiding the possibility of exacerbating mitochondrial oxidative damage through iron. It would be of interest to determine whether Mfrn1 levels are also decreased in other models of increased mitochondrial oxidative stress.
b.2) ABCB10 (ABC-me) in the heart
Mitochondria play a major role in heart physiology and disease [52]. The heart has high ATP demand and mitochondria are essential to support continuous cardiac contraction by maintaining the ATP pool [52]. A sudden increase in mitochondrial oxidative stress is a central mediator of cardiac damage and cell death under pathological conditions, such as in ischemia-reperfusion (caused by a temporal obstruction in the coronary artery, followed by re-perfusion and re-oxygenation of the tissue) [52]. We rationalized that this would be a relevant system in which we could study the role of ABCB10 in protecting from oxidative stress outside the context of erythropoiesis and hemoglobin synthesis. Indeed, heme synthesis by the mitochondria supports both cytochrome synthesis as well as providing substrate for the heme-oxygenase antioxidant system. [49,50]. In this regard, cytosolic Heme-Oxygenase 1 is required to protect the heart from ischemia-reperfusion [54]. Consistent with this hypothesis, the heart shows considerable expression levels of ABCB10 [76].
Thus, we used mice harboring one functional allele of ABCB10 (ABC-me +/−), as the KO (−/−) was embryonic lethal (see previous section). Hearts from ABCB10 +/− showed a 50% reduction in ABCB10 expression. However, this reduction did not impair heart function under resting conditions. Systolic (contraction) and diastolic (relaxation) pressure values were normal both in vivo and ex vivo, even under different working loads using Langendorff perfusions. Consistent with this, mitochondrial function was not impaired in isolated mitochondria from ABCB10 +/− hearts or in intact cardiomyocytes under basal conditions [53]. Furthermore, ABCB10 +/− hearts did not show signs of increased oxidative damage to proteins and production of superoxide in isolated mitochondria was unchanged [53]. This was also in agreement with the finding that, catalase and/or Sod2 expression were not increased in ABCB10 +/− hearts, suggesting that the lack of oxidative damage or cardiac dysfunction was not due to up-regulation in the expression of the main antioxidant defense mechanisms. Of note, Mfrn1 protein levels were not altered in ABCB10 +/− hearts, suggesting that reduction in ABCB10 expression levels is not associated with decreased Mfrn1 in the heart under resting conditions [53]. Thus, it was of interest to test how ABCB10 +/− hearts would respond to conditions of increased mitochondrial oxidative stress.
We subjected wild type and ABCB10 +/− hearts to 10 minutes of ischemia and 20 minutes of reperfusion. Ten minutes of ischemia results in bioenergetic and mechanical impairment that are linked to oxidative stress without promoting massive cell death. After ischemia-reperfusion, ABCB10 +/− diastolic function was severely impaired, together with a milder systolic dysfunction (Figure 3A). In parallel, ATP synthesis and oxygen consumption rates were decreased in mitochondria from ABCB10 +/− hearts after ischemiareperfusion, when compared to wild type hearts. Consistent with these data, total ATP levels were decreased by 50% in ABCB10 +/− hearts when compared to wild type hearts after ischemia-reperfusion, explaining their specific mechanical dysfunction. Acute treatment with a superoxide dismutase and catalase mimetic (EUK-207) (20 minutes of perfusion), prior to ischemia-reperfusion, completely prevented both the .bioenergetic and mechanical defects caused by inactivation of one allele of ABCB10 (Figure 3). This result demonstrated that decreased protection from oxidative stress in ABCB10 +/− hearts was causing the exacerbated cardiac dysfunction after ischemia-reperfusion [53].
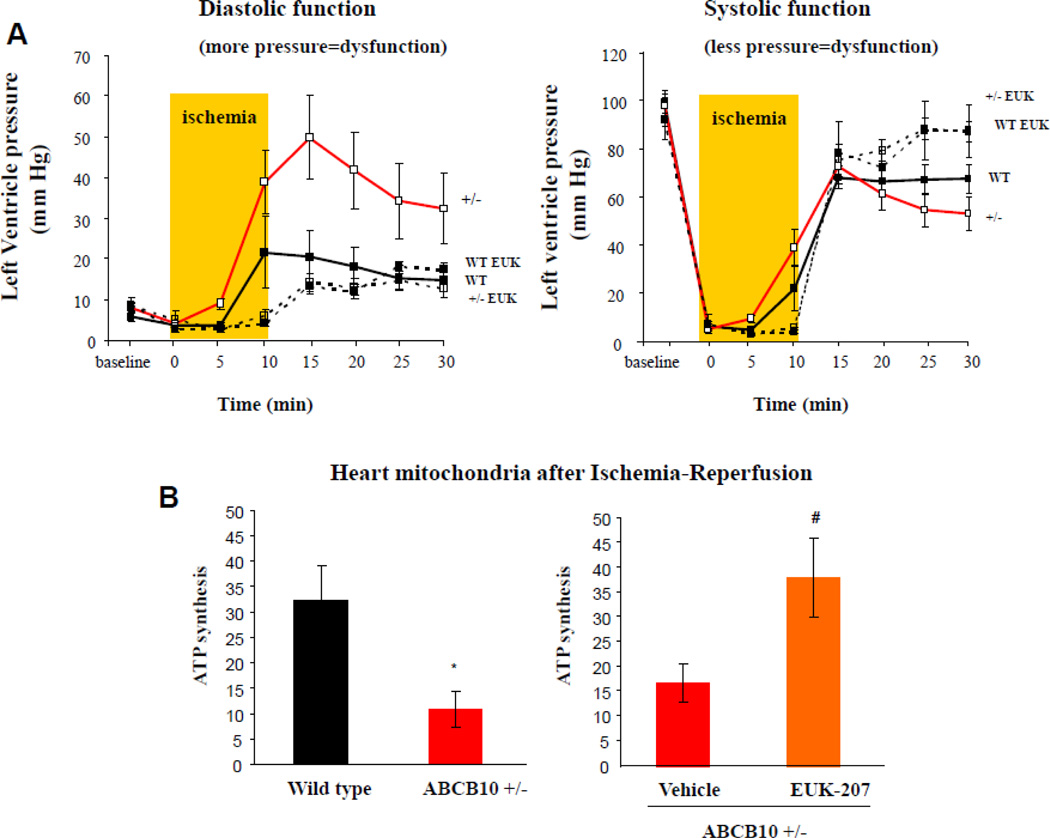
Figure 3. ABCB10 is required to protect from increased oxidative stress associated to cardiac ischemia-reperfusion.
A) Diastolic and systolic pressure in wild type (WT) and ABCB10 +/− hearts (red trace) during ischemia-reperfusion. Increased diastolic and decreased systolic pressures are symptomatic of impaired mechanical function. Pretreatment for 20 minutes with the antioxidant EUK-207, a superoxide dismutase and catalase mimetic, prevented these defects in ABCB10 +/− hearts (dashed open square traces). B) Mitochondrial ATP synthesis rates after ischemia-reperfusion of wild type and ABCB10 +/− hearts. The functional defect in ABCB10 +/− cardiac mitochondria was prevented by EUK-207 treatment. See reference 53 for a more detailed description.
Further experiments were carried out to investigate the site of oxidative damage in the ABCB10 +/− hearts. ABCB10 +/− cardiac mitochondria displayed a larger increase in lipid peroxidation after ischemia-reperfusion when compared to wild type hearts [53]. Outside the mitochondria, we found that the sarcoplasmic reticulum calcium ATPase (SERCA), required for proper diastolic function (as it is responsible for the calcium uptake from the cytosol to the endoplasmic reticulum, which allows heart relaxation), was sulfonylated in cysteine 674 [53]. This oxidative post-translational modification in cysteine 674 is known to inactivate SERCA function. Therefore, in addition to decreased mitochondrial ATP synthesis, SERCA inactivation likely contributed to diastolic dysfunction [53]. Both mitochondrial lipid peroxidation and SERCA sulfonylation induced by ABCB10 partial inactivation were completely prevented by the treatment with the superoxide dismutase/catalase mimetic EUK-207 [53]. Interestingly, a similar EUK compound was used to prevent some of the hematopoietic defects associated with Sod2 inactivation [25,26]. These results suggested that an increase in superoxide/hydrogen peroxide levels in ABCB10 +/− mitochondria could be causing this phenotype of poor recovery after ischemia-reperfusion. In addition, diffusion of hydrogen peroxide from the mitochondria could be responsible for SERCA inactivation.
This study demonstrated that ABCB10 protective function is conserved in nonhematopoetic tissues (tissues without hemoglobin synthesis or without very high heme synthesis rates). Furthermore, these in vivo models are compatible with the hypothesis formulated in the previous section by which lack of MDL1/ABCB10 could be causing oxidative damage (and in the case that ABCB10 harbors export activity):
Causing the accumulation of its substrate in the mitochondrial matrix, leading to dysfunction and ROS generation (through higher order structures and/or reaching toxic concentrations).
Lack of a signal exported from the mitochondria required to activate an antioxidant response and/or mitochondrial quality control mechanisms to counteract ROSmediated damage.
In the following section we will summarize the function of the rest of mitochondrial ABC transporters. All three have been also implicated in oxidative stress handling to some extent but the mechanisms are likely to be different from ABCB10.
4. UNDERSTANDING ABCB10 FUNCTION BY PHYLOGENETIC COMPARISON: MITOCHONDRIAL MEMBERS OF THE ABCB FAMILY AND CONSERVED FUNCTIONS
ABC transporters are the preferred agents of transport in bacteria. Close to a 5% of Escherichia coli K12 genome is constituted by genes encoding for components of ABC (ATP binding cassette) transporters, resulting in 78 different proteins with the ABC signature [37]. This relatively high percentage in E. coli suggests that ABC-mediated transport (import or export) could constitute one of the main molecular mechanisms selected during evolution for adaptation and communication of unicellular organisms with the environment (i.e. nutrient uptake, and excretion of toxic metabolic end-products).
However, this high requirement for ABC transporters in bacteria is not present in other unicellular organisms that share similar ecological niches. This suggests that the requirement for ABC proteins in E. coli is not related to the direct interaction of the unicellular organism with the ecological niche. For example, a eukaryotic unicellular organism such as Saccharomyces cerevisae expresses 29 ABC proteins (3 times less than E coli). Of these 29 ABC proteins, 72% are class 1 (with fused inter-membrane and ABC domains) and none are class 3 (the inter-membrane and ABC domains are carried by independent poly-peptide chains) [37]. This distribution of ABC proteins is exactly the opposite of the prokaryote E. coli K12, which expresses only a 9% of class 1 ABC proteins and an 81% of class 3 [37]. This profile in Saccharomyces cerevisae suggested that the majority of class 3 ABC proteins are not required for unicellular life. In all, one could conclude that ABC transport function is a preferred mechanism to constitute a fully functional prokaryotic organism, which would have a relative lower importance in eukaryotes.
On the other hand, the α-proteobacteria Rickettsia prowazekii only expresses 14 ABC proteins (6 class 1 and 6 class 3) [37]. This suggests that most of these class 3 ABC proteins expressed in E. coli are not required to constitute a functional prokaryotic unit. This low requirement for ABC transporters could be partially explained by the parasitic nature of Rickettsia prowazekii and thus, the more protected environment that confers living inside the host cell. Therefore, these 14 proteins in Rickettsia prowazekii might be the essential ABC proteins permitting basic prokaryotic physiological processes. Studying the functional conservation in eukaryotes and mammals of these 14 ABC proteins from Rickettsia prowazekii will provide very valuable information. It will be important to determine whether ABCB10 function is conserved between eukaryotic and prokaryotic cells, as mitochondria are thought to be developed through endosymbiosis of the α-proteobacteria Rickettsia prowazekii [4]. Consequently, the understanding of ABC proteins conferring the parasitic properties and/or allowing basic physiological processes of Rickettsia can potentially unravel molecular mechanisms of interaction between the mitochondria and the rest of the cell.
There are only 3 additional ABC proteins located in the mitochondria of mammalian cells and all of them are class 1 (as ABCB10): ABCB6, ABCB7 and ABCB8 [37]. BLAST protein sequence homology analysis shows that human ABCB6 and ABCB7 show high and similar levels of identity to Rickettsia prowazekii ABC protein ATM1 (57% identity, covering 98% of the human ABCB6 protein sequence and 49% identity, covering 79% in the case of ABCB7). In the case of human ABCB10 and ABCB8, the closest Rickettsia prowazekii ABC protein is Multi-drug resistance protein, but their similarity is lower (31% of identity, covering only 67% of ABCB10 sequence and 32% of identity, covering 71% of ABCB8 sequence). These data are consistent with phylogenic analysis of the mitochondrial ABC transporters, in which human ABCB6, ABCB7 and Saccharomyces cerevisae ATM1 cluster together, whereas ABCB10 and ABCB8 cluster separately [30,88,89] (see Figure 4). Of note, ABCB10 clusters together with yeast MDL1 (multidrug resistance like 1), whereas ABCB8 is in a separate cluster from ABCB10 and MDL1 [30,88,89].
Altogether, these findings suggest that ABCB8 and ABCB10 might play a more specialized eukaryotic function similar to yeast MDL1 or to Rickettsia Multidrug resistance protein, whereas ABCB6 and ABCB7 might play a more conserved function related to ATM1 that is likely common to prokaryotes and eukaryotes.
a) ABCB6/MTABC3
ABCB6 is located in the outer mitochondrial membrane and was shown to be involved in the import of porphyrins, including heme, coproporphyrinogen III, and protoporphoryrinogen IX inside the mitochondria, as its ATP binding domain is facing the cytosol [47,65]. However, the inner membrane protoporphoryrinogen IX importer has not been identified yet. Therefore, it was suggested that ABCB6 was essential for erythropoiesis, as coordinated protoporphyrinogen IX import through the outer and inner membrane is a step required for efficient heme biosynthesis and thus for hemoglobinization of erythroid cells [47].
These findings could be consistent with a highly conserved function of ABCB6 through evolution (and thus its high degree of identity with its prokaryotic orthologue), as heme is synthesized in Rickettsia prowazekii to support their aerobic metabolism. Indeed, according to KEGG pathway analysis and other BLAST studies [63,64], Rickettsia prowazekii does not express the prokaryotic orthologue of Uroporphyrinogen III synthase (named hemD), which would abrogate its ability to synthesize protoporphyrinogen IX [64]. Therefore, Rickettsia prowazekii would require the import of porphyrins from the host cell to complete heme synthesis and compensate the lack of hemD (only in that case that there is definitive proof that other unidentified enzyme with Uroporphyrinogen III synthase activity and completely unrelated to prokaryotic hemD did not exist). Furthermore, ABCB6 show ubiquitous expression, which is consistent with the fact that all cell types require heme synthesis to different extents [47,58]. In this regard, some studies demonstrated that ABCB6 expression was protective against different insults increasing oxidative stress, such as arsenite exposure [12,55]. The mechanism for this protection is that heme is required to assemble catalase in the peroxisome [22,49,50].
On the other hand, different lines of evidence suggest that ABCB6 might play other roles not directly related to heme synthesis and protoporphoryrinogen IX import in the mitochondria, such as providing multi-drug resistance activity to cancer cell lines [27,68,86]. In this regard, several studies have shown that ABCB6 is also located in the plasma membrane, ER and Golgi apparatus (as part of the secretory pathway) [41,43,65,82,85]. It was also shown that certain mutations in ABCB6 might cause an ocular disease named coloboma in humans [85]. In this study, ABCB6 was also detected in the ER and Golgi of retinal pigment epithelial cells, consistent with other studies showing that ABCB6 is not exclusively mitochondrial [85]. In addition, two recent reports challenged directly the physiological relevance of ABCB6 activity importing porphyrins, its mitochondrial localization and thus its role in hemoglobin synthesis [32,46]. The first study showed that ABCB6 is dispensable for erythropoiesis and hemoglobin synthesis [32]. Human subjects harboring mutations abrogating ABCB6 protein expression did not present any defect in erythropoiesis, hemoglobin synthesis or anemia [32]. They only presented a mild increase of intracellular porphyrin levels in circulating red blood cells and a decrease in the porphyrin plasma levels [32]. In addition, ABCB6 was detected in the plasma membrane of red blood cells [32,43]. These results suggest that ABCB6 located in the plasma membrane might be mediating the export of porphyrin from red blood cells to the serum. The second study demonstrates that ABCB6 is not located to the mitochondria and is not required for heme biosynthesis in differentiating K562 human erythroid cells. As a result, this study concludes that ABCB6 should not be considered as a mitochondrial ABC transporter [46].
It could also be possible that ABCB6 played an indirect role in protoporphyriogen IX mitochondrial import, by stabilizing and/or activating the unidentified porphyrin importer in the inner membrane (or others present in the outer membrane). It would be important to reconstitute purified ABCB6 in proteoliposomes, which would allow testing whether ABCB6 in isolation is sufficient to import porphyrins or requires the presence of other proteins. This technique would also facilitate the identification of additional potential substrates and thus the understanding of ABCB6 function not directly related to heme metabolism. In all, these data suggest that ABCB6 harbors specialized function/s in eukaryotic cells that might not be shared with its R. Prowazekii orthologue.
b) ATM1 and ABCB7 (ABC7)
ATM1 (ATP binding cassette Transporter Mitochondria 1) was identified in yeast and its discovery constituted the first description of a mitochondrial ABC transporter [51]. ATM1 is located in the inner mitochondrial membrane, with the ATP binding domain facing the matrix (therefore it should export molecules to the inter-membrane space) and functions as a homodimer [18,51]. Although the molecular entity/ies transported by yeast ATM1 or by its mammalian orthologue ABCB7 have not been identified, their function is known [18,19,73,75]: they both regulate iron/sulfur cluster assembly and iron homeostasis (and heme in the case of ABCB7, through interaction with ferrochelatase) [44,45,69,74,80]. In this regard, deletion of ATM1 or ABCB7 causes mitochondrial iron accumulation, oxidative damage to the mitochondria and cytosolic iron starvation, together with defective assembly of cytosolic iron/sulfur clusters [44,45,69,70]. This highly conserved function and mitochondrial sub-localization suggests that ABCB7 might conserve, to some extent, the function of its R. prowazekii orthologue (despite showing a lower % of identity when compared to ABCB6). Indeed, R. prowazekii lacks important proteins regulating iron transport and regulation [64]. Thus, it could be possible that the host cell was responsible for the handling of free iron, while R. prowazekii would export intermediates to the host cell cytosol through ATM1, free iron would be incorporated to this exported intermediate in the host cell and the final molecule containing iron (i.e iron/sulfur cluster) would be imported back into R. prowazekii. By avoiding handling free iron, the parasite R. prowazekii would have lower internal exposure to free iron toxicity, utilizing the host cell protective mechanisms and intermediate biosynthesis.
On the other hand, this hypothesis would not be consistent with the fact that the formation of mitochondrial iron/sulfur clusters does not require cytosolic steps in mammalian cells (in other words, the formation of iron/sulfur cluster in the mitochondria is not compartmentalized between mitochondria and cytosol). However, it could be possible that during the endosymbiotic evolution, the host cell transferred these functions to mitochondria (no longer parasitic, when compared to R. prowazekii), thereby to further minimize the toxic effects of free iron in the rest of the cell.
Consistent with ABCB7 function described in vitro, studies using mouse models abrogating ABCB7 activity showed that ABCB7 is essential for iron/sulfur cluster biogenesis and hematopoeisis in vivo [69,70]. Mutations in ABCB7 cause X-linked sideroblastic anemia with spinocerebellar ataxia (X-LSA/A) in humans and mouse models [1,9,56,72,75]. The sideroblastic anemia is caused by iron accumulation inside mitochondria of erythroid cells in the patients, together with defects in heme metabolism and iron sulfur cluster protein assembly. Therefore, the molecular mechanism of this disease is explained by an alteration in iron homeostasis. The cause for spinocerebellar ataxia in these patients is not known yet. This effect in non-hematopoietic tissues is consistent with ABCB7 expression among different cell types [73]. Thus, understanding the function of ABCB7 in brain, among other tissues, will be relevant to understand the development of the ataxia in XLSA/A patients. In this regard, the absence of ABCB7 did not cause lethality in the liver despite affecting iron/sulfur cluster formation, suggesting that the importance of ABCB7 function is different depending on the cellular type [69]. The identification of ABCB7 substrate/s will provide valuable information in order to understand not only ABCB7 function in iron metabolism, but also the specific relevance of ABCB7 activity in each tissue. Structure studies will be important to validate and confirm the candidate molecules (whether they fit the substrate binding site).
c) ABCB8 (M-ABC1/mABC1)
ABCB8 is expressed in 23 human tissues (data not shown by Hogue et al [36]), suggesting an ubiquitous presence and function in different tissues. Evidence gathered so far does not link this transporter to heme biosynthesis, which is consistent with its relatively higher degree of homology to multi-drug resistance like proteins (MDL1 in yeast or Multidrug resistance protein in R. prowazekii) when compared to ATM1. The main function attributed to yeast MDL1 is the export of peptides produced from inner membrane proteins degradation [87]. However, ABCB8 homology to MDL1 is lower when compared to ABCB10 (MDL1 and ABCB10 cluster together in phylogenetic analysis, ABCB8 separately) [88,89], which suggests that ABCB8 might play different roles than MDL1. Indeed, studies suggest that ABCB8 (M-ABC1/mABC1) constitutes the mitochondrial KATP channel and its over-expression protects from H2O2 induced cell death in neonatal rat cardiomyocytes [5,6]. This protective effect from oxidative stress could be explained by the cardioprotective role of mitochondrial KATP channel activity. In addition, different studies show that both ABCB8 and ABCB10 are part of the human breast and colorectal “cancer genomes” [77] and that ABCB8 is up-regulated in leukemic cells resistant to the anti-cancer drug doxorubicin [28]. Of note, knock-down of ABCB10 by siRNA did not decrease the resistance of melanoma cells to doxorubicin [24]. In marked contrast, down-regulation of ABCB8 using siRNA significantly reversed resistance of melanoma cells to the toxic effects of doxorubicin [24]. In addition, ABCB8 down-regulation resulted in increased doxorubicin-mediated damage to the mitochondrial DNA [24]. Importantly, doxorubicin was also found to accumulate inside the mitochondria of cell lines [2]. Therefore, it is possible that ABCB8 participates in the export of doxorubicin from the mitochondria, a function that would not be shared with ABCB10. Indeed, it was recently demonstrated that cardiac ABCB8 is specifically required to protect from the cardiotoxic effects of doxorubicin [39]. Furthermore, additional evidence shows that ABCB8 regulates mitochondrial export of GSH and iron in cardiac cells, which could also explain an additional role protecting from oxidative stress independently of its attributed to KATP channel activity [8]. It will be valuable to define the exact substrates of ABCB8 in order to understand the precise molecular mechanism by which ABCB8 regulates the transport of K+, iron, GSH and doxorubicin. This variety of potential ABCB8 substrates is consistent with its homology to multi-drug resistance like proteins, as they are able to transport a variety of different molecular entities (i.e. MDR1 is ABCB1, a member of the same family that ABCB8).
In order to determine ABCB8 substrate/s, experiments involving the purification of ABCB8 and reconstitution in proteoliposomes are required. These experiments will allow determining whether ABCB8 per se (in isolation) is able to transport K+, iron, GSH (reduced glutathione) and doxorubicin or whether it requires the presence of other proteins. These studies will provide definitive evidence on whether different candidate molecules can bind to and be transported by ABCB8.
d) Multi-drug resistance activity vs. heme/iron regulation
The 3 members of mitochondrial ABC transporters described in the previous section share different characteristics: the first one is that they are widely expressed across different tissues. This would suggest that they are regulating a physiological process relevant for most cell types (such as MDR activity or heme/iron metabolism). The second one is that they function as homodimers, and not as heterodimers. In principle, lack of heterodimer formation would suggest completely independent and sufficient functions of each protein. In this regard, phylogenic and functional analysis show that ABCB6 and ABCB7 might be involved in the regulation of iron and heme homeostasis (located in different membranes and orientation, but they cluster together in a phylogenic analysis), whereas ABCB8 function would be more related to multidrug resistance activity and peptide export (i.e. export of molecules that if they accumulate can result in mitochondrial damage and dysfunction; it clusters separately in a phylogenetic analysis).
However, this separation (ABCB6/ABCB7 vs. ABCB8) according to both phylogeny and main conserved functions is not completely accurate, as there is evidence showing that ABCB6 can act as a multi-drug resistance protein in some cancers, whereas ABCB8 might regulate iron export from the mitochondria [8,27,40,68,86]. The most important evidence is that yeast MDL1 (ABCB10 orthologue and ABCB8 to a lesser extent) is able to partially restore mitochondrial respiratory capacity caused by ATM1 (ABCB7 and ABCB6 orthologue) deletion in yeast, by reducing 50% the increase in mitochondrial iron content (and thus protecting from mitochondrial oxidative stress)[17]. Unexpectedly, MDL1 overexpression in a wild type background causes higher sensitivity to oxidative stress (in the same direction as ATM1 deletion, therefore the opposite than expected), without dramatically affecting iron homeostasis [17]. Thus, although it was expected that ABCB7 and ABCB6 would be the only mitochondrial ABC proteins regulating the oxidative status (indirectly because of their role regulating heme/iron metabolism), MDL1 and ABCB8 (the multi-drug resistance like proteins) can also regulate it. However, the main difference between MDL1 and ABCB8 is that ABCB8 overexpression can protect against oxidative stress (H2O2 exposure) in a wild type background, whereas MDL1 overexpression does exactly the opposite (sensitizes to H2O2 exposure). This paradoxical behavior suggests that MDL1 function would be protecting from oxidative stress only under specific conditions (such as ATM1 deletion). Thus, understanding ABCB10 (MDL1 closest mammalian orthologue) function can help to determine: a) the intimate functional relationship between mitochondrial multi-drug resistance activity, heme/iron metabolism and protection from oxidative stress, b) unraveling a potential novel target to treat pathologies involving abnormally increased mitochondrial oxidative stress, c) the physiology behind this paradoxical role of MDL1 in the context of yeast metabolism d) the functional relationship (if any) between ABCB6, ABCB7 and ABCB8.
5) CONCLUSIONS AND PERSPECTIVES
Recent evidence demonstrated that ABCB10 plays a protective role against increased oxidative stress in hematopoetic tissues and in the heart. The precise mechanisms are still unknown, but they are likely to be different from the rest of mitochondrial ABC transporters (ABCB6, ABCB7 and ABCB8). In this regard, the molecular mechanisms describing the function of these 3 transporters (ABCB6, ABCB7 and ABCB8) are also still unknown, but the physiological consequences of their modulation have been described, revealing important differences from ABCB10 function. In the case of ABCB6, recent evidence demonstrated that its function is not essential for erythropoesis and that abrogation of its expression does not cause any major symptoms in humans. Thus, ABCB6 might be playing a yet uncharacterized role in addition to porphyrin transport modulation. ABCB8 function is not completely understood. ABCB8 was suggested to be part of the mitochondrial KATP channel and to protect from oxidative stress in cardiac cells. In addition, recent evidence demonstrated that ABCB8 regulates iron and GSH export from the mitochondria, in addition to protecting from doxorubicin-induced mitochondrial damage in heart. Therefore, it is likely that ABCB8 indirectly modulates the oxidative status of the cell by several as yet uncharacterized multidrug resistance-like mechanisms, which involve K+, iron and GSH transport. ABCB7 mutations cause X-linked sideroblastic anemia with ataxia in humans, as a consequence of altered iron/sulfur cluster formation, to which erythroid cells are particularly sensitive. The exact mechanism by which ABCB7 regulates iron/sulfur cluster biogenesis is unknown, but it is likely that it exports an essential intermediate for cytosolic iron/sulfur cluster formation. This activity regulating heme/iron metabolism explains ABCB7 activity regulating oxidative status. Therefore, understanding the precise mechanism by which ABCB7 regulates iron/sulfur cluster formation will provide very valuable information towards the cure of XLSA. However, this is not an easy task, as different research groups have tried for a long time to identify ABCB7/ATM1 substrate without success.
ABCB10 is the only mitochondrial ABC transporter described to protect from increased oxidative stress associated with heme metabolism in higher eukaryotes, rather than being essential for heme synthesis or the formation of iron/sulfur clusters per se. This hypothesis is supported by the fact that some ABCB10 −/− erythroid cells are hemoglobinized and that this hemoglobinization is partially restored by antioxidant treatments. We expect that mutations/inactivation of ABCB10 might be contributing to congenital and/or drug-induced anemias related to increased mitochondrial oxidative stress in humans. Furthermore, we expect that ABCB10 inactivation will cause defects in other human tissues, particularly the ones sensitive to mitochondrial oxidative stress associated to heme metabolism, such as the heart, liver, brain and kidney. Thus, ABCB10 might be an interesting candidate to explain the adverse effect of some drugs in red blood cells formation (anemia) and in decreased recovery of cardiac function after ischemiareperfusion. In this regard, the future identification of ABCB10 substrate/s will facilitate the determination of drugs competing/inhibiting ABCB10 function by comparing their structures. Importantly, it will also determine whether ABCB10 activation (and/or preventing its inhibition) could be potentially used to efficiently protect from conditions causing increased mitochondrial oxidative stress, such as ageing, anemia, cardiac ischemiareperfusion or neurodegenerative diseases.
In all, the description of this novel mechanism protecting mitochondria from oxidative stress defined by ABCB10 could constitute an important step forward to understand and treat pathologies associated with increased mitochondrial oxidative stress.
ACKNOWLEDGEMENTS
M.L. was a recipient of a post doctoral fellowship from Fundación Ramόn Areces. O.S.S. was supported by National Institutes of Health funding grants R01HL071629–03 and R01DK074778. We thank Professor Daniel Dagan for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM. Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A) Hum. Mol. Genet. 1999;8:743. doi: 10.1093/hmg/8.5.743. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AB, Xiong G, Arriaga EA. Doxorubicin accumulation in individually electrophoresed organelles. J. Am. Chem. Soc. 2004;126:9168. doi: 10.1021/ja0492539. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 4.Andersson SG, Zomorodipour A, Andersson JO, Sicheritz-Ponten T, Alsmark UC, Podowski RM, Naslund AK, Eriksson AS, Winkler HH, Kurland CG. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 5.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11880. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardehali H, O'Rourke B, Marban E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circ. Res. 2005;97:740. doi: 10.1161/01.RES.0000186277.12336.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold I, Wagner-Ecker M, Ansorge W, Langer T. Evidence for a novel mitochondria-to-nucleus signalling pathway in respiring cells lacking i-AAA protease and the ABC-transporter Mdl1. Gene. 2006;367:74. doi: 10.1016/j.gene.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 8.Bayeva M, Ichikawa Y, Ghanefar M, Potini V, Sun L, Mutharasan K, Wu R, Kaplan J, Ardehali H. Abstract 16497: Characterization of ATP Binding Cassette Protein B8 (ABCB8) as a Mitochondrial Iron and Glutathione Exporter. Circulation. 2011;124:A16497. [Google Scholar]
- 9.Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, Lill R, Bishop DF. Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. Blood. 2000;96:3256. [PubMed] [Google Scholar]
- 10.Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic. Biol. Med. 2005;38:665. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Chang G. Multidrug resistance ABC transporters. FEBS Lett. 2003;555:102. doi: 10.1016/s0014-5793(03)01085-8. [DOI] [PubMed] [Google Scholar]
- 12.Chavan H, Oruganti M, Krishnamurthy P. The ATP-binding cassette transporter ABCB6 is induced by arsenic and protects against arsenic cytotoxicity. Toxicol. Sci. 2011;120:519. doi: 10.1093/toxsci/kfr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell. 2003;12:651. doi: 10.1016/j.molcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Abele R, Tampe R. Functional non-equivalence of ATP-binding cassette signature motifs in the transporter associated with antigen processing (TAP) J. Biol. Chem. 2004;279:46073. doi: 10.1074/jbc.M404042200. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Dailey HA, Paw BH. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood. 2010;116:628. doi: 10.1182/blood-2009-12-259614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J, Paw BH. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16263. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chloupkova M, LeBard LS, Koeller DM. MDL1 is a high copy suppressor of ATM1: evidence for a role in resistance to oxidative stress. J. Mol. Biol. 2003;331:155. doi: 10.1016/s0022-2836(03)00666-1. [DOI] [PubMed] [Google Scholar]
- 18.Chloupkova M, Reaves SK, LeBard LM, Koeller DM. The mitochondrial ABC transporter Atm1p functions as a homodimer. FEBS Lett. 2004;569:65. doi: 10.1016/j.febslet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 19.Csere P, Lill R, Kispal G. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett. 1998;441:266. doi: 10.1016/s0014-5793(98)01560-9. [DOI] [PubMed] [Google Scholar]
- 20.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De la Rosa MB, Nelson SW. An interaction between the Walker A and D-loop motifs is critical to ATP hydrolysis and cooperativity in bacteriophage T4 Rad50. J. Biol. Chem. 2011;286:26258. doi: 10.1074/jbc.M111.256305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de DC. Biochemical studies on the occurrence, biogenesis and life history of mammalian peroxisomes. J. Histochem. Cytochem. 1973;21:941. doi: 10.1177/21.11.941. [DOI] [PubMed] [Google Scholar]
- 23.Dean M, Allikmets R, Gerrard B, Stewart C, Kistler A, Shafer B, Michaelis S, Strathern J. Mapping and sequencing of two yeast genes belonging to the ATP-binding cassette superfamily. Yeast. 1994;10:377. doi: 10.1002/yea.320100310. [DOI] [PubMed] [Google Scholar]
- 24.Elliott AM, Al-Hajj MA. ABCB8 mediates doxorubicin resistance in melanoma cells by protecting the mitochondrial genome. Mol. Cancer Res. 2009;7:79. doi: 10.1158/1541-7786.MCR-08-0235. [DOI] [PubMed] [Google Scholar]
- 25.Friedman JS, Lopez MF, Fleming MD, Rivera A, Martin FM, Welsh ML, Boyd A, Doctrow SR, Burakoff SJ. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood. 2004;104:2565. doi: 10.1182/blood-2003-11-3858. [DOI] [PubMed] [Google Scholar]
- 26.Friedman JS, Rebel VI, Derby R, Bell K, Huang TT, Kuypers FA, Epstein CJ, Burakoff SJ. Absence of mitochondrial superoxide dismutase results in a murine hemolytic anemia responsive to therapy with a catalytic antioxidant. J. Exp. Med. 2001;193:925. doi: 10.1084/jem.193.8.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuya KN, Bradley G, Sun D, Schuetz EG, Schuetz JD. Identification of a new P-glycoprotein-like ATP-binding cassette transporter gene that is overexpressed during hepatocarcinogenesis. Cancer Res. 1997;57:3708. [PubMed] [Google Scholar]
- 28.Gillet JP, Efferth T, Steinbach D, Hamels J, de LF, Bertholet V, Remacle J. Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res. 2004;64:8987. doi: 10.1158/0008-5472.CAN-04-1978. [DOI] [PubMed] [Google Scholar]
- 29.Graf SA, Haigh SE, Corson ED, Shirihai OS. Targeting import dimerization of a mammalian mitochondrial ATP binding cassette (ABC) transporter ABCB10 (ABC-me) J. Biol. Chem. 2004;279:42954. doi: 10.1074/jbc.M405040200. [DOI] [PubMed] [Google Scholar]
- 30.Gray CH, Ines Borges-Walmsley M, Evans GJ, Walmsley AR. The pfr1 gene from the human pathogenic fungus Paracoccidioides brasiliensis encodes a half-ABC transporter that is transcribed in response to treatment with fluconazole. Yeast. 2003;20:865. doi: 10.1002/yea.1013. [DOI] [PubMed] [Google Scholar]
- 31.Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol. Cell. 2010;37:529. doi: 10.1016/j.molcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, Takahashi H, Tanaka M, Deybach JC, Puy H, Le GM, Sureau C, Pham BN, Le Pennec PY, Tani Y, Cartron JP, Arnaud L. ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat. Genet. 2012;44:170. doi: 10.1038/ng.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins CF. ABC transporters: physiology structure and mechanism--an overview. Res. Microbiol. 2001;152:205. doi: 10.1016/s0923-2508(01)01193-7. [DOI] [PubMed] [Google Scholar]
- 34.Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004;11:918. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 35.Hofacker M, Gompf S, Zutz A, Presenti C, Haase W, van der DC, Model K, Tampe R. Structural and functional fingerprint of the mitochondrial ATPbinding cassette transporter Mdl1 from Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:3951. doi: 10.1074/jbc.M609899200. [DOI] [PubMed] [Google Scholar]
- 36.Hogue DL, Liu L, Ling V. Identification and characterization of a mammalian mitochondrial ATP-binding cassette membrane protein. J. Mol. Biol. 1999;285:379. doi: 10.1006/jmbi.1998.2259. [DOI] [PubMed] [Google Scholar]
- 37.Holland IB, Cole SPC, Kuchler K, Higgins CF. ABC Proteins: From Bacteria to Man. Academic Press; 2002. [Google Scholar]
- 38.Hyde BB, Liesa M, Elorza AA, Qiu W, Haigh SE, Richey L, Mikkola HK, Schlaeger TM, Shirihai OS. The mitochondrial transporter ABC-me (ABCB10), a downstream target of GATA-1, is essential for erythropoiesis in vivo. Cell Death. Differ. 2012;19:1117. doi: 10.1038/cdd.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, Wu R, Khechaduri A, Jairaj NT, Ardehali H. Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc. Natl. Acad. Sci. U. S. A. 2012;109:4152. doi: 10.1073/pnas.1119338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichikawa Y, Bayeva M, Ghanefar M, Mutharasan RK, Wu R, Ardehali H. Abstract 16027: Mitochondrial ATP-Binding Cassette Protein-1 (mABC1) Protects Against Doxorubicin-Mediated Cardiotoxicity. Circulation. 2011;124:A16027. [Google Scholar]
- 41.Jalil YA, Ritz V, Jakimenko A, Schmitz-Salue C, Siebert H, Awuah D, Kotthaus A, Kietzmann T, Ziemann C, Hirsch-Ernst KI. Vesicular localization of the rat ATP-binding cassette half-transporter rAbcb6. Am. J. Physiol Cell Physiol. 2008;294:C579–C590. doi: 10.1152/ajpcell.00612.2006. [DOI] [PubMed] [Google Scholar]
- 42.Jones PM, George AM. Mechanism of ABC transporters: a molecular dynamics simulation of a well characterized nucleotide-binding subunit. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12639. doi: 10.1073/pnas.152439599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kakhniashvili DG, Bulla LA, Jr., Goodman SR. The human erythrocyte proteome: analysis by ion trap mass spectrometry. Mol. Cell Proteomics. 2004;3:501. doi: 10.1074/mcp.M300132-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Kispal G, Csere P, Guiard B, Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett. 1997;418:346. doi: 10.1016/s0014-5793(97)01414-2. [DOI] [PubMed] [Google Scholar]
- 45.Kispal G, Csere P, Prohl C, Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999;18:3981. doi: 10.1093/emboj/18.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiss K, Brozik A, Kucsma N, Toth A, Gera M, Berry L, Vallentin A, Vial H, Vidal M, Szakacs G. Shifting the Paradigm: The Putative Mitochondrial Protein ABCB6 Resides in the Lysosomes of Cells and in the Plasma Membrane of Erythrocytes. PLoS One. 2012;7:e37378. doi: 10.1371/journal.pone.0037378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 48.Lapinski PE, Neubig RR, Raghavan M. Walker A lysine mutations of TAP1 and TAP2 interfere with peptide translocation but not peptide binding. J. Biol. Chem. 2001;276:7526. doi: 10.1074/jbc.M009448200. [DOI] [PubMed] [Google Scholar]
- 49.Lazarow PB, de Duve DC. The synthesis and turnover of rat liver peroxisomes. IV. Biochemical pathway of catalase synthesis. J. Cell Biol. 1973;59:491. doi: 10.1083/jcb.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazarow PB, de Duve DC. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J. Cell Biol. 1973;59:507. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leighton J, Schatz G. An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J. 1995;14:188. doi: 10.1002/j.1460-2075.1995.tb06989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J. Mol. Cell Cardiol. 2001;33:1065. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 53.Liesa M, Luptak I, Qin F, Hyde BB, Sahin E, Siwik DA, Zhu Z, Pimentel DR, Xu XJ, Ruderman NB, Huffman KD, Doctrow SR, Richey L, Colucci WS, Shirihai OS. Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation. 2011;124:806. doi: 10.1161/CIRCULATIONAHA.110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54:778. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- 55.Lynch J, Fukuda Y, Krishnamurthy P, Du G, Schuetz JD. Cell survival under stress is enhanced by a mitochondrial ATP-binding cassette transporter that regulates hemoproteins. Cancer Res. 2009;69:5560. doi: 10.1158/0008-5472.CAN-09-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maguire A, Hellier K, Hammans S, May A. X-linked cerebellar ataxia and sideroblastic anaemia associated with a missense mutation in the ABC7 gene predicting V411L. Br. J. Haematol. 2001;115:910. doi: 10.1046/j.1365-2141.2001.03015.x. [DOI] [PubMed] [Google Scholar]
- 57.Manavalan P, Dearborn DG, McPherson JM, Smith AE. Sequence homologies between nucleotide binding regions of CFTR and G-proteins suggest structural and functional similarities. FEBS Lett. 1995;366:87. doi: 10.1016/0014-5793(95)00463-j. [DOI] [PubMed] [Google Scholar]
- 58.Mitsuhashi N, Miki T, Senbongi H, Yokoi N, Yano H, Miyazaki M, Nakajima N, Iwanaga T, Yokoyama Y, Shibata T, Seino S. MTABC3, a novel mitochondrial ATP-binding cassette protein involved in iron homeostasis. J. Biol. Chem. 2000;275:17536. doi: 10.1074/jbc.275.23.17536. [DOI] [PubMed] [Google Scholar]
- 59.Miyazaki E, Kida Y, Mihara K, Sakaguchi M. Switching the sorting mode of membrane proteins from cotranslational endoplasmic reticulum targeting to posttranslational mitochondrial import. Mol. Biol. Cell. 2005;16:1788. doi: 10.1091/mbc.E04-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moody JE, Millen L, Binns D, Hunt JF, Thomas PJ. Cooperative ATP- dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters. J. Biol. Chem. 2002;277:21111. doi: 10.1074/jbc.C200228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller M, Bakos E, Welker E, Varadi A, Germann UA, Gottesman MM, Morse BS, Roninson IB, Sarkadi B. Altered drug-stimulated ATPase activity in mutants of the human multidrug resistance protein. J. Biol. Chem. 1996;271:1877. doi: 10.1074/jbc.271.4.1877. [DOI] [PubMed] [Google Scholar]
- 62.Neefjes JJ, Momburg F, Hammerling GJ. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993;261:769. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- 63.Okuda S, Yamada T, Hamajima M, Itoh M, Katayama T, Bork P, Goto S, Kanehisa M. KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 2008;36:W423–W426. doi: 10.1093/nar/gkn282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panek H, O'Brian MR. A whole genome view of prokaryotic haem biosynthesis. Microbiology. 2002;148:2273. doi: 10.1099/00221287-148-8-2273. [DOI] [PubMed] [Google Scholar]
- 65.Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV, Szakacs G, Akiyama S, Gottesman MM. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry. 2007;46:9443. doi: 10.1021/bi700015m. [DOI] [PubMed] [Google Scholar]
- 66.Pevny L, Lin CS, D'Agati V, Simon MC, Orkin SH, Costantini F. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121:163. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 67.Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 68.Polireddy K, Chavan H, Abdulkarim BA, Krishnamurthy P. Functional significance of the ATP-binding cassette transporter B6 in hepatocellular carcinoma. Mol. Oncol. 2011;5:410. doi: 10.1016/j.molonc.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pondarre C, Antiochos BB, Campagna DR, Clarke SL, Greer EL, Deck KM, McDonald A, Han AP, Medlock A, Kutok JL, Anderson SA, Eisenstein RS, Fleming MD. The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic ironsulfur cluster biogenesis. Hum. Mol. Genet. 2006;15:953. doi: 10.1093/hmg/ddl012. [DOI] [PubMed] [Google Scholar]
- 70.Pondarre C, Campagna DR, Antiochos B, Sikorski L, Mulhern H, Fleming MD. Abcb7, the gene responsible for X-linked sideroblastic anemia with ataxia, is essential for hematopoiesis. Blood. 2007;109:3567. doi: 10.1182/blood-2006-04-015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N. Engl. J. Med. 2005;352:1992. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 72.Sato K, Torimoto Y, Hosoki T, Ikuta K, Takahashi H, Yamamoto M, Ito S, Okamura N, Ichiki K, Tanaka H, Shindo M, Hirai K, Mizukami Y, Otake T, Fujiya M, Sasaki K, Kohgo Y. Loss of ABCB7 gene: pathogenesis of mitochondrial iron accumulation in erythroblasts in refractory anemia with ringed sideroblast with isodicentric (X)(q13) Int. J. Hematol. 2011;93:311. doi: 10.1007/s12185-011-0786-y. [DOI] [PubMed] [Google Scholar]
- 73.Savary S, Allikmets R, Denizot F, Luciani MF, Mattei MG, Dean M, Chimini G. Isolation and chromosomal mapping of a novel ATP-binding cassette transporter conserved in mouse and human. Genomics. 1997;41:275. doi: 10.1006/geno.1997.4658. [DOI] [PubMed] [Google Scholar]
- 74.Senbongi H, Ling F, Shibata T. A mutation in a mitochondrial ABC transporter results in mitochondrial dysfunction through oxidative damage of mitochondrial DNA. Mol. Gen. Genet. 1999;262:426. doi: 10.1007/s004380051102. [DOI] [PubMed] [Google Scholar]
- 75.Shimada Y, Okuno S, Kawai A, Shinomiya H, Saito A, Suzuki M, Omori Y, Nishino N, Kanemoto N, Fujiwara T, Horie M, Takahashi E. Cloning chromosomal mapping of a novel ABC transporter gene (hABC7) a candidate for X-linked sideroblastic anemia with spinocerebellar ataxia. J. Hum. Genet. 1998;43:115. doi: 10.1007/s100380050051. [DOI] [PubMed] [Google Scholar]
- 76.Shirihai OS, Gregory T, Yu C, Orkin SH, Weiss MJ. ABC-me: a novel mitochondrial transporter induced by GATA-1 during erythroid differentiation. EMBO J. 2000;19:2492. doi: 10.1093/emboj/19.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 78.Szakacs G, Ozvegy C, Bakos E, Sarkadi B, Varadi A. Role of glycine-534 and glycine-1179 of human multidrug resistance protein (MDR1) in drugmediated control of ATP hydrolysis. Biochem. J. 2001;356:71. doi: 10.1042/0264-6021:3560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taguchi Y, Morishima M, Komano T, Ueda K. Amino acid substitutions in the first transmembrane domain (TM1) of P-glycoprotein that alter substrate specificity. FEBS Lett. 1997;413:142. doi: 10.1016/s0014-5793(97)00899-5. [DOI] [PubMed] [Google Scholar]
- 80.Taketani S, Kakimoto K, Ueta H, Masaki R, Furukawa T. Involvement of ABC7 in the biosynthesis of heme in erythroid cells: interaction of ABC7 with ferrochelatase. Blood. 2003;101:3274. doi: 10.1182/blood-2002-04-1212. [DOI] [PubMed] [Google Scholar]
- 81.Tarasova NI, Seth R, Tarasov SG, Kosakowska-Cholody T, Hrycyna CA, Gottesman MM, Michejda CJ, Transmembrane inhibitors of Pglycoprotein. an ABC transporter. J. Med. Chem. 2005;48:3768. doi: 10.1021/jm049065t. [DOI] [PubMed] [Google Scholar]
- 82.Tsuchida M, Emi Y, Kida Y, Sakaguchi M. Human ABC transporter isoform B6 (ABCB6) localizes primarily in the Golgi apparatus. Biochem. Biophys. Res. Commun. 2008;369:369. doi: 10.1016/j.bbrc.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 83.Urbatsch IL, Julien M, Carrier I, Rousseau ME, Cayrol R, Gros P. Mutational analysis of conserved carboxylate residues in the nucleotide binding sites of P-glycoprotein. Biochemistry. 2000;39:14138. doi: 10.1021/bi001128w. [DOI] [PubMed] [Google Scholar]
- 84.van Roermund CW, Visser WF, Ijlst L, van CA, Boek M, Kulik W, Waterham HR, Wanders RJ. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- 85.Wang L, He F, Bu J, Liu X, Du W, Dong J, Cooney JD, Dubey SK, Shi Y, Gong B, Li J, McBride PF, Jia Y, Lu F, Soltis KA, Lin Y, Namburi P, Liang C, Sundaresan P, Paw BH, Li DY, Phillips JD, Yang Z. ABCB6 Mutations Cause Ocular Coloboma. Am. J. Hum. Genet. 2012;90:40. doi: 10.1016/j.ajhg.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, Tsuruo T, Inazawa J. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- 87.Young L, Leonhard K, Tatsuta T, Trowsdale J, Langer T. Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science. 2001;291:2135. doi: 10.1126/science.1056957. [DOI] [PubMed] [Google Scholar]
- 88.Zhang F, Hogue DL, Liu L, Fisher CL, Hui D, Childs S, Ling V. M-ABC2 a new human mitochondrial ATP-binding cassette membrane protein. FEBS Lett. 2000;478:89. doi: 10.1016/s0014-5793(00)01823-8. [DOI] [PubMed] [Google Scholar]
- 89.Zutz A, Gompf S, Schagger H, Tampe R. Mitochondrial ABC proteins in health and disease. Biochim. Biophys. Acta. 2009;1787:681. doi: 10.1016/j.bbabio.2009.02.009. [DOI] [PubMed] [Google Scholar]