Abstract
Mast cells (MC) have been shown to mediate regulatory T-cell (Treg) dependent, peripheral allograft tolerance in both skin and cardiac transplants. Furthermore, Treg have been implicated in mitigating IgE mediated MC degranulation, establishing a dynamic, reciprocal relationship between MC and Treg in controlling inflammation. In an allograft tolerance model, it is now shown that intragraft or systemic MC degranulation results in the transient loss of Treg suppressor activities with the acute, T-cell dependent rejection of established, tolerant allografts. Upon degranulation, MC mediators can be found in the skin, Treg rapidly leave the graft, MC accumulate in the regional lymph node and the Treg are impaired in the expression of suppressor molecules. Such a dramatic reversal of Treg function and tissue distribution by MC degranulation underscores how allergy may causes the transient breakdown of peripheral tolerance and episodes of acute T-cell inflammation.
Introduction
Mast cells (MC) are best known for their role in allergies and protecting our body from parasitic, bacterial and viral infection(1). IgE antibody against the allergen or infectious agent binds to the high affinity IgE receptor on MC. Subsequent encounter with allergen leads to release of the MC granular content with heightened inflammation. While the historical role of MC has been as regulators of inflammatory responses, MC have been recently been implicated as regulators of tolerance(2, 3). The striking contrast in MC function is exemplified by their pro-inflammatory roles in nematode infection and allergies(1) or their role in mediating suppression, as in UV-B damage(4), mosquito bites(5) and graft tolerance(6, 7). Recently, the pivotal role of MC in the establishment of acquired tolerance to an allograft was shown(6, 7). It was hypothesized that the secretion of immunosuppressive mediators by MC was critical for sustaining tolerance.
Recently, the dynamic and reciprocal nature of MC-Treg interactions was shown. It was reported that Treg can suppress IgE mediated degranulation through OX40-OX40L interactions(8, 9) thereby showing a natural mechanism for MC stabilization. It is clear that the release of inflammatory mediators as a consequence of MC degranulation results in inflammation. How this impacts peripheral tolerance and Treg function is not known. Therefore, studies were designed to determine if degranulation of MC present in tolerant allografts would impact on allograft survival. Data presented show that IgE-mediated degranulation, either within the graft or systemically, breaks established peripheral tolerance and leads to T-cell mediated, acute rejection of the allograft. Degranulation causes release of MC intermediaries, a rapid migration of both Treg and MC from the graft as well as a transient demise in the expression of Treg suppressive cytokines. Such a dramatic reversal of Treg function and tissue distribution by MC degranulation underscores how allergy may cause the transient breakdown of peripheral tolerance and episodes of acute T-cell inflammation.
Material and methods
Mice
C57Bl/6, CB6F1 (C57Bl/6xBALB/c hybrid), C57BL/6-Ly5.2+, and C57BL/6-Rag-/- mice were purchased from the Jackson Laboratory. FoxP3/GFP reporter mice were provided by Dr. A. Rudensky (University of Washington School of Medicine, Seattle, WA)(23).
Skin graft model
Skin grafting was performed described previously(43). For dual grafting, the first graft was placed on the back near the base of the tail, whereas the second graft was placed on the back close to the neck two weeks later. Grafts were monitored for rejection for 30 days post-degranulation and were considered rejected when 80% of the original graft disappeared or became necrotic.
Degranulation
Chemical degranulation was done by application of 50μl Compound 40/80 (1mg/ml, Sigma) directly under the graft. “Active” immunization was achieved by 100μg of OVA/Alum (Pierce) i.p., 37 days prior to grafting or passive by transfer of IgE. For passive immunization 2 or 5μg of either OVA-specific IgE (clone 2C6; Serotec) or TNP-specific IgE (clone A3B1; cross-reactive with NP) was given intravenously 24h prior to degranulation, respectively. Degranulation was induced either locally (50μl of 1mg/ml OVA in PBS) or systemically (500μl of 1mg/ml OVA in PBS intraperitoneal) for mice that received OVA-IgE or were active immunized. Degranulation in mice that received NP-IgE was done by injecting 20ng of NP17-OVA/NP23-BSA locally. Blocking of degranulation was done by subcutaneous injection of 100μl of Cromolyn Sodium Salt (39mM in PBS, Sigma-Aldrich) 30 minutes prior to degranulation.
Cytokine profile of the graft
Grafts were collected, cut to small pieces in HBSS (6 grafts/ml) 18h post-degranulation and incubated for 1h at 37°C. Cytokines in the supernatants were determined by multiplex analysis(Biorad) and verified by ELISAs (IL4, IL6, IL10(Pharmingen), IL9(PeproTech) and TNFα(eBioscience)).
Induction of inflammation
Mice were either treated with 200μl complete Freund's adjuvant (Sigma), 50μg TLR4 agonist (LPS, E. coli 055:B5, Sigma) or 50μg TLR9 agonist (CpG; ODN-1826, Eurogentech) by intraperitoneal injection. For induction of local inflammation 8μl of 5mg/ml FITC(Sigma) in 1:1 acetone(Fisher scientific)/dibutylphthalate(Sigma) was applied directly onto the pre-shaved graft. Positive controls included degranulation after passive immunization or intraperitoneal injection of 50μg of agonistic αCD40 (FGK; BioExpress).
Immunohistochemistry
Tissues were snap-frozen and 8μm sections were fixed with methanol prior to staining with α-CD117-A647. Hoechst was used for nuclear staining. Slides were analyzed by confocal microscope (LSM510Meta, Zeiss) at a 100× magnification. To analyze granulated MC, slides were stained with toluidine blue(Sigma). Toluidine blue-positive MC numbers in skin were determined by counting the number of stained MC in seven randomly chosen fields at 100x magnification by two independent observers using an Olympus DP70 camera system operating on a BX60 transmitted microscope.
Cell isolation and analysis
Naïve T-cells were isolated from pooled spleen and LN. Prior to cell sorting, T-cells were pre-enriched by CD4 negative selection (biotin-selection kit, Stemcell). Isolation of graft T-cells from FoxP3-GFP mice was performed by digestion with DNAse, Liberase and Collagenase (10mg/ml, Roche) for 45 minutes at 37°C after weighing the grafts. Analysis was performed after co-staining with CD4(RM4-5, Pharmingen) by flow-cytometry. For determination of the total number of MC in the dLN, samples were stained with CD117(clone: 2B8) and FcεRI(MAR-1, eBioscience) and analyzed by flow-cytometry.
T-cell studies in vivo
At day 27 and 35 mice were treated with 300μl intraperitoneal and 50μl locally at the site of the graft with mixture of αCD4(GK1.5) and αCD8(2.43) antibodies (kind gift from Dr. MJ Turk, Dartmouth, NH, USA) to deplete T-cells. Depletion was confirmed at day 37. For the transfer of tolerance experiments, grafts dLN were collected at day 35 and T-cells purified by two rounds of CD4 negative depletion(StemCell). RAG-/- received an F1 graft two weeks prior to adoptive transfer of 1×106 purified T-cells by i.v. injection.
Functionality of regulatory T-cells in vitro and in vivo
One day post-degranulation FoxP3-GFP+ T-cells from the graft dLN were FACS sorted. For in vitro studies, Ly5.2+ naïve T-cells were purified by FACS sorting and labeled with carboxyfluorescein succinimidyl ester (5μM, CFSE). In total 5×104 were cultured with T-depleted irradiated Ly5.1+ splenocytes (3000rad) at different ratios of Treg:Tnaïve. After 3 days CFSE dilution, profiles were analyzed within the Ly5.2+ population. For in vivo functionality, GFP+/Ly5.1+ Treg were mixed at different ratios naïve, polyclonal T-cells and adoptively transferred into F1 pregrafted RAG-/-. Phenotypic analysis of Treg was performed by staining T-cells of the dLN after CD4 enrichment. All samples were stained with FoxP3 in either FITC or PE depending on the co-staining. FoxP3+ cells were analyzed for the expression of Ki67 (Ki67 staining kit, BD), CTLA4(UC10-4F10-11, Pharmingen), ICOS(15F9, eBioscience), CD62L(MEL-14, BioLegend), CD73(clone TY/11.8, eBioscience), CD103(2E7, eBioscience), GITR(YGITR 765, BioLegend), IL7Rα(CD127; A7R34, eBioscience). For RT-PCR analysis FoxP3+ Treg from the grafted FoxP3-GFP mouse were flow sorted. The primers used for estimates of gene expression were: TGF-β1 forward: 5′-ttgcttcagctccacagaga-3′, TGF-β1 reverse: 5′-tggttgtagagggcaaggac-3′, IL-10 forward: 5′-ggttgccaagccttatcgga-3′, IL-10 reverse: 5′-acctgctccactgccttgct-3′, Ebi3 forward: 5′-AGCAGCAGCCTCCTAGCCT-3′, Ebi3 reverse: 5′-gcggagtcggtacttgagag-3′, granzymeB forward: 5′-ctccacgtgctttcaccaaa-3′, granzymeB reverse: 5′-aggatccatgttgcttctgtagttag-3′.
Statistics
All statistics have been calculated using the prism 4.03 software package (GraphPad Software, San Diego California USA). Survival data were analyzed using the Kaplan-Meier method, with the Wilcoxon rank test and the log-rank test. Luminex data were analyzed by the immune monitoring laboratory of the Norris Cotton Cancer Center/Dartmouth Hitchcock Medical Center and are shown as mean +/- SD whereas other data are expressed as mean +/- SEM. The latter were analyzed by one-tailed ANOVA and post-tested by Tukey analysis. Statistical significance was calculated for a 95% confidence interval (P<0.05). The exact p-values are denoted in the figures.
Results
MC degranulation results in the rejection of an established tolerant allograft
Previously we showed that in mice tolerized with αCD154 and donor specific transfusion (DST) both Treg and MC accumulate in the tolerant allograft and are functionally critical for allograft persistence(7). Herein, it was asked if IgE-mediated MC degranulation and the release of inflammatory mediators would alter Treg function, distribution and impact on allograft persistent in the tolerant host.
Both tolerized and syngeneic control mice were treated as shown in figure 1A, unless stated differently(10-12). Within 15 days, all tolerized mice bearing an allogeneic skin graft in which MCs had been degranulated lost their grafts. Syngeneic mice maintained their grafts for the duration of the experiment regardless of treatment(Fig.1B). These results indicate that degranulation of MC facilitates the rejection of allogeneic skin grafts in tolerized hosts. Both active immunization with OVA-Alum 30 days prior to grafting, followed by challenge with OVA as well as chemically induced (compound 40/80)(13, 14) degranulation showed the same results as with passive immunization(Supplementary fig.1A and 1B, respectively). Cytokines known to be abundant after IgE mediated MC degranulation were increased in groups where MCs had been degranulated(Fig.1C), confirming that the MC released their granular content and established a pro-inflammatory Th2-biased micro-environment similar to that observed in allergies(15).
Figure 1. Degranulation of mast cell leads to acute rejection of established tolerant skin grafts.
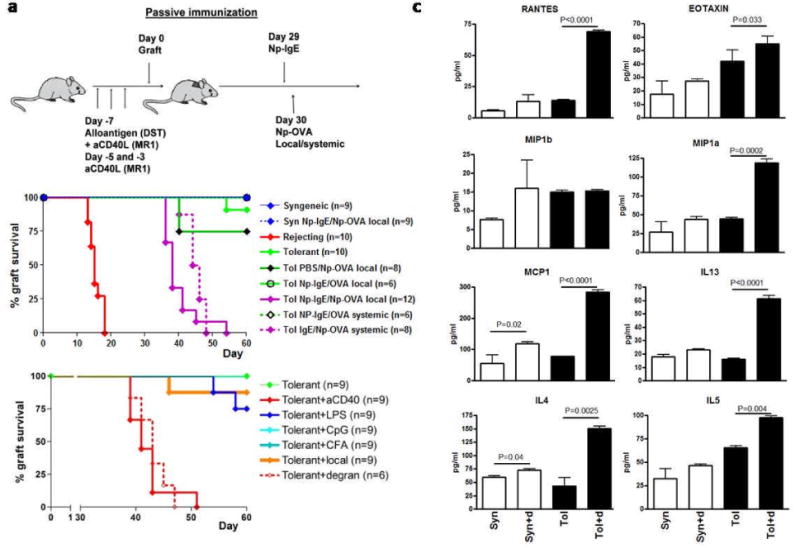
A. To induce tolerance, C57BL/6 mice were administered a regimen of donor specific transfusion (DST) of allogeneic splenocytes and anti-CD154 (200μg i.p.). Subsequently, mice received an allogeneic skin graft and survival was monitored. These mice maintain the graft for up to 100 days post-grafting, with day 0 designated as the day the mice received the graft. MCs were degranulated in mice 30 days after engraftment receiving the tolerance-inducing regiment and an allograft, in all studies described. At day 0 mice received either an CB6F1 graft (designated tolerant/tol) or a syngeneic (syn) B6 graft. Syngeneic and rejecting mice did not receive pretreatment. At day 29 mice were sensitized with 5μg of NP specific IgE (NP-IgE) and 24 hours later challenged with NP17-OVA or NP23-BSA, systemically (20ng/mouse intraperitoneal) or locally (2ng/mouse intragraft). Graft rejection was monitored for another 30 days. B. Survival of grafts for both systemic and local treatment. Controls included were either sensitized with PBS followed by local challenge with NP17-OVA or sensitized with NP-IgE followed by local challenge with 20ng of OVA. The total number of mice pooled from multiple independent experiments is shown in brackets. C. Tolerant and syngeneic with and without degranulation were collected 18 hours after degranulation and incubated in HBSS for 1h at 37°C. Supernatants were collected and tested for functionality in a proliferation assay (data not shown). Cytokine analysis was performed by multiplex and results were confirmed by ELISA. Shown are chemokines and cytokines known to be abundantly present under allergic conditions(15). D. In order to see whether degranulation induced rejection was solely the effect of inflammation various strategies were used to induce overt inflammatory responses. Mice were treated with 50μg TLR4 or TLR9 agonist, 200μl CFA or local by chemically induced inflammation. As positive controls agonistic CD40 and degranulation were included. Data shown is combined from two independent experiments.
Comparison of different inflammatory mediators in inducing graft rejection of tolerant allografts
A battery of inflammatory mediators was used to evaluate if any inflammatory insult under tolerizing conditions would inevitably lead to graft rejection. Mice were treated with inflammatory doses of the Toll-like receptor (TLR) 4 agonist, LPS; the TLR9 agonist, CpG-ODN 1826; Complete Freund's Adjuvant (CFA) or an agonistic αCD40 antibody. Finally, local, chemical-induced Th2-biased inflammation was induced using acetone/dibutylphthalate/FITC applied directly onto the graft(16-18). No acute rejection was observed in any of the groups tested except for the tolerant mice administered αCD40 antibody, as previously reported(19). These data demonstrate that the acute rejection observed after degranulation was not due to the introduction of overt inflammation per se(Fig.1D) but due to the nature of the inflammatory microenvironment created by degranulation.
Mast cell stabilization leads to prolonged allograft survival
To seek further evidence that breakdown of allograft tolerance was the effect of MC degranulation, degranulation was blocked by local intragraft injection of sodium cromoglycate (cromolyn)(20). The observed acute rejection after MC degranulation was completely blocked with the administration of cromolyn(Fig.2A) thereby confirming that the acute allograft rejection was the result of degranulation.
Figure 2. Mast cell stabilization protects against degranulation induced rejection and prolongs graft survival in general.
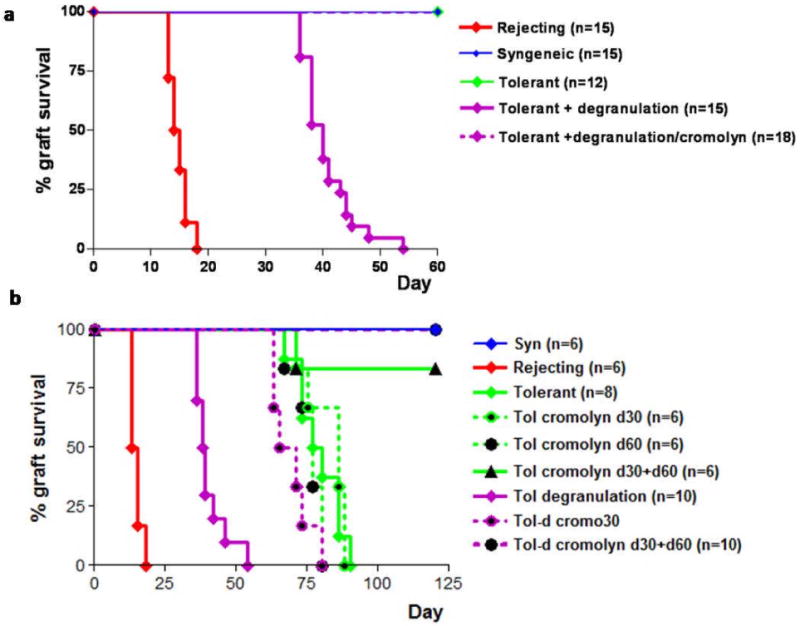
A. Mice were treated according figure 1A. However, 30 minutes prior to local challenge with 20ng of NP17-OVA one group received an intragraft injection of 100μl of a 39mM solution of cromolyn, a chemical known to stabilize MC thus preventing degranulation. The total number of mice pooled from three independent experiments is shown in brackets. B. Passively immunized mice were treated locally with Cromolyn at day 30 and/or day 60 post-grafting either with or without local degranulation. Grafts were monitored for a total of 120 days. The number of mice in each group, as shown in brackets is from multiple independent experiments. For both figure A and B the relevant syngeneic controls for this experiment did not show rejection for the entire duration of the experiments and are omitted from this figure for clarity.
In this system of allograft tolerance, peripheral tolerance begins to decay at day 70 post-grafting in tolerant mice(21). To address if limited allograft survival in tolerized mice was due to “low-grade” MC degranulation, cromolyn was applied to the allograft at day 30 and/or day 60 after engraftment. Single treatment did not impact graft survival. However, when performed on both days, allografts were maintained for >120 days even when allergic exacerbations were mimicked right after cromolyn treatment(Fig.2B). These results show that allograft survival in tolerized mice can be extended by the administration of cromolyn, and suggests that MC degranulation may contribute to the natural decay of tolerance in this system.
MC degranulation leads to an accumulation of MC in the draining lymph nodes
To define the cellular events occurring upon MC degranulation, tolerant and syngeneic skin grafts were collected at day 1 and 5 after degranulation. Degranulation leads to disappearance of MC from the allograft, as measured with by the number of c-Kit+ cells present within the graft(Fig.3A) and inversely correlated with the number of MC in the corresponding allograft-draining axillary and brachial LN as quantified by the number of c-KithighFcεRIhigh cells(Fig.3B). MC numbers were confirmed by toluidine blue staining of both graft and dLN(Supplementary fig.2). Taken together, these data suggest that upon degranulation, some MC degranulate and other MCs egress from the skin and enter the draining lymph.
Figure 3. Local IgE mediated degranulation leads to migration of MC from the graft to the draining LN.
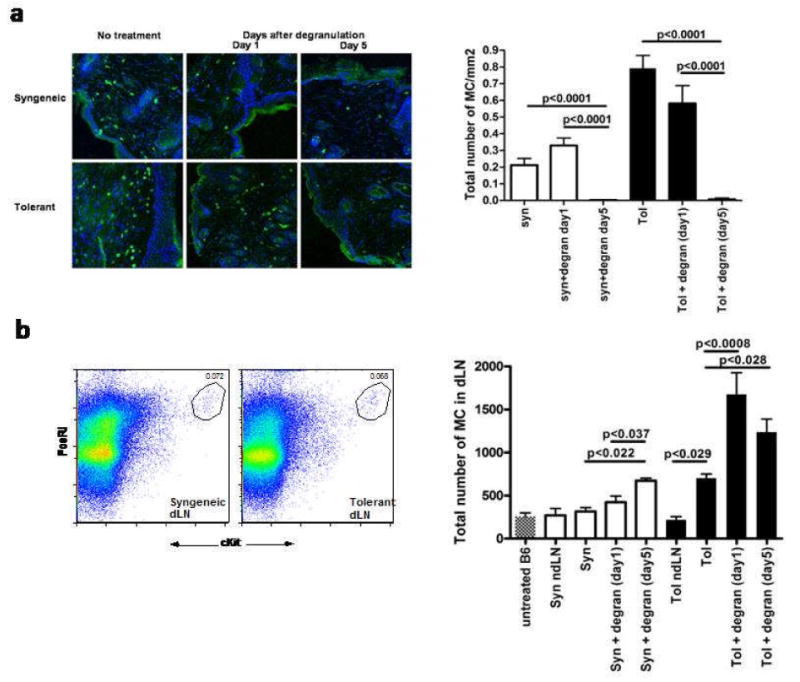
A. Grafts were degranulated locally after passive immunization. Confocal images of representative tolerant and syngeneic grafts with MC (cKit+FcεRI+) shown in green. Nuclear stain with Hoegst are shown in blue. Quantification of the number of MC in the skin was performed by counting 7 randomly chosen fields in a total of 6 individual mice. B. Flowcytometric analysis of the draining, pooled inguinal and axillary, lymph nodes. After digestion with DNAse/liberase single cell suspensions were counted and stained with cKit and FcεRI. A representative plot is showing MC in the gate after pre-enrichment with FcεRI-PE on the left. Cell counts were performed before and after enrichment to calculate the absolute number of MC (cKithighFcεRIhigh) in the dLN as shown on the right. Data is combined from 3 independent experiments with a total of 9 mice.
MC degranulation-mediated rejection is dependent on T-cells
In order to address whether the rejection observed after MC degranulation was dependent upon T-cells, CD4+ and CD8+ T-cells were eliminated with depleting antibodies. MC degranulation in the absence of T-cells did not lead to graft loss(Fig.4A). Similar results were obtained using compound 40/80 to degranulate the MC(Supplementary fig.3). This demonstrates that T-cells were mediating graft rejection after degranulation.
Figure 4. Break of peripheral tolerance by degranulation is T-cell dependent.
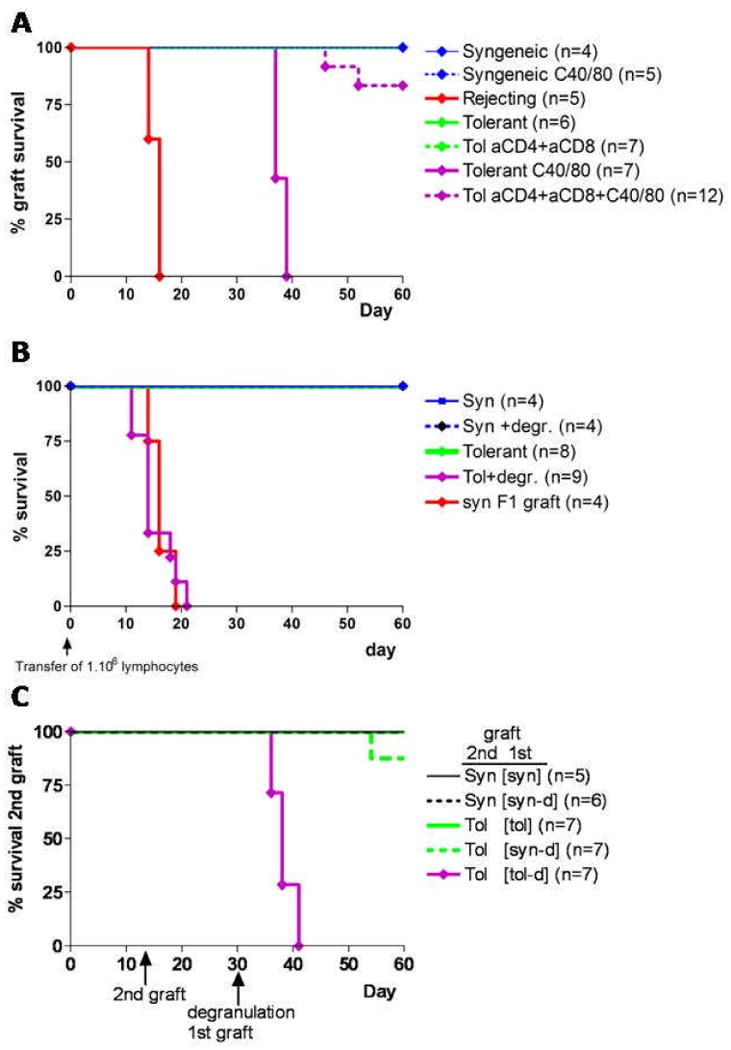
A. Graft survival after depletion of CD4 and CD8 T-cells with αCD4 and αCD8 antibodies (300μl intraperitoneal and 50μl local) 2 days prior to and 5 days after local degranulation at day 30. Grafts were monitored for another 30 days after degranulation and the data shown is pooled from two independent experiments. B. RAG-/- were grafted 2 weeks prior to adoptive transfer of 1.106 lymphocytes from the inguinal and axilliary draining LN of the indicated groups. One day after degranulation lymph nodes were isolated and T-cells were purified by two rounds of negative depletion yielded over 92% purity. Graft survival measurement starts at the day of transfer of lymphocytes. Pooled data is shown from in total 3 independent experiments. C. In order to see whether degranulation could lead to rejection of a graft in a distant location, mice received two graft two weeks apart. The first graft was placed close to the base of the tail and the second graft was in the neck to assure both grafts drained to separate lymph nodes. All mice receiving an allogeneic CB6F1 graft were pretreated with DST/αCD154. At day 29 mice with two accepted grafts were included in the study. After passive immunization the first graft was locally challenged. Graft survival of a secondary graft was monitored for another 30 days. In order to control for aspecific rejection due to antigen drainage to the second graft a control was included where the first graft was syngeneic, and thus would not reject by degranulation, and the second graft was a allogeneic CB6F1 graft. Treatment and source of the first graft is indicated in between squared brackets. Data is combined from multiple experiments and the total number of mice used is shown in round brackets.
MC degranulation results in a breakdown of T-cell tolerance
It is known that in this system a balance of allogeneic effector T-cells (Teff) and Treg controls graft rejection, and suggests that MC degranulation has an impact on this balance. To directly measure the intrinsic ability of T-cells from tolerant mice to mediate graft rejection or not, an adoptive transfer study was designed. Purified dLN T-cells from the different groups were transferred into RAG-/- mice bearing an allograft. Adoptive transfer of CD4+ T-cells from tolerized mice after local MC degranulation rejected the allografts in the secondary recipients(fig.4B). These data show that MC degranulation restores alloreactive T-cell responses in a tolerized mouse.
Regional degranulation results in the systemic decay of peripheral tolerance
Studies were designed to address whether local MC degranulation would facilitate a breakdown in allograft tolerance at the systemic level. To test this, tolerized mice received two skin allografts, one at day 0 and one at day 14. The first allograft (graft #1) was draining into the inguinal lymph nodes. The second graft (graft #2) drained to the brachial and axillary lymph nodes (day14)(22). At day 30 graft #1 was challenged after passive immunization and rejected at the expected kinetics. Interestingly, graft #2 was also rejected after a delay of about 1-2 days(Fig. 4C). As a control to ensure local degranulation of graft #1, a cohort of mice received a syngeneic graft #1 and an allogeneic graft #2. Local degranulation of the syngeneic graft did not lead to rejection of the allogeneic graft confirming allergen was not reaching the other graft. Together, these data demonstrate that local MC degranulation leads to a systemic breakdown of peripheral tolerance.
Degranulation facilitates Treg and MC loss from the graft and impairs Treg function
MCs and Treg are present in tolerant allografts and are required to maintain allograft tolerance(7). It was hypothesized that degranulation may change Treg composition and functionality, thereby altering graft longevity. Following degranulation, Treg were enumerated in the grafts by using Foxp3-GFP mice(23) as hosts. At day 1 following MC degranulation, a dramatic reduction of CD4+Foxp3+ was observed in allografts(Fig.5A). At this time point, MCs were still present in the allograft(Fig.3A), demonstrating that the efflux of Treg precedes the efflux of MC from the skin allograft following MC degranulation.
Figure 5. Mast cell degranulation leads to efflux of Treg from the graft and a transient block in regulatory T-cell function.
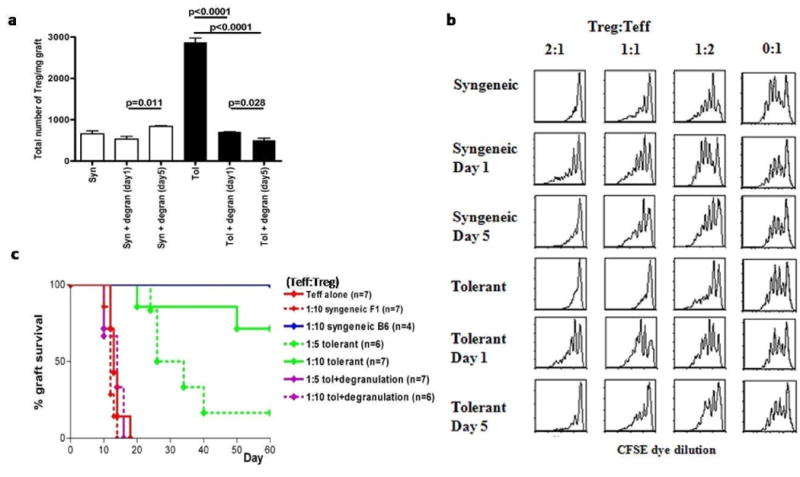
A. Quantification of CD4+FoxP3+ T-cells (Treg) in the grafts of tolerized and syngeneic mice with and without local degranulation after passive immunization. Grafts were collected and individually weighted before digestion with DNAse/Dispase/Collagenase. Single cell suspension were counted and stained with CD4 before analysis by flowcytometry. Total number of Treg/mg graft tissue is shown for a total of 6 mice from 2 independent experiments. B. Following degranulation of tolerized, allografted mice, Ly-5.2+ Treg were harvested from the dLNs of Foxp3-GFP mice and FACS-purified based on GFP expression at day 1 or day 5 after local degranulationto be used in a standard suppressor assay. Briefly, naïve CD4 T-cells from wildtype, C57Bl/6 mice (Teff cells), expressing Ly5.1 were purified and labeled with CFSE. FACS-purified Ly5.2 Treg based from either degranulated or non-degranulated hosts were mixed at different ratios with Teff cells, and Treg dependent suppression was measured by CFSE dye dilution. Grafting of mice was staggered in order to be able to isolate the Treg on the same day. Cells were flow sorted and mixed with congenically (Ly5.2+) marked CFSE labeled naïve polyclonal T-cells at the indicated ratios. After 4 days of culture the Ly5.2+ cells were analyzed for CFSE dye dilution. Shown are representative histograms of multiple independent experiments. C. Treg were purified from tolerized, allografted FoxP3+-GFP mice which were untreated or degranulated with allergen. As in figure 5B, 24 hours after degranulation, Treg from tolerized controls or degranulated, tolerized groups were FACS purified based on GFP expression. Treg from each of these groups were mixed at ratios with WT, Teff (Teff: Treg) to be used in an in vivo suppressor assay. RAG-/- mice were grafted 2 weeks prior to adoptive transfer of in total 1.106 lymphocytes. Grafts were monitored for rejection for 60 after transfer of the T-cells. Data is pooled from 3 independent experiments with the total number of mice shown in brackets.
In addition to the altered distribution of Treg, the in vitro and in vivo functions of Treg were also evaluated following MC degranulation. Treg function was measured in vitro by a suppressor assay. Treg from both tolerized, allografted mice and from those bearing syngeneic grafts suppressed WT Teff proliferation, as measured by CFSE dye dilution. However, Treg from mice whose MC were degranulated 1 day prior, were less effective at suppressing Teff proliferation(Fig.5B). The impairment in Treg suppression was transient, because Treg harvested at day 5 post-degranulation, regained their ability to suppress Teff indistinguishably from the non-degranulated controls. These results indicate that MC degranulation leads to a transient reduction in Treg function. Furthermore, Treg function was also impaired after MCs were degranulated in mice bearing syngeneic grafts, suggesting this is a general mechanism of immune regulation mediated by MC. Of note, Teff in the same groups as tested in Fig.5B could still be suppressed by WT-Treg(data not shown).
To examine whether Treg after MC degranulation are impaired in vivo, an in vivo Treg suppressor assay was performed. Treg were mixed with WT-Teff and adoptively transferred into pre-grafted RAG-/- mice. The majority of mice which received Treg from non-degranulated mice at a 1:10 Teff:Treg ratio, retained their allogeneic grafts for the duration of experiment. However, grafts were rejected when Treg were isolated after MC degranulation at the same ratio of Teff:Treg(Fig.5C). In sum, these data indicate that after MC degranulation, Treg transiently lose their ability to mediate graft survival.
MC degranulation leads to a reduced expression of key Treg immunomodulatory molecules
To define the underlying molecular mechanisms for the loss in Treg function after degranulation, the expression of key surface molecules and cytokines was analyzed. Comparative FACS analysis revealed no changes in the expression of markers involved in maturation/migration (CD62L, CD103), peripheral homeostasis (IL7αR), proliferation (Ki67) and contact dependent suppression (CTLA4, ICOS, CD73, GITR). Additionally, the level of FoxP3 expression was not changed as shown by histogram overlay and bar diagram of the MFI for the individual conditions(Fig.6A). Together, these results indicate that the prominent Treg markers noted are not affected by MC degranulation.
Figure 6. Degranulation blocks transcription of multiple important mediators used by Treg for suppression.
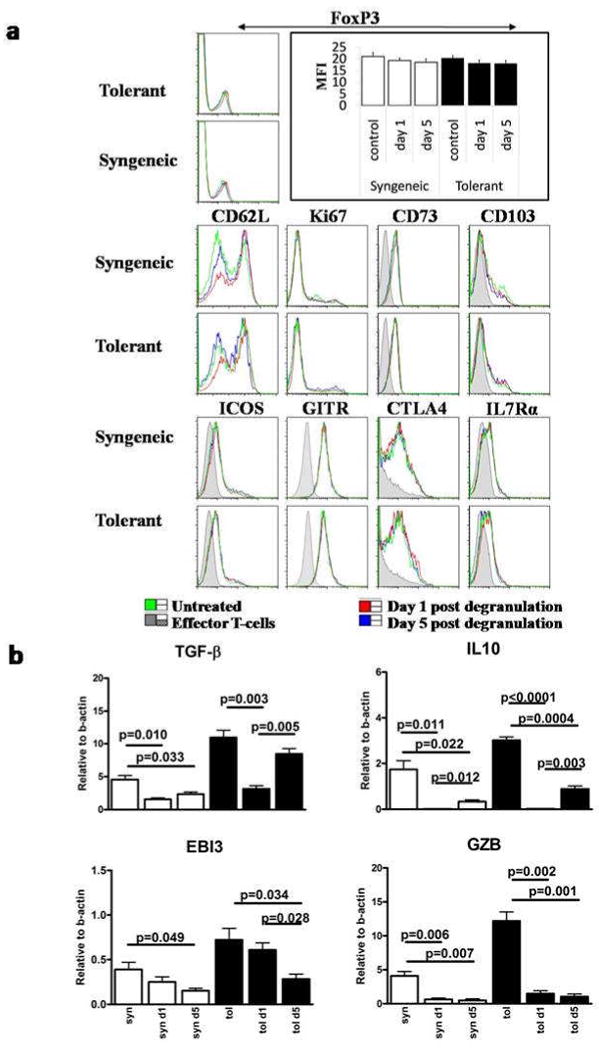
A. Phenotypic analysis of Treg isolated from the draining lymph nodes of treated and control mice by flowcytometry. T-cells were isolated from the draining, brachial and axillary, lymph nodes of the graft at day 1 and day 5 after degranulation and grafting was staggered in order to analyze all sample on the same day. Single cell suspensions were stained with CD4 and FoxP3 in combination with the shown markers. Histograms shown are from CD4+/FoxP3+ lymphocytes except for the FoxP3 histogram where all CD4+ cells within the lymphocyte gate are shown with the mean fluorescence intensities (MFI) of FoxP3 in the bar diagram. B. RT-PCR for TGFβ, IL10, EBI3 and GzB on flow sorted Treg of the dLN of FoxP3-GFP mice one and five days after degranulation. In every experiment mice were pooled in order to get sufficient numbers of Treg. Mean and SEM are calculated from triplicate wells.
The expression of suppressive mediators molecules (TGFβ, IL10, Granzyme B(GzB) and EBI3 (Ebstein Bar virus Induced gene 3)) secreted by FACS-purified FoxP3-GFP Treg were determined by RT-PCR. TGFβ mRNA expression was reduced over 50% in Treg harvested 24 hours after MC degranulation but was restored at day 5 post-degranulation when compared to Treg from non-degranulated controls. The mRNA levels of IL10 and GZB were nearly absent at 24 hours post-degranulation, with a modest recovery of expression for IL10 at 5 days post-degranulation. EBI3, however, was not impacted by MC degranulation at 24 hours post-challenge yet declined at day 5(Fig.6B). Taken together, these data suggest that MC degranulation leads to the loss of multiple suppressive mediators utilized by Treg which may account for the loss in peripheral tolerance upon degranulation.
Discussion
The studies presented significantly advance our knowledge of MC-Treg interactions and how degranulation impacts on the behavior and function of Treg. The data show 1) that antibody or chemically-induced MC degranulation, either regionally or systemically leads to a T-cell-dependent loss of tolerant skin allografts, 2) the “natural” loss of tolerant allografts can be reversed by the blockade of MC degranulation in vivo, 3) degranulation of MC causes a rapid change in the cellular composition of the tolerant microenvironment, with a loss in both MC and Treg, 4) regional degranulation ultimately results in systemic breakdown in T-cell tolerance, 5) MC degranulation causes a transient loss in Treg function.
Prior studies have documented the persistence of MC in tolerant allografts and their functional involvement in allograft survival. Histological examination indicated that these intragraft MC were not degranulated(6, 7). Based on these observations, it was suggested that the secretion of immunosuppressive mediators by MC was critical for maintaining the allograft(6, 7). In light of these findings, the impact of MC degranulation on the delicate balance of Treg and Teff under tolerant conditions was investigated. The various methods used for degranulation all led to acute T-cell-dependent allograft rejection.
MC-mediated graft loss could be blocked by using the MC stabilizing agent cromolyn providing additional proof that degranulation imparts an immunostimulatory impact leading to a breakdown in tolerance. Cromolyn has been effectively and safely used for over 30 years as a prophylactic treatment in allergies(24). A recent retrospective study demonstrated that blocking MC degranulation by a single application of cromolyn is effective in preventing allergies later in life. Most likely a permanent change in the composition of T-cell compartment was induced by the brief stabilization of the MC(25). Also preservation of transplant organs is prolonged by cromolyn by protecting the tissues from ex vivo, MC mediated, ischemia(26, 27). In the study presented, while a single application of cromolyn did not impact graft survival, a sequential treatment on day 30 and 60 post-engraftment prolonged allograft tolerance over 120 days. We suspect that in the absence of MC stabilization erosion of allograft tolerance over time as seen in this model could be partially explained by low levels of MC degranulation occurring even in the absence of allergens.
The regional or systemic delivery of allergen results in the tempered degranulation of MC with a significant proportion of MC retaining their granule content. MC degranulation resulted in the sequential egress of Treg and MC from the allograft. Unresolved is the role of MC graft egress and putative LN accumulation to the process of graft rejection. However, the lack of complete degranulation of the MC upon administration of allergen may be the result of the immunosuppressive impact that Treg exert on MC degranulation. Recent evidence has shown that Treg, can suppress MC degranulation by contact dependent OX40-OX40L interactions as well as by a contact independent manner through IL10 secretion in vitro(28, 29, 8). However, even in the absence of Treg, MC degranulation is likely never complete since only a 50% reduction of granulated MC has been reported after C40/80 treatment(30).
It has been previously reported that the adoptive transfer of Treg from untreated animals can suppress ongoing allergy(32, 33). This observation suggests a functional defect in the Treg compartment during allergy. No phenotypic differences were observed and the level of FoxP3 expression was similar at all analyzed time points. This is in contrast to a recent report that during rejection levels of FoxP3 of Treg are downregulated(34). Either this could be induced by using a different transplant model or degranulation leads to a different type of rejection as normal allograft rejection based on minor histocompatibility differences. Both in vitro and in vivo analyses revealed a temporary impaired ability of the Treg to suppress Teff responses after degranulation. This suggests a regulatory role for MC in Treg functionality. The underlying cause for the loss of Treg suppression may be due to the loss of expression of GZB as well as TGFβ, and/or IL10. Many studies have examined the function of Treg in atopic patients, but the findings from these studies are inconclusive(34-39). Some of the discrepancies from these studies can be explained, however, by the observation herein that there is only a brief window of time where the Treg lose their suppressive function after degranulation.
The findings over the past 5 years underscore the enormous plasticity of the MC compartment and their impact on acquired immunity and acquired immune privilege(40). While MC have been extensively described in type I hypersensitivity diseases as being pro-inflammatory, it is evident that MC control the development and persistence of peripheral tolerance and immunity(6, 7). Unlike T-cells, which can be decisively segregated into multiple effector and regulatory subsets, there are no defined subsets of functionally diverse MC(2). Emerging studies implicate MC as accessory cells for both Teff and Treg alike to mediate inflammation and suppression, respectively. The findings of this study are the first to show the reciprocal impact of MC degranulation on Treg function. The implications in allergy and in peripheral tolerance are significant. The clinical relevance for the presented data in regards to allograft transplant recipients becomes evident when the data from a retrospective study in patients who received a kidney transplant is taken into consideration. Atopic individuals, in this case allergic rhinitis, with a kidney transplant show more severe and acute episodes of rejection then individuals without any history of allergy(41). These data are concordant with our observation that MC degranulation breaks peripheral tolerance systemically.
In closing, with the increasing prevalence of allergies in Western countries,(42) the durability of allografts in atopic patients may suffer increasing jeopardy. Long term MC stabilization in transplant recipients could be beneficial in maintaining intact MC and functional Treg at the site of the graft and preventing temporal impairment of suppression due to degranulation. Since the regulation of Treg function seems to be a one component of MC function, defining MC products leading to down regulation of suppressive pathways in Treg will be a great asset for developing new treatment strategies in a variety of diseases where either loss of suppression needs to be restored or ablated. Altogether, MC seem to be able to regulate the immune response in general and Treg function in particular, either by supporting tolerance via secretion or by inducing inflammation via degranulation.
Supplementary Material
Supplementary figure 1: Degranulation by either compound 40/80 or by active immunization leads to acute rejection of established tolerant grafts. A. Mice were treated locally with 50μl of the degranulating agent compound 40/80 (C40/80; 1mg/ml) at day 30 post grafting. Grafts were followed for rejection for another 30 days. B. Mice were immunized intraperitoneally with 100μl OVA-Alum (1:1 emulsion of 1mg/ml OVA in Aluminium hydroxide) 37 days prior to grafting. Serum levels of IgE at day -7 were comparable to untreated control mice (data not shown). At day 30 post grafting local intragraft administration of 20ng OVA was used to induce antigen specific degranulation of the MC. Sensitized controls were challenged with 20ng of an irrelevant protein (BSA) whereas non-sensitized Alum treated animals received 20ng of OVA. Grafts were followed for another 30 days post-degranulation. The total number of mice shown in brackets is pooled from 2 independent experiments.
Supplementary Figure 2Degranulation under tolerant conditions is not complete Grafts and draining lymph nodes were collected from tolerized mice or from mice that were sensitized with NP-IgE at day 30 and challenged with NP17-OVA 24 hours later. Snap frozen tissues were cut and the 8μm thick sections were stained with toluidine blue. (200x magnification)
Supplementary figure 3: Chemically induced degranulation leads to a T-cell dependent break of tolerance. Graft survival after treatment with αCD4 and αCD8 antibodies (300μl intraperitoneal and 50μl local) 2 days prior to and 5 days after local chemical degranulation with 50μl C40/80 (1mg/ml) at day 30. Grafts were monitored for another 30 days after degranulation and the data shown is pooled from two independent experiments with the total number of mice shown in brackets.
Acknowledgments
The authors would like to thank Gary A. Ward for assistance in cell sorting, Jose R. Conejo-Garcia for the expertise in bright field microscopy and Kenneth A. Orndorff for assistance in confocal miscroscopy.
Funding: This work was supported by a grant from the National Institutes of Health (NIH AI048667)
Footnotes
Author Contributions: VdV conducted most of the experiments and wrote the manuscript. AW performed the toluidine blue staining and quantified the MC in histological slides. KB prepared and assisted with the surgeries. TW purified and supplied Np-IgE. MB, RE and RN provided discussions and final editing of the manuscript. VdV and RN designed and discussed the experiments. All authors have read and commented on the manuscript prior to submission.
Disclosure: The authors have no conflicting financial interests.
References
- 1.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19(1):31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cell : negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8(6):478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cell in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 4.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8(10):1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 5.Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176(7):4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 6.Boerma M, Fiser WP, Hoyt G, Berry GJ, Joseph L, Joseph J, et al. Influence of mast cell on outcome after heterotopic cardiac transplantation in rats. Transpl Int. 2007;20(3):256–265. doi: 10.1111/j.1432-2277.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cell are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 8.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T-cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29(5):771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendel I, Shevach EM. Activated T-cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology. 2006;117(2):196–204. doi: 10.1111/j.1365-2567.2005.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, et al. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest. 1996;97(6):1398–1408. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur A, Van Ness BG, Lynch RG. In vivo and in vitro regulation of IgE production in murine hybridomas. J Immunol. 1990;145(11):3610–3617. [PubMed] [Google Scholar]
- 12.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116(3):833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cell. Nature. 1988;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- 14.WoldeMussie E, Moran NC. Histamine release by compound 48/80: evidence for the depletion and repletion of calcium using chlortetracycline and 45calcium. Agents Actions. 1984;15(3-4):267–272. doi: 10.1007/BF01972361. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38(12-13):881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 16.Choquet-Kastylevsky G, Descotes J. Popliteal lymph node responses to acetone and ethanol differ from those induced by streptozotocin. Arch Toxicol. 2004;78(11):649–654. doi: 10.1007/s00204-004-0582-z. [DOI] [PubMed] [Google Scholar]
- 17.Chun KH, Imai Y, Higashi N, Irimura T. Migration of dermal cells expressing a macrophage C-type lectin during the sensitization phase of delayed-type hypersensitivity. J Leukoc Biol. 2000;68(4):471–478. [PubMed] [Google Scholar]
- 18.Takeshita K, Yamasaki T, Akira S, Gantner F, Bacon KB. Essential role of MHC II-independent CD4+ T-cells, IL-4 and STAT6 in contact hypersensitivity induced by fluorescein isothiocyanate in the mouse. Int Immunol. 2004;16(5):685–695. doi: 10.1093/intimm/dxh073. [DOI] [PubMed] [Google Scholar]
- 19.Buhlmann JE, Gonzalez M, Ginther B, Panoskaltsis-Mortari A, Blazar BR, Greiner DL, et al. Cutting edge: sustained expansion of CD8+ T-cells requires CD154 expression by Th cells in acute graft versus host disease. J Immunol. 1999;162(8):4373–4376. [PubMed] [Google Scholar]
- 20.Mazurek N, Berger G, Pecht I. A binding site on mast cell and basophils for the anti-allergic drug cromolyn. Nature. 1980;286(5774):722–723. doi: 10.1038/286722a0. [DOI] [PubMed] [Google Scholar]
- 21.Quezada SA, Fuller B, Jarvinen LZ, Gonzalez M, Blazar BR, Rudensky AY, et al. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102(5):1920–1926. doi: 10.1182/blood-2003-02-0586. [DOI] [PubMed] [Google Scholar]
- 22.the description I had was exactly pinpointing the location as used in medicine
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T-cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Murphy S, Kelly HW. Cromolyn sodium: a review of mechanisms and clinical use in asthma. Drug Intell Clin Pharm. 1987;21(1 Pt 1):22–35. doi: 10.1177/10600280870211p102. [DOI] [PubMed] [Google Scholar]
- 25.Korppi M. Disodium cromoglycate in asthma--worth to be re-appraised. Allergol Int. 2008;57(2):183. doi: 10.2332/allergolint.L-07-10. [DOI] [PubMed] [Google Scholar]
- 26.Barr ML, Carey JN, Nishanian GP, Roberts RF, Sakamaki Y, Darbinian SH, et al. Addition of a mast cell stabilizing compound to organ preservation solutions decreases lung reperfusion injury. J Thorac Cardiovasc Surg. 1998;115(3):631–636. doi: 10.1016/S0022-5223(98)70328-9. discussion 636-637. [DOI] [PubMed] [Google Scholar]
- 27.Vural KM, Oz MC, Liao H, Batirel HF, Pinsky DJ. Membrane stabilization in harvested vein graft storage: effects on adhesion molecule expression and nitric oxide synthesis. Eur J Cardiothorac Surg. 1999;16(2):150–155. doi: 10.1016/s1010-7940(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J, et al. Cutting edge: CD4 T-cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180(4):2039–2043. doi: 10.4049/jimmunol.180.4.2039. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180(5):2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 30.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14(5):536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 31.Rauter I, Krauth MT, Westritschnig K, Horak F, Flicker S, Gieras A, et al. Mast cell-derived proteases control allergic inflammation through cleavage of IgE. J Allergy Clin Immunol. 2008;121(1):197–202. doi: 10.1016/j.jaci.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T-cells is interleukin 10 dependent. J Exp Med. 2005;202(11):1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T-cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122(3):617–624. doi: 10.1016/j.jaci.2008.05.048. e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T-cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavani A, Nasorri F, Ottaviani C, Sebastiani S, De Pita O, Girolomoni G. Human CD25+ regulatory T-cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J Immunol. 2003;171(11):5760–5768. doi: 10.4049/jimmunol.171.11.5760. [DOI] [PubMed] [Google Scholar]
- 36.Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T-cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34(9):1364–1372. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 37.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T-cells in pediatric asthma. J Allergy Clin Immunol. 2007;119(5):1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363(9409):608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 39.Thunberg S, Akdis M, Akdis CA, Gronneberg R, Malmstrom V, Trollmo C, et al. Immune regulation by CD4+CD25+ T-cells and interleukin-10 in birch pollen-allergic patients and non-allergic controls. Clin Exp Allergy. 2007;37(8):1127–1136. doi: 10.1111/j.1365-2222.2007.02739.x. [DOI] [PubMed] [Google Scholar]
- 40.Tiemessen MM, Van Hoffen E, Knulst AC, Van Der Zee JA, Knol EF, Taams LS. CD4 CD25 regulatory T-cells are not functionally impaired in adult patients with IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2002;110(6):934–936. doi: 10.1067/mai.2002.128856. [DOI] [PubMed] [Google Scholar]
- 41.Graca L, Chen TC, Le Moine A, Cobbold SP, Howie D, Waldmann H. Dominant tolerance: activation thresholds for peripheral generation of regulatory T-cells. Trends Immunol. 2005;26(3):130–135. doi: 10.1016/j.it.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Seung LM, Lorincz AL. Incidence of acute renal transplant rejection in atopic individuals. Arch Dermatol. 1994;130(5):584–588. [PubMed] [Google Scholar]
- 43.Ring J, Kramer U, Schafer T, Behrendt H. Why are allergies increasing? Curr Opin Immunol. 2001;13(6):701–708. doi: 10.1016/s0952-7915(01)00282-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Degranulation by either compound 40/80 or by active immunization leads to acute rejection of established tolerant grafts. A. Mice were treated locally with 50μl of the degranulating agent compound 40/80 (C40/80; 1mg/ml) at day 30 post grafting. Grafts were followed for rejection for another 30 days. B. Mice were immunized intraperitoneally with 100μl OVA-Alum (1:1 emulsion of 1mg/ml OVA in Aluminium hydroxide) 37 days prior to grafting. Serum levels of IgE at day -7 were comparable to untreated control mice (data not shown). At day 30 post grafting local intragraft administration of 20ng OVA was used to induce antigen specific degranulation of the MC. Sensitized controls were challenged with 20ng of an irrelevant protein (BSA) whereas non-sensitized Alum treated animals received 20ng of OVA. Grafts were followed for another 30 days post-degranulation. The total number of mice shown in brackets is pooled from 2 independent experiments.
Supplementary Figure 2Degranulation under tolerant conditions is not complete Grafts and draining lymph nodes were collected from tolerized mice or from mice that were sensitized with NP-IgE at day 30 and challenged with NP17-OVA 24 hours later. Snap frozen tissues were cut and the 8μm thick sections were stained with toluidine blue. (200x magnification)
Supplementary figure 3: Chemically induced degranulation leads to a T-cell dependent break of tolerance. Graft survival after treatment with αCD4 and αCD8 antibodies (300μl intraperitoneal and 50μl local) 2 days prior to and 5 days after local chemical degranulation with 50μl C40/80 (1mg/ml) at day 30. Grafts were monitored for another 30 days after degranulation and the data shown is pooled from two independent experiments with the total number of mice shown in brackets.


