Abstract
Our knowledge on the function of mast cells (MC) as part of the immune system has expanded from ‘key cells in mediating allergy’ to ‘tunable regulators of the immune response’. Over the past years however, a large body of evidence has been presented indicating a more regulatory role for MC in the immune system by both contact dependent and independent mechanisms. Considering the vast amount of soluble mediators released by MC, it is not surprising that some are involved in the maintenance of peripheral tolerance and the control or even help to resolve ongoing inflammation. In this review we will focus on the immunosuppressive function of some of these mediators produced by MC in a wide variety of disease models.
Introduction
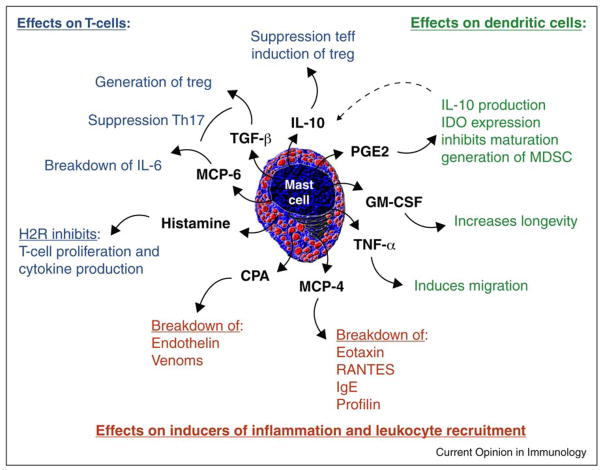
Cognizant of the enormous plasticity of MC, it is not surprising that MC can facilitate inflammation or tolerance depending upon the immunologic context [1]. Their function in mediation tolerance represents a new vision of MC partly on the basis of the discovery that MC are absolutely required to maintain immune tolerance [2]. How MC actually interact with other leukocytes to maintain peripheral tolerance has been the focus of recent research. With the capacity to produce a litany of soluble factors, and the expression of various surface molecules like MHC-II and several costimulatory molecules, MC can functionally regulate the immune response by contact independent and contact dependent mechanisms [3]. This review will focus on contact independent modes of immune regulation (Figure 1).
Figure 1.
MC induced contact independent mechanisms of immune suppression. Mast cells provide a wide range of mediators that can potentially suppress the immune response. Studies addressing these molecules have shown different modes of action targeting several aspect of inflammation. Broadly these can be categorized by (1) modulation of the inflammation inducing factors, like allergens or venoms, and recruitment of other leukocytes (red), (2) suppression of T-cell responses and/or induction of Treg (blue) or (3) changing DC function (green).
MC mediators are either stored in granules for immediate release or produced de novo. Recently, we showed that release of these mediators by IgE mediated degranulation as seen in allergies will break peripheral tolerance towards an established allograft, suggesting that immune suppression is mediated by either secretion or piece-meal degranulation [4••]. The latter process is a mechanism of releasing pre-formed mediators but does not require granule-to-plasma membrane fusion.
Cytokines
Mast cells can produce a wide variety of cytokines. Newly synthesized cytokines can be either Th1 associated (IFN-γ, IL-2, IL-3, and TNFα) or Th2 associated (IL-4, IL-5, IL-6, IL-10, and IL-13). Some cytokines are pre-made and stored in granules for immediate release upon activation (IL-4, TNFα, and GM-CSF) [5]. Of the long list of cytokines that can potentially be released by MC, IL-10 and TGFβ have received the most attention due to their already known role as immune-suppressive mediators produced by Treg.
Indeed it was found that MC derived IL-10 suppresses the skin pathology in both a model of hapten induced dermatitis and chronic UVB [6] and UVA [7] damage. Additionally, blocking migration of MC to the draining lymph nodes (dLN) of the UV damaged site abrogates UV induced immune suppression [8•]. Mosquito bites result in a similar inhibitory phenotype that is activation of MC and increased IL10 in the dLN [9], suggesting MC-derived IL10 might act local and in the dLN to suppress immunity. Additionally, IL10 down-regulates FcεRI expression thereby making MC less prone to IgE mediated degranulation [10–12]. Co-opting the immune suppression mediated by mast cell produced IL10 is also used by certain pathogens to evade the immune system. In humans the systemic dimorphic fungus paracoccidioides brasiliensis forms lesions that are characterized by a high number of IL10+ MC, especially in hot-spots of replication [13].
During the early stages of inflammation, the release of MIP-1α among others leads to the recruitment of mostly innate immune cells, among which are MC. Recently it has been shown that binding of CCR1, the receptor of MIP1α, leads to the production of IL6, TNFα and TGFβ [14]. With regard to the release of TGFβ by MC there is to our knowledge no literature available. However, many speculate on how very early events mediated by innate immune cells could influence the adaptive immune response. For instance, TGFβ promotes DC induced generation of antigen specific Treg [15]. Since Treg mediated suppression in growing tumors has been shown to be IL10/TGFβ dependent [16], it is not unrealistic that the early innate immune response to a seeding tumor is the release of MC derived IL10 and TGFβ. Especially in light of the recent report that MC derived TGFβ is required for proper wound healing after scalding [17].
MC derived TNFα on the other hand has been investigated in more detail. Membrane-bound TNFα is necessary for optimal migration of tissue DC to the dLN [18] whereas soluble TNFα is involved in the recruitment of DC to sites of inflammation [19]. The fact that MC produced TNFα controls DC migration empowers MC with enormous control over adaptive immunity. However, it is hard to predict whether the immunologic impact of MC produced TNFα is going to mediate inflammation or suppression. On the one hand, TNFα induces the production of IL-10 in monocytic cells during allergic exacerbations, which in return leads to suppression of IL-5, GM-CSF, and TNFα production [20]. On the other hand, it has been shown that TNFα is actually contributing to the severity of airway inflammation [21] and has been used as an indicator of ongoing allograft rejection [22]. With regard to the latter, this seems to be the case only in highly vascularized tissue like heart and kidney. In skin grafts we do not observe any difference in the production of tissue TNFα, between tolerant and rejecting allografts. In our own studies, MC produced TNFα in tolerant skin grafts appears to play an indispensable role in controlling graft derived DC migration (manuscript in preparation). While we typically think of TNFα as a mediator of DC maturation, ex vivo treatment of bone-marrow DC with TNFα rendered them maturation resistant. These DC were able to suppress autoimmunity when transferred by activation of auto-antigen specific Treg [23]. TNFα can signal through TNFR1 or TNFR2 of which the latter is preferentially expressed on hemapoietic cells, and has a high affinity for the membrane bound form of TNFα as used by MC to induce DC migration [18]. A recent study in a rheumatoid arthritis model clearly showed that in vivo, TNFR1 signaling is responsible for the induction of inflammation whereas the less well studied TNFR2 mediates immune suppression [24]. Thus, it seems there is a paradoxical role for TNFα which is most likely based not only on the source, the location and the timing of release but also on the difference in signaling through TNFR1 or TNFR2 as has been shown in autoimmunity [25]. It is therefore not surprising that MC, being able to present membrane TNFα and secrete TNFα, can have both positive and negative immune modulatory roles.
Another molecule produced by MC that can exert either pro-inflammatory or anti-inflammatory effects is granulocyte macrophage stimulating factor (GM-CSF). It has been known that GM-CSF can increase the survival of transplants [23] suggesting a immunosuppressive function but on the other hand is used successfully as adjuvant for anti-tumor therapy [26]. Recently, it was shown that DC cultured in low levels of GM-CSF stay immature and induced T-cell anergy [27]. We observed blocking GM-CSF locally within a tolerant skin graft leads to rejection of an established allograft. Additionally, our studies have shown that GM-CSF−/− MC reconstituted MC-deficient mice could not be tolerized suggesting that MC derived GM-CSF in this model plays an important role in the establishment of tolerance towards an allogeneic graft (manuscript in preparation). Tumor studies have taught us that GM-CSF can facilitate the accumulation of immunosuppressive myeloid derived suppressor cells (MDSC) and siRNA interference of GM-CSF production by a mammary tumor cell line showed a decrease in the MDSC and improved anti-tumor immunity [28]. GM-CSF seems to have its main effect on the CD8α− DC where it not only increases the absolute number of CD8α− DCs but also renders them maturation resistant. These DC were potent inducers of antigen specific FoxP3+ and IL10+ Treg which could suppress auto-immunity [29]. Thus, most studies show that low levels of GM-CSF have clear immunosuppressive capacities, whereas high levels of GM-CSF obtained by GM-CSF transduced high producing allogeneic tumor cells would suggest pro-inflammatory properties.
Mast cell specific proteases
The amount of proteases stored in MC is staggering, and therefore could have profound impact on the local immune response when released. The MC-specific proteases are divided in three main groups on the basis of their cleavage specificities: tryptases, chymases and MC-carboxypeptidase A (CPA) (extensively reviewed in [30]). Most members have a broad spectrum of substrates. The exact function of the proteases is still to be unraveled, but the recent generation of MC protease knock-out mice will enable us to define the role of these enzymes in vivo.
Asthma, in contrast to eosinophilic bronchitis is characterized by a large number of MC present in the airway smooth muscles (ASM) and large amounts of tryptases are found in lung biopsies of asthmatic patients. The predominant protease found in human asthmatics is beta-tryptase. Recently it has been shown that beta-tryptase can cleave both eotaxin and RANTES, recruitment factors for eosinophils, basophils and Th2 cells, explaining the low abundance of these cells in the ASM [31]. Earlier studies showed that alpha-chymases have specificity for profilin, the major allergen in pollen, thereby removing the IgE epitope needed for MC degranulation [32]. Furthermore, recently added to the known substrates of beta-tryptase which include certain allergens, like grass and birch pollen [33] was interestingly IgE [34•]. This shows that human tryptases and chymases can actively suppress allergic inflammation by reducing antigenicity and leukocyte recruitment. On the flip-side, this mechanism is used by for instance parasites to evade the IgE mediated immune responses [35] (and reviewed in [36]). Mouse MCP-4, a MC chymase, shows close resemblance to the human chymase and mice lacking MCP-4 show more severe allergic responses then their WT counterpart confirming the protective role of this chymase in the development of allergic airway responses [37].
From infectious disease models, it was shown that the murine MC specific tryptase MCP6 was necessary to bridge the innate with the adaptive immunity to clear helminth or bacterial infections like Trichinella spiralis [38] and Klebsiella pneumoniae [39]. In these studies it was shown that in the absence of MCP6, recruitment of eosinophils to the site of infection was impaired. Further research by our lab revealed that besides previously published increase of MCP1 and MCP5 [2] also MCP6 was highly upregulated in accepted allografts at day 30 post-grafting. This seems contradicatory however, one of the known substrates of MCP6, being IL6, was inversely correlated with the presence of MCP6. Furthermore, MCP6−/− cannot be tolerized and reject their grafts before day 30 (manuscript submitted). Reducing IL6 could also suppress the development of Th17 T-cells when TGFβ is present and could thus be involved in dampening autoimmune inflammation. Therefore, MCP6 may regulate inflammation and tolerance by reduction of the local IL6 concentration.
Finally, MC-CPA seems to have an important role in the protection towards, at least certain, snake venom toxins [40] and the vasocontriciting peptide endothelin 1 [41]. The latter plays an important role in the development of sepsis, bacterial infections, certain allergies and auto-immune disorders [42]. Recently, the CPA−/− mice were developed without disturbing the presence of CPA dependent chymase MCP5 [41]. We also observe an increase of CPA expression in tolerant skin allografts (unpublished observation). However, whether CPA has an immune suppressive function in this model is not known.
Altogether, mast cell specific proteases may play a role in the regulation of adaptive immunity by cleaving of cytokines/allergens and regulating extravasation. Their role in inflammation, and moreover tolerance is just starting to be resolved.
Histamine
Histamine is undoubtedly the most studied mediator released by MC due to its profound effects in allergies. In general, histamines are regarded as inducers of inflammation and the four known receptors are widely expressed. Histamine receptor 2 (H2R) has been implicated in dampening the immune response [43,44]. Binding of histamine to H2R on T-cells and intraepithelial lymphocytes has been shown to suppress cytokine production and proliferation [45]. In this context, UVB irradiation leads to histamine release by MC and increases H2R expression. As such, UVB irradiation has an MC dependent H2R dependent immune-suppressive effect [46]. H2R receptor is also involved in the suppression of the development of autoimmunity and gradual loss of H2R expression on MC in lupus-like lesions was observed [47]. Thus, even though histamine in general leads to inflammation, histamine binding to H2R mediates immune-suppression. This could provide a negative feedback loop to balance histamine induced pro-inflammatory immune response. However, substantially greater insights are needed to address the immune suppressive role of histamine.
Arachidonic acid metabolites
Another MC derived group of mediators that have shown a potential function as either pro-inflammatory or anti-inflammatory are the arachidonic acid metabolites. Although PGD2, LTC4, and LTB4 are involved in the onset, progression and resolution of allergic inflammation [48] especially PGE2 seems to have profound immuno-suppressive abilities. Even though PDG2 is considered the main prostaglandin produced by MC, recently it was shown that deletion of PGE2 in MC abrogated jet fuel induced immune suppression [47]. This was surprising since keratinocytes were considered the main producers of PGE2 in the skin. Moreover, UVB irradiation and application of naproxen induce PGE2 production [49]. PGE2 induces IL10 production in DC [50], indoleamine 2,3-dioxygenase expression [51] and seem to inhibit DC maturation [52]. All of these tolerogenic features can be blocked by COX-2, the enzyme synthesizing prostaglandins, inhibitors or NSAIDs. Additionally, PGE2 can induce the generation of MDSC and has been proposed as an additional mechanism of immune suppression during tumor development [53]. Inhibition of PGE2 has profound effects on tumor growth and induced anti-tumor immunity [54]. Considering that many tumors produce PGE2, the question how MC PGE2 might play a role in the establishment of tumors remains to be elucidated. However, like with TGFβ, it could very well be that MC derived PGE2 sets the stage for the initial establishment of an immunosuppressive niche.
Concluding remarks
The studies cited and the comments presented only scratch the surface of the innumerable ways in which MC can mediate inflammation and tolerance. Many mediators produced by MC are seemingly schizophrenic in nature, playing both pro-inflammatory and anti-inflammatory roles depending on their context and environment. As such it was suggested that MC are tunable in that MC behavior is dependent upon the circumstances in which they are activated [55]. Given the large number of mediators produced by MC, to unravel their pro-inflammatory and anti-inflammatory functions is a daunting task, however with the generation of novel mouse models where specific genes can be deleted in only the MC [56••,57••] compartment, the first steps are being taken. These approaches will allow us to specifically address the influence of the various MC derived mediators on the development of immunity and tolerance.
Acknowledgments
VCV was funded by Dartmouth College Fellowship and the research was supported by the National Institute of Health (NIH AI048667).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 3.de Vries VC, Pino-Lagos K, Elgueta R, Noelle RJ. The enigmatic role of mast cells in dominant tolerance. Curr Opin Organ Transpl. 2009;14:332–337. doi: 10.1097/MOT.0b013e32832ce87a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ. Mast cell degranulation breaks peripheral tolerance. Am J Transpl. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. The impact of allergies on peripheral tolerance was unclear. This paper links degranulation as seen in allergies to inflammation, and indirectly that secretion/piece meal degranulation as the most likely mechanism of MC mediated immunosuppression. Additionally, blocking MC degranulation with drugs used in allergies prevented not only allograft rejection but also prolonged acceptance compared to the standard tolerogenic treatment. This suggests that non-IgE mediated slow degranulation of the MC presence might be the underlying cause of rejection of long-term accepted solid organ transplants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 6.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 7.Mikita N, Kanazawa N, Yoshimasu T, Ikeda T, Li HJ, Yamamoto Y, Furukawa F. The protective effects of ultraviolet A1 irradiation on spontaneous lupus erythematosus-like skin lesions in MRL/lpr mice. Clin Dev Immunol. 2009;2009:673952. doi: 10.1155/2009/673952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Byrne SN, Limon-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–4655. doi: 10.4049/jimmunol.180.7.4648. This study ties several studies on UVA, UVB and snake venom induced MC mediated immune suppression together. With the exception of some UVA data, it was shown that suppression was MC dependent and IL10 dependent. This study elegantly shows that the migration of IL10 producing MC to the draining lymph nodes is required for the immunosuppressive effects of UVB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176:4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie SR, DeMartino RR, Zhu J, Chong HJ, Ramirez C, Shelburne CP, Bouton LA, Bailey DP, Gharse A, Mirmonsef P, et al. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- 11.Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, Kashyap MV, Barnstein BO, Fischer-Stenger K, Schwartz LB, et al. TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol. 2005;174:5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 13.Pagliari C, Fernandes ER, Guedes F, Alves C, Sotto MN. Role of mast cells as IL10 producing cells in paracoccidioidomycosis skin lesions. Mycopathologia. 2006;162:331–335. doi: 10.1007/s11046-006-0069-y. [DOI] [PubMed] [Google Scholar]
- 14.Fifadara NH, Aye CC, Raghuwanshi SK, Richardson RM, Ono SJ. CCR1 expression and signal transduction by murine BMMC results in secretion of TNF-alpha, TGFbeta-1 and IL-6. Int Immunol. 2009;21:991–1001. doi: 10.1093/intimm/dxp066. [DOI] [PubMed] [Google Scholar]
- 15.Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, Steinman RM, Suthanthiran M. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25-T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 17.Shiota N, Nishikori Y, Kakizoe E, Shimoura K, Niibayashi T, Shimbori C, Tanaka T, Okunishi H. Pathophysiological role of skin mast cells in wound healing after scald injury: study with mast cell-deficient W/W(V) mice. Int Arch Allergy Immunol. 2010;151:80–88. doi: 10.1159/000232573. [DOI] [PubMed] [Google Scholar]
- 18.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 19.Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, Staats HF, Abraham SN. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa Y, Duru EA, Ameredes BT. Role of IL-10 in the resolution of airway inflammation. Curr Mol Med. 2008;8:437–445. doi: 10.2174/156652408785160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Goldstein DR. IL-6 and TNF-alpha synergistically inhibit allograft acceptance. J Am Soc Nephrol. 2009;20:1032–1040. doi: 10.1681/ASN.2008070778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol. 2005;174:7433–7439. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- 24.Bluml S, Binder NB, Niederreiter B, Polzer K, Hayer S, Tauber S, Schett G, Scheinecker C, Kollias G, Selzer E, et al. Anti-inflammatory effects of TNF on hematopoietic cells in the development of erosive arthritis. Arthritis Rheum. 2010;62(6):1608–1619. doi: 10.1002/art.27399. [DOI] [PubMed] [Google Scholar]
- 25.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- 26.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24:419–424. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Berger TG, Schulze-Koops H, Schafer M, Muller E, Lutz MB. Immature and maturation-resistant human dendritic cells generated from bone marrow require two stimulations to induce T cell anergy in vitro. PLoS One. 2009;4:e6645. doi: 10.1371/journal.pone.0006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 29.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a–dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 31.Pang L, Nie M, Corbett L, Sutcliffe A, Knox AJ. Mast cell beta-tryptase selectively cleaves eotaxin and RANTES and abrogates their eosinophil chemotactic activities. J Immunol. 2006;176:3788–3795. doi: 10.4049/jimmunol.176.6.3788. [DOI] [PubMed] [Google Scholar]
- 32.Mellon MB, Frank BT, Fang KC. Mast cell alpha-chymase reduces IgE recognition of birch pollen profilin by cleaving antibody-binding epitopes. J Immunol. 2002;168:290–297. doi: 10.4049/jimmunol.168.1.290. [DOI] [PubMed] [Google Scholar]
- 33.Rauter I, Krauth MT, Flicker S, Gieras A, Westritschnig K, Vrtala S, Balic N, Spitzauer S, Huss-Marp J, Brockow K, et al. Allergen cleavage by effector cell-derived proteases regulates allergic inflammation. FASEB J. 2006;20:967–969. doi: 10.1096/fj.05-3999fje. [DOI] [PubMed] [Google Scholar]
- 34•.Rauter I, Krauth MT, Westritschnig K, Horak F, Flicker S, Gieras A, Repa A, Balic N, Spitzauer S, Huss-Marp J, et al. Mast cell-derived proteases control allergic inflammation through cleavage of IgE. J Allergy Clin Immunol. 2008;121:197–202. doi: 10.1016/j.jaci.2007.08.015. Before this study was presented it was already known that MC proteases could reduce the effects of allergic inflammation by cleavage of a wide array of allergens. However, this study added to this MC mediated regulation by showing proteolytic cleavage of IgE. The functionality of proteases is still poorly understood but with this study provides evidence of a novel negative feedback loop provided by MC to suppress inflammation during. [DOI] [PubMed] [Google Scholar]
- 35.Aslam A, Quinn P, McIntosh RS, Shi J, Ghumra A, McKerrow JH, Bunting KA, Dunne DW, Doenhoff MJ, Morrison SL, et al. Proteases from Schistosoma mansoni cercariae cleave IgE at solvent exposed interdomain regions. Mol Immunol. 2008;45:567–574. doi: 10.1016/j.molimm.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 36.McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol. 2006;1:497–536. doi: 10.1146/annurev.pathol.1.110304.100151. [DOI] [PubMed] [Google Scholar]
- 37.Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, Wernersson S. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 38.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 40.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 41.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Clouthier DE, Yanagisawa MM, Tsai M, Galli SJ. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka S, Ichikawa A. Recent advances in molecular pharmacology of the histamine systems: immune regulatory roles of histamine produced by leukocytes. J Pharmacol Sci. 2006;101:19–23. doi: 10.1254/jphs.fmj06001x5. [DOI] [PubMed] [Google Scholar]
- 44.Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 45.Takagaki K, Osawa S, Horio Y, Yamada T, Hamaya Y, Takayanagi Y, Furuta T, Hishida A, Ikuma M. Cytokine responses of intraepithelial lymphocytes are regulated by histamine H(2) receptor. J Gastroenterol. 2009;44:285–296. doi: 10.1007/s00535-009-0019-9. [DOI] [PubMed] [Google Scholar]
- 46.McGlade JP, Gorman S, Lenzo JC, Tan JW, Watanabe T, Finlay-Jones JJ, Thomas WR, Hart PH. Effect of both ultraviolet B irradiation and histamine receptor function on allergic responses to an inhaledantigen. J Immunol. 2007;178:2794–2802. doi: 10.4049/jimmunol.178.5.2794. [DOI] [PubMed] [Google Scholar]
- 47.Furukawa F, Yoshimasu T, Yamamoto Y, Kanazawa N, Tachibana T. Mast cells and histamine metabolism in skin lesions from MRL/MP-lpr/lpr mice. Autoimmun Rev. 2009;8:495–499. doi: 10.1016/j.autrev.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8:335–349. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- 49.Paz ML, Ferrari A, Weill FS, Leoni J, Maglio DH. Time-course evaluation and treatment of skin inflammatory immune response after ultraviolet B irradiation. Cytokine. 2008;44:70–77. doi: 10.1016/j.cyto.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Harizi H, Gualde N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell Mol Immunol. 2006;3:271–277. [PubMed] [Google Scholar]
- 51.von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, Fiore F, Roth U, Beyer M, Debey S, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 52.Sa-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JM, Francischetti IM. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J Immunol. 2007;179:1497–1505. doi: 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- 53.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 54.Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, Kim JH, Shin C, Shim JJ, In KH, et al. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother. 2009;32:22–28. doi: 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- 55.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 56••.Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, Roers A. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17:307–315. doi: 10.1007/s11248-007-9153-4. Both studies developed mice which harbor the cre gene under the promoter of MC specific proteases. The currently used kitW/W-v and kitW-sh/W-sh harbor mutations in the cKit gene which eventually results in the absence of MC. However, these strains also have other defects that might influence the results. With the development of these mice, researchers will be able to specifically delete floxed genes of interest only in the MC compartment or deleting MC by introducing and cre-inducible diphtheria toxin receptor. These mice will most likely become the future gold-standard in MC research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Musch W, Wege AK, Mannel DN, Hehlgans T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis. 2008;46:163–166. doi: 10.1002/dvg.20378. [DOI] [PubMed] [Google Scholar]