We decided to reexamine early postnatal synaptic innervation in relation to ongoing studies of the development of physiological, morphological, and molecular features of hair cells in the mouse utricular macula.1,2 Physiological studies 3,4 demonstrated that mammalian type I and type II hair cells have distinctively different potassium conductances. Type I hair cells have a conductance termed gK,L for low-voltage-activated K+ conductance because it turns on at about −90 mV. This conductance is absent at birth, but begins to be expressed at PD4 in normal, excised utricles. Coincident with this event, calyces begin to form. The two events are not inextricably linked because gK,L is expressed at PD4 even in maculae that have been cultured from PD1. Thus, ongoing synaptic innervation does not appear to be necessary for the expression of this conductance. Another motivation for this study was to provide baseline data for spaceflight experiments in which mammals are born or develop in microgravity.
An early study by Favre and Sans5 was the only quantitative developmental data available for vestibular hair cells. Their data indicated that the number of synaptic ribbons in type I hair cells decreased in cat crista by 93% between birth and adulthood. The neonatal cat appears to be equivalent to our PD4 stage in that about 50% of the type I hair cells possess calyces. The 93% reduction led these authors to conclude that synaptic ribbons in type I hair cells “regressed and disappeared in the adult because they were no longer needed,” since the calyx could provide a form of electrotonic transmission. In a previous study,6 we showed that there were about 10–20 synaptic ribbons per type I hair cell regardless of region; thus, we were curious about whether the type I hair cell started out with 100–200 ribbons and lost 93% of them by adulthood.
One problem with examining synaptic innervation in relation to hair-cell type at early postnatal stages, is that morphologically it has proven difficult to distinguish type I hair cells without their primary characteristic, the calyx. The calyx has been the classic definition of a type I hair cell, but there are secondary attributes that are also present. For example, patch clamp experiments frequently use the shape of the hair cell to distinguish hair-cell types. Several studies have shown that stereociliar diameter and bundle size,7,8 as well as the apical surface convexity of a hair cell8 can also be used to distinguish the two types. We have previously measured some of these features in adult material, and then worked in reverse chronological order through PD28 and PD7 utricular hair cells whose calyces were present.1,9 In so doing, we found that these morphometric differences between hair-cell types were statistically significant. Thus, we felt comfortable extending the use of these “secondary attributes” to stages before the presence of a full calyx.
In the present study, samples were taken from Harlan ICR mice at postnatal days 0 (birth), 4, 7, 10, and 28. Three samples of 30 serial sections 70 nm thick were taken at each stage. Each series of sections was cut perpendicular to the striola and contained a cross section of all three regions of utriculus (medial extrastriola, striola, and lateral extrastriola). Photomontages at 5000 × magnification were assembled and used to collect data about the numbers, positions, and shapes of synaptic ribbons. Medial and lateral extrastriola were combined. Synaptic ribbon counts were done using the dissector method as described previously.6 So far, two samples from each stage have been examined.
Results are shown in Figure 1 and are compared to counts taken from adult chinchilla crista.6 Sample sizes are smaller in the striola, which constitutes only 10% of the epithelium in a transverse section, and indeed, we have no type II hair-cell equivalents yet at PD28. Nonetheless, we have so far examined the equivalent of 26 striolar and 97.5 extrastriolar hair-cells for a total of 123.5 fully reconstructed hair cells across all stages. While the results are still preliminary, they indicate that after an initial decrease between PDO and PD4, there is an orderly increase in numbers of ribbons over all stages, with PD28 mouse utricular hair cells being roughly comparable in numbers to the adult chinchilla crista. In the extrastriola, type II hair cells in particular tend toward increased numbers at older stages.
FIGURE 1.
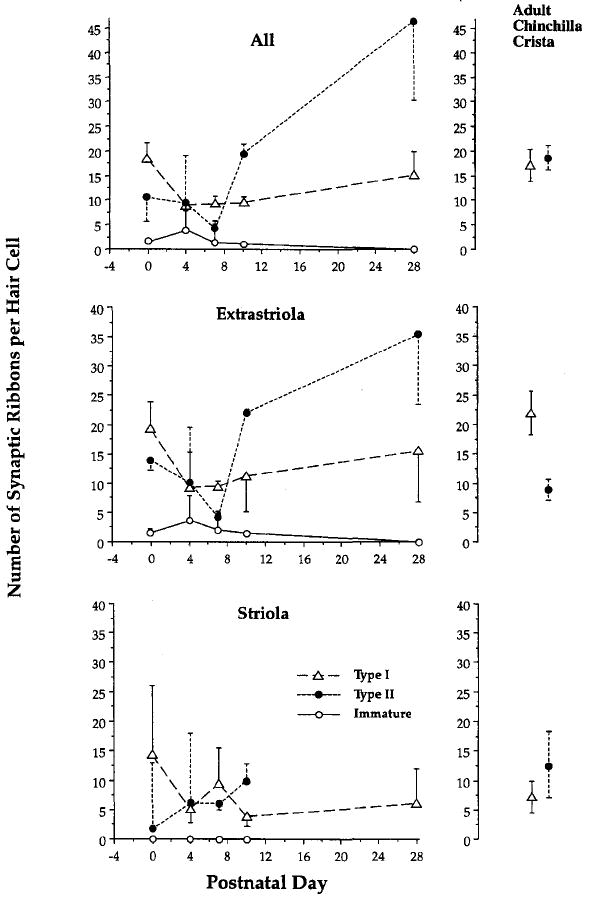
Development of synaptic innervation over the first postnatal month in mouse utricle. The key for the entire figure is given in the bottom (striola) panel. Values for adult chinchilla crista are shown at right; they are taken from reference 6 and represent all (top), the peripheral zone, equivalent to the extrastriola (center); and the central zone, equivalent to the striola (bottom).
At younger stages, we noticed greater numbers of ribbon clusters and pairs (not shown). Hollow and spherical ribbons were also more common in type II hair cells. In addition, we noticed more “free ribbons”l0 at PD4 and PD7, that is, ribbons floating free in the cytoplasm. Thus, we would agree with the results in the cochlea of Sobkowicz and colleagues,10 that these free ribbons probably represent misplaced and free ribbons liberated after the initial afferent denervation. Even with these “floating ribbons” included (≈ 5% of the total), numbers of ribbons tended to be less at PD4 than at later stages, indicating that synaptogenesis continues after this initial “pruning back” of innervation.
In conclusion: (1) approximately 14,500 hair-cell profiles have been examined, equivalent to about 125 complete hair cells; (2) synaptic innervation in the postnatal utricular macula appears to proceed at an orderly rate, after a decrease from neonatal stages; however, sample sizes are still small, and more samples need to be taken through larger portions of the striola; (3) hollow ribbons are more common in type II hair cells, as in the chinchilla crista, but not significantly more, suggesting that they are not per se an immature form; and (4) synaptic innervation is another marker of hair-cell development that may be specific for one hair-cell type versus another.
Acknowledgments
This work was supported by PHS Grant ROI DC-02290 and NASA Grant NAG 5-4593. We gratefully acknowledge the excellent technical assistance of Mr. Steve Price. Ms. Parveen Ahmed assisted in the early stages of this work.
References
- 1.Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci. 1998;18:7487–750l. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carranza A, Lysakowski A, Barritt LC, Vollrath MA, Himes DL, Beisel KW, Eatock RA. ARO 21st Midwinter Meet Abstr. ARO; St. Petersburg Beach, Fla: 1998. Expression of Kv subunits in rodent vestibular hair cells; p. 76. [Google Scholar]
- 3.Rennie KJ, Correia MJ. Potassium currents in mammalian and avian isolated type I semicircular canal hair cells. J Neurophysiol. 1994;71:317–329. doi: 10.1152/jn.1994.71.1.317. [DOI] [PubMed] [Google Scholar]
- 4.Rüsch A, Eatock RA. A delayed rectifier conductance in type I hair cells of the mouse utricle. J Neurophysiol. 1996;76:995–1004. doi: 10.1152/jn.1996.76.2.995. [DOI] [PubMed] [Google Scholar]
- 5.Favre D, Sans A. Morphological changes in afferent vestibular hair cell synapses during the postnatal development of the cat. J Neurocytol. 1979;8:765–775. doi: 10.1007/BF01206675. [DOI] [PubMed] [Google Scholar]
- 6.Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Compo Neurol. 1997;389:419–443. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson EH, Cotton JR, Grant JW. Structural variations in ciliary bundles of the posterior semicircular canal: quantitative anatomy and computational analysis. Ann N Y Acad Sci. 1996;781:85–102. doi: 10.1111/j.1749-6632.1996.tb15695.x. [DOI] [PubMed] [Google Scholar]
- 8.Lapeyre PNM, Guilhaume A, Cazals Y. Differences in hair bundles associated with type I and type II vestibular hair cells of the guinea pig saccle. Acta Otolaryngol (Stockh) 1992;112:635–642. doi: 10.3109/00016489209137453. [DOI] [PubMed] [Google Scholar]
- 9.Lysakowski A. Morphometric criteria for hair cell types. J Vestibular Res. 1996;S4:S86. [Google Scholar]
- 10.Sobkowicz HM, Rose JE, Scott GL, Levenick CV. Distribution of synaptic ribbons in the developing organ of Corti. J Neurocytol. 1986;15:693–714. doi: 10.1007/BF01625188. [DOI] [PubMed] [Google Scholar]


