Abstract
The mitochondrial life cycle consists of frequent fusion and fission events. Ample experimental and clinical data demonstrate that inhibition of either fusion or fission result in deterioration of mitochondrial bioenergetics. While fusion may benefit mitochondrial function by allowing the spreading of metabolites, protein and DNA throughout the network, the functional benefit of fission is not as intuitive. Remarkably, studies that track individual mitochondria through fusion and fission found that the two events are paired and that fusion triggers fission. On average each mitochondrion would go though ~5 fusion:fission cycles every hour. Measurement of Δψm during single fusion and fission events demonstrate that fission may yield uneven daughter mitochondria where the depolarized daughter less likely to become involved in a subsequent fusion and is more likely to be targeted by autophagy. Based on these observations we propose a mechanism by which the integration of mitochondrial fusion, fission and autophagy form a quality maintenance mechanism. According to this hypothesis pairs of fusion and fission allow for the reorganization and sequestration of damaged mitochondrial components into daughter mitochondria that are segregated from the networking pool and then becoming eliminated by autophagy.
Keywords: Mitochondria, Membrane potential, Fusion, Fission, Autophagy
Introduction
Mitochondrial dysfunction is suggested to play a central role in metabolic diseases such as diabetes and in a number of chronic conditions such as Alzheimer’s disease, Parkinson disease and aging. In these conditions accumulation of dysfunctional mitochondria leads to oxidative stress and impair cellular functions [1-4].
Diverse mechanisms enable the turnover of damaged or misfolded mitochondrial proteins. A mechanism that functions inside mitochondria is the inner membrane AAA protease that digests inner membrane and matrix proteins [5]. Turnover of outer membrane proteins may be mediated by E3 ubiquitin ligase system, as in the case of mitofusin-2, implicating the ubiquitin proteasome in processing of mitochondrial membrane proteins and mitochondrial architecture [6]. Finally, turnover of a mitochondrion as a whole organelle is mediated by mitochondrial autophagy or mitophagy. [7, 8].
While autophagy is commonly considered involved in cell death pathways [9], it has a number of other functions required for cellular adaptation. For example, autophagy allows for adaptive reduction in the mass of organelles as observed in liver cells during the shrinkage of the ER in response to withdrawal of cytochrome P450-inducing drugs. Autophagy also functions as a mechanism to provide nutrients under starvation as seen for example in the hibernating myocardium [10]. Selective elimination of mitochondria by autophagy occurs in specific settings such as erythrocyte maturation [11] and specific targeting of sperm mitochondria during oocyte fertilization [12]. Large scale autophagy of mitochondria has been described during apoptosis where opening of the PTP was found to be essential for mitochondrial autophagy to proceed [7].
Although the molecular flag(s) that specifically targets mitochondria for autophagy has not yet been identified in mammals, autophagy has been associated with the dissipation of mitochondrial membrane potential (Δψm) [7, 13-16]. This property makes autophagy a potential housekeeping process that targets dysfunctional mitochondria and maintains the bioenergetic efficiency of the cell. Inhibition of ATG genes in yeast resulted in higher levels of reactive oxygen species (ROS), reduced oxygen consumption, higher mitochondrial mutation rates and severe defects in mitochondrial degradation [17]. A similar phenotype was recently described in clonal (INS1) beta cells showing an increased sub-cellular heterogeneity in mitochondrial membrane potential, impaired oxygen consumption and accumulation of oxidized mitochondrial protein [16]. These observations suggest a rather individualistic and linear model of the mitochondrial life cycle: a mitochondrion gradually deteriorates to the point of Δψm dissipation, at which point it is targeted for recycling by autophagy.
Dynamics studies complicate the perceived mitochondrial life-cycle
The discovery of mitochondrial dynamics has made this scenario rather too simplistic. Based on recent findings in mammal models and yeast, mitochondria exist in networks that are continuously remodeled through fusion and fission [18-21]. Fusion events allow rapid diffusion of matrix proteins [22-27], with slower migration of inner and outer membrane components [22, 28].
Time-lapse imaging of mitochondrial fusion and fission indicates that it is a rapid process. Laser mediated photoactivation of matrix-targeted photoactivatable GFP (mtPA-GFP) allows for selective labeling of mitochondria which is then followed by the spread of the photoactivated mtPA-GFP to conjoined, non-photoactivated, mitochondria. When 10-20% of mitochondria within a cell are photoactivated, the probe equilibrates across the entire mitochondrial population within ~45 min [16, 24, 29]. Given that the turnover of mitochondrial proteins is in the range of hours to days [30], it is predicted that the mitochondrial population within a cell will be homogenous in protein content and, consequently, in function.
In view of these findings, mitochondrial dynamics are expected to impact mitochondrial turnover and thereby the bioenergetic efficiency of the mitochondria population within a cell. The goal of this review is to propose a hypothesis in which the combined functions of fusion, fission and autophagy constitute a quality control mechanism that allows the sequestration, sorting and elimination of functionally impaired mitochondrial components. We address the paradoxical generation of depolarized mitochondria within a constantly mixing population of mitochondria and discuss a mechanism that targets dysfunctional mitochondria for autophagy and not for rescue by fusion with the network. Detailed reviews on the molecular machinery of mitochondrial fusion and fission, protein turnover and autophagy are available in the these references [8, 31-36].
Life cycle of the mitochondrion
Reduced mitochondrial membrane potential (hereafter referred as mitochondrial depolarization) may be the result of a gradual or spontaneous deterioration, or alternatively, may occur as a result of a regulated event*. To investigate the events leading to appearance of depolarized mitochondria and the consequent mechanism(s) that target them to mitophagy or metabolic rescue, one must characterize the bioenergetic and biochemical properties of the life cycle of a mitochondrion.
A number of studies indicate that mitochondria go through continuous cycles of fusion and fission [19, 20, 40]. Although at a first glance this might appear to be in conflict with the tubular, web-like, structures described in HeLa cells and COS7 cells, a more detailed investigation showed that even in these connected webs mitochondria do not make a large continuum but rather continuously rewire the segments through fusion and fission [22, 41-43]. Long term single mitochondrion tracking showed that the frequency of fusion events in COS7 and INS1 cells is once every ~5-20 min per mitochondrion [16]. In plants and mammals this behavior has been described as having a pattern of kiss and run, indicating that fusion is a brief event (~100 sec in INS1 and COS7 cells) and is characteristically followed by fission [16, 27]. As a result, mitochondria spend most of their time in their post fission state as solitary units before entering a subsequent fusion. It is therefore suggested that the life cycle of mitochondria can be divided into two periods, the pre-fusion period (solitary period) and the post fusion period when the mitochondrion is connected to another (networked period).
The birth of a depolarized mitochondrion
Depolarized mitochondria may therefore be the result of a spontaneous depolarization during the solitary or networked periods or during the transition between them. A number of studies have reported on the monitoring of individual mitochondria over time and the observation of a specific depolarization event. Loew et al. provided one of the earliest quantitative measurements of individual mitochondria (as judged by imaging with the Δψm-dependent dye TMRE) that were tracked in the z-stack with high time resolution. They reported stable mitochondrial membrane potential for a period of 40-80 sec that could be followed by a drop of more than 15 mV [44]. This pioneering study was however limited by the lack of technology to assure that the detected mitochondrion did not go through fusion and/or fission events during the recording time. Since fission can occur without movement of the two daughter mitochondria, or involved only the inner (but not the outer) mitochondrial membrane [22, 45], it cannot be reliably identified by observation of separation of a mitochondrion into two segments. Similarly, the repositioning of a mitochondrion to become juxtaposed to another mitochondrion is not an indication that a fusion event occurred [22].
The use of photoactivatable proteins overcame a major technical difficulty of imaging individual organelles that move and change morphology within a complex architecture [46-48]. Simultaneous imaging of mitochondria stained with TMRE and expressing mtPA-GFP solved two major problems in monitoring biophysical activity of single mitochondria [22]. The first is the accurate determination of organelle boundaries that can easily be tracked despite movement within a dense mitochondrial architecture. The second is the use of mtPA-GFP with TMRE to derive a ratiometric value for Δψm that is independent of the exact focal plane. This approach spares laser radiation needed to image the entire z-axis and thereby reduces phototoxic damage. As a result, the recording period free of phototoxic damage can be extended from minutes to hours.
In COS7, INS1 and primary β-cells prolonged tracking (up to 2 hrs) revealed that mitochondria retain a stable Δψm during the solitary period [16, 49]. During most of the time (95% of the recording period) Δψm of the mitochondrion was within ±2.7 mV of its average baseline. This observation indicates that continuous deterioration in Δψm during the solitary phase is an unlikely (or infrequent) route for the generation of depolarized mitochondria under normal conditions.
Biophysical properties of individual fission events
In contrast to the remarkable stability of Δψm under control conditions during the solitary period, fission events are associated with major changes in Δψm. Most fission events yield daughter mitochondria with opposite Δψm deflections, usually greater than 5 mV [16]. These observations indicate that while fusion mixes the content of the parent mitochondria, fission generates functionally divergent daughters. In support of functional asymmetry of fission events is data generated by EM tomography showing that NO-toxicity generates asymmetrical daughter mitochondria during fission [50]. The reported membrane structures of the two daughters suggest that the two would have disparate respiratory capacity [51]. Nucleoids that contain mtDNA were also shown to occasionally distribute asymmetrically during fission events [27].
It is unclear if the dissimilarity of the daughters is a result of an active or passive process that sorts active from inactive components and sequester them to different segments within the mitochondrion. So far there are no data that oppose or support a “mitoskeleton” that would be involved in such function. An alternative possibility would be that the uneven distribution of functional components in the mitochondria is random and is mediated by diffusion.
In contrast to mitochondrial fusion, fission events do not require intact Δψm (see next section). This property is supported by numerous studies showing that depletion of ATP either by inhibiting ATP synthase [52, 53], collapsing Δψm [45, 54-56] or inhibiting Na/K ATPase [53] trigger general fragmentation of the mitochondrial web. Indeed, selective tracking of individual mitochondria with sustained depolarization revealed the occurrence of fission events. Remarkably, some of these events could regenerate a daughter mitochondrion with an intact Δψm, while the other daughter remains at a sustained depolarized level (unpublished data). Taking together, these data indicate that mitochondrial fission is a central metabolic event in the life cycle of mitochondria, being able to alter their energy state.
Fission as the autophagic checkpoint
Since mitochondrial fission occasionally generates depolarized mitochondria it is expected to feed autophagy [7, 14, 15]. Indeed, knockdown of FIS1 (by siRNA) or over expression of a dominant negative isoform of DRP1 (DRP1K38A), reduces mitochondrial autophagy [16, 50, 57]. This reduction is selective to mitochondria and was not accompanied by a reduction in ER mass in autophagosomes (APs), rate of APs formation or in lysosomal mass.
Manipulations in FIS1 and DRP1 expression level were consistent with fission having a role in mitochondrial autophagy. Frieden et al showed that overexpression of hFis1 reduced selectively the mitochondrial (but not ER) mass, consistent with fission increasing mitochondrial autophagy [60]. Arnoult et al showed that overexpression of DRP1 facilitated mitochondrial elimination under various pro-apoptotic stimuli [57]. Qualitative evidence linking mitochondrial morphology and mitophagy were obtained in several studies in the CNS. In neurons, exposure to nitric oxide donor (50-200 μm of SNOC) resulted in fragmentation of the mitochondrial web, increased fraction of structurally damaged mitochondria and selective increase in mitochondrial mass in APs [50]. A similar relationship between mitochondria fragmentation and autophagy is found in Alzheimer’s disease where a chronic and progressive oxidative stress correlates with mitochondrial fragmentation that is accompanied by a selective increase in mitochondrial mass in APs [61-63]. Common to these observations is a stressor-induced mitochondrial fission and fragmentation, and a selective increase of mitochondrial localization in autophagosomes.
Mitochondrial fission is more likely to be permissive for autophagy since only a minor fraction of fission events yield daughter mitochondria that will be eliminated by autophagy. For example, in an unstressed individual beta cell, a population of 300 mitochondria will generate 500-1000 fission events per hour, but less than 100 mitochondria–containing autophagosomes per hour. That reduction in mitochondrial size is not sufficient to induce autophagy is also exemplified by conditions of OPA1 over expression which result in reduced mitochondrial size as well as reduced autophagy [58, 59].
This raises the hypothesis that mitochondrial fragmentation, which can be induced by various insults, is a common stress response that permits the segregation and elimination of dysfunctional mitochondria from the web. However, the function of fusion as a rescue mechanism may not allow efficient segregation of dysfunctional mitochondria from the networking population. The emerging question is how mitochondrial fusion relates to depolarization and whether it acts to rescue damaged mitochondria.
Is Fusion a rescue mechanism?
Mitochondrial fusion was suggested as a complementation mechanism through which mitochondria compensate for certain metabolic depletions by transferring soluble as well as membranous components. While ample data supports that fusion allows for the diffusion of multiple components between the involved mitochondria [20, 27, 28], there is no evidence to indicate that this process functions to rescue mitochondria that are bioenergetically compromised. Several studies revealed the existence of potential mechanisms that would prevent fusion of mitochondria when mitochondrial membrane potential is dissipated by an uncoupler [45, 54, 55, 64-66].
Fusion is a selective and primarily exclusive mechanism rather than a rescue one
While the above observations conclude that depolarized mitochondria do not fuse with each other, more recent studies determined whether depolarized mitochondria can fuse with those that have intact Δψm. Simultaneous tracking of fission and Δψm shows that depolarized mitochondria generated during fission events are 6 times less likely to become involved in a consecutive fusion event within the next 10 minutes as compared to their sisters generated during the same events (“fission-mate”) [16]. This finding suggested that depolarized mitochondria may remain in the cell as non-fusing mitochondria.
By selectively tagging in vivo a group of mitochondria with mtPA-GFP and observing them over time, non-fusing mitochondria can be identified as those that fail to dilute their mtPA-GFP. Co-staining with TMRE or OPA1 antibody revealed that non-fusing mitochondria are depolarized by ~7 mV compared to average Δψm and have approximately 50% less OPA1 compared to the rest of mitochondrial web [16].
The biochemical mechanisms that link OPA1 processing to bioenergetic parameters have been studied by a number of groups. Herlan et al. were the first to show that mgm1 (OPA1 equivalent in yeast) undergo splicing (also referred as alternative topogenesis) to s- and l-isoforms in an ATP-dependent manner [67]. Indeed, consequent studies in mammalian models revealed that the l-isoforms of OPA1 undergo cleavage (or degradation) under mitochondrial depolarization or depletion in ATP [68-71]. Since both l- and s-isoforms of OPA1 are pre-requisite for mitochondrial fusion [70], a decrease in the driving force for ATP synthesis (i.e., Δψm depolarization) triggers mitochondrial fragmentation by regulating OPA1 isoforms. A number of proteases have been implicated in OPA1 cleavage, including Rbd1/Pcp1 in yeast and presenilin-associated rhomboid-like protease (PARL), m-AAA Paraplegin, and AFG3L2 proteases in mammals [69, 72, 73]. These proteases have been found to be regulated both by ATP levels and by Δψm, implicating mitochondrial energetic status as a regulator of OPA1 processing. Recent studies by Baricault et al. have shown that OPA1 processing in mammalian cells may involve direct interaction of OPA1 with respiratory chain complexes [74, 75].
Taken together, these findings suggest that mitochondrial fusion is primarily a selective and exclusive process rather than an unselective rescue mechanism.
Remarkably however, certain mtDNA mutations have been shown to spread across the mitochondrial network through fusion events. In a heteroplasmic cell, exchange of mitochondrial DNA may allow for complementation and recovery of function [76]. Ono et al. isolated two types of respiration-deficient cell lines with pathogenic mutations in mitochondrial tRNAIle or tRNALeu(UUR) genes from patients with mitochondrial diseases [77]. Following PEG induced fusion of the two cell lines, the coexistence of their mitochondria within hybrids restored their normal morphology and respiratory enzyme activity. These observations do not necessarily contradict the selective fusion hypothesis since the effect of each of these mutations on mitochondrial membrane potential and OPA1 is unknown, and it is unclear if fusion of depolarized mitochondria occurs in these cells. Future studies need to unravel why and how under certain circumstances mtDNA inter-mitochondrial complementation compensates for the mutated mtDNA in a manner that skip the selective fusion.
Fusion, fission and autophagy as a bioenergetic quality control mechanism
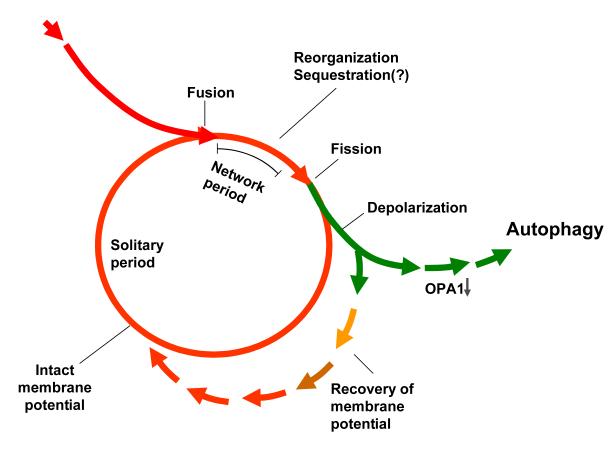
The observation that mitochondrial fusion is a Δψm-dependent process ensures that potentially dysfunctional organelles will avoid fusion, while intact mitochondria benefit from sharing of metabolites. Segregation of dysfunctional mitochondria from the fusing population shifts their balance from fusion to fission resulting in the generation of small and depolarized mitochondria. The finding that autophagy targets depolarized mitochondria places autophagy at the end of the axis of quality control as a receiver of the segregation output. Figure 1 summarizes schematically how the combination of fusion, fission and autophagy act as a quality control mechanism. This view suggests that the absolute rate of fission events per se (and not merely the balance between fusion and fission) determines the efficacy of the proposed quality control axis. The higher the fission frequency, the higher is the probability that dysfunctional units will be segregated and eliminated. In this respect it should be emphasized that while in the absence of stress inducers most fission events occur as part of a fusion-fission cluster [16, 27], under stress, fission events will occur independently of fusion events. This may facilitate the segregation of damaged mitochondria in conditions that are characterized by increased damage to mitochondria or those that require adaptation of mitochondria through positive selection.
Figure 1. A Schematic model of the mitochondrion’s life cycle and the roles of mitochondrial dynamics and autophagy in the segregation of dysfunctional mitochondria.
The mitochondrion cyclically shifts between a post fusion state (Network) and a post fission state (Solitary). Fusion is brief and triggers fission. Following a fission event, the daughter mitochondrion may either maintain intact membrane potential (red line), or depolarize (green line). If it depolarizes, it is unlikely to re-engage in further fusion events for the entire depolarization interval. In the case mitochondrial depolarization is transient and Δψm resumes, fusion capacity is restored (green to red short arrows). However, if Δψm depolarization is sustained, reduction in OPA1 follows and elimination by autophagy occurs.
Enrichment arm of the quality control mechanism – a theory
Thus far we have been describing the selectivity characteristics of fusion as a key component of the quality control axis. However a number of observations suggest the possibility that mitochondrial fusion might play an active role in the quality control.
Although fusion allows for the equilibration of mtPA-GFP between the fusing pair of mitochondria [22, 24], membranous components and DNA do not necessarily become equilibrated [27, 50]. Therefore, fusion may lead to functionally uneven distribution of components involved in respiration and to the generation of dissimilar daughter mitochondria. The daughter that ends up with higher content of impaired components may become depolarized and therefore segregated from the networking population, then removed by autophagy. Parallel to removing a daughter enriched in impaired components, such process would also result in the enrichment of the remaining daughter with undamaged material.
The hypothesis of enrichment by redistribution, fission and autophagy offers a mechanism for the maintenance of the bioenergetics efficiency of a cell and may function to prevent aging related deterioration. However, a number of key points are yet to be studied and assessed. These include the mechanism by which mitochondria redistribute material in an uneven manner, prior to fission. This process might be stochastic or may involve active sorting of material between the two ends of a mitochondrion tubular structure. Moreover, it is yet unclear why this mechanism would not work in a number of mitochondrial diseases where heteroplasmy remains stable with age even when the mutation is compromising the respiratory function and can lead to cellular death. Future investigation on the mechanisms underlying fission-induced asymmetry is a key for testing additive pathways of how mitochondrial fusion benefit mitochondrial metabolic quality.
Footnotes
Extensive work has been done on depolarization that occurs during apoptosis and by acute insult. This form of depolarization and its mechanisms are out of the scope of the current review and are detailed elsewhere [15, 37-39].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Refrences
- [1].Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–68. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–49. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- [3].Simmons RA, Suponitsky-Kroyter I, Selak MA. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to beta-cell failure. J Biol Chem. 2005;280:28785–91. doi: 10.1074/jbc.M505695200. [DOI] [PubMed] [Google Scholar]
- [4].Tanaka M, Kovalenko SA, Gong JS, Borgeld HJ, Katsumata K, Hayakawa M, Yoneda M, Ozawa T. Accumulation of deletions and point mutations in mitochondrial genome in degenerative diseases. Ann N Y Acad Sci. 1996;786:102–11. doi: 10.1111/j.1749-6632.1996.tb39055.x. [DOI] [PubMed] [Google Scholar]
- [5].Arnold I, Langer T. Membrane protein degradation by AAA proteases in mitochondria. Biochim Biophys Acta. 2002;1592:89–96. doi: 10.1016/s0167-4889(02)00267-7. [DOI] [PubMed] [Google Scholar]
- [6].Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–7. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- [8].Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- [10].May D, Gilon D, Djonov V, Itin A, Lazarus A, Gordon O, Rosenberger C, Keshet E. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc Natl Acad Sci U S A. 2008;105:282–287. doi: 10.1073/pnas.0707778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takano-Ohmuro H, Mukaida M, Kominami E, Morioka K. Autophagy in embryonic erythroid cells: its role in maturation. Eur J Cell Biol. 2000;79:759–64. doi: 10.1078/0171-9335-00096. [DOI] [PubMed] [Google Scholar]
- [12].Shitara H, Kaneda H, Sato A, Inoue K, Ogura A, Yonekawa H, Hayashi JI. Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis. Genetics. 2000;156:1277–84. doi: 10.1093/genetics/156.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kissova I, Deffieu M, Manon S, Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem. 2004;279:39068–74. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- [14].Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12:1613–21. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- [15].Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–53. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang Y, Qi H, Taylor R, Xu W, Liu LF, Jin S. The role of autophagy in mitochondria maintenance: characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Autophagy. 2007;3:337–46. doi: 10.4161/auto.4127. [DOI] [PubMed] [Google Scholar]
- [18].Westermann B. Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 2002;3:527–31. doi: 10.1093/embo-reports/kvf113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 2002;12:178–84. doi: 10.1016/s0962-8924(01)02246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death and Differentiation. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- [21].Griffin EE, Detmer SA, Chan DC. Molecular mechanism of mitochondrial membrane fusion. Biochim Biophys Acta. 2006;1763:482–9. doi: 10.1016/j.bbamcr.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [22].Twig G, Graf SA, Wikstrom JD, Mohamed H, Haigh SE, Elorza A, Deutsch M, Zurgil N, Reynolds N, Shirihai OS. Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. Am J Physiol Cell Physiol. 2006;291:C176–84. doi: 10.1152/ajpcell.00348.2005. [DOI] [PubMed] [Google Scholar]
- [23].Partikian A, Olveczky B, Swaminathan R, Li Y, Verkman AS. Rapid diffusion of green fluorescent protein in the mitochondrial matrix. J Cell Biol. 1998;140:821–9. doi: 10.1083/jcb.140.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol. 2004;164:493–9. doi: 10.1083/jcb.200309082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jakobs S, Schauss AC, Hell SW. Photoconversion of matrix targeted GFP enables analysis of continuity and intermixing of the mitochondrial lumen. FEBS Lett. 2003;554:194–200. doi: 10.1016/s0014-5793(03)01170-0. [DOI] [PubMed] [Google Scholar]
- [26].Jakobs S. High resolution imaging of live mitochondria. Biochim Biophys Acta. 2006;1763:561–75. doi: 10.1016/j.bbamcr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [27].Arimura S, Yamamoto J, Aida GP, Nakazono M, Tsutsumi N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc Natl Acad Sci U S A. 2004;101:7805–8. doi: 10.1073/pnas.0401077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Busch KB, Bereiter-Hahn J, Wittig I, Schagger H, Jendrach M. Mitochondrial dynamics generate equal distribution but patchwork localization of respiratory Complex I. Mol Membr Biol. 2006;23:509–20. doi: 10.1080/09687860600877292. [DOI] [PubMed] [Google Scholar]
- [29].Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004;166:1027–39. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–9. [PubMed] [Google Scholar]
- [31].Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–9. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- [32].Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–36. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- [33].Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–63. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- [34].Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–7. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- [35].Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–13. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- [36].Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. Embo J. 2008;27:306–14. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006;1757:1297–300. doi: 10.1016/j.bbabio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [38].Lee WK, Thevenod F. A role for mitochondrial aquaporins in cellular life-and-death decisions? Am J Physiol Cell Physiol. 2006;291:C195–202. doi: 10.1152/ajpcell.00641.2005. [DOI] [PubMed] [Google Scholar]
- [39].Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- [40].Chen H, Chan DC. Mitochondrial dynamics in mammals. Curr Top Dev Biol. 2004;59:119–44. doi: 10.1016/S0070-2153(04)59005-1. [DOI] [PubMed] [Google Scholar]
- [41].Diaz G, Falchi AM, Gremo F, Isola R, Diana A. Homogeneous longitudinal profiles and synchronous fluctuations of mitochondrial transmembrane potential. FEBS Letters. 2000;475:218–224. doi: 10.1016/s0014-5793(00)01683-5. [DOI] [PubMed] [Google Scholar]
- [42].Collins TJ, Bootman MD. Mitochondria are morphologically heterogeneous within cells. Journal of Experimental Biology. 2003:1993–2000. doi: 10.1242/jeb.00244. [DOI] [PubMed] [Google Scholar]
- [43].Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogenous within cells. EMBO J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Loew LM, Tuft RA, Carrington W, Fay FS. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J. 1993;65:2396–407. doi: 10.1016/S0006-3495(93)81318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, Rojo M. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6:853–9. doi: 10.1038/sj.embor.7400488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- [47].Lippincott-Schwartz J, Patterson GH. Development and use of fluorescent protein markers in living cells. Science. 2003;300:87–91. doi: 10.1126/science.1082520. [DOI] [PubMed] [Google Scholar]
- [48].Patterson GH, Schwartz J. Lippincott. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–7. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- [49].Wikstrom JD, Katzman SM, Mohamed H, Twig G, Graf SA, Heart E, Molina AJ, Corkey BE, de Vargas LM, Danial NN, Collins S, Shirihai OS. beta-Cell mitochondria exhibit membrane potential heterogeneity that can be altered by stimulatory or toxic fuel levels. Diabetes. 2007;56:2569–78. doi: 10.2337/db06-0757. [DOI] [PubMed] [Google Scholar]
- [50].Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–11. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006;1763:542–8. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [52].De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15:678–83. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- [53].Pletjushkina OY, Lyamzaev KG, Popova EN, Nepryakhina OK, Ivanova OY, Domnina LV, Chernyak BV, Skulachev VP. Effect of oxidative stress on dynamics of mitochondrial reticulum. Biochim Biophys Acta. 2006;1757:518–24. doi: 10.1016/j.bbabio.2006.03.018. [DOI] [PubMed] [Google Scholar]
- [54].Ishihara N, Jofuku A, Eura Y, Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun. 2003;301:891–8. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- [55].Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–54. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kennedy ED, Maechler P, Wollheim CB. Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes. 1998;47:374–80. doi: 10.2337/diabetes.47.3.374. [DOI] [PubMed] [Google Scholar]
- [57].Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, Sheng M, Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15:2112–8. doi: 10.1016/j.cub.2005.10.041. [DOI] [PubMed] [Google Scholar]
- [58].Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–92. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- [59].Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem. 2004;279:22704–14. doi: 10.1074/jbc.M312366200. [DOI] [PubMed] [Google Scholar]
- [61].Baloyannis SJ. Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis. 2006;9:119–26. doi: 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- [62].Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–32. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- [63].Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy. 2007;3:614–5. doi: 10.4161/auto.4872. [DOI] [PubMed] [Google Scholar]
- [64].Mattenberger Y, James DI, Martinou JC. Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin. FEBS Lett. 2003;538:53–9. doi: 10.1016/s0014-5793(03)00124-8. [DOI] [PubMed] [Google Scholar]
- [65].Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–95. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- [66].Meeusen SL, Nunnari J. How mitochondria fuse. Curr Opin Cell Biol. 2005;17:389–94. doi: 10.1016/j.ceb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- [67].Herlan M, Bornhovd C, Hell K, Neupert W, Reichert AS. Alternative topogenesis of Mgm1 and mitochondrial morphology depend on ATP and a functional import motor. J Cell Biol. 2004;165:167–73. doi: 10.1083/jcb.200403022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–64. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–77. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–55. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281:37972–9. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- [72].Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–75. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- [73].Duvezin-Caubet S, Koppen M, Wagener J, Zick M, Israel L, Bernacchia A, Jagasia R, Rugarli EI, Imhof A, Neupert W, Langer T, Reichert AS. OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell. 2007;18:3582–90. doi: 10.1091/mbc.E07-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Baricault L, Segui B, Guegand L, Olichon A, Valette A, Larminat F, Lenaers G. OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res. 2007;313:3800–8. doi: 10.1016/j.yexcr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [75].Zanna C, Ghelli A, Porcelli AM, Karbowski M, Youle RJ, Schimpf S, Wissinger B, Pinti M, Cossarizza A, Vidoni S, Valentino ML, Rugolo M, Carelli V. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain. 2008;131:352–67. doi: 10.1093/brain/awm335. [DOI] [PubMed] [Google Scholar]
- [76].Sato A, Nakada K, Hayashi J. Mitochondrial dynamics and aging: Mitochondrial interaction preventing individuals from expression of respiratory deficiency caused by mutant mtDNA. Biochim Biophys Acta. 2006;1763:473–81. doi: 10.1016/j.bbamcr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [77].Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–5. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]