Abstract
Background
During the 2009 influenza pandemic, uncertainty surrounding the seriousness of human infections with the H1N1pdm09 virus hindered appropriate public health response. One measure of seriousness is the case fatality risk, defined as the probability of mortality among people classified as cases.
Methods
We conducted a systematic review to summarize published estimates of the case fatality risk of the pandemic influenza H1N1pdm09 virus. Only studies that reported population-based estimates were included.
Results
We included 77 estimates of the case fatality risk from 50 published studies, about one-third of which were published within the first 9 months of the pandemic. We identified very substantial heterogeneity in published estimates, ranging from less than 1 to more than 10,000 deaths per 100,000 cases or infections. The choice of case definition in the denominator accounted for substantial heterogeneity, with the higher estimates based on laboratory-confirmed cases (point estimates= 0–13,500 per 100,000 cases) compared with symptomatic cases (point estimates= 0–1,200 per 100,000 cases) or infections (point estimates=1–10 per 100,000 infections). Risk based on symptomatic cases increased substantially with age.
Conclusions
Our review highlights the difficulty in estimating the seriousness of infection with a novel influenza virus using the case fatality risk. In addition, substantial variability in age-specific estimates complicates the interpretation of the overall case fatality risk and comparisons among populations. A consensus is needed on how to define and measure the seriousness of infection before the next pandemic.
In April 2009 the World Health Organization declared a formal “public health emergency of international concern,” marking the start of an international public health response to the first influenza pandemic of the 21st Century. One of the immediate priorities was to quantify the transmissibility of the new pandemic influenza A(H1N1pdm09) virus (denoted H1N1pdm09 hereafter) and the seriousness of infection with this virus, because these two epidemiologic measures in combination determine the severity of the pandemic in the absence of control measures. 1, 2 Whereas a number of transmissibility estimates, based on the reproduction number R, were published with broad agreement from the early stages of the pandemic, 3 there was far greater difficulty in estimating the seriousness of infections. In the report of the World Health Organization’s Review Committee on the functioning of the 2005 International Health Regulations in relation to H1N1pdm09, Fineberg et al. 4 identified “the absence of a consistent, measurable and understandable depiction of severity of the pandemic” as one of the major shortcomings of the international public health response.
One measure of the seriousness of infection is the “CFR,” classically the case fatality rate or ratio.5 We prefer “risk” to describe this probability, namely the conditional probability of mortality among classified “cases.” Strictly speaking, the case fatality risk is neither a rate (because there is no unit of time in the denominator) nor a ratio, which applies principally to a relationship between two measures of the same kind (for example, an odds ratio). There are complications in defining and estimating the case fatality risk, associated with both the numerator (deaths) and the denominator (persons classified as cases).1, 6 We reviewed published estimates of the case fatality risk of H1N1pdm09 to identify technical challenges in its estimation and to offer recommendations for estimating this measure in the future.
METHODS
Search Strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 7 Studies reporting estimates of the case fatality risk of H1N1pdm09 were retrieved from Medline (PubMed) and Embase on 26 April 2013. We searched for articles using the following free search terms in ‘All fields’:
-
#1
‘fatalit*’ OR ‘case-fatality*’ OR ‘severity’ OR ‘mortality’ OR ‘death’ OR ‘lethal*’ OR ‘virulence’
-
#2
‘influenza’ OR ‘flu’ OR ‘H1N1*’ OR ‘pH1N1*’ OR ‘pdmH1N1*’ OR ‘nH1N1*’
-
#3
#1 AND #2
The search was limited to studies published after 1 April 2009 (subsequent to the emergence of H1N1pdm09) through 26 April 2013. Additional relevant studies identified by the authors were manually retrieved from the database.
Study Selection
The titles of all papers identified by the search strategy were independently screened by two authors (J.Y.W. and B.J.C.). Abstracts of potentially relevant papers and the full text of manuscripts were reviewed for eligibility. Articles in all languages were selected for assessment if at least one statistical estimate of the case fatality risk for H1N1pdm09 was presented and described in the article. Eligible articles reported one or more population-based estimates of the case fatality risk. We excluded studies that reported only estimates in hospitalized patients or in population subgroups such as pregnant women or those at higher risk of severe illness if infected (e.g. persons with chronic health conditions).
Definition of Case Fatality Risk
We defined the case fatality risk as the conditional probability of death associated with H1N1pdm09 for cases that met a specified case definition. The case fatality risk for a population is estimated as the number of H1N1pdm09-associated deaths divided by the number of H1N1pdm09 cases in that population. The numerator could be counts or estimates of the number of deaths among laboratory-confirmed cases, the number of deaths among symptomatic cases, or indirect estimates of the total number of deaths associated with H1N1pdm09. The denominator could be counts or estimates of the number of laboratory-confirmed H1N1pdm09 cases, the number of symptomatic H1N1pdm09 cases, or the number of infections. We classified the denominator of the CFR as the estimated number of infections only when it was estimated based on serologic surveillance or included explicit adjustment for asymptomatic infections.
Data Extraction
All data were extracted onto a standardized form. The primary data were the estimates of the case fatality risk, the estimates or counts of the number of H1N1pdm09-associated deaths (numerator), and the estimates or counts of the number of H1N1pdm09 cases (denominator). Whenever available, we extracted case fatality risks stratified by age group. Although age groupings differed across studies, we defined children as those up to age 19, adults as those 20–64 and elderly as those 65 years or older.
Statistical Analysis
Statistical heterogeneity was assessed by the I2 statistic, with higher values signifying a greater degree of variation.8 Due to very substantial heterogeneity, we did not make pooled estimates of the case fatality risk. All analyses were conducted with R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) and the metafor package.9
RESULTS
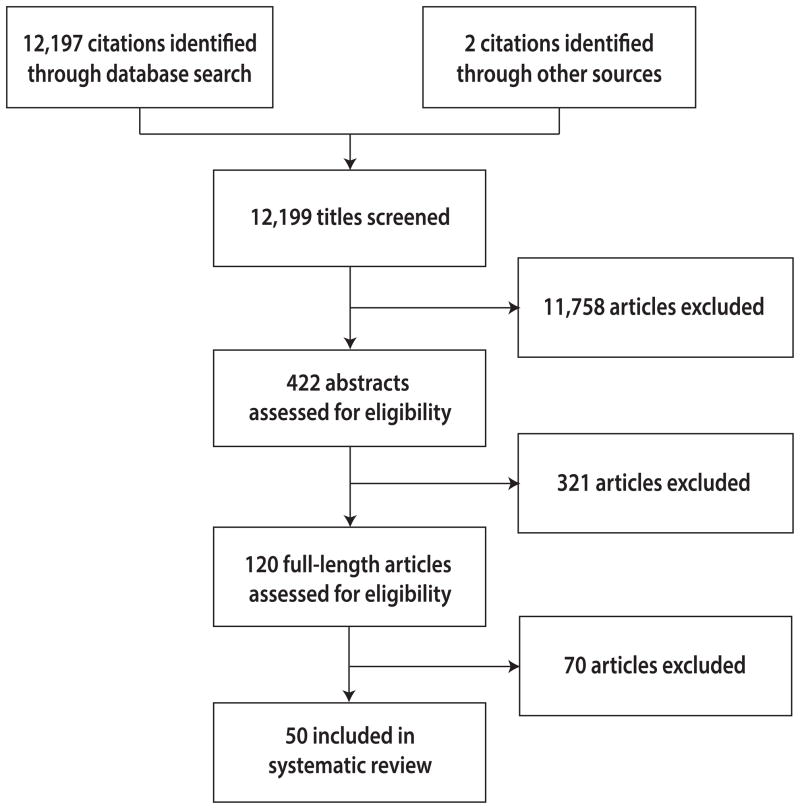
Of the 12,197 papers initially identified, we examined 120 full-length articles, of which 70 were subsequently excluded (eTable, http://links.lww.com/EDE/A708) (Figure 1).6, 10–58 The 50 articles we included, reporting a total of 77 case fatality risk estimates, are summarized in the Table. One-third (16/50) of the studies were published within the first nine months of the pandemic (Figure 2). The lag time between the end of the particular study period and the publication date increased over time (median = 236 days) (eFigure 2, http://links.lww.com/EDE/A708). In total, our analysis was based on reports from 32 countries or regions, specifically Abu Dhabi, Argentina, Australia, Canada, Chile, Colombia, European Union (EU), French Guiana, Germany, Greece, Guadeloupe, Hong Kong, India, Japan, Korea, Martinique, Mauritius, Mexico, New Zealand, Nepal, Netherlands, New Caledonia, Peru, Pacific Island countries, Reunion Island, Singapore, Spain, St. Martin, Taiwan, Thailand, United Kingdom (UK) and the United States (including metropolitan Atlanta). In addition, three publications estimated the case fatality risk worldwide,54 for 45 specified countries,43 and for developed countries.57 Illustrating one of the potential sources of heterogeneity, the number of cases or infections adopted as denominators ranged from 172 to 61,000,000 (eFigure 1, http://links.lww.com/EDE/A708).
Figure 1.
Flow diagram of study selection.
Table 1.
Table Summary of case fatality risk studies of influenza A (H1N1-2009) included in the systematic review.
| Referencesa | Country | Study period | Death definition | Case definition | Case fatality risksb | (95% CI) |
|---|---|---|---|---|---|---|
| Estimated infections as denominatorc | ||||||
| Bandaranayake 2010a 15 | New Zealand | Apr 09 – Sep 09 | Confirmed deaths | Serology | 4.5 | na |
| Chen 2011 19 | Taiwan | Jul 09 – Aug 10 | Confirmed deaths | Serology | 1 | (0.6–1.4) |
| McVernon 2010 39 | Australia | Apr 09 – Dec 09 | Confirmed deaths | Serology | 10 | na |
| Presanis 2011a 46 | UK | Jun 09 – Aug 09 | Confirmed deaths | Deaths to hospitalizations × hospitalizations to symptomatic cases × symptomatic cases to infection | 5 | (4–8) |
| Presanis 2011c 46 | UK | Sep 09 – Feb 10 | Confirmed deaths | Deaths to hospitalizations × hospitalizations to symptomatic cases × symptomatic cases to infection | 9 | (4–14) |
| Riley 2011 48 | Hong Kong | Jul 09 – Feb 10 | Confirmed deaths | Serology | 7.6 | (6.2–9.5) |
| Steens 2011 51 | Netherlands | Sep 09 – Apr 10 | Confirmed deaths | Serology | 4.7 | (3.2–9.2) |
| Sypsa 2011b 52 | Greece | Aug 09 – Feb 10 | Confirmed deaths | ILI × LAB, adjusting for the sensitivity of LAB test and proportion of infections that are asymptomatic | 6.3 | (5.3–7.5) |
| Wong 2013a 6 | Hong Kong | May 09 – Dec 09 | Excess deaths | Serology | 8.2 | (0.1–17.3) |
| Wong 2013b 6 | Hong Kong | May 09 – Dec 09 | Confirmed deaths | Serology | 5.8 | (3.9–7.8) |
| Wu 2010 58 | Hong Kong | Apr 09 – Dec 09 | Confirmed deaths | Serology | 4.4 | (3.2–17) |
| Estimated symptomatic cases as denominatord | ||||||
| Abdalla 2011 10 | USA | Apr 09 – Apr 10 | Confirmed deaths | ILI | 20.4 | na |
| Baker 2009 13 | New Zealand | Jun 09 – Aug 09 | Confirmed deaths | ILI × LAB, adjusting for health-care seeking behaviour | 5 | (3–11) |
| Bandaranayake 2010b 15 | New Zealand | Apr 09 – Sep 09 | Confirmed deaths | Serology, scaling down to include only individuals with symptoms | 8.2 | na |
| Bandaranayake 2011 16 | New Zealand | Jan 10 – Oct 10 | Confirmed deaths | Serology, scaling down to include only individuals with symptoms | 8.5 | na |
| Brooks-Pollock 2011 17 | UK | Jul 09 – Nov 09 | Confirmed deaths | ILI × LAB, adjusting for health-care seeking behaviour | 17 | na |
| Chowell 2011a 20 | Mexico | Apr 09 – Dec 09 | ILI deaths | ILI | 1200 | (1100–1200) |
| Cutter 2010 21 | Singapore | Jun 09 – Oct 09 | Confirmed deaths | ILI of ARI cases × LAB + non-ILI of ARI cases × LAB | 6.7 | na |
| Dawood 2010 22 | Australia | Jun 09 – Aug 09 | Confirmed deaths | ILI × LAB | 9.4 | (7.1–13.2) |
| Donaldson 2009 24 | UK | Jun 09 – Nov 09 | Confirmed deaths | ILI × LAB + ANTIVIRAL service × LAB, adjusting for health-care seeking behaviour | 26 | (11–66) |
| Doshi 2012 25 | USA | Aug 09 – Sep 09 | P&I deaths | ILI | 24 | na |
| Echevarria-Zuno 2009a 26 | Mexico | Apr 09 – Jul 09 | Confirmed deaths | ILI | 100 | na |
| Flahault 2009a 27 | New Caledonia | Aug 09 | Confirmed deaths | Estimated cases, adjusting for health-care seeking behaviour | 10 | na |
| Flahault 2009b 27 | Mauritius | Aug 09 | Confirmed + suspected deaths | Estimated cases, adjusting for health-care seeking behaviour | 10 | na |
| Fraser 2009 28 | Mexico | Mar 09 – Apr 09 | Confirmed + suspected deaths | Confirmed cases among tourists, backcalculating from confirmation to infection | 440 | (370–520) |
| Godoy 2011 31 | Spain | Jun 09 – May 10 | Confirmed deaths | ILI | 30 | (10–40) |
| Hadler 2010a 34 | USA | May 09 – Jun 09 | Confirmed deaths | ILI, adjusting for background ILI using LAB and emergency department visit data | 8.6 | (6.1–15.1) |
| Hadler 2010b 34 | USA | May 09 – Jun 09 | Confirmed deaths | ILI, adjusting for background ILI using emergency department visit data | 5.4 | (4.7–6.5) |
| Kamigaki 2009 35 | Japan | Jul 09 – Dec 09 | Confirmed deaths | ILI, adjusting by medical institutions proportion | 0.7 | na |
| Kim 2011 36 | South Korea | Aug 09 – Nov 09 | Confirmed deaths | ILI × LAB, adjusting by medical institutions proportion | 16 | na |
| Larrieu 2011a 38 | Martinique | Aug 09 – Jan 10 | Confirmed deaths | ILI | 5 | na |
| Larrieu 2011b 38 | Guadeloupe | Aug 09 – Jan 10 | Confirmed deaths | ILI | 31 | na |
| Larrieu 2011c 38 | French Guiana | Aug 09 – Jan 10 | Confirmed deaths | ILI × LAB | 17 | na |
| Larrieu 2011d 38 | St. Martin | Aug 09 – Jan 10 | Flu-related deaths | ILI × LAB | 0 | na |
| Nishiura 2010g 43 | Japan | Jul 09 – Jan 10 | Confirmed deaths | ILI | 0.94 | (0.8–1.08) |
| Pebody 2010 44 | UK | Apr 09 – Mar 10 | Confirmed deaths | ILI × LAB + ANTIVIRAL service × LAB, adjusting for health-care seeking behaviour | 40 | (20–100) |
| Presanis 2009a 45 | USA | Apr 09 – Jul 09 | Confirmed deaths | Deaths to hospitalizations × hospitalizations to medically attended cases × medically attended cases to symptomatic cases + deaths to medically attended cases × medically attended cases to symptomatic cases | 48 | (26–96) |
| Presanis 2009b 45 | USA | Apr 09 – Jul 09 | Confirmed deaths | ILI | 7 | (5–9) |
| Presanis 2011b 46 | UK | Jun 09 – Aug 09 | Confirmed deaths | Deaths to hospitalizations × hospitalizations to symptomatic cases | 15 | (10–22) |
| Presanis 2011d 46 | UK | Sep 09 – Feb 10 | Confirmed deaths | Deaths to hospitalizations × hospitalizations to symptomatic cases | 25 | (13–40) |
| Renault 2011 47 | Reunion Island | Jul 09 – Oct 09 | Confirmed deaths | ARI × LAB, adjusting for health-care seeking behaviour | 7 | na |
| Sachedina 2010 49 | UK | Jun 09 – Mar 10 | Confirmed deaths | ILI × LAB + ANTIVIRAL service × LAB, adjusting for health-care seeking behaviour | 19 | (7–51) |
| Simon Mendez 2011 50 | Spain | May 09 – Mar 10 | Confirmed deaths | ILI × LAB | 43 | (38–48) |
| Sypsa 2011a 52 | Greece | Aug 09 – Feb 10 | Confirmed deaths | ILI × LAB, adjusting for the sensitivity of LAB test | 17.5 | (14.6–20.8) |
| Wilson 2009a 57 | Developed countries | Apr 09 – Jun 09 | Confirmed deaths | Confirmed cases, adjusting by a multiplier of 10–30 | 10–30 | na |
| Wilson 2009b 57 | Developed countries | Apr 09 – Jun 09 | Confirmed deaths | ILI x LAB | 2–3 | na |
| Wilson 2009c 57 | Developed countries | Apr 09 – Jun 09 | Excess deaths | 5%–10% of the US population <65y | 4–60 | na |
| Wilson 2009d 57 | Developed countries | Apr 09 – Jun 09 | Confirmed deaths | 5%–30% of the Canadian population | 0.4–3 | na |
| Laboratory-confirmed cases as denominatore | ||||||
| Adhikari 2011 11 | Nepal | Apr 09 – May 10 | Confirmed deaths | Confirmed cases | 1740 | na |
| Ahmed 2012 12 | Abu Dhabi | May 09 – Aug 09 | Confirmed deaths | Confirmed cases | 0 | na |
| Balaganesakumar 2013 14 | India | Jan 10 – Dec 10 | Confirmed deaths | Confirmed cases | 5500 | na |
| Castro-Jimenez 2009 18 | Colombia | May 09 – Jul 09 | Confirmed deaths | Confirmed cases | 3800 | na |
| Chowell 2011b 20 | Mexico | Apr 09 – Dec 09 | ILI deaths | Confirmed cases | 5000 | (4700–5300) |
| de Silva 2009 23 | Thailand | Jun 09 – Jul 09 | Confirmed deaths | Confirmed cases | 580 | na |
| Echevarria-Zuno 2009b 26 | Mexico | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases | 900 | na |
| Garske 2009a 29 | USA | Apr 09 – Jul 09 | Confirmed deaths | Confirmed + probable cases, adjusting for delay from onset to death | 680 | (590–780) |
| Garske 2009b 29 | Mexico | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 1230 | (1030–1470) |
| Garske 2009c 29 | Canada | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 430 | (300–580) |
| Garske 2009d 29 | UK | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 240 | (130–410) |
| Garske 2009e 29 | EU | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 200 | (110–320) |
| Glinsky 2009 30 | Argentina | Jul 09 | Confirmed deaths | Confirmed cases | 13500 | na |
| Gomez 2009 32 | Peru | May 09 – Sep 09 | Confirmed deaths | Confirmed cases | 1710 | na |
| Gu 2013 33 | Japan | Jul 09 – Aug 10 | Influenza-associated encephalopathy deaths | Influenza-associated encephalopathy cases | 3600 | na |
| Kool 2013 37 | Pacific Island countries | Apr 09 – Aug 10 | Confirmed deaths | Confirmed cases | 1000 | na |
| Mishra 2010 40 | India | Aug 09 – Oct 09 | Confirmed deaths, adjusting by hospitalization rate | Confirmed cases | 860 | na |
| Nishiura 2009a 41 | USA | Apr 09 – Jun 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 1230 | (210–3760) |
| Nishiura 2009b 41 | Canada | Apr 09 – Jun 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 180 | (50–410) |
| Nishiura 2010a 42 | Argentina | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 2330 | (1970–2720) |
| Nishiura 2010b 42 | Canada | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 400 | (330–470) |
| Nishiura 2010c 42 | Chile | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 250 | (180–330) |
| Nishiura 2010d 42 | Mexico | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 5580 | (5150–6020) |
| Nishiura 2010e 42 | USA | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases, adjusting for delay from onset to death | 250 | (220–290) |
| Nishiura 2010f 43 | 45 countries | Jul 09 – Jan 10 | Confirmed deaths | Confirmed cases | 690 | (650–730) |
| Tuite 2010 53 | Canada | Apr 09 – Jun 09 | Confirmed deaths | Confirmed cases | 300 | (100–500) |
| Vaillant 2009 54 | Worldwide | Apr 09 – Jul 09 | Confirmed deaths | Confirmed cases | 600 | (100–5100) |
| WHO. 2009 55 | Mexico | Apr 09 – May 09 | Confirmed deaths | Confirmed cases | 2000 | na |
| Wilking 2010 56 | Germany | Apr 09 – Mar 10 | Confirmed deaths | Confirmed + suspected cases | 110 | (100–130) |
na indicates not available; ILI, influenza-like illness consultations; LAB, laboratory specimens positive for influenza; ARI, acute respiratory infection consultations; ANTIVIRAL, antiviral dugs authorizations.
Letters following the year of publication (e.g., Bandaranayake 2010a) indicate one of multiple analyses, which are described in this table.
Case fatality risks are expressed as number of deaths per 100,000 cases or infections.
Overall: point estimates ranged from 1 to 10 deaths per 100,000 infections
Overall: point estimates ranged from 0 to 1,200 deaths per 100,000 cases
Overall: point estimates ranged from 0 to 13,500 deaths per 100,000 cases
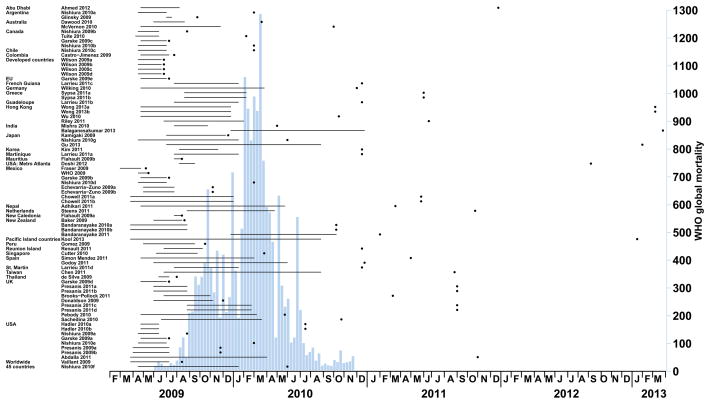
Figure 2.
Study dates (solid horizontal lines), eventual publication dates of the studies included in the review (points) compared with the histogram of confirmed H1N1pdm09 deaths reported to the World Health Organization (underlying histogram). See Table for details of each study.
Overall, the case fatality risk estimates based on laboratory-confirmed cases were higher than the other estimates and published earlier (Figure 3). Estimates based on laboratory-confirmed cases ranged from 0 to 13,500 deaths per 100,000 cases, with very substantial heterogeneity [I2=99.97%]. Most (25/29) of the risk estimates in this category fell within the range of 100 to 5,000 deaths per 100,000 cases.
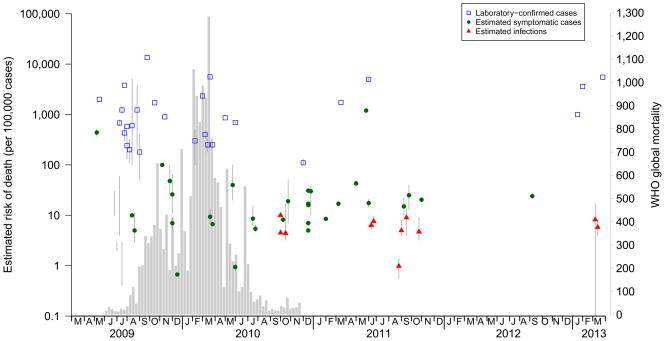
Figure 3.
Estimated risk of death by eventual publication dates of the studies included in the review (points with 95% CI) compared with the histogram of confirmed H1N1pdm09 deaths reported to the World Health Organization (underlying histogram).
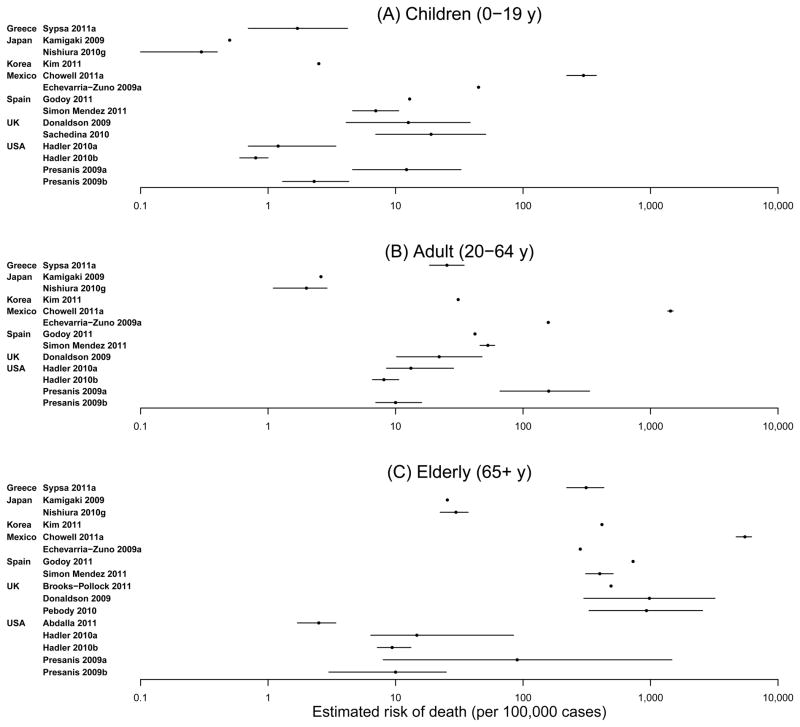
There was also substantial heterogeneity among the 37 risk estimates based on symptomatic cases, ranging from 0 to 1,200 deaths per 100,000 [I2=99.98%]. Most of the estimates in this category fell in the range of 5 to 50 deaths per 100,000 cases. In age-stratified analyses, risk estimates rose monotonically with age, from approximately one death per 100,000 symptomatic cases in children to approximately 1,000 deaths per 100,000 symptomatic cases in the elderly, although with substantial variation in the estimates within each age group (Figure 4).
Figure 4.
Age-specific estimated risk of death.
In 14% (11/77) of the case fatality risk estimates, the denominator was based on an estimated number of infections. Among those 11 studies, 8 used denominators based on population serological studies, while the others used modelling or multiplier approaches. Those risks tended to remain stable over time, although no such estimates were published within the first year of the pandemic (Figure 3). Estimates ranged from 1 to 10 deaths per 100,000 infections, with substantial heterogeneity [I2=94.46%]. The majority of studies adopted laboratory-confirmed deaths as the numerator (Table), while one study estimated that the number of “excess” deaths attributable to H1N1pdm09 was higher than the number of deaths of laboratory-confirmed cases, leading to a generally higher case fatality risk based on the excess-deaths numerator compared with the confirmed-deaths numerator.6 The increase in age-specific risk estimates by age based on infection were similar to those based on symptomatic cases (data not shown).
The highest case fatality risk estimates were observed in Argentina,30, 42 Mexico,20, 42 and Colombia.18 All of these studies reported case fatality risks among laboratory-confirmed cases. Apart from differences in case definition, it is not clear why estimates during the early stage of the pandemic were so high in Mexico (ranging from 100 to 5,580 deaths per 100,000).20, 26, 28, 29, 42, 55
DISCUSSION
There is very substantial heterogeneity in published estimates of case fatality risk for H1N1pdm09, ranging from <1 to >10,000 per 100,000 infections (Figure 3). Large differences were associated with the choice of case definition (denominator). Because influenza virus infections are typically mild and self-limiting, and a substantial proportion of infections are subclinical and do not require medical attention, it is challenging to enumerate all symptomatic cases or infections.2, 45 In 2009, some of the earliest available information on fatality risk was provided by estimates based primarily on confirmed cases. However, because most H1N1pdm09 infections were not laboratory-confirmed, the estimates based on confirmed cases were up to 500 times higher than those based on symptomatic cases or infections (Figure 3). The consequent uncertainty about the case fatality risk — and hence about the severity of H1N1pdm09 — was problematic for risk assessment and risk communication during the period when many decisions about control and mitigation measures were being made.
Whereas our review has focused on the case fatality risk, there are other measures of seriousness of infection that can be useful in assessing the risk associated with pandemic and seasonal influenza viruses. For example, the outbreak of H1N1pdm09 in a New York school in April–May 2009 was informative because none of the more than 800 students and staff with influenza-like illness had severe illness,59 implying a low risk of hospitalization among infected cases. At the other end of the spectrum of disease, a description of the risk of mortality among hospitalized patients could be affected by flexible thresholds for admission depending on demand and capacity. A comprehensive seriousness profile could include age-specific estimates of the risks of illness, hospitalization and mortality on a per-infection basis. In addition, while transmissibility and seriousness profiles can allow prediction of the number of serious illnesses, the availability of health care resources would also affect the likely impact of an epidemic or pandemic.60
Arguably one of the ideal estimates of seriousness of infection is the infection fatality risk,6 i.e. the case fatality risk based on infections as denominator. This risk estimate can be compared across populations without concerns over differences in symptom perceptions and reporting, health-care seeking behaviors, or laboratory-testing capacity.61 Estimates of the infection fatality risk can also be used in combination with estimates of transmissibility to directly inform predictions of population impact.62 The use of the term “infection fatality” differentiates this risk estimate from the case fatality risk since the asymptomatic, undetected and undiagnosed infections included in the denominator would not appear as “cases” under typical case definitions. However, few estimates of the infection fatality risk were available for H1N1pdm09, and none was available early in the pandemic (Figure 3). Serologic studies,63 or estimates of the cumulative incidence of symptomatic infections in a population adjusted for the proportion of infections that are asymptomatic,52, 64 can be used to estimate the denominator for the infection fatality risk. However, one has to define how to estimate infection rates from serologic data, and there is not yet consensus on the best approach.
Given that estimates of the infection fatality risk are unlikely to be available early enough for decision-making in a pandemic, a more feasible solution may be to measure the case fatality risk among symptomatic cases. One obvious limitation is that differences in the definition of a symptomatic case are likely to affect comparability and limit generalizability. In our review the case fatality risk estimates for symptomatic cases covered a wide range (eFigure 1, http://links.lww.com/EDE/A708). In practice, estimation of fatality risk among symptomatic cases could be based on outpatient surveillance data in combination with laboratory data, and with adjustment for the proportion of symptomatic cases not presenting for medical attention. These may vary by age, sex, location, and other factors.13, 17, 24, 44, 45, 49, 52 Such estimates based on symptomatic cases may provide timely but imprecise estimates of seriousness for risk assessment.
In addition to differences in case fatality risk estimates due to the differences in case definition (denominator), the definition of the numerator is also an important issue. Almost all of the studies in our review based the numerator on deaths among patients with laboratory-confirmed influenza infection. In contrast, most estimates of the population impact of seasonal influenza epidemics have been based on estimation of the number of excess deaths associated with influenza (i.e. estimated deaths), with the greatest annual impact in the elderly — despite influenza virus infections rarely being confirmed in this age group.65, 66 The use of excess deaths rather than laboratory-confirmed deaths in the numerator of the infection fatality risk would theoretically be justified because the denominator includes all infections and not only those with a positive laboratory result. For a similar reason, deaths of patients with laboratory-confirmed infection might be a more appropriate numerator for the case fatality risk based on symptomatic case denominators.
In addition to the differences in risk estimates due to differences in numerators and denominators, our review also identified substantial variability by age, ranging from approximately one death per 100,000 symptomatic cases in children to 1,000 deaths per 100,000 symptomatic cases in the elderly (Figure 4). This variability complicates the interpretation of the overall risk and the comparison of risk among countries, because overall risk would depend on the age structure of the population and the age distribution of infections.67, 68 Age-standardization is an accepted method for comparing incidence rates among the populations of various countries,69 but no similar approach has yet been discussed or recommended for comparison of case fatality risks.
Further limitations of our review are that most of the included estimates corresponded to the initial epidemics of H1N1pdm09 in 2009 (Figure 2). Fewer data are available on possible changes in the case fatality risk after that period, although there have been reports of up to four epidemic waves in some countries.20, 70 We excluded studies that focused on specific risk groups (e.g. those with underlying diseases) to improve consistency among studies. There was also substantial heterogeneity among estimates of the case fatality risk, depending particularly on case definition (eFigure 1, http://links.lww.com/EDE/A708). We could not determine whether these differences were artefactual or real, perhaps related to differences in the prevalence of underlying risk factors for severe illness in various populations.71, 72 Further work could attempt to identify additional factors that explain this heterogeneity, for example by exploring very different estimates of risk apparently based on the same case definition (e.g., from Argentina,30, 42 Mexico20, 26, 28 and the United States29, 41, 42).
Results of this review point to possible improvements in future studies of the case fatality risk. First, there is a problem in using confirmed cases as the denominator of CFR for influenza, given that most infections are mild and do not present for medical attention. Because it is not feasible to diagnose all suspected cases with laboratory testing except at the very beginning of a pandemic,2 it is unrealistic that risk estimates based on confirmed cases can be consistently calculated and remain directly comparable over time, age groups, and location. We suggest avoiding entirely the use of case fatality risk based on confirmed cases. The case fatality risk based on symptomatic cases would provide a more reliable early assessment of seriousness for seasonal influenza or the next influenza pandemic. Second, estimation of seriousness in real-time is complicated by delays in reporting and analysis. Estimation of the case fatality risk based on confirmed deaths and symptomatic cases may be possible if relevant models can be prepared in advance and quickly fitted to available data during the pandemic. We have previously discussed real-time estimation of the cumulative incidence of infection based on serologic data.73 This would form the denominator of the infection fatality risk, but, as noted previously, this is unlikely to be available early in the pandemic.
In preparation for the next influenza pandemic, it is essential to reach a consensus on how to define and measure the seriousness of infection (an important indicator of the severity of the pandemic), and whether the analysis can be based entirely on estimates of age-specific risk of death among cases. The consistent estimates of the infection fatality risk at around 1 to 10 deaths per 100,000 infections identified in our review (Figure 3) may represent the seriousness of H1N1pdm09 in developed countries where data were available. Similar estimates for seasonal influenza viruses, however, are not available for comparison, and neither are estimates from less developed countries in which the seriousness profile would likely be higher.74
An alternative assessment of seriousness of infection may be to compare an absolute value of case fatality risk against an arbitrarily defined threshold value.1 In either case, the experience from 2009 indicates that explicit and consistent definitions are required to permit comparisons. As highlighted by the Fineberg report,4 updated pandemic plans must clarify how severity will be estimated and interpreted. Improvements in measuring seriousness of infection, perhaps within a multidimensional metric not limited to the case fatality risk, could be a valuable objective of the new World Health Organization Pandemic Influenza Preparedness Framework. Our review also identified substantial delays between study completion dates and publication dates. A mechanism for early sharing of data that does not interfere with subsequent publication would be welcome.
Supplementary Material
Acknowledgments
DKMI receives research funding from F. Hoffman-La Roche. BJC has received research funding from MedImmune Inc., and consults for Crucell NV.
SOURCES OF FUNDING
This work received financial support from the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), the Research Fund for the Control of Infectious Disease, Food and Health Bureau, Government of the Hong Kong SAR (grant no. HK-12-04-02), and the Area of Excellence Scheme of the University Grants Committee of Hong Kong (grant no. AoE/M-12/06).
We thank Vicky Fang and Lincoln Lau for technical support. We thank Eric Lau and Peng Wu for helpful discussions.
Footnotes
CONFLICTS OF INTEREST
The authors report no other potential conflicts of interest.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
References
- 1.Van Kerkhove MD, Asikainen T, Becker NG, et al. Studies needed to address public health challenges of the 2009 H1N1 influenza pandemic: insights from modeling. PLoS Med. 2010;7(6):e1000275. doi: 10.1371/journal.pmed.1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipsitch M, Hayden FG, Cowling BJ, et al. How to maintain surveillance for novel influenza A H1N1 when there are too many cases to count. Lancet. 2009;374(9696):1209–11. doi: 10.1016/S0140-6736(09)61377-5. [DOI] [PubMed] [Google Scholar]
- 3.Boelle PY, Ansart S, Cori A, et al. Transmission parameters of the A/H1N1 (2009) influenza virus pandemic: a review. Influenza Other Respi Viruses. 2011;5(5):306–16. doi: 10.1111/j.1750-2659.2011.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Implementation of the International Health Regulations (2005) Report of the review committee on the functioning of the International Health Regulations (2005) in relation to pandemic (H1N1) 2009. 2011 [Google Scholar]
- 5.Nishiura H. Case fatality ratio of pandemic influenza. Lancet Infect Dis. 2010;10(7):443–4. doi: 10.1016/S1473-3099(10)70120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong JY, Wu P, Nishiura H, et al. The infection fatality risk of pandemic influenza A(H1N1) in Hong Kong in 2009. Am J Epidemiol. 2013;177(8):834–40. doi: 10.1093/aje/kws314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 9.metafor: Meta-analysis package for R [computer program]. Version 1.6-0. 2011.
- 10.Abdalla E, HabteMariam T, Nganwa D, et al. Epidemiology of influenza A 2009 H1N1 virus pandemic in the U.S. J Health Care Poor Underserved. 2011;22(4 Suppl):39–60. doi: 10.1353/hpu.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari BR, Shakya G, Upadhyay BP, et al. Outbreak of pandemic influenza A/H1N1 2009 in Nepal. Virol J. 2011;8:133. doi: 10.1186/1743-422X-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed F, Al Hosani F, Al Mannaie A, et al. Early outcomes of pandemic influenza (H1N1) 2009 surveillance in Abu Dhabi Emirate, May–August 2009. East Mediterr Health J. 2012;18(1):31–6. doi: 10.26719/2012.18.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Baker MG, Wilson N, Huang QS, et al. Pandemic influenza A(H1N1)v in New Zealand: the experience from April to August 2009. Euro Surveill. 2009;14(34) doi: 10.2807/ese.14.34.19319-en. pii:19319. [DOI] [PubMed] [Google Scholar]
- 14.Balaganesakumar SR, Murhekar MV, Swamy KK, et al. Risk factors associated with death among influenza A (H1N1) patients, Tamil Nadu, India, 2010. Journal of postgraduate medicine. 2013;59(1):9–14. doi: 10.4103/0022-3859.109481. [DOI] [PubMed] [Google Scholar]
- 15.Bandaranayake D, Huang QS, Bissielo A, et al. Risk factors and immunity in a nationally representative population following the 2009 influenza A(H1N1) pandemic. PLoS One. 2010;5(10):e13211. doi: 10.1371/journal.pone.0013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandaranayake D, Jacobs M, Baker M, et al. The second wave of 2009 pandemic influenza a(H1N1) in New Zealand, January–October 2010. Euro Surveill. 2011;16(6) pii: 19788. [PubMed] [Google Scholar]
- 17.Brooks-Pollock E, Tilston N, Edmunds WJ, et al. Using an online survey of healthcare-seeking behaviour to estimate the magnitude and severity of the 2009 H1N1v influenza epidemic in England. BMC Infect Dis. 2011;11:68. doi: 10.1186/1471-2334-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro-Jimenez MA, Castillo-Pabon JO, Rey-Benito GJ, et al. Epidemiologic analysis of the laboratory-confirmed cases of influenza A(H1N1)v in Colombia. Euro Surveill. 2009;14(30) doi: 10.2807/ese.14.30.19284-en. pii:19284. [DOI] [PubMed] [Google Scholar]
- 19.Chen CJ, Lee PI, Chang SC, et al. Seroprevalence and severity of 2009 pandemic influenza A H1N1 in Taiwan. PLoS One. 2011;6(9):e24440. doi: 10.1371/journal.pone.0024440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowell G, Echevarria-Zuno S, Viboud C, et al. Characterizing the epidemiology of the 2009 influenza A/H1N1 pandemic in Mexico. PLoS Med. 2011;8(5):e1000436. doi: 10.1371/journal.pmed.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutter JL, Ang LW, Lai FY, et al. Outbreak of pandemic influenza A (H1N1-2009) in Singapore, May to September 2009. Ann Acad Med Singapore. 2010;39(4):273–10. [PubMed] [Google Scholar]
- 22.Dawood FS, Hope KG, Durrheim DN, et al. Estimating the disease burden of pandemic (H1N1) 2009 virus infection in Hunter New England, Northern New South Wales, Australia, 2009. PLoS One. 2010;5(3):e9880. doi: 10.1371/journal.pone.0009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Silva UC, Warachit J, Waicharoen S, et al. A preliminary analysis of the epidemiology of influenza A(H1N1)v virus infection in Thailand from early outbreak data, June–July 2009. Euro Surveill. 2009;14(31) doi: 10.2807/ese.14.31.19292-en. pii: 19292. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson LJ, Rutter PD, Ellis BM, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doshi SS, Stauffer KE, Parker Fiebelkorn A, et al. The Burden and Severity of Illness Due to 2009 Pandemic Influenza A (H1N1) in a Large US City During the Late Summer and Early Fall of 2009. Am J Epidemiol. 2012;176(6):519–26. doi: 10.1093/aje/kws137. [DOI] [PubMed] [Google Scholar]
- 26.Echevarria-Zuno S, Mejia-Arangure JM, Mar-Obeso AJ, et al. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet. 2009;374(9707):2072–9. doi: 10.1016/S0140-6736(09)61638-X. [DOI] [PubMed] [Google Scholar]
- 27.Flahault A. First estimation of direct H1N1pdm virulence: From reported non consolidated data from Mauritius and New Caledonia. PLoS Curr. 2009;1:RRN1010. doi: 10.1371/currents.RRN1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324(5934):1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garske T, Legrand J, Donnelly CA, et al. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009;339:b2840. doi: 10.1136/bmj.b2840. [DOI] [PubMed] [Google Scholar]
- 30.Glinsky GV. Real-time case fatality analysis points to emerging evidence of increasing severity of pandemic (H1N1) 2009. Cell Cycle. 2009;8(19):3057–62. doi: 10.4161/cc.8.19.9817. [DOI] [PubMed] [Google Scholar]
- 31.Godoy P, Pumarola T, Martinez A, et al. Surveillance of the pandemic influenza (H1N1) 2009 in Catalonia: results and implications. Rev Esp Salud Publica. 2011;85(1):37–45. doi: 10.1590/S1135-57272011000100005. [DOI] [PubMed] [Google Scholar]
- 32.Gomez J, Munayco C, Arrasco J, et al. Pandemic influenza in a southern hemisphere setting: the experience in Peru from May to September, 2009. Euro Surveill. 2009;14(42) doi: 10.2807/ese.14.42.19371-en. pii: 19371. [DOI] [PubMed] [Google Scholar]
- 33.Gu Y, Shimada T, Yasui Y, et al. National surveillance of influenza-associated encephalopathy in Japan over six years, before and during the 2009–2010 influenza pandemic. PLoS One. 2013;8(1):e54786. doi: 10.1371/journal.pone.0054786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadler JL, Konty K, McVeigh KH, et al. Case fatality rates based on population estimates of influenza-like illness due to novel H1N1 influenza: New York City, May–June 2009. PLoS One. 2010;5(7):e11677. doi: 10.1371/journal.pone.0011677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamigaki T, Oshitani H. Epidemiological characteristics and low case fatality rate of pandemic (H1N1) 2009 in Japan. PLoS Curr. 2009;1:RRN1139. doi: 10.1371/currents.RRN1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HS, Kim JH, Shin SY, et al. Fatal cases of 2009 pandemic influenza A (H1N1) in Korea. J Korean Med Sci. 2011;26(1):22–7. doi: 10.3346/jkms.2011.26.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kool JL, Pavlin BI, Musto J, et al. Influenza surveillance in the Pacific Island countries and territories during the 2009 pandemic: an observational study. BMC Infect Dis. 2013;13:6. doi: 10.1186/1471-2334-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larrieu S, Rosine J, Ledrans M, et al. Epidemic of influenza A(H1N1) 2009 in the French overseas territories of the Americas: epidemiological surveillance set up and main results, April 2009–January 2010. Bull Soc Pathol Exot. 2011;104(2):119–24. doi: 10.1007/s13149-010-0111-7. [DOI] [PubMed] [Google Scholar]
- 39.McVernon J, Laurie K, Nolan T, et al. Seroprevalence of 2009 pandemic influenza A(H1N1) virus in Australian blood donors, October – December 2009. Euro Surveill. 2010;15(40) doi: 10.2807/ese.15.40.19678-en. pii: 19678. [DOI] [PubMed] [Google Scholar]
- 40.Mishra AC, Chadha MS, Choudhary ML, et al. Pandemic influenza (H1N1) 2009 is associated with severe disease in India. PLoS One. 2010;5(5):e10540. doi: 10.1371/journal.pone.0010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiura H, Klinkenberg D, Roberts M, et al. Early epidemiological assessment of the virulence of emerging infectious diseases: a case study of an influenza pandemic. PLoS One. 2009;4(8):e6852. doi: 10.1371/journal.pone.0006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishiura H. The relationship between the cumulative numbers of cases and deaths reveals the confirmed case fatality ratio of a novel influenza A (H1N1) virus. Jpn J Infect Dis. 2010;63(2):154–6. [PubMed] [Google Scholar]
- 43.Nishiura H. The virulence of pandemic influenza A (H1N1) 2009: an epidemiological perspective on the case-fatality ratio. Expert Rev Respir Med. 2010;4(3):329–38. doi: 10.1586/ers.10.24. [DOI] [PubMed] [Google Scholar]
- 44.Pebody RG, McLean E, Zhao H, et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill. 2010;15(20) pii: 19571. [PubMed] [Google Scholar]
- 45.Presanis AM, De Angelis D, Hagy A, et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6(12):e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Presanis AM, Pebody RG, Paterson BJ, et al. Changes in severity of 2009 pandemic A/H1N1 influenza in England: a Bayesian evidence synthesis. BMJ. 2011;343:d5408. doi: 10.1136/bmj.d5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renault P, Thouillot F, Do C, et al. Epidemic of influenza A(H1N1) 2009 in Reunion Island: epidemiological data. Bull Soc Pathol Exot. 2011;104(2):108–13. doi: 10.1007/s13149-010-0113-5. [DOI] [PubMed] [Google Scholar]
- 48.Riley S, Kwok KO, Wu KM, et al. Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study. PLoS Med. 2011;8(6):e1000442. doi: 10.1371/journal.pmed.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet. 2010;376(9755):1846–52. doi: 10.1016/S0140-6736(10)61195-6. [DOI] [PubMed] [Google Scholar]
- 50.Simon Mendez L, de Mateo Ontanon S, Larrauri Camara A, et al. Transmissibility and severity of the pandemic influenza A (H1N1) 2009 virus in Spain. Gac Sanit. 2011;25(4):296–302. doi: 10.1016/j.gaceta.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Steens A, Waaijenborg S, Teunis PF, et al. Age-dependent patterns of infection and severity explaining the low impact of 2009 influenza A (H1N1): evidence from serial serologic surveys in the Netherlands. Am J Epidemiol. 2011;174(11):1307–15. doi: 10.1093/aje/kwr245. [DOI] [PubMed] [Google Scholar]
- 52.Sypsa V, Bonovas S, Tsiodras S, et al. Estimating the disease burden of 2009 pandemic influenza A(H1N1) from surveillance and household surveys in Greece. PLoS One. 2011;6(6):e20593. doi: 10.1371/journal.pone.0020593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuite AR, Greer AL, Whelan M, et al. Estimated epidemiologic parameters and morbidity associated with pandemic H1N1 influenza. CMAJ. 2010;182(2):131–6. doi: 10.1503/cmaj.091807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaillant L, La Ruche G, Tarantola A, et al. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14(33) doi: 10.2807/ese.14.33.19309-en. pii: 19309. [DOI] [PubMed] [Google Scholar]
- 55.Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009;84(21):185–9. [PubMed] [Google Scholar]
- 56.Wilking H, Buda S, von der Lippe E, et al. Mortality of 2009 pandemic influenza A(H1N1) in Germany. Euro Surveill. 2010;15(49) doi: 10.2807/ese.15.49.19741-en. pii: 19741. [DOI] [PubMed] [Google Scholar]
- 57.Wilson N, Baker MG. The emerging influenza pandemic: estimating the case fatality ratio. Euro Surveill. 2009;14(26) pii: 19255. [PubMed] [Google Scholar]
- 58.Wu JT, Ma ES, Lee CK, et al. The infection attack rate and severity of 2009 pandemic H1N1 influenza in Hong Kong. Clin Infect Dis. 2010;51(10):1184–91. doi: 10.1086/656740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lessler J, Reich NG, Cummings DA, et al. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med. 2009;361(27):2628–36. doi: 10.1056/NEJMoa0906089. [DOI] [PubMed] [Google Scholar]
- 60.Rudge JW, Hanvoravongchai P, Krumkamp R, et al. Health system resource gaps and associated mortality from pandemic influenza across six Asian territories. PLoS One. 2012;7(2):e31800. doi: 10.1371/journal.pone.0031800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.SteelFisher GK, Blendon RJ, Ward JR, et al. Public response to the 2009 influenza A H1N1 pandemic: a polling study in five countries. Lancet Infect Dis. 2012;12(11):845–50. doi: 10.1016/S1473-3099(12)70206-2. [DOI] [PubMed] [Google Scholar]
- 62.Baguelin M, Hoek AJ, Jit M, et al. Vaccination against pandemic influenza A/H1N1v in England: a real-time economic evaluation. Vaccine. 2010;28(12):2370–84. doi: 10.1016/j.vaccine.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Kelly H, Peck HA, Laurie KL, et al. The age-specific cumulative incidence of infection with pandemic influenza H1N1 2009 was similar in various countries prior to vaccination. PLoS One. 2011;6(8):e21828. doi: 10.1371/journal.pone.0021828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lau LL, Nishiura H, Kelly H, et al. Household transmission of 2009 pandemic influenza A (H1N1): a systematic review and meta-analysis. Epidemiology. 2012;23(4):531–42. doi: 10.1097/EDE.0b013e31825588b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simonsen L, Reichert TA, Viboud C, et al. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165(3):265–72. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 66.Thompson WW, Moore MR, Weintraub E, et al. Estimating influenza-associated deaths in the United States. Am J Public Health. 2009;99 (Suppl 2):S225–30. doi: 10.2105/AJPH.2008.151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melegaro A, Jit M, Gay N, et al. What types of contacts are important for the spread of infections?: using contact survey data to explore European mixing patterns. Epidemics. 2011;3(3–4):143–51. doi: 10.1016/j.epidem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Hollingsworth TD, Klinkenberg D, Heesterbeek H, et al. Mitigation strategies for pandemic influenza A: balancing conflicting policy objectives. PLoS Comput Biol. 2011;7(2):e1001076. doi: 10.1371/journal.pcbi.1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad OB, Boschi-Pinto C, Lopez AD. Age standardization of rates: a new WHO standard. GPE Discussion Paper No. 31. Geneva: World Health Organisation; 2001. [Google Scholar]
- 70.Chowell G, Echevarria-Zuno S, Viboud C, et al. Recrudescent wave of pandemic A/H1N1 influenza in Mexico, winter 2011–2012: Age shift and severity. PLoS Curr. 2012;4:RRN1306. doi: 10.1371/currents.RRN1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Kerkhove MD, Vandemaele KA, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang YH, Zhao Y, Li N, et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nat Commun. 2013;4:1418. doi: 10.1038/ncomms2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu JT, Ho A, Ma ES, et al. Estimating infection attack rates and severity in real time during an influenza pandemic: analysis of serial cross-sectional serologic surveillance data. PLoS Med. 2011;8(10):e1001103. doi: 10.1371/journal.pmed.1001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12(9):687–95. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.