Abstract
Our recent work has shown that older adults are disproportionately impaired at bimanual tasks when the two hands are moving out of phase with each other [Bangert, A. S., Reuter-Lorenz, P. A., Walsh, C. M., Schachter, A. B., & Seidler, R. D. Bimanual coordination and aging: Neurobehavioral implications. Neuropsychologia, 48, 1165–1170, 2010]. Interhemispheric interactions play a key role during such bimanual movements to prevent interference from the opposite hemisphere. Declines in corpus callosum (CC) size and microstructure with advancing age have been well documented, but their contributions to age deficits in bimanual function have not been identified. In the current study, we used structural magnetic resonance and diffusion tensor imaging to investigate age-related changes in the relationships between callosal macrostructure, microstructure, and motor performance on tapping tasks requiring differing degrees of interhemispheric interaction. We found that older adults demonstrated disproportionately poorer performance on out-of-phase bimanual control, replicating our previous results. In addition, older adults had smaller anterior CC size and poorer white matter integrity in the callosal midbody than their younger counterparts. Surprisingly, larger CC size and better integrity of callosal microstructure in regions connecting sensorimotor cortices were associated with poorer motor performance on tasks requiring high levels of interhemispheric interaction in young adults. Conversely, in older adults, better performance on these tasks was associated with larger size and better CC microstructure integrity within the same callosal regions. These findings implicate age-related declines in callosal size and integrity as a key contributor to bimanual control deficits. Further, the differential age-related involvement of transcallosal pathways reported here raises new questions about the role of the CC in bimanual control.
INTRODUCTION
Coordinated bimanual actions are ubiquitous in everyday life. Consider tying your shoes in the morning, one of the most automatic movements an adult performs every day. Each hand works independently during this task to accomplish a unified goal. Bimanual movements involve coordinated motion of the two hands in time and space but also often rely on the ability of movements performed with each hand to be different without interfering with the other (Perrig, Kazennikov, & Wiesendanger, 1999). The neurophysiological mechanisms underlying the production of such orchestrated behaviors have not been fully elucidated.
Previous work has reported a “bimanual advantage”; tapping is less variable during synchronous bimanual as compared with unimanual tapping (Ivry & Hazeltine, 1999; Helmuth & Ivry, 1996). Furthermore, bimanual finger tapping is most stable and accurate when movements are performed with the hands moving either in phase (both fingers are tapping simultaneously) or antiphase (fingers are moving 180° out of phase) across a range of frequencies (Swinnen, 2002; Kelso, 1984). Bimanual tapping becomes more difficult at phase delays that divide the total response cycle into unequal subintervals, particularly when delays are extreme (termed asynchronous out-of-phase bimanual actions; Semjen & Ivry, 2001). Young and older adults perform almost identically with the hands moving in phase or antiphase across a range of frequencies. However, our recent work (Bangert, Reuter-Lorenz, Walsh, Schachter, & Seidler, 2010) and that of others (Lee, Almeida, & Chua, 2002; Swinnen et al., 1998) have demonstrated that older adults are disproportionately impaired at bimanual control when the two hands are moving out of phase (at unequal subintervals) with each other, indicating that older adults may have difficulty with interference arising between the two hands. The underlying cause(s) of bimanual control deficits in older adults remains undetermined, but the specific nature of the out-of-phase bimanual control deficit points toward callosal mechanisms.
Interhemispheric interactions play an integral role in bimanual control (Shim et al., 2005; Serrien, Nirkko, & Wiesendanger, 2001). Studies evaluating individuals with corpus callosum (CC) pathology (e.g., partial callosotomy or multiple sclerosis) have shown that although the total number of fibers between motor cortices is relatively few in number, they have the capability to strongly influence motor behavior (Bonzano et al., 2008; Lenzi et al., 2007; Kennerley, Diedrichsen, Hazeltine, Semjen, & Ivry, 2002; Eliassen, Baynes, & Gazzaniga, 1999, 2000). This inter-hemispheric communication can be either facilitatory or inhibitory (Chen, Yung, & Li, 2003); however, multiple lines of research indicate that callosal connections between the two motor cortices have primarily inhibitory effects (Lenzi et al., 2007; De Gennaro et al., 2004; Netz, 1999). Thus, it might be expected that larger size and higher microstructural integrity of these tracts would be associated with better performance under conditions that require increased interhemispheric inhibition (IHI).
IHI occurs when the upper limbs are moving out of phase; however, inhibition is down-regulated when the limbs perform simultaneous movements (Stinear & Byblow, 2002). Similar to bimanual out-of-phase tasks, unimanual movement (Sohn, Jung, Kaelin-Lang, & Hallett, 2003) and unimanual force production (Vercauteren, Pleysier, Van Belle, Swinnen, & Wenderoth, 2008) also have net inhibitory effects on the ipsilateral motor cortex. Further, in comparison with bimanual in-phase tapping, interhemispheric coupling (assessed by electroencephalography coherence) has been shown to increase during unimanual tapping and bimanual antiphase tapping (Serrien, 2008). Conversely, intercerebellar coupling, as measured via magneto-encephalography coherence, is restricted to simultaneous bimanual tapping conditions, implying that intercerebellar coupling and not transcallosal communication is key for the execution of simultaneous bimanual movements (Pollok, Butz, Gross, & Schnitzler, 2007). Furthermore, callosotomy patients retain the bimanual advantage during bimanual simultaneous tapping (Ivry & Hazeltine, 1999). Finally, Tuller and Kelso (1989) found that split-brain patients make more pronounced errors under bimanual out-of-phase conditions as compared with healthy controls and revert back to more stable in-phase and antiphase patterns. Taken together, these findings suggest that repetitive simultaneous bimanual actions do not rely on interhemispheric communication via the CC (either facilitatory or inhibitory) to the same extent as unimanual and bimanual out-of-phase movements. This task-specific IHI is presumably to prevent interference from the opposite hemisphere or “sensorimotor overflow” (cf. Hoy, Fitzgerald, Bradshaw, Armatas, & Georgiou-Karistianis, 2004). Considering that the CC undergoes significant degeneration with age (Ota et al., 2006; Salat et al., 2005; Pfefferbaum et al., 2000), it follows that age-related deficits in bimanual control in these conditions could be due in part to degradation of the CC.
A fast-growing body of literature indicates that not only is the quantity of white matter reduced in older adults, but the quality is compromised as well (for a review, see Seidler et al., 2010; Sullivan, Rohlfing, & Pfefferbaum, 2010). The use of conventional MRI allows for macroscopic measurements of regional brain volume, whereas diffusion tensor imaging (DTI) allows assessment of white matter microstructure. Numerous studies have reported that older adults have extensive reductions in both CC volume (Langan et al., 2010; Muller-Oehring, Schulte, Raassi, Pfefferbaum, & Sullivan, 2007) and microstructural integrity (Sullivan et al., 2010; Ota et al., 2006; Salat et al., 2005; Pfefferbaum et al., 2000). Sullivan, Pfefferbaum, Adalsteinsson, Swan, and Carmelli (2002) noted that declines in CC white matter microstructure were related to declines in behavioral performance on tasks requiring interhemispheric transfer efficiency. Moreover, less lateralized task processing has been shown to be associated with reductions in CC cross-sectional area in older adults (Muller-Oehring et al., 2007). It has also been shown that older adults have prolonged interhemispheric transit times for sensorimotor tasks (Reuter-Lorenz & Stanczak, 2000; Jeeves & Moes, 1996); therefore, this age-related callosal degeneration has the potential to significantly affect motor behavior.
The impact of age differences in CC structure on bi-manual control remains unclear. The purpose of the present study was to investigate age differences in the relationship between callosal structural measures and performance of motor tasks requiring varying degrees of interhemispheric interaction. In accordance with previous work, we hypothesized that older adults would demonstrate decreased motor performance on tasks requiring high interhemispheric inhibitory demands (out-of-phase bimanual tapping) compared with their young adult counterparts. Furthermore, we hypothesized that older adults would have decreased callosal size and integrity of CC microstructure, as assessed by DTI. Because of the advantages of bihemispheric processing in older adults (cf. Reuter-Lorenz & Lustig, 2005), we expected to find that larger callosal size and better integrity of CC microstructure would correlate with better performance on all motor tasks in older adults. However, we expected this relationship would only exist in young adults on tasks with high interhemispheric interaction demands.
METHODS
Participants
Fourteen young adults (9 men; mean ± SD age = 23.1 ± 3.2 years) and 16 older adults (9 men; 71.9 ± 5.2 years) participated in this study. Participants were strongly right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). This experiment was approved by the Medical Institutional Review Board (IRBMED) of the University of Michigan. Participants gave their informed written consent before starting the experiment and were compensated for their participation.
Experimental Procedures
We performed testing over 2 days, separated by less than a week. On the first day of testing, participants completed a series of neuropsychological tasks and practiced the motor tapping tasks. The second day of testing took place while participants lay supine in an MRI scanner. We acquired structural MRI and DTI along with motor tapping data as described in detail in the following sections.
Neuropsychological Assessments and Health Questionnaires
Participants completed a number of paper-and-pencil tests. These tests assessed the presence of neurological deficits, general health, and general cognitive performance (Table 1). Assessments included health history questionnaires, the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975; evaluates general cognitive ability) and the Dysexecutive Questionnaire (Wilson, Evans, & Emslie, 1996; assesses dysexecutive behavior or difficulty in performing tasks associated with frontal lobe function).
Table 1.
Neuropsychological Test Performance
| Group | Young Adults | Older Adults | Significance |
|---|---|---|---|
| Age | |||
| Mean | 23.1 | 71.9 | p < .001 |
| SD | 3.18 | 5.2 | |
| Edinburgh Handedness | |||
| Mean | 86.1 | 80.5 | p > .1 |
| SD | 12.18 | 21.75 | |
| Mini-Mental State Examination | |||
| Mean | 29.5 | 28.87 | p > .1 |
| SD | 0.85 | 1.48 | |
| Dysexecutive Questionnaire | |||
| Mean | 21.6 | 17.2 | p > .3 |
| SD | 10.59 | 12.0 | |
Tapping Procedure
Participants performed five different finger-tapping conditions similar to those already reported by Bangert et al. (2010). However, the individuals presented in this report are a separate cohort from those that have previously been published. These tapping conditions were chosen because they require a range of interhemispheric interactions:
Simultaneous bimanual (0 msec between-hand lag; low interhemispheric interaction)
Right-hand unimanual (moderate interhemispheric interaction)
Left-hand unimanual (moderate interhemispheric interaction)
Right-leads-left (RL) bimanual (180 msec between-hand lag; high interhemispheric interaction)
Left-leads-right (LR) bimanual (180 msec between-hand lag; high interhemispheric interaction)
In all conditions, participants tapped with their index finger(s) at 1 Hz. Tasks were presented and analyzed using LabView 6.1 (National Instruments, Austin, TX). Participants were asked to focus on a fixation crosshair in the center of the monitor. Red circles appeared lateral to the fixation cross, indicating when a tap should take place and with which hand. For example, during right-hand unimanual tapping, a red circle would appear on the right side of the fixation cross at the target rate of 1 Hz. Participants were instructed not to use the circle appearance as a cue to tap; instead, they were to tap in synchrony with its onset. In the out-of-phase conditions, the following finger was paced at a 180-msec delay relative to the leading finger, but each within-hand tapping rate remained at 1 Hz. The 180-msec phase delay was chosen because it is among the most difficult patterns to maintain (Semjen & Ivry, 2001). For all tapping conditions, unimanual stability was defined as the standard deviation of the intertap interval for a single hand. For all bimanual tapping conditions, bimanual coupling was defined as the standard deviation of the between-hand lag. In both measures, a lower value is indicative of better performance. These are standard measures used to describe tapping performance for unimanual stability (Bangert et al., 2010; Ivry & Hazeltine, 1999; Helmuth & Ivry, 1996) and bimanual coupling (Bangert et al., 2010; Kennerley et al., 2002).
Each tapping condition consisted of four 30-sec tapping trials (30 taps per hand), with 20 sec of visual fixation at the start and end of each of these trials. A tapping trial was considered an outlier if the average unimanual stability and/or bimanual coupling was more than 2.5 standard deviations from the overall group mean. If a participant had more than one trial identified as an outlier within a tapping condition (out of four trials), that particular condition was removed from the participant’s motor performance data set. In cases where a participant had a condition removed, that participant’s data were still included for the remaining tapping conditions.
Imaging Procedure
Whole-brain high-resolution structural MR images were collected on a 3.0-T MRI scanner (General Electric, Waukesha, WI) using a spoiled gradient-echo sequence (124 slices, field of view = 24 cm, voxel size = 0.94 × 0.94 × 1.4 mm, repetition time = 10.2 msec, echo time = 3.4 msec). Diffusion tensor images were collected using a single-shot spin-echo with forward-spiral readout (40 slices, slice thickness = 3.2 mm, echo time/repetition time = 60 msec/ 6000 msec, field of view = 220 × 220 mm). Diffusion gradients were applied in six directions (b = 1000 s/mm2; four averages). Eight reference images (b = 0 s/mm2) were acquired (Basser & Pierpaoli, 1998) and averaged. Using the averaged images with b = 0 and b = 1000 s/mm2, the diffusion tensor was calculated. Diagonalization of the diffusion tensor yields the eigenvalues λ1, λ2, and λ3 as well as the eigenvectors that define the predominant diffusion direction. Fractional anisotropy (FA; Basser & Pierpaoli, 1996) images were constructed off-line using a custom MATLAB program (MATLAB, R2006b; MathWorks Inc., Natick, MA). Each participant’s average b = 0 image was coregistered with their high-resolution image using Statistical Parametric Mapping software (SPM2; Wellcome Department of Cognitive Neurology, London, UK), and the resulting transformation was applied to the FA image. Radial diffusivity was calculated for each participant by taking the mean of the second and third eigenvalues (λ2 + λ3) / 2. FA is a rotationally invariant index that ranges from 0 (isotropic) to 1 (anisotropic). Therefore, higher FA values can be interpreted as reflecting higher white matter integrity (Basser & Pierpaoli, 1996). Conversely, lower radial diffusivity is interpreted as being indicative of better white matter tract microstructure (Sullivan et al., 2010).
CC Structural Measures
A custom MATLAB program was used to manually outline the CC from a midsagittal slice taken of a high-resolution T1 image. Another custom MATLAB program was then used to divide the CC into seven subregions (Figure 1) as previously described by Witelson (1989). These seven subregions approximately correspond to distinct anatomical connections of the caudal/orbital prefrontal cortices (rostrum; Region 1); prefrontal cortices (genu; Region 2); premotor cortices (rostral truncus; Region 3); cingulate motor, presupplementary, and SMAs (anterior intermediate truncus; Region 4); primary motor cortices (posterior intermediate truncus; Region 5); primary sensory cortices (isthmus; Region 6); and superior temporal and posterior parietal cortices and occipital and inferior temporal cortices (splenium; Region 7; Hofer & Frahm, 2006). Tractography work by Wahl et al. (2007) found the mean Talairach coordinates of normalized hand callosal motor fibers were located at 0, −14.3, 17.6 mm (x,y,z) in a group of young adult participants. For all participants in the current study, the location of the anterior commissure was visually identified to allow determination of the coordinate position from Wahl et al. Although Hofer and Frahm (2006) outline a scheme with five CC segments, we maintained all seven CC subregions from the Witelson segmentation to increase the sensitivity for determining relationships between regions of the callosal midbody and motor performance.
Figure 1.
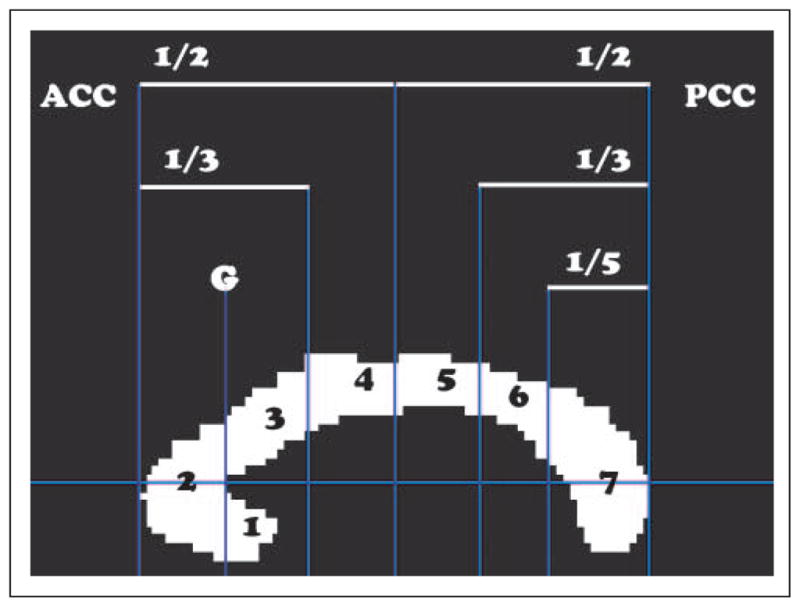
The cross-sectional area of the midsagittal CC was parsed into seven subregions: (1) rostrum, (2) genu, (3) rostral truncus, (4) anterior intermediate truncus, (5) posterior intermediate truncus, (6) isthmus, and (7) splenium.
All callosal measurements were made by four independent raters. Interrater reliability was assessed with Krippendorff’s alpha (Hayes & Krippendorff, 2007). The mean value across raters was used as the cross-sectional area measurements in subsequent analyses. These seven callosal subregions were also used as masks for calculating regional FA and radial diffusivity from the DTI data. The cross-sectional area of each CC subregion was normalized to intracranial area (ICA) to control for any differences in size because of age, gender, or other factors. The same custom MATLAB program was used to calculate ICA; an outline was drawn along the interior border of the skull with a straight line connecting the nasion and the inion. The same midsagittal slice was used to measure the CC and the ICA for each participant.
Data Analysis
Statistical analyses of behavioral measures were performed using the Statistical Package for the Social Sciences (Version 15.0; SPSS Inc., Chicago, IL). Mixed model repeated measures ANOVAs were used to analyze the motor performance data: Tapping condition was included as the within-subjects variable and age group as the between-subjects variable. Mixed model repeated measures ANOVA were also used to analyze CC size and microstructure: CC region was included as the within-subjects variable and age group as the between-subjects variable. Significance was set at α = .05. The Huynh–Feldt epsilon was computed to test for sphericity, and we interpreted corrected p values in cases of violation. Significant main effects were subjected to post hoc independent and paired t tests and Bonferroni-corrected for multiple comparisons. Independent samples t tests were used to compare group performance on the neuropsychological tests and additional measures; all t tests were two-tailed, unless otherwise noted. In callosal subregions that had significant group differences in either macro- or microstructure, we performed linear regression analyses to investigate relationships between tapping performance and callosal metrics. Further, for all significant linear regressions (separated by age group), we tested whether the correlations for each age group had different strengths (i.e., was the correlation coefficient equal to or different from the other age group). Finally, we performed linear regression of all callosal metrics within each callosal subregion. All data are presented as mean ± SD unless otherwise noted.
RESULTS
Neuropsychological Assessments
No group differences were seen in handedness or on the Mini-Mental State Examination and the Dysexecutive Questionnaire (see Table 1).
Motor Tapping Performance
Unimanual Stability
For all tapping conditions, no differences in performance ( p > .5) were noted between dominant and nondominant hands in either group. Therefore, data for the two hands were combined within each condition. There were significant main effects of group ( p < .01) and tapping condition ( p < .001) but no Group × Condition interaction ( p > .1). Post hoc pairwise comparisons for the tapping condition main effect revealed that variability was significantly lower ( p < .01) in the simultaneous bimanual condition (M = 56.1 msec) than that in the unimanual condition (M = 63.4 msec), replicating the bimanual advantage in both age groups (Bangert et al., 2010; Helmuth & Ivry, 1996). Variability was also significantly lower during simultaneous bimanual tapping compared with the bimanual out-of-phase LR condition (M = 66.8 msec, p < .001) but not the RL condition (M = 61.0 msec, p > .09). No difference ( p > .2) in unimanual stability was found between the unimanual and the out-of-phase conditions (RL and LR). Finally, unimanual variability was significantly higher ( p < .05) for LR versus RL out-of-phase bimanual tapping.
Bimanual Coupling
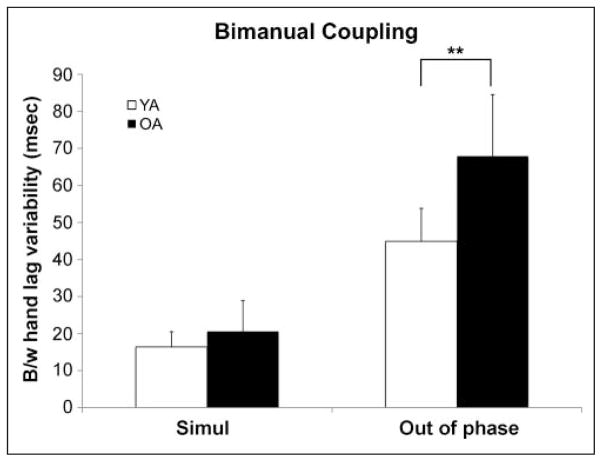
No differences were noted in bimanual coupling between the two out-of-phase tapping conditions for either group ( p > .3), nor did any participants demonstrate transitions to in-phase tapping; therefore, the conditions were combined within groups resulting in two conditions for comparison: bimanual simultaneous and bimanual out of phase. There were significant main effects of group ( p < .001) and tapping condition ( p < .001) and a Group × Condition interaction ( p < .001) (Figure 2). Post hoc t tests revealed that older adults had significantly greater variability during out-of-phase tapping compared with young adults (young adults: M = 44.9 msec, SD = 7.5 msec; older adults: M = 67.9 msec, SD = 16.6 msec; p < .001). No difference was noted between groups during simultaneous tapping (young adults: M = 16.4 msec, SD = 4.1 msec; older adults: M = 20.6 msec, SD = 8.3 msec; p > .1). These results replicate our recent work reporting disproportionately poorer performance by older adults on motor tasks that require out-of-phase bimanual control with a new cohort of participants (Bangert et al., 2010).
Figure 2.
Bimanual coupling (mean ± 1 SD). Data show an Age × Task interaction with older adults disproportionately impaired at maintaining the appropriate phase delay between hands during out-of-phase tapping. B/w = between; Simul = simultaneous. **p < .01.
It is worth noting that performance was stable across all four trials within each tapping condition. Therefore, we are confident that no learning-related improvement occurred over the course of the four trials, nor does it appear that performance differences between groups can be explained by a slower learning curve in the older adult group.
CC Metrics
Paralleling Hofer and Frahm’s (2006) results, we found that the location of hand callosal motor fibers identified by Wahl et al. (2007) fell within CC Subregion 5 for all young and older adults (see Figure 3A and B, respectively).
Figure 3.
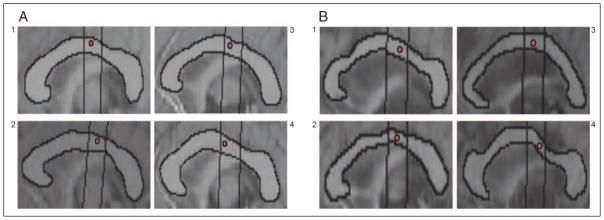
(A) Coordinate from Wahl et al. (2007) identified as the average location where fibers connecting hand regions of the primary motor cortex pass through the CC in four representative young adults and (B) four representative older adults. In all participants, this coordinate fell within CC Subregion 5.
Cross-sectional Area
Interrater reliability of the CC cross-sectional area measurements within Subregion 1 (rostrum) was poor (Krippendorff’s alpha = .34). However, measurements within the remaining six CC subregions (2–7) were highly reliable (Krippendorff’s alpha > .90). Because of the large variability among measures of CC Subregion 1 both within and across raters, the rostrum was excluded from further analyses. Thus, although age differences and relationships between task performance and CC metrics of the rostrum may exist, the current study was unable to reliably assess this.
For callosal cross-sectional area, there was a significant main effect of group ( p < .01) and a Group × Region interaction ( p < .001). Post hoc t tests indicated that young adults had significantly larger cross-sectional area in callosal Subregion 2 ( p < .001) and Subregion 4 ( p < .01) (Figure 4A). No significant effect of gender was found in either age group; thus, normalization of callosal size to ICA appears to be a satisfactory method to control for sex differences (Muller-Oehring et al., 2007).
Figure 4.
CC Subregions 2–7 (mean ± 1 SD). (A) Age × Region interaction; young adults had significantly larger cross-sectional area (normalized to ICA) in CC Subregions 2 and 4. (B) Main effect of age; young adults had significantly greater FA than older adults. (C) Age × Region interaction; young adults had significantly lower radial diffusivity in CC Subregions 3–6. X-sectional = cross-sectional; ICA = intracranial area; FA = fractional anisotropy; rad diff = radial diffusivity; X-sect = cross-sectional. **p < .01; ***p < .001.
Diffusion Tensor Imaging
Because of technical problems, DTI data from only 13 young adults and 10 older adults were available for analysis. There were significant main effects of group ( p < .004) and callosal region ( p < .001) but no Group × Region interaction ( p > .1) for FA (Figure 4B).
There were significant main effects of group ( p < .001) and callosal region ( p < .001) and a significant Group × Region interaction ( p < .01) for radial diffusivity. Post hoc t tests revealed significantly lower radial diffusivity in young adult callosal Subregions 3 through 6 ( p < .001 for all regions; Figure 4C).
None of the callosal metrics were significantly correlated with age within each age group ( p > .10). Thus, in agreement with previous work (Sullivan et al., 2001), the relationships described in this report are not primarily being driven by age alone but rather changes in callosal structure.
Relationships between CC Structure and Motor Performance
For all callosal regions where CC metrics significantly differed between groups, we correlated motor tapping performance with the appropriate structural metrics associated with that region. Thus, cross-sectional area within callosal Regions 2 and 4 and radial diffusivity within callosal Subregions 3 through 6 were correlated with unimanual stability and bimanual coupling for each age group. Because no Age × Group interaction was noted in FA, we correlated motor tapping with FA within all CC subregions. Correlations for each callosal metric were Bonferroni corrected for multiple comparisons: cross-sectional area (critical α = .05/2; .025), FA (critical α = .05/6; .0083), and radial diffusivity (critical α = .05/4; .0125).
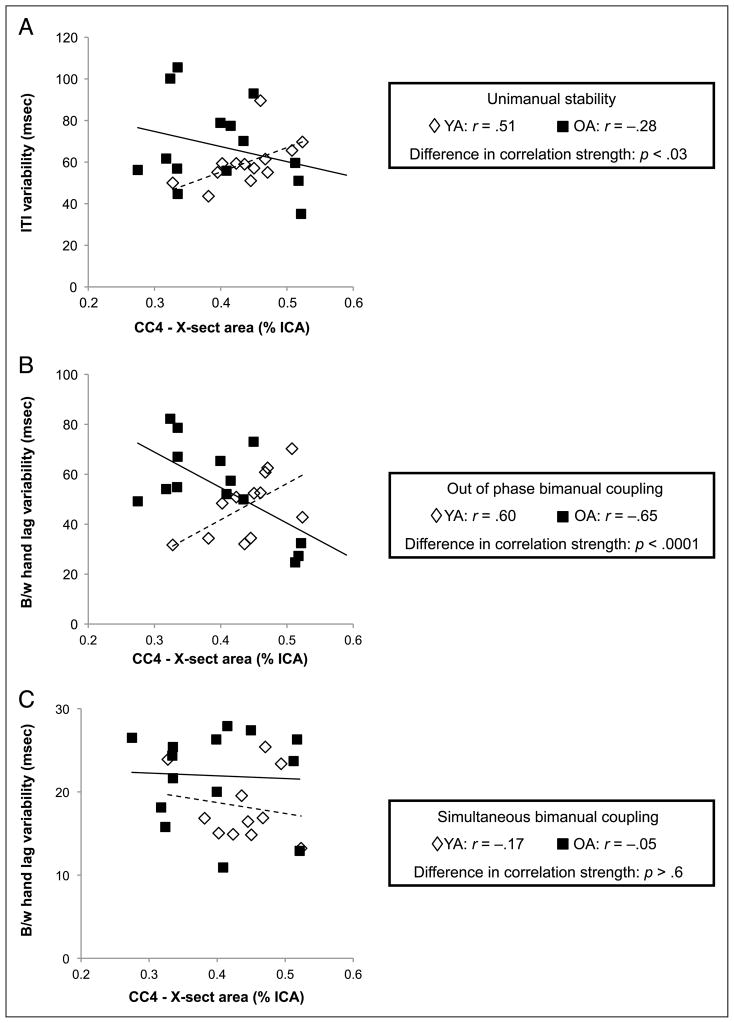
Cross-sectional area within callosal Subregions 2 and 4 were correlated with unimanual stability and bimanual coupling for each age group separately. The size of the genu (Subregion 2) showed no relationship with motor performance in either group. In young adults, larger cross-sectional area of CC Subregion 4 was correlated with poorer performance on out-of-phase bimanual coupling (r = 0.60, p < .02), and there was a trend toward the same relationship with unimanual tapping in this group (r = 0.51, p < .04; not significant when corrected for multiple comparisons). Conversely, in older adults, larger callosal size within Subregion 4 was associated with better performance on out-of-phase bimanual coupling (r = −0.65, p < .01). An age group difference in correlation strength was found for the relationships between unimanual tapping ( p < .03) and out-of-phase bimanual tapping ( p < .0001) with the size of callosal Subregion 4 (Figure 5A and B, respectively). It is important to note that no such relationship exists between CC4 size and performance on bimanual simultaneous tapping (Figure 5C).
Figure 5.
(A) Age differences in the relationship between cross-sectional area in CC Subregion 4 (composed of transcallosal fibers connecting SMA) and unimanual tapping. (B) Similar relationships exist between CC4 size and bimanual coupling during out-of-phase tapping. (C) There is no relationship for either age group between CC4 size and bimanual coupling during simultaneous tapping. Solid lines indicate linear regression fit for older adults, whereas dashed lines indicate linear regression fit for young adults. ITI = intertap interval; X-sect = cross-sectional; ICA = intracranial area; B/w = between.
Diffusion Tensor Imaging
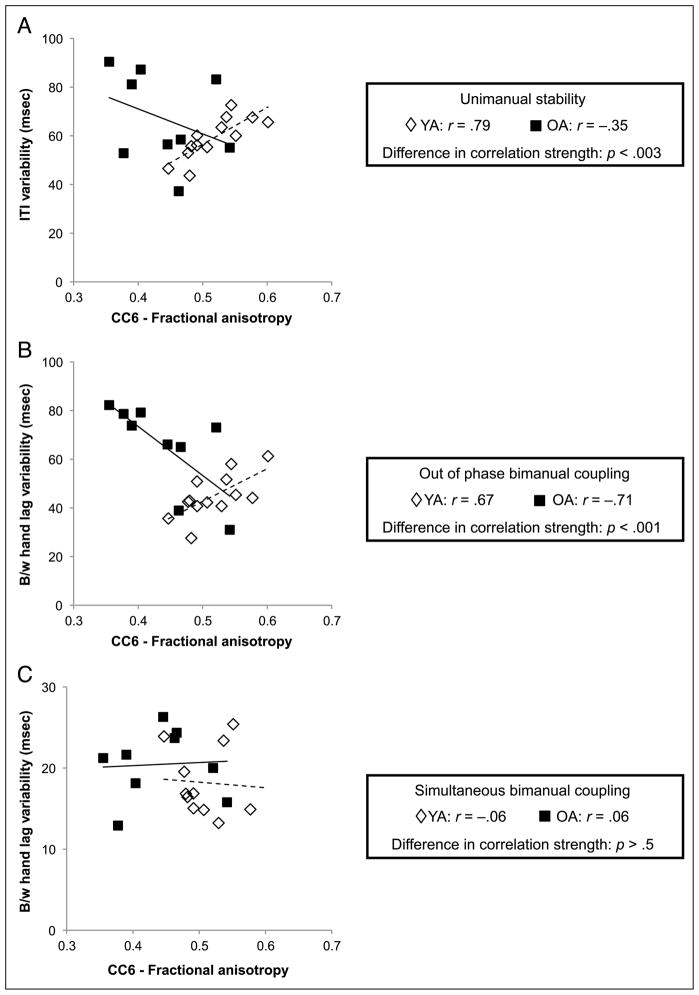
FA within callosal Subregions 2 through 7 was tested for correlation with unimanual stability and bimanual coupling for each age group separately. No relationships in either age group were found between motor performance and FA within callosal Subregion 2, 3, 4, 5, or 7. However, similar to relationships with callosal macrostructure, poorer performance in young adults during unimanual (r = 0.79, p < .002) and out-of-phase bimanual coupling (r = 0.67, p < .01) was correlated with greater FA of callosal Subregion 6 (connecting somatosensory areas). On the other hand, although not significant when corrected for multiple comparisons, better performance during out-of-phase bimanual coupling (r = −0.71, p < .02) was associated with higher FA within CC Subregion 6 in older adults. An age group difference in correlation strength was found for the relationships between FA of CC Subregion 6 and both unimanual tapping ( p < .003) and out-of-phase bimanual tapping ( p < .001; Figure 6A and B, respectively). Similar to callosal size, no such relationship exists between CC6 microstructure and performance on bimanual simultaneous tapping (Figure 6C).
Figure 6.
(A) Age differences in the relationship between FA of CC Subregion 6 (composed of transcallosal fibers connecting primary somatosensory cortex) and unimanual tapping. (B) Similar relationships exist between FA in CC6 and bimanual coupling during out-of-phase tapping. (C) There is no relationship for either age group between FA in CC6 and bimanual coupling during simultaneous tapping. Solid lines indicate linear regression fit for older adults, whereas dashed lines indicate linear regression fit for young adults. ITI = intertap interval; B/w = between.
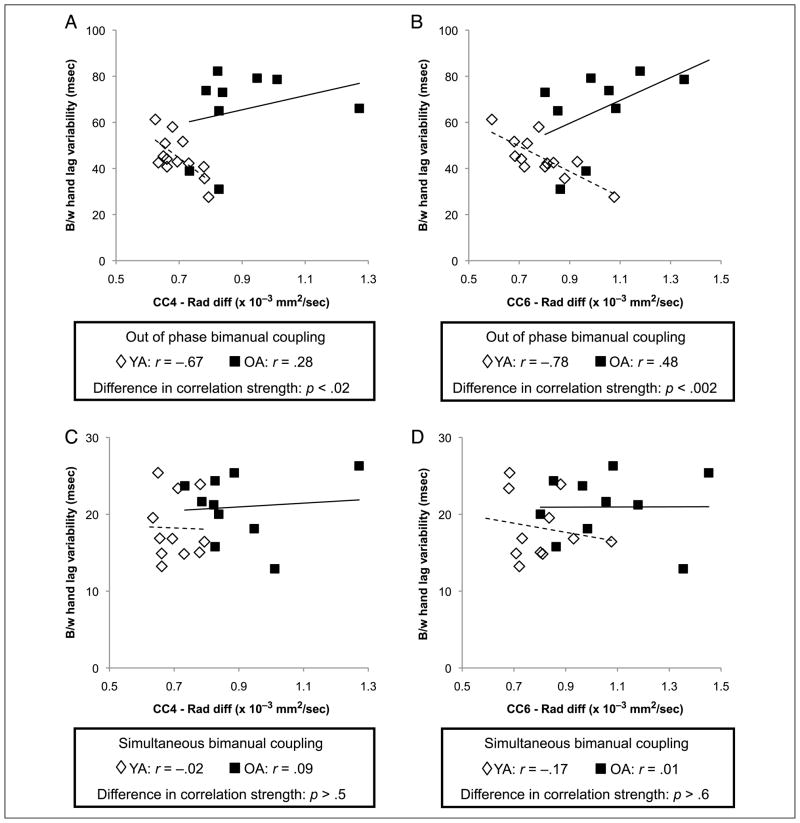
No relationship between motor performance and radial diffusivity within CC Subregion 3 was found for either group. In young adults, lower radial diffusivity (indicating better integrity of fiber microstructure) within CC Subregion 4 (r = −0.67, p < .007) and Subregion 6 (r = −0.78, p < .001) was correlated with poorer out-of-phase bimanual coupling. For older adults, there were trends toward significant relationships between radial diffusivity within CC Subregion 5 (r = 0.57, p < .06) and Subregion 6 (r = 0.48, p < .1) and out-of-phase bimanual coupling, again revealing that better CC microstructure was associated with better bimanual coupling. An age group difference in correlation strength was found for relationships between out-of-phase bimanual coupling and radial diffusivity within CC Subregion 4 ( p < .02; Figure 7A) and Subregion 6 ( p < .002; Figure 7B) and a trend toward an age group difference in CC Subregion 5 ( p < .06). Again, no association was found between bimanual simultaneous tapping and radial diffusivity of CC Subregion 4 (Figure 7C) or CC Subregion 6 (Figure 7D).
Figure 7.
(A) Age differences in the relationship between bimanual coupling during out-of-phase tapping and radial diffusivity in CC Subregion 4 (composed of transcallosal fibers connecting SMA). (B) Similar relationships exist between radial diffusivity in CC Subregion 6 (composed of fibers connecting somatosensory cortex) and bimanual coupling during out-of-phase tapping. (C) There is no relationship for either age group between radial diffusivity in CC4 and bimanual coupling during simultaneous tapping. (D) Nor is there a relationship between bimanual simultaneous tapping and radial diffusivity within CC6 for either age group. Solid lines indicate linear regression fit for older adults, whereas dashed lines indicate linear regression fit for young adults. Rad diff = radial diffusivity; B/w = between.
Relationships between CC Metrics of Macro- and Microstructure
Linear regression analysis of cross-sectional area of the callosal subregions and all DTI metrics are presented in Table 2. Normalized cross-sectional area and FA were not significantly correlated with each other within any CC subregions. Thus, at least with respect to the CC, larger area is not necessarily reflective of better white matter tract microstructure. Cross-sectional area and radial diffusivity were negatively correlated with each other in CC Subregions 2 and 4. This is interpreted to indicate that within these two subregions, larger callosal area is associated with better fiber microstructure. Furthermore, radial diffusivity and FA were negatively correlated with each other in all CC subregions.
Table 2.
Relationships between Callosal Subregion Cross-sectional Area and DTI Metrics
| CC2 r |
CC3 r |
CC4 r |
CC5 r |
CC6 r |
CC7 r |
|
|---|---|---|---|---|---|---|
| X-sect. area and FA | 0.35 | 0.11 | 0.37 | 0.27 | 0.33 | −0.14 |
| X-sect. area and Rad. diff | −0.63* | −0.20 | −0.54** | −0.22 | −0.39 | −0.07 |
| FA and Rad. diff | −0.81* | −0.81* | −0.80* | −0.77* | −0.91* | −0.86* |
X-sect. area = cross-sectional area; FA = fractional anisotropy; Rad. diff = radial diffusivity.
p < .001.
p < .01.
DISCUSSION
Young adults were significantly better at maintaining unimanual stability than their older counterparts, replicating previous work (Bangert et al., 2010). When coupling the two hands during bimanual tapping, young adults demonstrated better control than their older counterparts on out-of-phase tapping tasks, but no group difference was noted on simultaneous tapping. Therefore, this task-specific group difference was restricted to movements requiring elevated levels of IHI. We suggest that these motor deficits could be the result of age-related CC degradation. Larger CC size and better CC microstructure of subregions connecting sensorimotor cortical areas was correlated with poorer performance on motor tasks requiring increased interhemispheric communication in young adults. Conversely, on these same tasks, larger CC size and better fiber integrity were associated with better performance in older adults. The observed relationships were restricted to subregions of the CC composed of fibers connecting the SMA, the primary motor cortex, and the primary somatosensory cortex. Further, no relationships between callosal metrics and performance on the bimanual simultaneous tapping task were found in either age group. These results indicate age, CC subregion, and task-specific relationships between motor performance and callosal structure. Although the structure–function relationship during unimanual and out-of-phase tapping for the young adult group is surprising, we think it can be understood by considering the role of inhibitory interhemispheric interactions in these tasks.
IHI is the result of excitatory axons crossing the CC and acting on local inhibitory interneurons in the contra-lateral cortex (Chen et al., 2003). It has been shown that IHI is important to prevent interference between the two hands and is significantly greater during unimanual and bimanual out-of-phase actions compared with bimanual simultaneous movements (Vercauteren et al., 2008; Sohn et al., 2003; Stinear & Byblow, 2002). Providing further confirmation, individuals who have undergone callosotomy are still able to move the two hands in synchrony when they perform discrete, simultaneous actions (Kennerley et al., 2002; Ivry & Hazeltine, 1999), suggesting that this type of bimanual control is still possible in the absence of callosal inhibitory (or facilitatory) communication. In the current study, we found multiple associations between callosal metrics and performance on unimanual and bimanual out-of-phase tapping but no significant relationships between bimanual simultaneous performance and any callosal metrics. Thus, our current data fit well with the existing literature, indicating that transcallosal inhibitory control is important during unimanual and bimanual out of phase but not bimanual simultaneous movements. In addition to connections between primary motor cortices, our study emphasizes interhemispheric connections between the SMA and the somatosensory cortices (SS1) as key transcallosal fiber pathways involved in unimanual and complex bimanual control. We suggest that these findings may be indicative of interhemispheric overflow during the planning (SMA) and/or feedback processing (SS1) stages of movement.
Somatosensory cortices and medial motor areas have been implicated as serving prominent roles during complex bimanual movements. Gerloff and Andres (2002) described an extended cortical network involved in bimanual motor performance with emphasis placed on the bilateral primary sensorimotor cortex, supplementary motor, and cingulate motor areas. Geffen et al. (1994) suggest that sensory feedback information carries significant temporal information and that it is the loss of this component that most impairs out-of-phase bimanual movement. Furthermore, using paired-pulse TMS, recent work has shown that the SS1 exerts an inhibitory influence on the contralateral M1 (Ni et al., 2009). There are also dense homotopic transcallosal connections within the hand area of the SMA (Rouiller et al., 1994). The SMA substantially influences M1 activity in both hemispheres during the execution of visually paced unimanual and bimanual movements (Grefkes, Eickhoff, Nowak, Dafotakis, & Fink, 2008). Specifically, the SMA exerts an inhibitory influence over the contralateral M1 significantly suppressing its activity. Using repetitive TMS to disrupt activity of the SMA, it has been demonstrated that temporal control during bimanual antiphase tapping is preferentially degraded, as compared with in-phase tapping (Serrien, Strens, Oliviero, & Brown, 2002). The authors suggest that deterioration of interhemispheric coupling due to SMA stimulation likely reduces IHI resulting in poorer motor performance on tasks requiring a higher level of IHI. Thus, in addition to direct connections between the primary motor cortices, both the SS1 and the SMA exert an inhibitory influence on the contralateral M1, particularly for tasks such as the unimanual and the out-of-phase bimanual tapping tasks presented in the current study.
Young Adults: CC and Bimanual Control
How then can we explain the counterintuitive relationship in young adults whereby greater callosal size and integrity are associated with selectively poorer performance on tasks requiring greater IHI? Perhaps young adults with greater CC size and white matter integrity are unable to effectively prevent motor overflow from one hemisphere to the other, resulting in too much interhemispheric cross talk and poorer motor performance. Conversely, those with larger CC volume and better microstructure may be affected by excessive mutual inhibition during out-of-phase bimanual tasks, leading to poorer motor performance because each hemisphere is less able to activate the proper motor commands. Without physiological metrics, it is not possible for us to determine whether a larger CC size is associated with more (or less) interhemspheric inhibition than a smaller CC in either young or older adults. The inclusion of physiological metrics of callosal function was beyond the scope of the current study, and further work is clearly necessary to fully elucidate the relationships between structural and neurophysiological function of the CC and their role in bimanual control.
Although several studies have demonstrated that for speeded motor responses higher callosal fiber integrity is beneficial to task performance in young adults (Muetzel et al., 2008; Johansen-Berg, Della-Maggiore, Behrens, Smith, & Paus, 2007; Madden et al., 2004), the relationship between white matter tract microstructure and performance appears to be more complex. Better performance during simultaneous in-phase (Bonzano et al., 2008) and antiphase bimanual coordination tasks ( Johansen-Berg et al., 2007) is associated with higher CC microstructure within anterior and midbody regions of the CC in young adults. The current study does not replicate the findings of Bonzano et al. (2008), as we show no relationship between bimanual in-phase tapping and CC metrics. However, it should be noted that Bonzano et al. only reported this relationship in individuals with multiple sclerosis, a group with significant callosal deterioration, and not in control participants. Johansen-Berg et al. (2007) instructed participants to tap at the fastest antiphase frequency they could maintain, whereas our task requires participants to pace to an external stimulus, and thus it is a paced task as opposed to a speeded task. Considering that the bimanual tasks in the current manuscript rely heavily on timing, some insight may be provided from research in expert musicians, individuals with exquisite bimanual control.
Previous neuroimaging research has revealed that long-term training within critical developmental periods has the potential to induce regionally specific neural adaptations. The anterior half of the CC (i.e., CC1–CC4) was found to be larger in adult musicians who began training before the age of 7 years when compared with control participants (Schlaug, Janck, Huang, Staiger, & Steinmetz, 1995). In addition, FA is significantly greater in the genu of musicians (Schmithorst & Wilke, 2002), although no differences in CC macro- or microstructure have been noted within the midbody or posterior callosal regions of musicians. Surprisingly, both inter- and intrahemispheric inhibition are reduced in musicians (Nordstrom & Butler, 2002; Ridding, Brouwer, & Nordstrom, 2000). It is interesting that cortical inhibition is less effective in musicians, who have extraordinary control of independent finger movements. However, it is unclear whether the inhibitory differences noted in musicians represent an adaptive change related to exceptional control of finger movements or a maladaptive change brought about by overuse of the hand from extensive training (Nordstrom & Butler, 2002). The conflicting findings that musicians with superior bimanual control and larger callosal size have reduced IHI may partially explain the results of our young adult participants. We suggest that for speeded motor responses in untrained young adults, faster and more efficient interhemispheric processing may be beneficial to task performance. Whereas for tasks requiring increased IHI and precise temporal control, larger CC size and higher white matter microstructural integrity may be detrimental because of the complex balance of excitatory and inhibitory processes required for these tasks.
Older Adults: Age-related Changes in the CC and Bimanual Control
In agreement with a large body of literature (Langan et al., 2010; Sullivan et al., 2001, 2010; Davis et al., 2009; Ota et al., 2006; Madden et al., 2004), we observed decreased callosal size and microstructure in the older adult CC. In areas with significant age differences in structural measures, larger callosal size and better integrity of CC microstructure were associated with better performance in older adults on tasks with high interhemispheric interaction demands. These results parallel previous work showing that older adults who maintain callosal size and microstructure demonstrate better performance on cognitive and motor tasks (Sullivan et al., 2001, 2010; Davis et al., 2009; Muller-Oehring et al., 2007).
Bilateral brain activation patterns have been associated with better task performance in older adults, suggesting that increased interhemispheric interaction may aid performance (cf. Reuter-Lorenz & Lustig, 2005). The bilateral brain activation patterns observed in older adults are potentially related to the growing body of literature demonstrating that older adults appear to have decreased IHI as compared with young adults (Talelli, Ewas, Waddingham, Rothwell, & Ward, 2008; Talelli, Waddingham, Ewas, Rothwell, & Ward, 2008). The specificity of age deficits during out-of-phase bimanual movement (Bangert et al., 2010) implicates IHI as a contributing factor. Using paired-pulse TMS, it has been shown that older adults have reduced IHI between the motor cortices compared with young adults (Talelli, Ewas, et al., 2008; Talelli, Waddingham, et al., 2008). This phenomenon is not limited to motor regions of the cortex; Muller-Oehring et al. (2007) found that smaller size of the CC’s genu was related to a decreased ability to inhibit task-irrelevant information in older adults. Furthermore, older adults do not modulate inhibitory control to meet task demands to the same extent as young adults (Fujiyama, Garry, Levin, Swinnen, & Summers, 2009; Talelli, Ewas, et al., 2008; Talelli, Waddingham, et al., 2008). The degree of change in IHI from rest to muscle contraction is also associated with the extent of brain activation in the ipsilateral sensorimotor cortex during unimanual actions (“motor overflow”; Talelli, Waddingham, et al., 2008). This may provide a partial explanation for the disproportionately poorer performance on out-of-phase bimanual tasks by older adults; however, because of the lack of physiological data in the current manuscript, it is unknown if there is selective degeneration of either excitatory or inhibitory interhemispheric connections with aging. For example, it is possible that excitatory interhemispheric pathways decline at a faster rate with age. As a result, sensorimotor overflow that may be experienced in young adults with larger callosal size has the possibility of being significantly reduced in older adults with larger CC size, resulting in task-specific improved performance. Thus, understanding of the age-related changes in physiological function of the CC is an important question for future studies to address.
DTI Metrics
The individual contributions of myelin and axonal membranes to FA are not fully understood. Evidence suggests that axonal membranes play the primary role and myelination can modulate the degree of anisotropy (Gulani, Webb, Duncan, & Lauterbur, 2001). For example, FA is reduced significantly in demyelinating disease (multiple sclerosis; Bonzano et al., 2008) and in conditions of premyelination (children; Barnea-Goraly et al., 2005). However, radial diffusivity has been shown to increase by 75% in myelin-deficient rats, indicating that it is impacted to a greater extent by myelin degradation than FA (Gulani et al., 2001). In agreement with these findings in animal models, recent work has indicated that age-related increases in radial diffusivity likely reflect myelin deterioration in healthy older adults (Zahr, Rohlfing, Pfefferbaum, & Sullivan, 2009). In accord with these previous studies, we show larger age group differences in radial diffusivity measures as opposed to FA, although these two DTI measures are significantly correlated with each other within each CC subregion. Of particular interest, we found increased radial diffusivity in CC Subregions 3 through 6 of older adults. These are all CC subregions containing fibers that connect sensorimotor cortices. These fibers are noted as being large, heavily myelinated fibers in the healthy adult brain (Aboitiz, Scheibel, Fisher, & Zaidel, 1992). Furthermore, we found that cross-sectional area of CC subregions was only correlated with radial diffusivity within CC Subregions 2 and 4, the same two subregions that were significantly different in size between age groups. Our findings support the idea that radial diffusivity is a highly sensitive measure of age-related changes in callosal microstructure; however, although previous work has described preferential degradation of anterior callosal regions with aging, we present a picture of decreased anterior CC size and decreased CC midbody microstructure.
Although there is overlap between CC metrics in the current study, these measurements likely provide different physiological information about the CC. There are two types of fibers within the CC, the previously described large diameter fibers that mediate sensory-motor coordination and the small diameter fibers that connect association areas (Aboitiz et al., 1992; cf. Bloom & Hynd, 2005). The small diameter fibers are more numerous and have been shown to be a reflection of individual difference in callosal size. Thus, a loss of these small diameter fibers in callosal subregions such as the genu may account for the decreased CC size observed in older adults. Furthermore, it has been suggested that these small diameter fibers are important for maintaining the balance between excitation and inhibition between the cerebral hemispheres (Yazgan, Wexler, Kinsbourne, Peterson, & Leckman, 1995). Our results demonstrate that the size of the CC is not necessarily related to fiber microstructure; cross-sectional area and FA were not correlated with each other within any callosal subregions. This highlights the importance of the use of techniques such as DTI to investigate the integrity of white matter tracts in addition to measuring the size of callosal structures.
A limitation of the current study is the use of only six DTI diffusion gradient directions. Recent studies have demonstrated that increasing the number of gradient directions can improve the reliability of white matter microstructure measurements (Giannelli et al., 2009; Ni, Kavcic, Zhu, Ekholm, & Zhong, 2006). However, our results (both FA and radial diffusivity) are quite similar to previously published work (Sullivan et al., 2001, 2010; Davis et al., 2009). Furthermore, the fact that we found similar relationships between motor performance and callosal size bolsters our DTI findings.
Conclusion
We found that older adults had significantly smaller callosal size and poorer integrity of callosal microstructure compared with young adults. The observed age group differences in callosal size and integrity suggest that deterioration of macro- and microstructure within callosal regions connecting sensorimotor cortical areas have the potential to significantly impact motor performance. In young adults, larger size and better integrity of CC microstructure within regions connecting sensorimotor cortical areas was associated with poorer performance on tasks requiring increased IHI (e.g., out-of-phase bimanual tapping). Conversely, larger size and better microstructure of these same callosal regions were associated with better performance in older adults. However, older adults demonstrated disproportionately poorer performance than young adults on motor tasks requiring increased IHI. These findings strongly implicate age-related declines in size and integrity of CC microstructure as key contributors to bimanual control deficits. Furthermore, these findings raise new questions about the role of the CC in bimanual control by indicating differential age-related involvement of transcallosal pathways. The proposed interpretations would be in conflict if there were substantial overlap between young and older adults in relation to CC macro- and microstructure. Although there is some overlap for size, this is not the case in terms of microstructure. Our results suggest that there may be an optimal CC size and level of microstructural integrity for performance of out-of-phase bimanual tasks, which shifts as a function of age.
Acknowledgments
The authors thank N. Rademacher, M. Chapekis, A. Boonin, and D. Goble for their assistance with this study. This work was supported by the National Institutes of Health (grant no. T32-AG00114-21, CMW and BWF; grant no. T32-AG000030ASB), the University of Michigan Office of the Vice President for Research, and the University of Michigan National Institutes of Health Claude D. Pepper Older Americans Independence Center (AG08808 pilot grant and human subjects cores).
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Research. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Bangert AS, Reuter-Lorenz PA, Walsh CM, Schachter AB, Seidler RD. Bimanual coordination and aging: Neurobehavioral implications. Neuropsychologia. 2010;48:1165–1170. doi: 10.1016/j.neuropsychologia.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance, Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magnetic Resonance in Medicine. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychology Review. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. Journal of Neuroscience. 2008;28:3227–3233. doi: 10.1523/JNEUROSCI.4076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. Journal of Neurophysiology. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Cristiani R, Bertini M, Curcio G, Ferrara M, Fratello F, et al. Handedness is mainly associated with an asymmetry of corticospinal excitability and not of transcallosal inhibition. Clinical Neurophysiology. 2004;115:1305–1312. doi: 10.1016/j.clinph.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Eliassen JC, Baynes K, Gazzaniga MS. Direction information coordinated via the posterior third of the corpus callosum during bimanual movements. Experimental Brain Research. 1999;128:573–577. doi: 10.1007/s002210050884. [DOI] [PubMed] [Google Scholar]
- Eliassen JC, Baynes K, Gazzaniga MS. Anterior and posterior callosal contributions to simultaneous bimanual movements of the hands and fingers. Brain. 2000;123:2501–2511. doi: 10.1093/brain/123.12.2501. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, Levin O, Swinnen SP, Summers JJ. Age-related differences in inhibitory processes during interlimb coordination. Brain Research. 2009;1262:38–47. doi: 10.1016/j.brainres.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Geffen GM, Jones DL, Geffen LB. Interhemispheric control of manual motor activity. Behavioural Brain Research. 1994;64:131–140. doi: 10.1016/0166-4328(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Andres FG. Bimanual coordination and interhemispheric interaction. Acta Psychologica. 2002;110:161–186. doi: 10.1016/s0001-6918(02)00032-x. [DOI] [PubMed] [Google Scholar]
- Giannelli M, Cosottini M, Michelassi MC, Lazzarotti G, Belmonte G, Bartolozzi C, et al. Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. Journal of Applied Clinical Medical Physics. 2009;11:2927. doi: 10.1120/jacmp.v11i1.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008;41:1382–1394. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magnetic Resonance in Medicine. 2001;45:191–195. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Communication Methods and Measures. 2007;1:77–89. [Google Scholar]
- Helmuth LL, Ivry RB. When two hands are better than one: Reduced timing variability during bimanual movements. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:278–293. doi: 10.1037//0096-1523.22.2.278. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited-comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou-Karistianis N. Investigating the cortical origins of motor overflow. Brain Research Reviews. 2004;46:315–327. doi: 10.1016/j.brainresrev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Hazeltine E. Subcortical locus of temporal coupling in the bimanual movements of a callosotomy patient. Human Movement Science. 1999;18:345–375. [Google Scholar]
- Jeeves MA, Moes P. Interhemispheric transfer time differences related to aging and gender. Neuropsychologia. 1996;34:627–636. doi: 10.1016/0028-3932(95)00157-3. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36(Suppl 2):T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso JA. Phase transitions and critical behavior in human bimanual coordination. American Journal of Physiology. 1984;246:R1000–R1004. doi: 10.1152/ajpregu.1984.246.6.R1000. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB. Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nature Neuroscience. 2002;5:376–381. doi: 10.1038/nn822. [DOI] [PubMed] [Google Scholar]
- Langan J, Peltier S, Bo J, Fling BW, Welsh RC, Seidler RD. Functional implications of age differences in motor system connectivity. Frontiers in Systems Neuroscience. 2010;4:17. doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD, Almeida QJ, Chua R. Spatial constraints in bimanual coordination: Influences of effector orientation. Experimental Brain Research. 2002;146:205–212. doi: 10.1007/s00221-002-1179-5. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Conte A, Mainero C, Frasca V, Fubelli F, Totaro P, et al. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: A functional and anatomical study. Human Brain Mapping. 2007;28:636–644. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel MA, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Raassi C, Pfefferbaum A, Sullivan EV. Local–global interference is modulated by age, sex and anterior corpus callosum size. Brain Research. 2007;1142:189–205. doi: 10.1016/j.brainres.2007.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J. Asymmetry in transcallosal inhibition. Electroencephalography and Clinical Neurophysiology, Supplement. 1999;51:137–144. [PubMed] [Google Scholar]
- Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR, American Journal of Neuroradiology. 2006;27:1776–1781. [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh I, Castillo G, Hoque T, et al. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cerebral Cortex. 2009;19:1654–1665. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Butler SL. Reduced intracortical inhibition and facilitation of corticospinal neurons in musicians. Experimental Brain Research. 2002;144:336–342. doi: 10.1007/s00221-002-1051-7. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, et al. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Perrig S, Kazennikov O, Wiesendanger M. Time structure of a goal-directed bimanual skill and its dependence on task constraints. Behavioural Brain Research. 1999;103:95–104. doi: 10.1016/s0166-4328(99)00026-1. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magnetic Resonance in Medicine. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pollok B, Butz M, Gross J, Schnitzler A. Intercerebellar coupling contributes to bimanual coordination. Journal of Cognitive Neuroscience. 2007;19:704–719. doi: 10.1162/jocn.2007.19.4.704. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: Reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L. Differential effects of aging on the functions of the corpus callosum. Developmental Neuropsychology. 2000;18:113–137. doi: 10.1207/S15326942DN1801_7. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Nordstrom MA. Reduced interhemispheric inhibition in musicians. Experimental Brain Research. 2000;133:249–253. doi: 10.1007/s002210000428. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Experimental Brain Research. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Janck L, Huang Y, Staiger JF, Steinmetz H. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: A diffusion tensor imaging study. Neuroscience Letters. 2002;321:57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, et al. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neuroscience and Biobehavioral Reviews. 2010;34:13. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semjen A, Ivry RB. The coupled oscillator model of between-hand coordination in alternate-hand tapping: A reappraisal. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:251–265. doi: 10.1037//0096-1523.27.2.251. [DOI] [PubMed] [Google Scholar]
- Serrien DJ. Coordination constraints during bimanual versus unimanual performance conditions. Neuropsychologia. 2008;46:419–425. doi: 10.1016/j.neuropsychologia.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Wiesendanger M. Role of the corpus callosum in bimanual coordination: A comparison of patients with congenital and acquired callosal damage. European Journal of Neuroscience. 2001;14:1897–1905. doi: 10.1046/j.0953-816x.2001.01798.x. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens L, Oliviero A, Brown P. Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neuroscience Letters. 2002;328:89–92. doi: 10.1016/s0304-3940(02)00499-8. [DOI] [PubMed] [Google Scholar]
- Shim JK, Kim SW, Oh SJ, Kang N, Zatsiorsky VM, Latash ML. Plastic changes in interhemispheric inhibition with practice of a two-hand force production task: A transcranial magnetic stimulation study. Neuroscience Letters. 2005;374:104–108. doi: 10.1016/j.neulet.2004.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn YH, Jung HY, Kaelin-Lang A, Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Experimental Brain Research. 2003;148:176–185. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Disinhibition in the human motor cortex is enhanced by synchronous upper limb movements. Journal of Physiology. 2002;543:307–316. doi: 10.1113/jphysiol.2002.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuro Report. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional brain change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cerebral Cortex. 2002;12:438–445. doi: 10.1093/cercor/12.4.438. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiology of Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: From behavioural principles to neural-network interactions. Nature Reviews Neuroscience. 2002;3:348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Jardin K, Verschueren S, Meulenbroek R, Franz L, Dounskaia N, et al. Exploring interlimb constraints during bimanual graphic performance: Effects of muscle grouping and direction. Behavioural Brain Research. 1998;90:79–87. doi: 10.1016/s0166-4328(97)00083-1. [DOI] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage. 2008;40:1772–1781. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Experimental Brain Research. 2008;186:59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller B, Kelso JA. Environmentally-specified patterns of movement coordination in normal and split-brain subjects. Experimental Brain Research. 1989;75:306–316. doi: 10.1007/BF00247936. [DOI] [PubMed] [Google Scholar]
- Vercauteren K, Pleysier T, Van Belle L, Swinnen SP, Wenderoth N. Unimanual muscle activation increases interhemispheric inhibition from the active to the resting hemisphere. Neuroscience Letters. 2008;445:209–213. doi: 10.1016/j.neulet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, et al. Human motor corpus callosum: Topography, somatotopy, and link between microstructure and function. Journal of Neuroscience. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BA, Evans JJ, Emslie H. The development of an ecologically valid test for assessing patients with dysexecutive syndrome. Neuropsychological Rehabilitation. 1996;8:213–228. [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Yazgan MY, Wexler BE, Kinsbourne M, Peterson B, Leckman JF. Functional significance of individual variations in callosal area. Neuropsychologia. 1995;33:769–779. doi: 10.1016/0028-3932(95)00018-x. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. Neuroimage. 2009;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]