Abstract
The link between cyclobenzaprine (Flexeril) administration and serotonin syndrome (SS) is subject to debate. Establishing such a connection is difficult because of the limited number of case reports available and the almost complete ignorance of its preclinical pharmacology. In this context, evidence is provided here that cyclobenzaprine blocks the serotonin and norepinephrine transporters and binds to another set of five serotonin receptors. SS should be considered when indicative signs occur in the context of cyclobenzaprine use.
Any therapeutic agent can produce adverse effects.1 Side effects that occur only rarely, have a long latency, or manifest exclusively in specific subpopulations are likely to remain undetected during clinical trials and are discovered only once the drug is on the market, through pharmacovigilance2 and postmarketing analyses.3 These adverse effects may be due to idiosyncratic or otherwise uncharacterized pharmacology that was not identified during the research and development process.4,5
The recognition of direct links between (i) the interaction of a drug with a particular protein, pathway, organelle, or other in vivo component and (ii) certain side effects has motivated the systematic testing of molecules across groups of safety-relevant targets during the early phases of drug discovery.5 This has contributed significantly to the reduction in the risk of failure late in the drug-optimization process. However, knowledge in this area is still very incomplete and varies widely among individual drugs,6 which further hampers our understanding of how drugs act on “targets” and “antitargets.”7
Serotonin syndrome (SS) is a rare, potentially lethal event resulting from excessive central and peripheral serotonergic activity.8 SS is characterized by altered mental status, autonomic instability, and neuromuscular abnormalities. Many drugs have been linked to SS,9 which typically develops in patients within hours of initiating treatment, after dosage increase or overdose, or when combining two or more serotonergic drugs. Substances associated with SS include monoamine oxidase inhibitors, tricyclic antidepressants, selective serotonin reuptake inhibitors, opioids, and antibiotics.8
In this context, the role of cyclobenzaprine (Flexeril) as a causal agent of SS has been subject to debate.10–12 Despite its wide use as a skeletal muscle relaxant, little is known about its pharmacological profile. Cyclobenzaprine is a 5-HT2 receptor antagonist;13 moreover, it moderately inhibits the Toll-like receptor 4 (ref. 14) and aldehyde oxidase15 and is a substrate of the cytochrome P450 isoforms 1A2 and 3A4.16 Several case reports have suggested a causal relationship between cyclobenzaprine and SS.10,11 Therefore, we aimed to identify additional potential SS-relevant targets for which cyclobenzaprine might have affinity and that may provide pharmacological support to the link between cyclobenzaprine and SS.
RESULTS
The increasing availability of drug–target interaction data has promoted the development of computational approaches that link drugs and targets to clinical outcomes.17 Some of these methods predict the affinity profiles of small molecules across thousands of targets (currently 4,643 proteins) and thus represent an efficient method of prioritizing the targets against which a small-molecular-structure drug should be tested.18 Accordingly, cyclobenzaprine was profiled against ligand-based models available for 4,643 proteins. The results, summarized in Table 1, suggest cyclobenzaprine’s action on several targets previously linked to SS, namely, serotonin transporter and the 5-HT2A and 5-HT2C receptors.10,19 In addition, affinity predictions for five phylogenetically related targets of relevance for SS—the 5-HT2B,20 5-HT6, and 5-HT7 receptors;21 the norepinephrine transporter; and the dopamine transporter (DAT)—were also made.10,22
Table 1.
Computationally predicted (in silico) and experimentally determined (in vitro) binding affinities (Kj, in nmol/l) of cyclobenzaprine to three monoamine transporters and five serotonin receptor subtypes
 |
 |
 |
||
|---|---|---|---|---|
| Transporter/receptor | In silico | In vitro | In vitro | In vitro |
| Serotonin transporter | 251 | 108 | 4a | 1a |
| Norepinephrine transporter | 126 | 36 | 22a | 52a |
| Dopamine transporter | 7,943 | 5,489 | 3,250a | 8,500a |
| Serotonin 5-HT2A receptor | 20 | 29 | 4a | 119a |
| Serotonin 5-HT2B receptor | 200 | 154 | 39.8 | N/A |
| Serotonin 5-HT2C receptor | 62 | 57 | 6a | 120a |
| Serotonin 5-HT6 receptor | 40 | 145 | 103a, 89b | 190a |
| Serotonin 5-HT7 receptor | 69 | 151 | 126a, 398b | 1,000a |
Subsequent in vitro testing of cyclobenzaprine on these targets confirmed that, except for DAT, the affinities of cyclobenzaprine for the other molecules were all within the submicromolar range (Table 1). A good correspondence was found between the experimental affinities and our predictions, with a root mean square deviation of 0.34 log units. The average affinity of the compound for the three 5-HT2 receptor subtypes agrees with the nonspecific inhibition constant (Ki) of 62 nmol/l for 5-HT2 receptors reported earlier. 12 In particular, the affinity of cyclobenzaprine for the 5-HT2A receptor subtype was the most potent interaction identified. The corresponding dose–response curve is provided in Figure 1.
Figure 1.
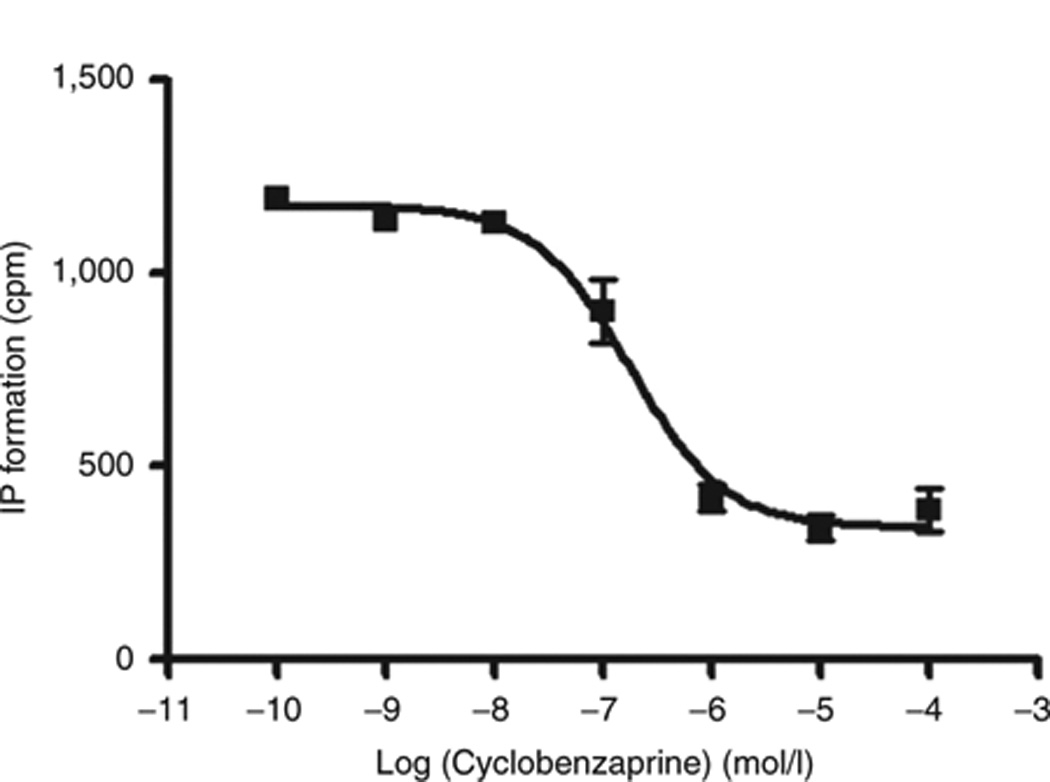
Concentration–response curve of the action of cyclobenzaprine on 1μmol/l 5HT-activated 5-HT2A receptors, obtained by measuring the release of inositol phosphate (IP). The antagonistic potency (expressed as Kb) of cyclobenzaprine for this receptor was 28.78 nmol/l ± 2.49. Mean values ± SEM (vertical bars) of triplicate measurements are shown. Kb, equilibrium dissociation constant.
The structure of cyclobenzaprine is closely related to those of the tricyclic antidepressants amitriptyline and imipramine. Cyclobenzaprine differs from amitriptyline by just one double bond in the seven-membered ring, whereas imipramine contains an additional nitrogen atom in the same ring. These structural differences are relevant because imipramine is linked to SS whereas amitriptyline does not appear to be associated with it.10 Thus, for comparative purposes, Table 1 shows the affinities of amitriptyline and imipramine for the same eight targets for which we report the affinity values of cyclobenzaprine. Data for the two tricyclic antidepressants were extracted from the databases of the Psychoactive Drug Screening Program22 and the International Union of Basic and Clinical Pharmacology.23 No relevant bioactivity data for cyclobenzaprine are currently available in these public repositories, substantiating the novelty of the data reported here.
DISCUSSION
Cyclobenzaprine reaches plasma concentration levels between 0.003 and 0.046 mg/l,24 which is equivalent to values between 10 and 150 nmol/l . With the exception of DAT, the affinities of cyclobenzaprine for all the targets tested (Table 1) are within the range of its therapeutic levels. The steady-state plasma concentrations of cyclobenzaprine in elderly subjects and individuals with mild hepatic insufficiency can be twice as high as those in young subjects and healthy controls, respectively,25 setting the upper limit of therapeutically relevant affinities at 300 nmol/l. A 10-fold overdose might even lead to a cyclobenzaprine-induced DAT blockade.
A comparative analysis of the affinity profiles of cyclobenzaprine, amitriptyline, and imipramine on the eight targets studied is not conclusive (Table 1). Even though they could be considered qualitatively similar, there are differences in the relative potencies of the drugs for several targets. For example, cyclobenzaprine is two orders of magnitude less potent than imipramine on serotonin transporter; by contrast, it shows higher affinities for 5-HT2A, 5-HT2C, and 5-HT7. However, because these measurements were obtained from different laboratories and are therefore subject to interexperimental variation, strict comparisons using these values cannot be made. Regardless of how accurate these profiles are, the affinities of cyclobenzaprine for the aforementioned targets are consistent with the therapeutic levels achieved by this drug and are likely to lead to increased agonist activity at serotonergic neuronal junctions. In summary, the data from Table 1 provide a pharmacological basis for a causal relationship between cyclobenzaprine—taken alone or in combination with other serotonergic drugs—and SS.
Several case reports link cyclobenzaprine, in combination with known serotonergic drugs, to possible cases of SS. For example, a 27-year-old woman taking escitalopram (Lexapro) developed somnolence following an overdose of cyclobenzaprine.9 She became stuporous and developed fever, nystagmus, tachycardia, diaphoresis, tremors, muscle rigidity, and hyperreflexia. The symptoms resolved over a period of 2 days following treatment with cyproheptadine, nonspecific therapies, and discontinuation of cyclobenzaprine and escitalopram.9 In another case, a 70-year-old woman who was taking phenelzine (Nardil) developed confusion, agitation, tremulousness, tachycardia, diaphoresis, and fever following a third dose of cyclobenzaprine.10 The symptoms resolved over a period of 3 days following withdrawal of phenelzine and cyclobenzaprine.10 Finally, a 53-year-old man who was taking duloxetine (Cymbalta), pregabalin (Lyrica), bupropion (Wellbutrin), and opioids developed diaphoresis, tachycardia, agitation, tremors, and spontaneous, sustained clonus shortly after cyclobenzaprine was added to his regimen.10 Symptoms resolved over several days, following intubation, sedation, and withdrawal of cyclobenzaprine and duloxetine.10 Cyclobenzaprine-induced blockade of norepinephrine transporter alone can cause tachycardia, and coadministration with duloxetine and escitalopram is likely to increase synaptic concentrations of serotonin, causing agonist saturation at all receptor subtypes. Coadministration with phenelzine may lead to increased hyperkinetic neuromuscular symptoms. In the absence of additional data, however, ascertaining the pharmacodynamic consequences of cyclobenzaprine administration is difficult because no single receptor has been implicated in the clinical tableau of SS;7 moreover, extrapolating directly from these preclinical binding studies and clinical effects is not possible. Among cyclobenzaprine’s prevalent side effects, xerostomia is likely to be caused by blockade of the muscarinic M1 and M3 receptors,26 and drowsiness is caused by histamine H1-receptor antagonism.6
Why is the clinical association between cyclobenzaprine and SS not clearly recognized? The first difficulty in establishing such a relationship, apart from the limitations of retrospective case reports, is the absence of a definitive diagnostic test.27 At least one of the aforementioned cases has been criticized as not being indicative of SS, citing various physical “diagnostic” criteria.11 Although most scientists and clinicians currently believe that there is a pathophysiologic state of serotonin excess associated with various physical manifestations, and there are animal models that confirm an increase in neurotransmitters of the central nervous system in correlation with certain clinical effects following exposure to serotonergic agents,28 this condition does not have a definitive diagnostic test in humans. Current “diagnostic” criteria, such as the Hunter Serotonin Toxicity Criteria,29 are ultimately based on the clinical impressions of an experienced observer. They cannot have true sensitivity or specificity, and a definitive diagnosis of SS in any clinical case cannot be made, or disputed, on this basis. The second difficulty in establishing a relationship between cyclobenzaprine and SS is the logistical challenge of developing a sufficiently large retrospective series or a prospective study. Further difficulties include the apparent rarity of the interaction and the probable misattribution of symptoms and signs to other agents of exposure. In 2009, 10,184 human exposures to cyclobenzaprine were reported at various poison centers in the United States, 5,787 of them being polysubstance exposures. Twenty-six deaths involved cyclobenzaprine, in each instance as part of a polysubstance exposure. In addition, 45,238 human exposures to selective serotonin reuptake inhibitors and hundreds of thousands of exposures to other substances with serotonergic activity were reported. However, only 62 cases in the database were coded as SS.30 This may be secondary to the actual rarity of the condition, the difficulty in establishing the diagnosis, coding issues, or other limitations of the database. The observed symptoms and signs may also be the direct effect of many of these substances. Thus, when cyclobenzaprine is combined with a serotonergic drug, the symptoms and signs of SS might simply be attributed to other conditions or substances of exposure.29 Furthermore, no retrospective or prospective studies on this subject are currently available.
CONCLUSION
Cyclobenzaprine is a potent blocker of the transporters of the monoamines serotonin and norepinephrine. It has low micromolar affinity for the DAT and exerts its pharmacological action over the 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, and 5-HT7 receptor subtypes with affinities that are within the therapeutic concentration range. This study provides preclinical pharmacological evidence that supports a causative relationship between cyclobenzaprine and the occurrence of SS.
METHODS
In silico target profiling
The affinities of cyclobenzaprine for the different targets were predicted using a similarity-based approach to target profiling.19 This method relies on the assumption that small molecules provide a complementary description to the target binding site. Molecular descriptors that capture the structural and pharmacophoric features of all molecules for which affinity data are publicly available provide a mathematical description of each target from a ligand’s perspective. On this basis, the affinity of a compound for a given target can be estimated by inverse distance–weighting interpolation of the experimental affinities from all neighboring molecules found within a predetermined applicability domain.19 On the basis of the ligand-based target models defined from all the pharmacological data available in chemogenomic databases, each small molecule can be currently processed against 4,643 such models. The output returns a list of the targets for which affinity is predicted for each query molecule. Further details on this methodology are provided as Supplementary Materials and Methods online.
Radioligand binding assays
Cyclobenzaprine (Sigma Aldrich, St Louis, MO; purity >98%) was assayed in vitro using concentration–response competition curves at all targets predicted in silico, by assaying six different concentrations (range 10 nmol/l–100 μmol/l). The –log value of the inhibition constant (pKi) was calculated using the Cheng–Prusoff equation: Ki = IC50/(1 + (L)/KD), where IC50 is the concentration of the compound that displaces 50% of the bound radioligand, (L) is the concentration of free radioligand, and KD is the dissociation constant of each radioligand. The IC50 values were obtained by nonlinear data fitting with Prism 2.1 (GraphPad, San Diego, CA). The procedures and conditions for the competition binding experiments carried out with five subtypes of the human serotonin receptor (5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, and 5-HT7) and three human neurotransmitter transporters (the serotonin transporter, the norepinephrine transporter, and DAT) are provided in detail as Supplementary Materials and Methods online.
Supplementary Material
ACKNOWLEDGMENTS
J.M. acknowledges the financial support provided by the Instituto de Salud Carlos III and the Spanish Ministerio de Ciencia e Innovacion (project reference BIO2008-02329). This work was supported, in part, by grants 1R21GM095952-01 and 5U54MH084690-03 from the National Institutes of Health and by the Villum Foundation CDSB (T.I.O.). All in vitro data for cyclobenzaprine in Table 1 were determined at the high throughput screening facilities at the University of Santiago de Compostela, Spain. Ki determinations were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, contract HHSN-271-2008-00025-C (http://pdsp.med.unc.edu).
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
CONFLICT OF INTEREST
T.I.O. is the founder and CEO of Sunset Molecular Discovery LLC, a company that produces and markets chemical bioactivity databases. J.M. is the president of Chemotargets SL, a company that provides services in the area of in silico pharmacology. S.A.S. declared no conflict of interest.
References
- 1.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 2.Härmark L, van Grootheest AC. Pharmacovigilance: methods, recent developments and future perspectives. Eur. J. Clin. Pharmacol. 2008;64:743–752. doi: 10.1007/s00228-008-0475-9. [DOI] [PubMed] [Google Scholar]
- 3.Hauben M, Bate A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discov. Today. 2009;14:343–357. doi: 10.1016/j.drudis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 5.Whitebread S, Hamon J, Bojanic D, Urban L. Keynote review: in vitro safety pharmacology profiling: an essential tool for successful drug development. Drug Discov. Today. 2005;10:1421–1433. doi: 10.1016/S1359-6446(05)03632-9. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Serna R, Mestres J. Chemical probes for biological systems. Drug Discov. Today. 2011;16:99–106. doi: 10.1016/j.drudis.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Broccatelli F, Carosati E, Cruciani G, Oprea TI. Transporter-mediated efflux influences CNS side effects: ABCB1, from antitarget to target. Mol. Inf. 2010;29:16–26. doi: 10.1002/minf.200900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer EW, Shannon M. The serotonin syndrome. N. Engl. J. Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 9.Sun-Edelstein C, Tepper SJ, Shapiro RE. Drug-induced serotonin syndrome: a review. Expert Opin. Drug Saf. 2008;7:587–596. doi: 10.1517/14740338.7.5.587. [DOI] [PubMed] [Google Scholar]
- 10.Day LT, Jeanmonod RK. Serotonin syndrome in a patient taking Lexapro and Flexeril: a case report. Am. J. Emerg. Med. 2008;26:1069.e1–1069.e3. doi: 10.1016/j.ajem.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Keegan MT, Brown DR, Rabinstein AA. Serotonin syndrome from the interaction of cyclobenzaprine with other serotoninergic drugs. Anesth. Analg. 2006;103:1466–1468. doi: 10.1213/01.ane.0000247699.81580.eb. [DOI] [PubMed] [Google Scholar]
- 12.Gillman PK. Is there sufficient evidence to suggest cyclobenzaprine might be implicated in causing serotonin toxicity? Am. J. Emerg. Med. 2009;27:509–510. doi: 10.1016/j.ajem.2009.03.001. author reply 510. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Hasegawa Y, Ono H. Cyclobenzaprine, a centrally acting muscle relaxant, acts on descending serotonergic systems. Eur. J. Pharmacol. 1996;311:29–35. doi: 10.1016/0014-2999(96)00402-5. [DOI] [PubMed] [Google Scholar]
- 14.Hutchinson MR, et al. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience. 2010;168:551–563. doi: 10.1016/j.neuroscience.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obach RS, Huynh P, Allen MC, Beedham C. Human liver aldehyde oxidase: inhibition by 239 drugs. J. Clin. Pharmacol. 2004;44:7–19. doi: 10.1177/0091270003260336. [DOI] [PubMed] [Google Scholar]
- 16.Wang RW, Liu L, Cheng H. Identification of human liver cytochrome P450 isoforms involved in the in vitro metabolism of cyclobenzaprine. Drug Metab. Dispos. 1996;24:786–791. [PubMed] [Google Scholar]
- 17.Oprea TI, et al. Associating drugs, targets and clinical outcomes into an integrated network affords a new platform for computer-aided drug repurposing. Mol. Inf. 2011;30:100–111. doi: 10.1002/minf.201100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal D, Mestres J. In silico receptorome screening of antipsychotic drugs. Mol. Inf. 2010;29:543–551. doi: 10.1002/minf.201000055. [DOI] [PubMed] [Google Scholar]
- 19.Gillman PK. A review of serotonin toxicity data: implications for the mechanisms of antidepressant drug action. Biol. Psychiatry. 2006;59:1046–1051. doi: 10.1016/j.biopsych.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Diaz SL, Maroteaux L. Implication of 5-HT(2B) receptors in the serotonin syndrome. Neuropharmacology. 2011;61:495–502. doi: 10.1016/j.neuropharm.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur. J. Neurosci. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- 22.Jensen NH, Roth BL. Massively parallel screening of the receptorome. Comb. Chem. High Throughput Screen. 2008;11:420–426. doi: 10.2174/138620708784911483. [DOI] [PubMed] [Google Scholar]
- 23.Harmar AJ, et al. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucl. Acids Res. 2009;37:D680–D685. doi: 10.1093/nar/gkn728. < http://www.iuphar-db.org>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz M, Schmoldt A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie. 2003;58:447–474. [PubMed] [Google Scholar]
- 25.Winchell GA, King JD, Chavez-Eng CM, Constanzer ML, Korn SH. Cyclobenzaprine pharmacokinetics, including the effects of age, gender, and hepatic insufficiency. J. Clin. Pharmacol. 2002;42:61–69. doi: 10.1177/0091270002042001007. [DOI] [PubMed] [Google Scholar]
- 26.Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol. Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 27.Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med. J. Aust. 2007;187:361–365. doi: 10.5694/j.1326-5377.2007.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 28.Nisijima K, Yoshino T, Ishiguro T. Risperidone counteracts lethality in an animal model of the serotonin syndrome. Psychopharmacology (Berl.) 2000;150:9–14. doi: 10.1007/s002130000397. [DOI] [PubMed] [Google Scholar]
- 29.Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635–642. doi: 10.1093/qjmed/hcg109. [DOI] [PubMed] [Google Scholar]
- 30.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2008 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin. Toxicol. (Phila) 2009;47:911–1084. doi: 10.3109/15563650903438566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


