Abstract
The metal ion copper is a cofactor essential for maintaining normal biological and physical functions in human beings. High copper levels have been found in variety of tumor tissues and are involved in tumor angiogenesis processes. The ubiquitin-proteasome system plays an important role in cell growth and apoptosis and has been shown as a novel target for cancer therapy. We previously reported that some organic copper complexes can inhibit the proteasomal chymotrypsin-like activity and induce apoptosis in human cancer cells and xenograft models. In the current study, we investigated the effect of oxidation status of copper, Cu(I) or Cu(II), on inhibition of proteasome activity, induction of apoptosis, and induction of reactive oxygen species (ROS) in human cancer cells. We report four major findings here: i) both Cu(I) and Cu(II) could inhibit the chymotrypsin-like activity of purified 20S proteasome, but Cu(I) was more potent than Cu(II), ii) purified 20S proteasome protein was able to reduce Cu(II) to Cu(I), suggesting that Cu(I) is the oxidation status of copper that directly reacts with the proteasome, iii) when complexed with the copper ligand neocuproine, Cu(I) showed higher ability to induce ROS production in cancer cells, compared with Cu(II), iv) addition of a ROS scavenger in the cancer cell culture-blocked copper-induced ROS generation, but did not overcome copper-mediated proteasome-inhibitory and cell death-inducing events, demonstrating the ROS-independent proteasome-inhibitory property of copper complexes.
Keywords: copper, oxidation status, proteasome inhibition, apoptosis, ROS
Introduction
Copper (Cu) is an essential trace metal for mammalians and human beings (1,2). One of the important physiological functions of Cu is to serve as a cofactor in a process of formation of new blood vessels, termed as angiogenesis (3,4). Tumor growth invasion and metastasis all depend on angiogenesis. It has been shown that tumors can not grow >1-2 mm3 without forming new blood vessels (5). High tissue levels of copper have been found in many types of human cancers, including breast, prostate, colon, lung and brain (5-9). The ubiquitin-proteasome-dependent degradation system plays an important regulatory role in processes of cell growth and apoptosis. Development of proteasome inhibitors as novel anti-cancer agents is currently under intensive investigation. Previously we found that certain organic copper ligands can react with cupric copper (CuCl2) and form complexes. These complexes are potent proteasome inhibitors and can induce apoptotic cell death in cancer cells in vitro and in vivo (10-13). In the current study, we further investigated which oxidation states of copper, Cu(I) or Cu(II), serve as a proteasome inhibitor and an apoptosis inducer in cancer cells. Under cell-free conditions, we found that purified 20S proteasome could reduce Cu(II) to Cu(I) and that both Cu(I) and Cu(II) could inhibit the activity of a purified 20S proteasome, but Cu(I) is more potent that Cu(II). Data from cell culture studies showed that when Cu(I) or Cu(II) was mixed with neocuproine (NC), a copper-binding compound, both copper mixtures could inhibit proteasome activity and induce apoptosis in tumor cells, and Cu(I) mixture was slightly more potent. We also found that the Cu(I) mixture was a strong ROS inducer and that a ROS scavenger could block the ROS generation produced by Cu(I) mixture but could not overcome its proteasome-inhibitory activity. Our data suggest that the inhibition of proteasome activity by copper complexes might be mainly through the direct binding of copper to the proteasome, and that oxidative stress or ROS generation may be only partially responsible for the copper-induced tumor cell death.
Materials and methods
Reagents and antibodies
Purified rabbit 20S proteasome, fluorogenic peptide substrate was obtained from Calbiochem (San Diego, CA). BCS (bathocuproinedisulfonic acid disodium salt), hydroxide peroxide, NC (neocuproine, 2,9-dimethyl-1,10-phenanthroline), copper chloride and AH (ascorbic acid) were obtained from Sigma. RPMI-1640, DMEM/F-12, penicillin and streptomycin were purchased from Invitrogen. Mouse monoclonal antibody against human poly(ADP-ribose) polymerase (PARP) was purchased from BD Biosciences Pharmingen (San Diego, CA). Mouse monoclonal antibody against ubiquitin (P4D1), goat polyclonal antibodies against actin (C-11) and IκB-α (C15), and secondary antibodies were from Santa Cruz Biotechnology, Inc. The antibody against proteasomal ß5 subunit was from BIOMOL International (Plymouth Meeting, PA, USA). The Image-iT™ live green ROS detection kit was purchased from Molecular Probes (Eugene, OR, USA).
Cell cultures and whole cell extract preparation
Human Jurkat T cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. MDA-MB-231 human breast cancer cells were obtained from American Type Culture Collection (Manassas, VA) and grown in DMEM/F-12 supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. All cells were maintained at 37°C and 5% CO2. A whole cell extract was prepared as described previously (10).
Color change and precipitate formation reactions
NC, AH, CuCl and CuCl2 were dissolved in DMSO to a final concentration of 50 mM. CuCl or CuCl2 was then mixed with NC or AH in a 1:4 ratio or with NC and AH in a 1:4:4 ratio, followed by examination of color change, as an indicator of complex formation.
Trypan blue exclusion assay
The trypan blue dye exclusion assay was performed by mixing 20 μl of 0.4% trypan blue dye and 20 μl of Jurket T cells treated with different concentration of NC-copper complexes before injecting into a hemocytometer and counting. The number of cells that absorbed the dye and those that exclude the dye were counted, from which the percentage of non-viable cell number to total cell number was calculated.
Inhibition of the proteasomal chymotrypsin-like activity in cell extracts
Whole cell extracts (5 μg) of treated MDA-MB-231 cells were incubated for 2 h at 37°C in 100 μl of assay buffer (50 mM Tris-HCl, pH 7.5) with 10 μmol/l fluorogenic peptide substrate Suc-LLVY-AMC for the proteasomal chymotrypsin-like activity. After incubation, production of hydrolyzed AMC groups was measured with a Wallac Victor 3 Multiabel Counter with an excitation filter of 365 nm and emission filter of 460 nm. Changes in fluorescence were calculated against DMSO-treated control.
Measurement of the reduction of Cu(II) to Cu(I)
Reduction of Cu(II) to Cu(I) generation was determined by using BCS, according to the published method (14), based on the specific absorption of Cu(I)-BCS complex at 480 nm wavelength. Purified 20S proteasome (35 ng) was mixed with CuCl2 or CuCl and 100 μM of BCS in PBS, pH 7.3, and then incubated at 37°C for 2 h. The absorption of each sample was measured by a spectrophotometer at 480 nm wavelength.
Western blot analysis
The cell extracts were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Western blot analysis was done using indicated specific antibodies, following by visualization with the enhanced chemiluminescence reagent.
Visualization of ROS in live tumor cells
The Image-iT live green ROS detection kit was used to visualize ROS in live MDA-MB-231 cells. Carboxy-H2DCFDA [5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate] is a cell-permeable non-fluorescent indicator reagent. When the carboxy-H2DCFDA permeates into live cells, it can be deacetylated by intracellular esterases and oxidized by cellular ROS, and then it is converted to the fluorescent form DCF by emitting bright green color. Tert-butyl hydroperoxide (TBHP), a ROS inducer, was used as positive control. A Zeiss Axiovert 25 microscope was used for capturing images of oxidized fluorescein (green fluorescence), nuclei (blue fluorescence, Hoechst 33342) and cellular morphology.
Results
Both NC-CuCl and NC-CuCl2 were able to inhibit proteasome activity and induce cell death in human leukemia Jurkat T cells
Previously we found that some copper ligands could react with cupric copper chloride (CuCl2) and formed organic copper complexes (13). These complexes were able to inhibit the cellular proteasome activity and induce apoptotic cell death in cancer cells in vitro and in vivo (10,12,13,15). In the current study we examined whether cuprous copper (CuCl) when mixed with a copper ligand could play the similar biological role as CuCl2 in cancer cells. Because of the unstable nature of Cu(I) species, comparison of effect of oxidation status of inorganic copper in cells remains difficult. Therefore, we used neocuproine (NC), a copper ligand to form organic copper complexes with CuCl or CuCl2, respectively in the current experiment. It has been reported that NC can bind with inorganic copper, especially cuprous copper, to form a complex and present color changes from no color to light orange (16). To test the reaction of NC with cuprous and cupric copper chloride, we mixed NC and CuCl or CuCl2 solution in a 4:1 molar ratio in DMSO. Slight color change was observed in NC-CuCl mixture (data not shown), indicating that a chemical reaction had occurred. CuCl is not stable and is easily oxidized and converted to CuCl2. To protect CuCl from being oxidized to CuCl2, we added ascorbic acid (AH) as a reducer in a 1:4 molar ratio. (17). When NC was mixed with AH and CuCl (NC-AH-CuCl) or with AH and CuCl2 (NC-AH-CuCl2), dramatic color changes were observed. There is no color change observed in the mixtures of NC-AH, AH-CuCl or AH-CuCl2 (data not shown). We then used the mixtures of NC-CuCl-AH (AH was always added in NC-CuCl complex if not mentioned) and NC-CuCl2 for further investigation using cancer cell culture.
We hypothesized that NC-CuCl and NC-CuCl2 may have similar biological effects on proteasome inhibition and apoptosis induction in cancer cells. First of all, we tested whether these complexes were capable of inhibiting the proteasome activity in intact tumor cells. Human leukemia Jurkat T cells were treated with NC-CuCl2 or NC-CuCl-AH, with NC, AH, CuCl2, CuCl alone or DMSO as controls at indicated concentrations. After 3 h of treatment, cells were incubated with fluorogenic peptide substrate for additional 2 h and then proteasome chymotrypsin (CT)-like activity was measured. The results showed that both NC-CuCl2 and NC-CuCl-AH significantly inhibited the proteasome activity in intact Jurkat T cells, as indicated by decreased levels of the proteasomal chymotrypsin-like activity (Fig. 1A). Moreover, the NC-CuCl-AH was more potent than that of NC-CuCl2, measured as 25 vs. 14% inhibition at 1 μM and 60 vs. 49% inhibition at 10 μM concentrations of NC-CuCl-AH and NC-CuCl2, respectively (Fig. 1A). In contrast, the treatments with NC, AH, CuCl2 or CuCl alone had no effects on proteasome activity (Fig. 1A).
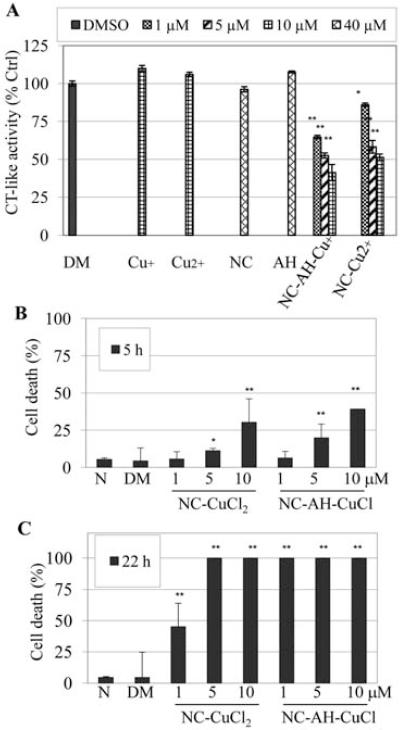
Figure 1.
Both of NC-CuCl and NC-CuCl2 mixtures could inhibit proteasome activity and reduce cell death in Jurkat T cells. (A) Human leukemia Jurkat T cells were treated with indicated doses of CuCl (Cu+), CuCl2 (Cu2+), NC, AH alone or NC mixtures with CuCl or CuCl2 for 5 h, followed by measurement of proteasome chymotrypsin (CT)-like activity. (B and C) Jurkat T cells were treated with indicated concentrations of NC and copper mixtures for 5 (B) or 22 h (C), followed by trypan blue dye exclusion assay. Treatment with DMSO (DM) or no-treatment (N) was used as controls. *P<0.05, **P<0.01, bars, SD, mean of three experiments.
Cytotoxicity against Jurkat T cells was then measured by trypan blue dye exclusion assay. Jurkat T cells were treated with different concentrations of NC-CuCl-AH or NC-CuCl2 for 5 or 22 h (Fig. 1B and C). The results showed that both copper complexes could induce cell death. Compared with NC-CuCl2, NC-CuCl-AH was more potent at all concentrations tested after 5 h of treatment, for example, 40% cell death by NC-CuCl-AH at 10 μM compared to 30% cell death by NC-CuCl2 at the same concentration (Fig. 1B). After 22 h of treatment, all the cells treated with 1 μM NC-CuCl-AH were dead. Treatment with NC-CuCl2 mixture at the same concentration resulted in only 45% cell death (Fig. 1C).
Treatment with NC-CuCl2 and NC-CuCl-AH results in proteasome inhibition and apoptotic cell death in breast cancer cells
We then examined the effects of NC-CuCl2 and NC-CuCl-AH on solid tumor cells. Human breast cancer MDA-MB-231 cells were treated with NC-CuCl2, or NC-CuCl-AH or DMSO at indicated concentrations for 19 h (Fig. 2). After each treatment, proteins were extracted and used for measurement of proteasome activity by decreasing levels of cellular proteasomal chymotrypsin-like activity and accumulation of ubiquitinated proteins and proteasome target protein IκB-α.
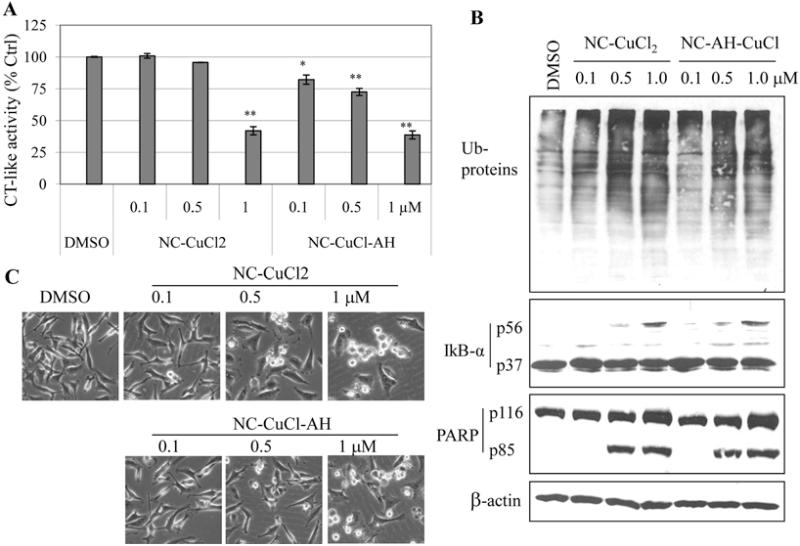
Figure 2.
Proteasome-inhibitory and apoptosis-inducing effects of NC-CuCl2, NC-AH-CuCl on breast cancer cells. MDA-MB-231 cells were treated with indicated concentrations of NC-CuCl2, NC-AH-CuCl for 19 h, followed by measurement of proteasome activity (A), Western blot analysis (B) and cellular morphological changes (magnification, ×100) (C). DMSO (DM) was used as solvent control. *P<0.05, **P<0.01, bars, SD, mean of three experiments.
We found that both complexes could inhibit proteasomal chymotrypsin-like activity and that NC-CuCl-AH was more potent than NC-CuCl2 at all concentrations tested (Fig. 2A). Also, levels of ubiquitinated proteins were accumulated by treatment with both complexes at all concentrations tested (Fig. 2B). The ubiquitinated form (56 kDa) of IκB-α protein was observed mainly in the cells treated with 1 μM of NC-CuCl2 or NC-CuCl-AH (Fig. 2B).
It has been reported that inhibition of proteasome chymotrypsin-like activity in cancer cells is associated with apoptosis induction (18,19). To investigate whether the complexes have apoptosis-inducing activity, morphologic changes and apoptosis-associated PARP cleavage were studied. Cellular apoptotic morphology changes (shrunken and blebbing) were observed starting at 0.5 μM and were more severe at 1 μM treatment with both complexes (Fig. 2C). Consistent with apoptosis induction, PARP cleavage fragment p85 was observed in the cells treated with both complexes at 0.5 and 1 μM (Fig. 2B). Both complexes have similar activity in inducing apoptosis.
Purified 20S proteasome is able to reduce Cu(II) to Cu(I) and both CuCl and CuCl2 can inhibit the activity of purified 20S proteasome
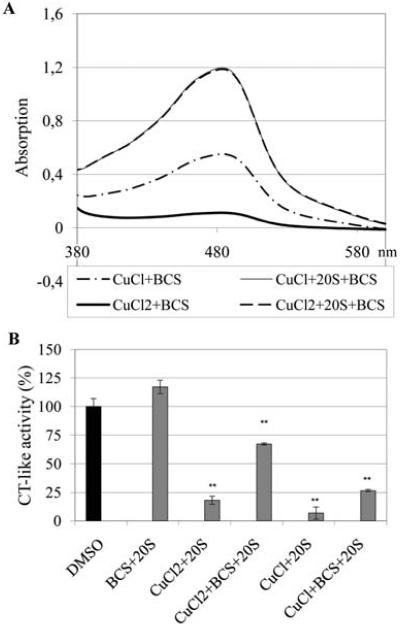
So far, all obtained data above have shown that both NC-CuCl and NC-CuCl2 have similar effects on proteasome inhibition and cell death induction and NC-CuCl is slightly more potent than NC-CuCl2. However, it is still unclear what oxidation status of copper interacts with the proteasome complex. Some transition metal ions, mainly iron and copper, at reducing conditions, are able to catalyze the formation of hydroxyl radicals by Fenton-type reactions. If the proteasome inhibition caused by Cu(II) occurs via Fenton Reactions, we would expect that 20S proteasome could reduce Cu(II) to Cu(I), and the hydroxyl radicals could repeatedly be formed by recycling Cu(II) back to Cu(I). Indeed, when we incubated a purified 20S proteasome with cupric copper chloride at 37°C for 2 h in the presence of the Cu(I) indicator bathocuproinedisulfonic acid (BCS), we detected formation of a Cu(I) species, as indicated by the appearance of a peak with maximal absorbance at 480 nm, a characteristic of the BCS-Cu(I) complex (Fig. 3A) (14). In contrast, after incubation of BCS with Cu(II) without purified 20S proteasome, just a slight increase in absorbance at 480 nm was observed, and BCS and Cu(II) alone did not cause any increase in the absorbance at 480 nm (Fig. 3A). Those results clearly demonstrate that purified 20S proteasome is able to reduce Cu(II) to Cu(I), further suggesting that Cu(I) could directly react with 20S proteasome and consequently inhibit the proteasome activity. In order to measure the proteasome activity in the same experiment, the fluorogenic peptide substrate Suc-LLVY-AMC for proteasome was added to all the reaction mixtures above and incubated for 1 h, followed by determination of released fluorogenic AMC. The results showed that both CuCl and CuCl2 alone could inhibit the activity of purified 20S proteasome (Fig. 3B). Again, CuCl is more potent than CuCl2. The data also showed that the proteasome-inhibitory ability of both CuCl and CuCl2 was significantly decreased when incubated with BCS (Fig. 3B). It indicates that direct binding of copper to proteasome may play a critical role in its proteasome-inhibitory activity since BCS is a copper ligand which could compete with the proteasome for binding with copper.
Figure 3.
Purified 20S proteasome reduces Cu(II) to Cu(I). (A) Cu(I) chloride (CuCl) (10 μM) or Cu(II) chloride (CuCl2) was mixed with Cu(I) indicator BCS (100 μM) with or without purified 20S proteasome (35 ng) and incubated at 37°C for 2 h, followed by measuring absorption of each sample in a spectrophotometer. (B) The solutions in Fig. 3A were incubated with fluorogenic peptide substrate Suc-LLVY-AMC (20 μM) for 1 h at 37°C, followed by measuring the proteasome chymotrypsin (CT)-like activity. **P<0.01, bars, SD, mean of three experiments.
Both NC-copper complexes induce the production of ROS in breast cancer MDA-MB-231 cells, but NC-CuCl-AH is a much stronger ROS inducer
Production of ROS in cells is well known to be related to apoptotic cell death (20). A previous report demonstrated that copper and neocuproine complex induces apoptosis in astrocytes through oxidative stress and JNK activation (20). In the current study we found that NC and copper complexes induced apoptosis in MDA-MB-231 cells (Fig. 2). In order to determine whether generation of ROS play a role in proteasome inhibition and cell death induction caused by NC-CuCl and NC-CuCl2, we used an Image-iT assay system that enables to detect ROS in live cells (21,22). The ROS inducer TBHP was used as a positive control in this assy. The more ROS generates in cells, the more intensive fluorescent green will be visualized. In this study, MDA-MB-231 cells were treated with indicated concentrations of NC-CuCl-AH, NC-CuCl2 or TBHP (positive control) for 1, 2 or 4 h. The results showed that both of the copper complexes were able to induce intracellular ROS production after 1 h of treatment (Fig. 4A). However, after 2 and 4 h of treatment much more ROS production was accumulated in the cells treated with NC-AH-CuCl, compared with NC-CuCl2 treatment (Fig. 4A). The results showed that NC-AH-CuCl was a much stronger ROS inducer than NC-CuCl2.
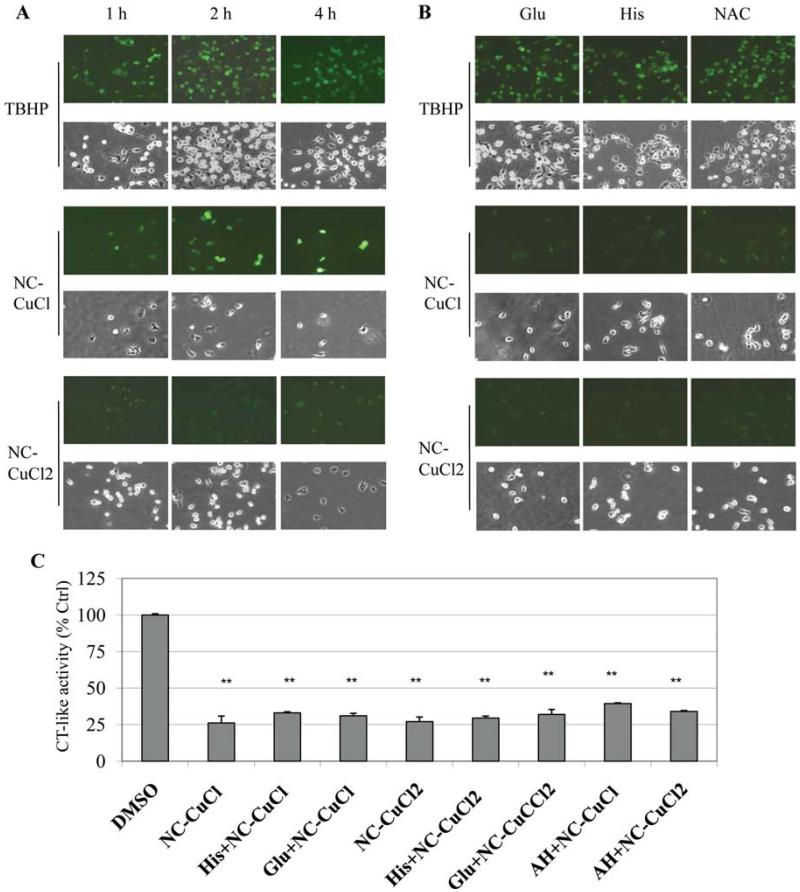
Figure 4.
NC-CuCl induces more ROS compared with NC-CuCl2, and ROS scavengers are able to block ROS induction but can not overcome NC-Cu-induced proteasome inhibition. (A) MDA-MB-231 cells were treated with 10 μM of NC-AH-CuCl or NC-CuCl2 for 1, 2 or 4 h, followed by ROS stain (green color) assay and observed under fluorescence and phase contrast microscopy. Treatment with 200 μM of TBHP for 1.5 h was used as positive control. (B) MDA-MB-231 cells were pre-treated with 200 μM of glutamine (Glu), histodine (His) or NAC for 30 min and post-treated with 10 μM of NC-AH-CuCl or NC-CuCl2 for 4 h, followed by ROS stain assay and photography. Post-treatment with 200 μM of TBHP for 1.5 h was used as positive control. Cellular morphological changes were visualized by phase-contrast imaging (magnification, ×100). (C) Measurement of the proteasome chymotrypsin (CT)-like activity in aliquots of the cells treated for 4 h from above experiments in Fig. 4A and B. **P<0.01, bars, SD, mean of five experiments.
ROS scavengers block NC-copper-induced ROS generation in cancer cells, but can not inhibit the proteasome-inhibitory activity of the NC-copper complex
To investigate the relationship between accumulation of cellular ROS production and the proteasome inhibition induced by NC-copper complexes, MDA-MB-231 cells were pre-treated with the ROS scavengers glutathione (Glu), histidine (His) or N-acetyl-L-cysteine (NAC) (23-25) for 30 min, and then co-treated with NC-CuCl-AH or NC-CuCl2 for 4 h, followed by ROS image detection assay (Fig. 4B) and analysis of proteasome inhibition by the chymotrypsin-like activity assay (Fig. 4C). The results showed that either Cu(I)- or Cu(II)-induced ROS in the cells pretreated with Glu, His or NAC was almost completely eliminated (Fig. 4B), however these ROS scavengers could not overcome proteasome inhibition induced by NC-Cu mixtures (Fig. 4C). Furthermore, these ROS scavengers did not reduce the cell death induced by NC-copper complexes (Fig. 4B). These findings indicated that the inhibition of proteasome activity by NC-Cu mixtures might be mainly through copper-proteasome direct binding. The cytotoxicity and apoptosis induced by NC-Cu mixtures is associated with proteasome inhibition but not ROS induction.
Proteasome-inhibitory activity of NC-Cu(I) may involve direct interaction with proteasome
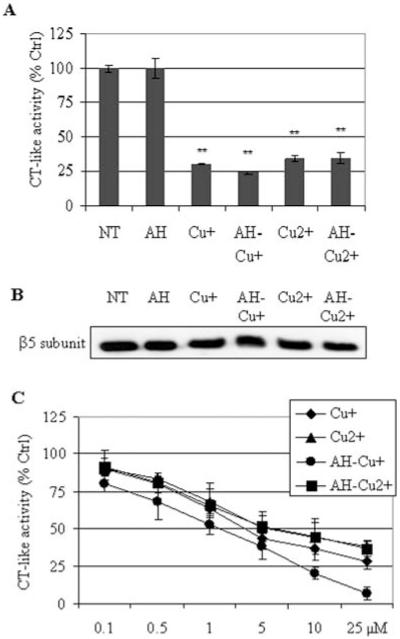
We hypothesized that Cu(I)-proteasome interaction may be responsible for the observed proteasome inhibition. To test this hypothesis, a purified human 20S proteasome was pre-incubated with CuCl or CuCl2 for 12 h. The free copper was removed by multiple wash-spins in an Ultrafree-MC centrifugal filter unit (MW cut-off 10 kDa). The 20S proteasome solution from each sample was then incubated with fluorogenic peptide substrates Suc-LLVY-AMC for 2 h, followed by measurement of proteasome activity. The data showed that both CuCl and CuCl2 could inhibit proteasome activity and consistently Cu(I) was more potent than Cu(II) (Fig. 5A). The results of Western blot in Fig. 5B confirmed that the amount of purified proteasome ß5 protein in each sample was very similar. A similar experiment using different concentrations of CuCl or CuCl2 showed that direct inhibition of purified 20S proteasome by Cu(I) or Cu(II) was dose-dependent (Fig. 5C).
Figure 5.
Cu(I) and Cu(II) inhibit the activity of purified 20S proteasome probably by direct interaction. (A) The purified 20S proteasome was incubated with 10 μM of CuCl (Cu+) or CuCl2 (Cu2+), or their mixtures with AH for overnight at 4°C. After removing the unbound copper from the proteasome by multiple wash-spins in an Ultrafree-MC centrifugal filter unit (MW cut-off 10 kDa), 2 μl of the 20S proteasome solution from each sample was incubated with fluorogenic peptide substrates Suc-LLVY-AMC for 2 h, followed by measurement of CT-like activity. (B) The amount of proteasome ß5 subunit in each sample was measured by Western blot analysis. (C) Purified 20S proteasome was pre-incubated with different concentrations of Cu(I), Cu(II) or their mixtures with AH for 4 h at 37°C and then post-incubated with fluorogenic peptide substrates Suc-LLVY-AMC for 2 h, followed by measurement of CT-like activity. **P<0.01, bars, SD, mean of three experiments.
Discussion
After revealing that copper is one of the pro-cancer factors and can boost angiogenesis in tumor tissue, scientists have tested whether reduction of copper level in cancer patients can present an anti-cancer effect. Results from clinical trials showed significant anti-angiogenic and antitumor effect of tetrathiomolybdate (TM), a copper-chelator, in the malignant pleural mesothelioma and the advanced kidney cancer patients (26,27). Our group has examined a series of copper-binding compounds and tested their antitumor activity. We found that among these compounds disulfiram and clioquinol possess proteasome-inhibitory and apoptosis-inducing properties when they are mixed with copper (10,14). We also examined tumor tissue from mice by X-ray fluorescence microscopy and found that Cu(I) was predominant in the growing tumor tissue while increased Cu(II) was found in tumors treated with clioquinol (28). Therefore, a new question has been raised: which oxidation states of copper have anti-tumor activity when reacted with a copper ligand.
In the current study we mixed neocuproine (NC) a copper ligand with CuCl or CuCl2. We also used ascorbic acid (AH) as a reducer in NC-CuCl mixture to protect Cu(I) from oxidation. First, we tested NC-Cu(I) and NC-Cu(II) for their proteasome-inhibitory property. In the in vitro assay by using purified 20S proteasome we found that both copper mixtures inhibited proteasome activity and NC-Cu(I) was slightly more potent than NC-Cu(II). We further tested the proteasomal-inhibitory effect of both mixtures on cultured human breast cancer cells and the results were consistent with the findings in vitro. It has been reported that the majority of copper in cells and tissues is Cu(I) and that copper is reduced to Cu(I), when transported into a cell, by the copper transporter on cell membrane (29).
Generation of ROS and its involvement in induction of various cancer cell apoptosis has been well investigated (6,30). We hypothesized that Cu(I) and Cu(II) may have different effects on ROS generation and thereby on cell apoptosis. Our findings showed that indeed NC-Cu(I) was a potent ROS inducer, compared with NC-Cu(II). However, the generation of ROS by NC-Cu(I) was not needed for its proteasome-inhibitory activity since ROS scavengers could block ROS generation produced by NC-Cu(I) but could not overcome its proteasomal-inhibitory activity. One of the possible mechanisms for NC-Cu(I) to inhibit proteasome activity may be direct binding of Cu(I) to the active site of proteasome ß5 subunit. Further experiments are needed to confirm whether ROS is involved in the cell death induced by copper complexes.
In summary, we have provided evidence that trace metal copper in different oxidation states have different biological effects in cancer cells. Copper plays an important role as pro-cancer factor in tumor tissues especially in tumor angiogenesis and metastasis. Our findings demonstrated that when complexed with a copper ligand, copper preferentially in its reduced status could be converted to a proteasome inhibitor and an apoptosis inducer. The findings not only help us to further understand the biological effects of copper in different oxidation/reduction states in cells, but also provide a rationale for synthesis of copper-containing complexes as potential anticancer reagents.
Acknowledgements
This research is partially supported by Karmanos Cancer Institute of Wayne State University (to Q.P. Dou), National Cancer Institute/NIH (1R01CA120009, 3R01CA120009-04S1, and 1R21CA139386 to Q.P. Dou), and the National Cancer Institute/NIH Cancer Center Support Grant (to Karmanos Cancer Institute). This research was also partially supported by a Scholarship from the Chinese Scholarship Council (to Y. Xiao and X. Zhang). We thank Ms. Carol Maconochie for critical reading of the manuscript.
References
- 1.Aggett PJ, Fairweather-Tait S. Adaptation to high and low copper intakes: its relevance to estimated safe and adequate daily dietary intakes. Am J Clin Nutr. 1998;67:S1061–S1063. doi: 10.1093/ajcn/67.5.1061. [DOI] [PubMed] [Google Scholar]
- 2.Labbe S, Thiele DJ. Pipes and wiring: the regulation of copper uptake and distribution in yeast. Trends Microbiol. 1999;7:500–505. doi: 10.1016/s0966-842x(99)01638-8. [DOI] [PubMed] [Google Scholar]
- 3.Fox SB, Gasparini G, Harris AL. Angiogenesis: pathological, prognostic, and growth-factor pathways and their link to trial design and anticancer drugs. Lancet Oncol. 2001;2:278–289. doi: 10.1016/S1470-2045(00)00323-5. [DOI] [PubMed] [Google Scholar]
- 4.Gourley M, Williamson JS. Angiogenesis: new targets for the development of anticancer chemotherapies. Curr Pharm Des. 2000;6:417–439. doi: 10.2174/1381612003400867. [DOI] [PubMed] [Google Scholar]
- 5.Ryan CJ, Wilding G. Angiogenesis inhibitors. New agents in cancer therapy. Drugs Aging. 2000;17:249–255. doi: 10.2165/00002512-200017040-00001. [DOI] [PubMed] [Google Scholar]
- 6.Huang YL, Sheu JY, Lin TH. Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem. 1999;32:131–136. doi: 10.1016/s0009-9120(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 7.Nayak SB, Bhat VR, Upadhyay D, Udupa SL. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian J Physiol Pharmacol. 2003;47:108–110. [PubMed] [Google Scholar]
- 8.Rizk SL, Sky-Peck HH. Comparison between concentrations of trace elements in normal and neoplastic human breast tissue. Cancer Res. 1984;44:5390–5394. [PubMed] [Google Scholar]
- 9.Turecky L, Kalina P, Uhlikova E, Namerova S, Krizko J. Serum ceruloplasmin and copper levels in patients with primary brain tumors. Klin Wochenschr. 1984;62:187–189. doi: 10.1007/BF01731643. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Peng F, Cui QC, et al. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Front Biosci. 2005;10:2932–2939. doi: 10.2741/1749. [DOI] [PubMed] [Google Scholar]
- 12.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem Pharmacol. 2004;67:1139–1151. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Rahman A, Shahabuddin, Hadi SM, Parish JH, Ainley K. Strand scission in DNA induced by quercetin and Cu(II): role of Cu(I) and oxygen free radicals. Carcinogenesis. 1989;10:1833–1839. doi: 10.1093/carcin/10.10.1833. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Cui QC, Yang H, et al. Clioquinol, a therapeutic agent for Alzheimer’s disease, has proteasome-inhibitory, androgen receptor-suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts. Cancer Res. 2007;67:1636–1644. doi: 10.1158/0008-5472.CAN-06-3546. [DOI] [PubMed] [Google Scholar]
- 16.Zhu BZ, Chevion M. Copper-mediated toxicity of 2,4,5-trichlorophenol: biphasic effect of the copper(I)-specific chelator neocuproine. Arch Biochem Biophys. 2000;380:267–273. doi: 10.1006/abbi.2000.1919. [DOI] [PubMed] [Google Scholar]
- 17.Bhat SH, Azmi AS, Hanif S, Hadi SM. Ascorbic acid mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties. Int J Biochem Cell Biol. 2006;38:2074–2081. doi: 10.1016/j.biocel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 18.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 19.Lopes UG, Erhardt P, Yao R, Cooper GM. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 20.Chen SH, Lin JK, Liu SH, Liang YC, Lin-Shiau SY. Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol Sci. 2008;102:138–149. doi: 10.1093/toxsci/kfm292. [DOI] [PubMed] [Google Scholar]
- 21.Khanna S, Roy S, Parinandi NL, Maurer M, Sen CK. Characterization of the potent neuroprotective properties of the natural vitamin E alpha-tocotrienol. J Neurochem. 2006;98:1474–1486. doi: 10.1111/j.1471-4159.2006.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto T, Ikeda Y, Teramoto A. Protective effect of L-histidine (singlet oxygen scavenger) on transient forebrain ischemia in the rat. No To Shinkei. 1997;49:612–618. [PubMed] [Google Scholar]
- 24.Lawton DG, Gorman C, Lowe PN. Small G protein characterisation by isothermal titration calorimetry. Biochem Soc Trans. 1997;25:S510. doi: 10.1042/bst025510s. [DOI] [PubMed] [Google Scholar]
- 25.Rattan AK, Arad Y. Temporal and kinetic determinants of the inhibition of LDL oxidation by N-acetylcysteine (NAC) Atherosclerosis. 1998;138:319–327. doi: 10.1016/s0021-9150(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 26.Pass HI, Brewer GJ, Dick R, Carbone M, Merajver S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann Thorac Surg. 2008;86:383–389. doi: 10.1016/j.athoracsur.2008.03.016. discussion 390. [DOI] [PubMed] [Google Scholar]
- 27.Redman BG, Esper P, Pan Q, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 28.Barrea RA, Chen D, Irving TC, Dou QP. Synchrotron X-ray imaging reveals a correlation of tumor copper speciation with Clioquinol’s anticancer activity. J Cell Biochem. 2009;108:96–105. doi: 10.1002/jcb.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izeradjene K, Douglas L, Tillman DM, Delaney AB, Houghton JA. Reactive oxygen species regulate caspase activation in tumor necrosis factor-related apoptosis-inducing ligand-resistant human colon carcinoma cell lines. Cancer Res. 2005;65:7436–7445. doi: 10.1158/0008-5472.CAN-04-2628. [DOI] [PubMed] [Google Scholar]