INTRODUCTION
Hip fracture is the most significant complication of osteoporosis in terms of mortality, loss of independent function and health and social costs. While areal BMD is a strong predictor of future hip fracture (1), differences in the shape of the proximal femur are also predictive of both mechanical strength and fracture risk (2). Though while many determinants of areal BMD have been established, there are relatively few predictors of proximal femoral geometry (3). There is a growing body of evidence that environmental influences during periods of early development (4) lead to persisting changes in adult bone mass (5,6) and risk of fracture (7) that appear to be independent of genetic factors (8). We have previously reported that weight during first year of life predicted bioengineering estimates of proximal strength using two dimensional DXA measurements (9). However, such two dimensional assessments are prone to rotational artifact and in addition DXA is unable to distinguish the different contributions from the trabecular vs. cortical compartments. Here we report a novel association between self-reported weight at birth with geometric three dimensional measurements of the proximal femur, both cortical and trabecular, in a well defined cohort of elderly men.
METHODS
Study population
The Osteoporotic Fractures in Men (MrOS) Study enrolled 5,995 men at six U.S. clinical centers (Birmingham AL, Minneapolis MN, Palo Alto CA, Pittsburgh PA, Portland OR and San Diego CA) from March 2000 through April 2002. Eligible participants were community-dwelling, at least 65 years of age, able to walk without assistance from another person, and had not had bilateral hip replacement surgery.
All MrOS participants completed the baseline self-administered questionnaire and attended the baseline visit when skeletal, anthropometric and other measures were obtained. Details of the MrOS recruitment and study design have been published elsewhere (11). The institutional review board at each site approved the study protocol and written informed consent was obtained from all participants.
At the baseline visit participants were asked “Approximately how much did you weigh at birth?” and allowed to select 6 possible responses: less than 3 pounds; 3 to 4.9 pounds; 5 to 6.9 pounds; 7.0 to 8.9 pounds; more than 9 pounds and don't know. Of baseline recorded data, we used measured height (measured with a wall-mounted Harpenden stadiometer (Holtain Ltd, DyFed, United Kingdom)) and body weight (measured with a balance beam scale (except for the MrOS Portland site, which used a digital scale) and quantitative computed tomography (QCT) measurements of femoral neck axis length, cross sectional area and volumetric BMD (vBMD) which are described in detail below.
Femoral geometry and vBMD assessment
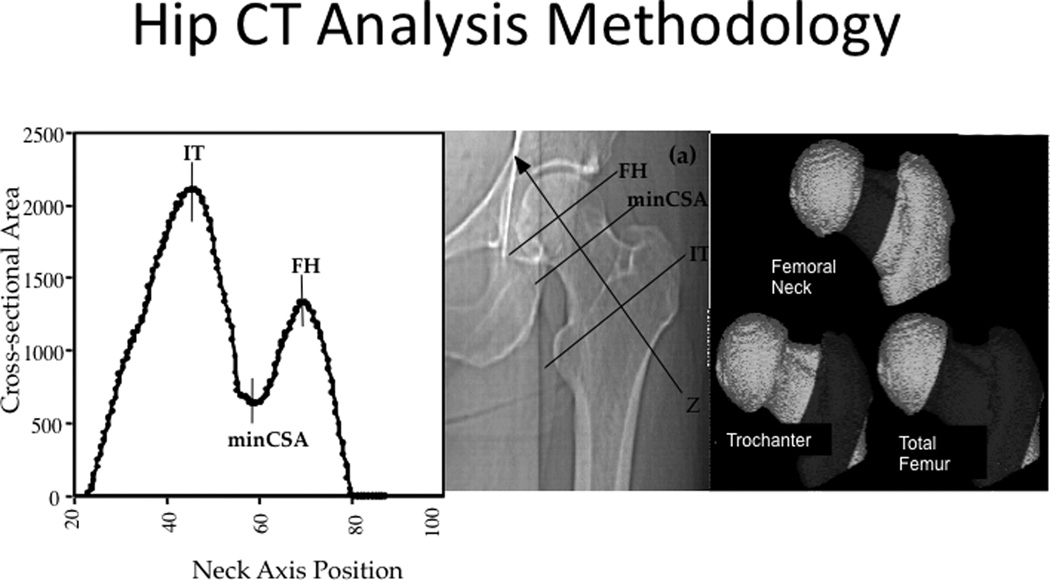
QCT measurements were acquired for the first 650 participants recruited at each clinic site and all men from minority backgrounds. The imaging of the proximal femur was performed with 80 kVp, 280 mA, 3-mm slice thickness, 512 × 512 matrix in spiral reconstruction mode. Each scan was initiated at the proximal femoral head and continued to 3.5 cm below the lesser trochanter. Calibration standards were made with known hydroxyapatite concentrations (150, 75, and 0 mg/cm3; Image Analysis)(10). The method of analyzing the QCT has been described previously (11) and is summarized here. The left hip image was reformatted in the neutral axis and the periosteal border was delineated using a region growing algorithm. The maximum and minimum cross sectional areas (CSA) of the femoral neck (FN) were identified (Figure 1) and the neck axis length was measured as the distance between the minimum and maximum CSA. The FN region was defined as 75% of the distance from the minimum to the maximum CSA. The Intertrochanteric (IT) region was defined medially by the lateral aspect of the FN region and laterally at a distance equal to 45% of the computed neck length lateral to the position of the maximum CSA. The shaft region was defined as 3 cm below the lesser trochanter in a 10 mm transverse section. A peel retaining the same shape as the periosteal boundary was then performed to define a sub cortical margin.
Figure 1.
Regions of femoral neck, trochanter and total hip using QCT images reformatted along the neutral axis.
The following measurements were then derived at each of the FN, IT and shaft regions: the integral volume (equal to volume of tissue within the periosteal boundary); the cortical volume and volumetric density was measured using all voxels of the outer peel of bone with a vBMD of ≥ 0.35 g/cm3; the marrow volume was calculated as the integral volume subtracted by cortical volume; the percentage of cortical vs. marrow volume was calculated using the integral volume as the denominator. To compute the neck-shaft angle, we first computed the projection of the femoral neck axis on the coronal plane, and calculated the angle of the neck axis with respect to the axis of the CT imaging table (the longitudinal axis of the patient). The angle of the femoral shaft was taken by reconstructing three 1-mm thick transverse sections perpendicular to the neutral axis of the femoral shaft. The most inferior section was the most inferior cross-section of the scan. The other two sections were located 10mm and 20 mm superior to the most inferior cross-section. The periosteal boundary of the shaft was computed in each cross-section, and from that boundary, we computed the area-weighted centroid of the shaft bone. A three dimensional shaft axis was obtained by fitting a line through the three centroids. We calculated the projection of the 3D shaft axis onto the coronal plane, and then computed the angle of that axis with respect to the axis of the CT imaging table. The neck-shaft angle was taken as the difference of the neck angle and the shaft angle with respect to the table axis.
To prevent extreme observations biasing the estimates, we report estimates from distributions trimmed at 3 SD (11).
Statistical analysis
Due to small sample numbers we pooled the lowest three birth weight (BW) groups together to <7 lbs. We compared men with BW < 7 pounds (lower BW; n=501) and ≥ 9 pounds (higher BW; n=262) with those weighing 7–8.9 pounds (medium BW; n= 1,068) as the referent group using T-tests to compare differences in the study subject characteristics and linear regression models adjusting for current age, height and body mass index (BMI). Due to BW differences between ethnic groups and the small numbers of non-whites in the higher BW group, we analyzed QCT data for Caucasian/White subjects and included all non Caucasians into a non-white group separately.
RESULTS
Of the 3786 men with proximal femur QCT data, 1831 (48.3%) reported a BW; 14 men reported a BW of less than 3 pounds, 44 men 3 to 4.9 pounds, 443 men 5 to 6.9 pounds; 1,068 men 7.0 to 8.9 pounds; 262 men more than 9 pounds. The mean age of the 1831 men who had both BW and QCT measurements was 73 yrs (SD 5.9). The characteristics at baseline characteristics are shown in Table 1. Those without a self reported birth weight (n=1,270) were significantly older, taller and had a lower BMI than the referent middle BW group. They were also significantly more likely to be non-white.
Table 1.
Characteristics of MrOS participants who had a QCT of the pelvis at baseline by self-reported birth weight
| Self-reported BW | ||||
|---|---|---|---|---|
| Low | Middle | High | Not reported | |
| < 7 lbs | 7–9 lbs | > 9 lbs | ||
| N | 501 | 1068 | 262 | 1,270 |
| Age (yrs) | 73.0 (5.7) | 72.9 (5.5) | 73.5 (5.9) | 74.1 (6.1)*** |
| Non White (%) | 14.4%*** | 8.3% | 7.6% | 16.8%*** |
| Previous Fragility fracture | 14.0% | 12.4% | 12.6% | 11.8% |
| Hip fracture | 1.8% | 1.8% | 1.9% | 2.2% |
| Height (cm) | 172 (7)*** | 175 (7) | 177 (7)*** | 173 (7)*** |
| BMI (kg/m2) | 26.8 (3.5)*** | 27.5 (3.7) | 28.1 (3.5)* | 27.0 (3.8)** |
Legend: Mean (SD) shown, significance
<0.05,
<0.01,
<0.001 compared with referent middle BW group.
White men
Compared with middle BW men, lower BW men were significantly shorter [172.5 (6.6) cm vs 174.7 (6.5) cm, p<0.001] and higher BW men were significantly taller [176.6 (6.8) cm, p<0.001]. Similar relationships were seen with adult BMI and lower BW [26.8 (3.5) kg/m2 vs middle BW: 27.52 (3.7) kg/m2 vs, p<0.001] and higer BW [28.1 (3.6) g/m2, p=0.02]. The QCT measurements between BW groups and the associations, unadjusted and after adjusting for adult height and BMI are shown in Table 2.
Table 2.
QCT measurement differences between birth weight categories compared to middle birth weight in Caucasian subjects
| Lower birth weight (n= 495) |
Higher birth weight (n=305) |
||||
|---|---|---|---|---|---|
| Site | Geometric Measurement | Crude | Adjusted | Crude | Adjusted |
| Femoral Neck | Axis length | 0.11 [0.0001,0.22] | 0.16 [0.04,0.27] | 0.16 [0.02,0.30] | 0.11 [−0.03,0.25] |
| Neck Shaft Angle | 0.07 [−0.05,0.19] | 0.05 [−0.07,0.17] | −0.02 [−0.17,0.13] | 0.01 [−0.14,0.16] | |
| Cross-Sectional Area | −0.26 [−0.37,−0.14] | −0.14 [−0.24,−0.03] | 0.28 [0.15,0.42] | 0.11 [−0.01,0.23] | |
| Integral Volume | −0.05 [−0.16,0.07] | 0.05 [−0.06,0.16] | 0.24 [0.10,0.38] | 0.14 [0.01,0.27] | |
| Cortical Volume | −0.01 [−0.12,0.10] | 0.08 [−0.02,0.19] | 0.17 [0.03,0.31] | 0.07 [−0.07,0.20] | |
| Marrow Volume | −0.06 [−0.17,0.05] | 0.01 [−0.10,0.13] | 0.23 [0.09,0.37] | 0.15 [0.01,0.29] | |
| Trochanter | Cross-Sectional Area | −0.36 [−0.47,−0.25] | −0.20 [−0.30,−0.10] | 0.28 [0.15,0.42] | 0.11 [−0.01,0.23] |
| Integral Volume | −0.16 [−0.27,−0.05] | −0.03 [−0.13,0.07] | 0.31 [0.17,0.45] | 0.17 [0.04,0.30] | |
| Cortical Volume | −0.05 [−0.16,0.06] | 0.06 [−0.05,0.17] | 0.22 [0.08,0.36] | 0.11 [−0.03,0.24] | |
| Marrow Volume | −0.20 [−0.31,−0.09] | −0.08 [−0.18,0.02] | 0.30 [0.16,0.44] | 0.18 [0.05,0.30] | |
| Femoral Shaft | Cross-sectional Area | −0.14 [−0.26,−0.02] | 0.02 [−0.09,0.13] | 0.31 [0.16,0.46] | 0.17 [0.04,0.31] |
| Cortical Thickness | −0.13 [−0.25,−0.01] | −0.07 [−0.19,0.05] | 0.05 [−0.09,0.20] | −0.01 [−0.16,0.13] | |
| Total hip | Integral vBMD | −0.14 [−0.25,−0.03] | −0.03 [−0.12,0.07] | 0.25 [0.12,0.38] | 0.11 [−0.007,0.23] |
| Cortical vBMD | 0.01 [−0.10,0.12] | 0.05 [−0.06,0.15] | −0.01 [−0.14,0.13] | −0.05 [−0.18,0.08] | |
Legend: Standardized beta coefficient (95% CI) from linear regression models with middle birth weight group as the referent group both crude and adjusted for height and BMI.
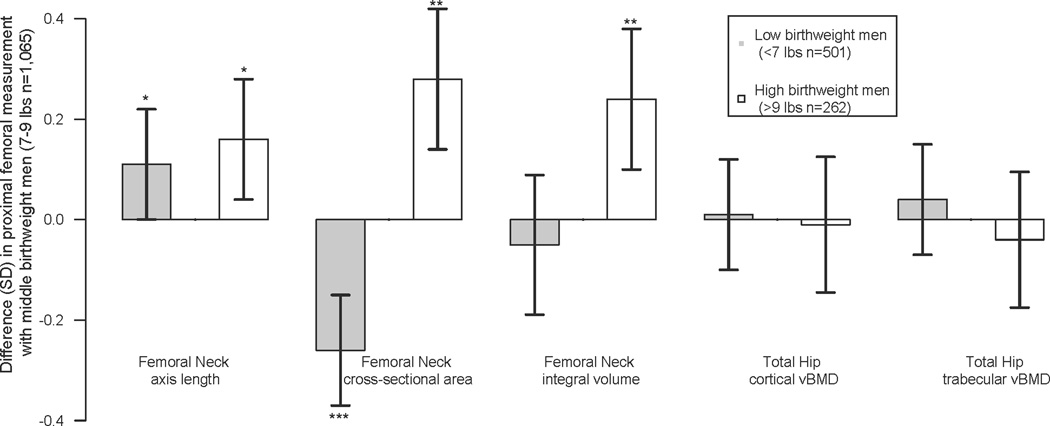
There were significant (p<0.05) differences in adult femoral geometry by reported BW (Figure 2) and the difference in CSA and length were not concordant by birth weight group. At the femoral neck site, compared with middle BW men, higher BW men had a longer femoral neck length (+0.16SD; p=0.028). Higher BW men also had a wider CSA (+0.24 SD, p=0.001) than middle BW men. Associations between higher birth weight and adult femoral neck length and CSA were no longer statistically significant after adjusting for height and BMI (p=0.12 and 0.09 respectively). As anticipated, higher BW men had a larger integral neck volume (+0.25 SD, p=0.001) compared with middle birth weight men. Of the two compartments, the increase in integral volume appeared to be due to increases in both marrow volume (+0.23 SD, p=0.001) and cortical volume (+0.17 SD, p=0.018).
Figure 2.
Differences in adult proximal femoral geometry and volumetric density in low and high birth weight Caucasian men compared the to the referent middle birth weight men
Legend: SD change (95% CI) for Femoral neck (FN) geometry and total hip (TOT HIP) volumetric BMD of the cortex (CvBMD) and marrow (MvBMD) compartments.
* <0.05; ** <0.01, ***<0.001
In contrast, lower BW men had narrower femoral cross sectional area (CSA) (−0.24 SD, p<0.001) even after adjusting for height and BMI (p=0.01) but unexpectedly had a longer femoral neck for their height (+0.11 SD, p=0.05), and this resulted in no overall difference in the integral volume at the femoral neck (−0.04 SD, p=0.41).
At the trochanteric sites the higher BW men had a larger CSA (0.28 SD, p<0.001), integral volume (0.31 SD, p<0.001), cortical volume (0.22 SD, p=0.002) and marrow volume (0.30 SD, p<0.001). The lower BW men had a narrow CSA (−0.36 SD, p<0.001) and reduced integral volume (−0.16 SD, p=0.005) which appeared to be due to reductions in marrow volume (−0.19 SD, p<0.001) but not cortical (−0.05 SD, p=0.38) volume. The femoral shaft below the lesser trochanter was wider in the higher BW men (0.31 SD, p=0.004) with no difference in cortical thickness (0.05 SD, p=0.48), while the lower BW had similar shaft diameters (−0.14 SD, p=0.081) to the referent group, with a reduction in cortical thickness (−0.13 SD, p=0.041).
Finally, neither cortical nor trabecular vBMD at the femoral neck, trochanter or shaft regions differed (p>0.05) by self reported BW.
Non White men
Compared with middle BW men, higher BW men were taller (177.0 ± 8.7 vs 172.6 ± 7.0; p=0.02) but did not differ by BMI (28.9 ± 3.8 vs middle BW: 27.7 ± 3.4; p=0.17). Lower BW men were shorter (169.0 ± 6.6; p=0.002), and were non-significantly thinner (BMI: 26.5 ± 4.0; p=0.051), when compared to middle BW subjects.
The number of men in the higher BW group was small (n=20) and there were no statistically significant differences in hip proximal geometry of bone density. Lower BW men had non-significantly lower femoral neck cross-sectional area (−0.28 SD, p=0.08), with no difference in femoral length observed (−0.0 SD, p=0.9). At trochanteric sites, they had significantly lower cross-sectional area (−0.51 SD, p<0.001). Trochanter integral volume was reduced in lower BW men (−0.33 SD, p=0.04), with non-significant reduction in cortical (−0.16, p=0.32) and significant reduction in marrow volume (−0.38 SD, p=0.02). At the femoral shaft, cross-sectional area (−0.53 SD, p=0.003) and cortical thickness (−0.29 SD, p=0.07) were smaller amongst the lower BW subjects. After adjusting for current height and BMI the above associations between birth weight and hip geometry were no longer statistically significant (p≥0.1). Cortical or trabecular vBMD did not significantly differ by BW group.
DISCUSSION
We report here the novel association between reduced birth weight and reductions of both femoral neck cross sectional area and width with an absolute increase in neck length in Caucasian men. Both of these geometric features have been associated with an increased risk of future fracture. In previous reports from the SOF study, increased neck length was associated with an increase in hip fracture risk (2). Our previous study of the association between femoral shape and early life growth also demonstrated a positive association between birth size and femoral cross sectional area but no association with neck length (9). The explanation for this discrepancy may result from the error of measuring neck length from the two dimensional DXA scans, and not measuring neck length along the longest axis of the neck. The benefit of the three dimensional QCT images is that each image was reformatted along the longest axis, and this provides a more accurate true neck length measurement. The lack of association in Caucasian men between self-reported BW and measures of volumetric bone density is consistent with previous studies using a UK community based cohort of men of similar age and range of birth weights who had peripheral QCT of the tibia and radius (12). However in that study, increasing birth weight was positively associated with not only area but also length at both tibia and radius.
The mechanisms underlying this association could either represent unknown confounders, such as a genetic polymorphisms that predict or may be associated with both hip geometry and birth size. At present, birth weight is thought to have a heritability of only 25 – 40% (13). In addition, within healthy identical twins, discordance in adult bone mass was found to be significantly associated with birth weight suggesting a relatively small genetic component compared with environmental influences during early life (8). It is thought that a low self reported birth weight is associated with poor environment in utero for the developing fetus. Studies from the Europe (5,6,14,15), America (16) and Australia (17) have supported the hypothesis that size in early life is associated with adult bone mass. Cohorts from Finland have shown these associations also apply to fracture risk (7,18). Mother-offspring studies have confirmed these observations, highlighting the importance of the maternal environment on the bone mass of the child. The "developmental origins" hypothesis proposes that in utero environment triggers specific fetal adaptations such as changes in metabolism, alterations in hormone production and in tissue-sensitivity to hormones, as well as the relative growth rates of different anatomical compartments and structures (4). These adaptations lead to persisting changes in the body’s structure, physiology and metabolism, and are often associated with reduction in fetal growth rate and small body size at birth. Subsequent environmental exposures during infancy, childhood and adult life may modify or condition the later risk of disease. One of the candidate factors is vitamin D. Studies have shown a positive association between maternal vitamin D status in late pregnancy and differences in the geometry of the fetal distal femur as measured by ultrasound (19), the subsequent bone mass of the child (20); further neonatal rickets maps ecologically to rates of adult hip fracture (21). Insufficient maternal vitamin D status result in changes in placental calcium transport and the fetal skeletal development. (22).
This study has a number of strengths including a sophisticated QCT measurements of the proximal femur and a very well characterized cohort of elderly men. However, there are also some potential limitations including the use of self-reported vs. documented birth weight. It is to be expected the recalled birth weight is less accurate than actual birth weight abstracted from clinical records, however studies comparing birth weight with phenotypes that develop in later adult life, rarely have the availability of such records. While some have questioned the value of self reported birth weight (23), other studies have shown the self-reported birth weight is accurate (24,25). Self-reported birth weight has been used to explore associations between the intrauterine environment and adult life in a wide range of adult diseases. The question whether self reported birth weight leads to random versus systematic error has been less well described. The methods for collecting birth weight in the study by Allen et al.(26) were similar to MrOS in that the participants were allowed to answer “Don’t know” rather than offer a guess, which may further reduce the error. In the study by Allen et al (26), the independent predictors of an accurate self-reported birth weight within 4 oz were found to be being younger at time of self report and being the eldest child. Also from this study, being able to report birth weight was determined by having a living mother and a lower recorded birth weight and while non-manual working was a predictor of birth weight reporting in uni-variate models, it was no longer significant in the multivariate model. Finally, for statistical reasons we grouped those with birth weight of < 7 lbs and we recognize this threshold is not considered low birth weight in current clinical medicine (27). However, few low birth weight babies born during the period of time which the men of this cohort were born would have survived (28). A survival bias due to the selective survival of healthier low birth weight men would lead to an underestimation of the association between birth weight and bone size and, given the dramatically increased survival of such low birth weight men currently, the association of low birth weight and poorer health outcomes may be magnified in the future (29).
In this study, we have demonstrated that while low size at birth is associated with an increase in femoral neck length with a decreased neck area compared to middle birth size in men. Additional research describing the relationship between the early developmental factors and lifetime fracture risk should be pursued; such research may inform primary preventative strategies for fracture prevention.
Acknowledgements
NIHR Musculoskeletal BRU, University of Oxford for supporting MK Javaid; NIH grants K24-AR04884, AR45654, Ar052000
References
- 1.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18(11):1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner KG, Cummings SR, Nevitt MC, Pressman A, Jergas M, Genant HK. Hip axis length and osteoporotic fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1995;10(3):506–508. doi: 10.1002/jbmr.5650100323. [DOI] [PubMed] [Google Scholar]
- 3.Rivadeneira F, Houwing-Duistermaat JJ, Beck TJ, Janssen JA, Hofman A, Pols HA, Van Duijn CM, Uitterlinden AG. The influence of an insulin-like growth factor I gene promoter polymorphism on hip bone geometry and the risk of nonvertebral fracture in the elderly: the Rotterdam Study. J Bone Miner Res. 2004;19(8):1280–1290. doi: 10.1359/JBMR.040405. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, Barker D. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res. 1995;10(6):940–947. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- 6.Cooper C, Fall C, Egger P, Hobbs R, Eastell R, Barker D. Growth in infancy and bone mass in later life. Ann Rheum Dis. 1997;56(1):17–21. doi: 10.1136/ard.56.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int. 2001;12(8):623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 8.Antoniades L, MacGregor AJ, Andrew T, Spector TD. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology (Oxford) 2003;42(6):791–796. doi: 10.1093/rheumatology/keg227. [DOI] [PubMed] [Google Scholar]
- 9.Javaid MK, Lekamwasam S, Clark J, Dennison EM, Syddall HE, Loveridge N, Reeve J, Beck TJ, Cooper C. Infant growth influences proximal femoral geometry in adulthood. J Bone Miner Res. 2006;21(4):508–512. doi: 10.1359/jbmr.051214. [DOI] [PubMed] [Google Scholar]
- 10.Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23(8):1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall LM, Lang TF, Lambert LC, Zmuda JM, Ensrud KE, Orwoll ES. Dimensions and volumetric BMD of the proximal femur and their relation to age among older U.S. men. J Bone Miner Res. 2006;21(8):1197–1206. doi: 10.1359/jbmr.060506. [DOI] [PubMed] [Google Scholar]
- 12.Oliver H, Jameson KA, Sayer AA, Cooper C, Dennison EM. Growth in early life predicts bone strength in late adulthood: The Hertfordshire Cohort Study. Bone. 2007;41(3):400–405. doi: 10.1016/j.bone.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000;107(3):375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 14.Laitinen J, Kiukaanniemi K, Heikkinen J, Koiranen M, Nieminen P, Sovio U, Keinanen-Kiukaanniemi S, Jarvelin MR. Body size from birth to adulthood and bone mineral content and density at 31 years of age: results from the northern Finland 1966 birth cohort study. Osteoporos Int. 2005;16(11):1417–1424. doi: 10.1007/s00198-005-1857-9. [DOI] [PubMed] [Google Scholar]
- 15.te Velde SJ, Twisk JW, van Mechelen W, Kemper HC. Birth weight and musculoskeletal health in 36-year-old men and women: results from the Amsterdam Growth and Health Longitudinal Study. Osteoporos Int. 2004;15(5):382–388. doi: 10.1007/s00198-003-1554-5. [DOI] [PubMed] [Google Scholar]
- 16.Yarbrough DE, Barrett-Connor E, Morton DJ. Birth weight as a predictor of adult bone mass in postmenopausal women: the Rancho Bernardo Study. Osteoporos Int. 2000;11(7):626–630. doi: 10.1007/s001980070085. [DOI] [PubMed] [Google Scholar]
- 17.Jones G, Dwyer T. Birth weight, birth length, and bone density in prepubertal children: evidence for an association that may be mediated by genetic factors. Calcif Tissue Int. 2000;67(4):304–308. doi: 10.1007/s002230001148. [DOI] [PubMed] [Google Scholar]
- 18.Javaid MK, Eriksson JG, Kajantie E, Forsen T, Osmond C, Barker DJP, Cooper C. Growth in childhood predicts hip fracture risk in later life. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1224-3. [DOI] [PubMed] [Google Scholar]
- 19.Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K. Low Maternal Vitamin D Status and Fetal Bone Development: Cohort Study. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 21.Paulozzi LJ. Does inadequate diet during childhood explain the higher high fracture rates in the Southern United States? Osteoporos Int. 21(3):417–423. doi: 10.1007/s00198-009-0997-8. [DOI] [PubMed] [Google Scholar]
- 22.Martin R, Harvey NC, Crozier SR, Poole JR, Javaid MK, Dennison EM, Inskip HM, Hanson M, Godfrey KM, Cooper C, Lewis R. Placental calcium transporter (PMCA3) gene expression predicts intrauterine bone mineral accrual. Bone. 2007;40(5):1203–1208. doi: 10.1016/j.bone.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 23.Andersson SW, Niklasson A, Lapidus L, Hallberg L, Bengtsson C, Hulthen L. Poor agreement between self-reported birth weight and birth weight from original records in adult women. Am J Epidemiol. 2000;152(7):609–616. doi: 10.1093/aje/152.7.609. [DOI] [PubMed] [Google Scholar]
- 24.Troy LM, Michels KB, Hunter DJ, Spiegelman D, Manson JE, Colditz GA, Stampfer MJ, Willett WC. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int J Epidemiol. 1996;25(1):122–127. doi: 10.1093/ije/25.1.122. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson M, Williams MA, White E, Daling JR, Holt VL, Malone KE, Self SG, Moore DE. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147(2):136–140. doi: 10.1093/oxfordjournals.aje.a009425. [DOI] [PubMed] [Google Scholar]
- 26.Allen DS, Ellison GT, dos Santos Silva I, De Stavola BL, Fentiman IS. Determinants of the availability and accuracy of self-reported birth weight in middle-aged and elderly women. Am J Epidemiol. 2002;155(4):379–384. doi: 10.1093/aje/155.4.379. [DOI] [PubMed] [Google Scholar]
- 27.WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldenberg RL, Culhane JF. Low birth weight in the United States. Am J Clin Nutr. 2007;85(2):584S–590S. doi: 10.1093/ajcn/85.2.584S. [DOI] [PubMed] [Google Scholar]
- 29.Erickson K, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Birth weight and cognitive performance in older women: the Rancho Bernardo study. Arch Womens Ment Health. 13(2):141–146. doi: 10.1007/s00737-009-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]