Abstract
The ventral tegmental area (VTA) plays an important role in reward and motivational processes that facilitate the development of drug addiction. Glutamatergic inputs into the VTA contribute to dopamine (DA) neuronal activation related to reward and response-initiating effects in drug abuse. Previous investigations indicate that alpha1-adrenoreceptors (α1-AR) are primarily localized at presynaptic elements in the ventral midbrain. Studies from several brain regions have shown that presynaptic α1-AR activation enhance glutamate release. Therefore, we hypothesized that glutamate released onto VTA-DA neurons is modulated by pre-synaptic α1-AR. Recordings were obtained from putative VTA-DA cells of male Sprague-Dawley rats (28–50 days postnatal) using voltage clamp techniques. Phenylephrine (10 µM) and methoxamine (80 µM), both α1-AR agonists, increased AMPA receptor-mediated excitatory postsynaptic currents (EPSCs) amplitude evoked by electrical stimulation of afferent fibers (p<0.05). This effect was blocked by the α1-AR antagonist prazosin (1 µM). Phenylephrine decreased the paired-pulse ratio and increased spontaneous EPSCs frequencies but not their amplitudes suggesting a presynaptic locus of action. No changes in miniature EPSCs (0.5 µM TTX) were observed after phenylephrine’s application which suggest that α1-AR effect was action potential dependent. Normal extra- and intracellular Ca2+ concentration seems necessary for the α1-AR effect since phenylephrine in low Ca2+ ACSF and depletion of intracellular Ca2+ stores with thapsigargin (10 µM) failed to increase the AMPA EPSCs amplitude . Chelerythrine (1 µM, PKC inhibitor) but not Rp-cAMPS (11 µM, PKA inhibitor) blocked the α1-AR activation effect on AMPA EPSCs, indicating that a PKC intracellular pathway is required. These results demonstrated that presynaptic α1-ARs activation modulates glutamatergic inputs that affect VTA-DA neurons excitability. α1-ARs action might be heterosynaptically localized at glutamatergic fibers terminating onto VTA-DA neurons. It is suggested that drug-induced changes in α1-AR could be part to the neuroadaptations occurring in the mesocorticolimbic circuitry during the addiction process.
Keywords: Dopamine neurons, Glutamate release, alpha1-adrenoreceptos, Ventral Tegmental Area
Introduction
Dopamine (DA) neurons projecting from the ventral tegmental area (VTA) to cortical and ventral forebrain structures are the source of the so called mesocorticolimbic system (Dahlstrom and Fuxe, 1964, Ungerstedt, 1971, Lammel et al., 2011). Activation of VTA DA neurons has been implicated in motivated behaviors as well as in mediating the reinforcing actions of drugs of abuse (Schultz and Schultz, 2002, Kauer, 2004, Grace et al., 2007). Extensive evidence demonstrates a noradrenergic innervation and synaptic modulation of VTA DA neurons. Several tracing studies have shown noradrenergic inputs from locus coeruleus and other pontine structures making extrasynaptic and synaptic contacts into VTA DA neurons (Jones et al., 1977, Liprando et al., 2004, Geisler and Zahm, 2005, Mejias-Aponte et al., 2009).
The presence of alpha-1 adrenoreceptors (α1-ARs) has been demonstrated in the VTA area (Greene et al., 2005). α1-ARs were found to be primarily localized at pre-synaptic elements in the VTA region (Rommelfanger et al., 2009). α1-ARs are Gq-protein-coupled receptors that participate in the development of stressors and anxiety responses, and in addiction-related behaviors (Cecchi et al., 2002, Hague et al., 2003, Jimenez-Rivera et al., 2006, Greenwell et al., 2009). Noradrenergic inputs facilitate VTA DA neuronal transmission and induce changes in burst firing via α1-ARs (Grenhoff et al., 1993, Grenhoff and Svensson, 1993, Grenhoff et al., 1995, Paladini and Williams, 2004). Since bursting activity in the VTA is dependent on glutamate transmission (Lodge and Grace, 2006) it is logical to postulate that α1-ARs might be important in the presynaptic control of glutamate release onto VTA cells. However, to date, there is no direct evidence demonstrating such mechanism.
Diverse brain nuclei send glutamatergic inputs to the VTA. The prefrontal cortex (PFC), seems to be the primary source of glutamatergic innervation of this structure (Carr and Sesack, 2000, Omelchenko and Sesack, 2007). However, the lateral hypothalamus, medial habenula, bed nucleus of the stria terminalis (BNST), laterodorsal and pedunculo pontine tegmental nuclei also send glutamate inputs into the VTA (Murase et al., 1993, Charara et al., 1996, Georges and Aston-Jones, 2001, 2002, Omelchenko and Sesack, 2005, Lodge and Grace, 2006, Gao et al., 2007, Geisler et al., 2007, Omelchenko and Sesack, 2007, 2009). Recently, the presence of local glutamatergic neurons has been demonstrated (Yamaguchi et al., 2007, Dobi et al., 2010).
The VTA contain mainly dopaminergic neurons that are under the influence of these glutamatergic inputs (Cameron et al., 1997). Electrophysiological studies have demonstrated that glutamate-induced excitation can change cell firing, pacemaker and bursting activity in VTA DA neurons (Murase et al., 1993, Georges and Aston-Jones, 2002, Lodge and Grace, 2006). Glutamate release onto this area contribute to changes in cognition, stress and reward, and at the same time, are critical to the effects induced by drugs of abuse (Ungless et al., 2001, Saal et al., 2003, You et al., 2007, Wise, 2009).Thus, the role of glutamate inputs onto VTA DA neurons are of utmost importance in controlling the normal physiological and pathophysiological activity of these cells.
Here we demonstrate that activation of presynaptic α1-ARs facilitates glutamate release onto VTA DA neurons. This effect involves a selective activation of the PKC intracellular pathway.
EXPERIMENTAL PROCEDURES
Animals and Slice preparation
Sprague-Dawley male rats between 28 and 50 postnatal days were anesthetized with a 90 mg/kg i.p. chloral-hydrate injection of (Sigma, St Louis, MO, USA) and their brains rapidly removed. Sagital slices (220 µM) containing the VTA were cut using a vibratome (VT1000S, Leica, Germany). The rat midbrain was placed into an ice-cold oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): 127 NaCl; 2.5 KCl; 1.25 NaH2PO4 ; 25 NaHCO3 ; 2 CaCl2 ; 1 MgCl2 ; 25 D(+)-glucose, and saturated with 95%O2–5%CO2 gas mixture to a pH=7.4. Slices were transferred to an intermediate chamber and incubated at 32°C in the same solution for 45-min before the initiation of electrophysiological recordings. MK-801 (10 µM, Tocris, Ellisville, MO, USA) was added to the incubation solutions to block N-methyl-D-aspartate (NMDA)-mediated excitotoxicity. All animal procedures conformed to the guidelines approved by the Institutional Animal Care and Use Committee of the Medical Sciences Campus - University of Puerto
Electrophysiological recordings
VTA slices were totally submerged in a recording chamber (500 µL) with ACSF superfusion at 1–2 ml/min at 32°C. Picrotoxin (100 µM, Sigma, St Louis, MO, USA) was added to the ACSF during recording procedures to block GABAA receptor- mediated inhibitory postsynaptic currents (IPSC’s). Whole cell voltage clamp recordings were obtained from visually identified neurons in the VTA using an infrared microscope with differential interference contrast (DIC) optics, (BX51WI Olympus, Japan). Recordings were acquired through data acquisition software (pClamp 10, Molecular Devices, Sunnyvale, CA). All recordings were performed in putative DA neurons identified by the presence of a large hyperpolarization-activated cation current (Ih >200 pA), evoked by 1-s hyperpolarizing steps from −60 to −130 mV. Ih is present in about 84% VTA DA neurons and VTA GABA cells do not express this conductance (Margolis et al., 2006). Therefore, the contribution of non-dopaminergic neurons to the experimental recording performed in this study is likely to be not significant. Whole-cell voltage clamp recordings were made at a holding potential of −70 mV unless indicated. Borosilicate glass patch pipettes (O.D.1.5 mm,I.D:1,0 mm WPI, Sarasota, FL) were pulled to a final resistance of 3–6 MΩ and were filled with (in mM): 115 CH3SO4K (Methyl potassium sulfate); 20 KCl ; 1.5 MgCl2; 5 HEPES; 1 EGTA; 2 ATP; 0.2 GTP; 10 Creatine Phosphate (CP); pH 7.25, 290 mOsm. (Na) GTP, (Mg) ATP and (Na) CP were added fresh daily. Data were collected through a Multiclamp 700B amplifier (Axon Instruments, Foster City, CA, USA), filtered at 1 kHz, digitized at 5 kHz using Digidata 1440A (Axon Instruments, Foster City, CA, USA), and stored in a PC computer and analyzed off line using GraphPad Prism 5 (GraphPad Software, Inc) software. Pipette’s Liquid junction potential was offset compensated using standard Multiclamp 700B circuitry. The seal’s quality used was typically 4–6 GO. Series resistance was not compensated and was monitored during the entire experiment. Data was discarded if changes of more than 15% occurred.
Recording of Synaptic Currents
A bipolar stainless steel stimulating electrode (FHC Inc, Bowdoin, ME) was placed approximately 100 µm rostral to the recording electrode and used to stimulate afferents at 0.1Hz by applying a brief (400 µs; low pass filter 1 KHz, digitized 5 KHz) electrical pulse (100–300 µA). AMPA-mediated Excitatory Post-Synaptic Currents (EPSCs) were recorded at −70 mV. All EPSCs shown in figures are averages of 5 current traces for the treatment under inspection. AMPA EPSCs’ amplitudes were calculated by taking a 1 ms window around the peak of the EPSC and comparing this to a 5 ms window immediate before the stimulation artifact. Peak EPSCs’ amplitudes were average during control recordings. This value was used to normalize control and treatment recordings. This procedure allowed expressing data as percentages of the control condition for appropriate statistical comparisons. Paired stimuli were given with a 50 ms interstimulus interval. Paired Pulse Ratio (PPR) was calculated as the ratio of the first and second EPSC’s. Spontaneous AMPA EPSC’s (sEPSCs) and miniature AMPA EPSC’s (mEPSC) were recorded. Tetrodotoxin (TTX, 0.5 µM, Alomone Laboratories, Jerusalem, Israel) was added to the ACSF to isolate mEPSCs that are not dependent on presynaptic action potentials. sEPSCs and mEPSCs were recorded at −70 mV, filtered at 1 kHz and digitized at 5 kHz using pCLAMP 10 software (Molecular Devices, Sunnyvale, CA, USA). For a given cell, sEPSCs and mEPSCs were collected (1 sweep for each condition, 3min/sweep) for a control and phenylephrine’s period. The recorded sEPSCs and mEPSCs were analyzed afterward using Mini Analysis program 6.0.7 (Synaptosoft Inc.Decatur, GA). Detection criteria were set at >6 pA, <1.3 ms rise time, and <0.1 ms decay time. The choice of this cutoff amplitude for acceptance of sEPSCs and mEPSCs was made to obtain a high signal-to-noise ratio. Then, each event was also visually inspected to prevent noise disturbance of the analysis.
Data Analysis
All data are presented as mean ± SEM. Statistical significance was assessed using Student’s paired t-test or One-Way ANOVA with Newman-Keuls as post hoc analysis, except when examining the significance of horizontal shifts to the cumulative probability distribution plots obtained from single cell recordings. For the latter case we used the Kolmogorov–Smirnov (K–S) test. P values are reported throughout the text and significance was set as p<0.05.
Drugs
Pharmacological agents used in this study: Phenylephrine hydrochloride ([R]-[–]-1-[3-Hydroxyphenyl]-2-methylaminoethanol hydrochloride), methoxamine hydrochloride (α-[1-Aminoethyl]-2,5-dimethoxybenzyl alcohol hydrochloride), prazosin hydrochloride (1-[4-Amino-6,7-dimethoxy-2-quinazolinyl]-4-[2-furanylcarbonyl]piperazine hydrochloride), chelerythrine chloride (1,2-dimethoxy-12-methyl[1,3]benzodioxolo[5,6-c]phenanth ridinium chloride tetrodotoxin citrate), Rp-cAMPS (Rp-adenosine 3’,5’-cyclic monophosphorothioate triethylammonium salt hydrate) were purchased from Sigma (St Louis, MO, USA). Thapsigargin (3S,3aR,4S,6S,6AR,7S,8S,9bS)-6- (Acetyloxy)-2,3,3a,4,5,6,6a,7,8,9b-decahydro-3,3a-dihydroxy-3,6,9-trimethyl-8-[[(2Z)-2-methyl-1-oxo-2-butenyl]oxy]-2-oxo-4-(1-oxobutoxy)azuleno[4,5-b]furan-7-yl octanoate) was purchased from Tocris (Ballwin, MO). All substances were diluted in fresh ACSF until completely mixed, then transferred to separate graduated reservoirs connected to the chamber. The effects on current amplitude were measured within 5 min after the beginning of the flow (1–2 ml/min).
RESULTS
In order to asses if the activation of α1-ARs alters glutamatergic transmission onto VTA DA neurons whole cell recordings of AMPA EPSCs were performed on putative DA neurons identified by the presence of large Ih (> 200 pA), slow spontaneous activity and relatively regular inter-spike intervals (Grace and Bunney, 1983, Grace and Onn, 1989). We confirmed that this evoked current was due to AMPA receptor activation by blocking the response with the potent and selective AMPA receptor antagonist NBQX (30 M, data not shown). EPSCs were electrically evoked in the presence of the GABAA receptor antagonist, picrotoxin (100 µM).
α1-AR activation increases excitatory synaptic transmission at VTA DA cells
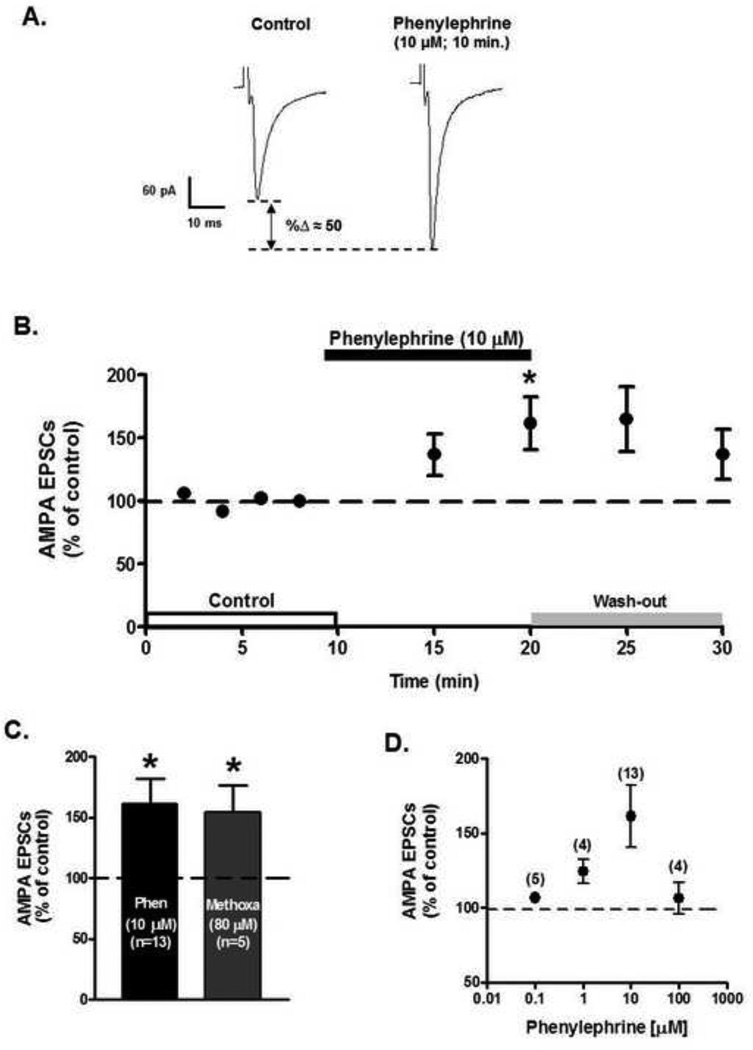
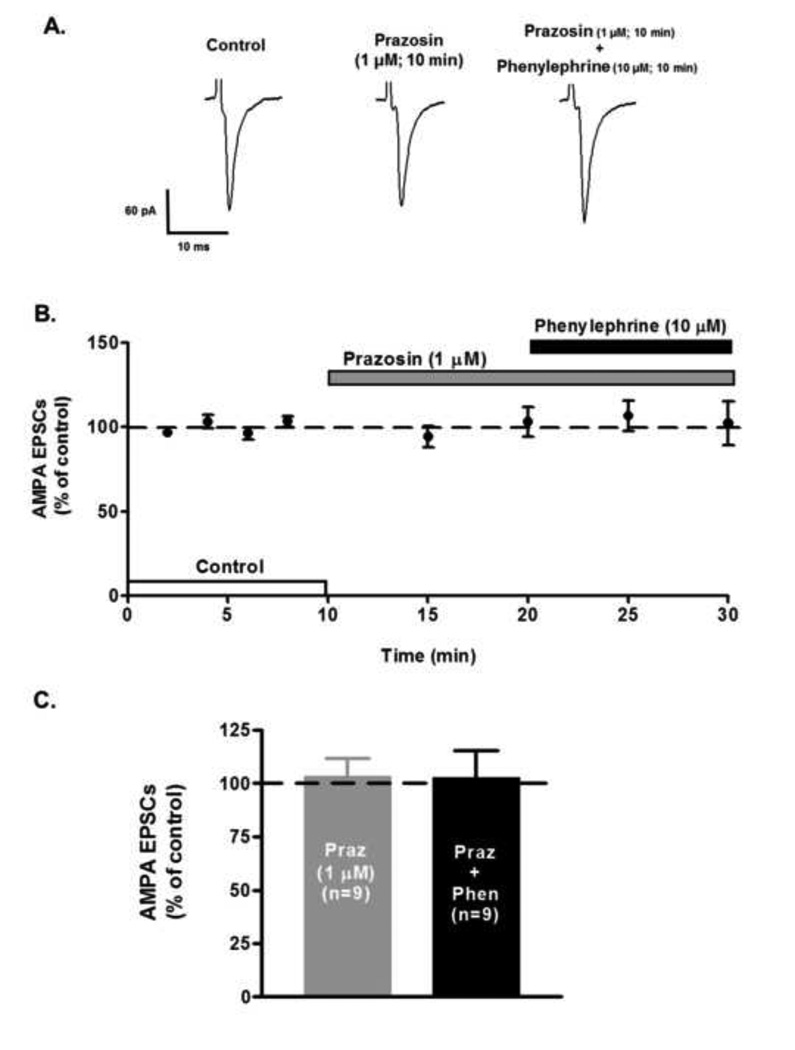
Bath application of the selective α1-AR agonist, phenylephrine (10 µM), during 10 minutes, but not for 5 minutes, increased AMPA EPSCs peak amplitude to 161.4 ± 20.7% of control (n=13; ANOVA F2,36 =4.08, p<0.05, Fig. 1B). The phenylephrine’s effect on AMPA EPSCs had a slow wash-out (10 minutes). Similarly, superfusion of methoxamine (80 µM), another α1-AR agonist, significantly increased AMPA EPSCs peak amplitude to 154.57 ± 22.14% after 10 minutes’ superfusion (n=5; ANOVA F2,12 = 5.43, p<0.05; Fig 1C). Phenylephrine’s excitatory action was dose-dependent over the concentration of 0.1 and 100 µM (Fig. 1D). Phenylephrine’s effect on AMPA EPSCs recordings was blocked in the presence of the α1-AR antagonist, prazosin (1 µM) (n=9; ANOVA F4,40 = 0.28, p=0.88, Fig. 2) strongly suggesting that activation of α1-ARs increases AMPA receptor-mediated synaptic transmission at VTA DA neurons.
Figure 1.
Bath application of phenylephrine (10 µM) increases AMPA EPSCs amplitude in putative VTA DA neurons. A. Representative recordings from a neuron illustrating that phenylephrine superfusion (10 µM, 10 min), induces a significant increment in AMPA EPSCs amplitude in a putative VTA DA neuron voltage clamped at −70 mV. B. Summary time course of the effects of phenylephrine bath application on AMPA EPSCs amplitude recorded from 13 putative VTA DA neurons at 8 min of control (2 min intervals), 5 and 10 min phenylephrine (10 µM) and 5 and 10 min washout. A 10 min phenylephrine application increases the AMPA EPSCs amplitude. Slow washout of phenylephrine’s response was observed. C. Bar graph showing that on average phenylephrine (n=13) and methoxamine (n=5) application resulted in an ~ 50% increase in AMPA EPSCs amplitude. No significant differences were observed between controls (100.1 ± 2.3%). D. Dose-response curve of phenylephrine’s effect on AMPA EPSCs. Phenylephrine-induced increase was dose-dependent over the concentration range of 0.1 – 100 µM. At 100 µM it seems to desensitize the receptor. Parenthesis indicates numbers of cells in each experiment. *p < 0.05, One-way ANOVA, Newman-Keuls post-hoc.
Figure 2.
α1-AR antagonist, prazosin, blocks phenylephrine’s effect on AMPA EPSCs. A. Representative recordings from a neuron showing that the α1-AR antagonist prazosin (1 µM) completely abolishes the phenylephrine-induced increase in AMPA EPSCs amplitude. B. Summary time course illustrating prazosin antagonistic actions. Note that prazosin alone has no effect on EPSCs amplitude. Each point represents the mean ± SEM of n=9. C. Prazosin superfusion leaves AMPA EPSCs amplitude unaltered. Phenylephrine (10 µM) and prazosin (1 µM) co-superfusion prevents phenylephrine-induced increase of AMPA EPSCs amplitude.
α1-AR activation facilitates presynaptic glutamate release
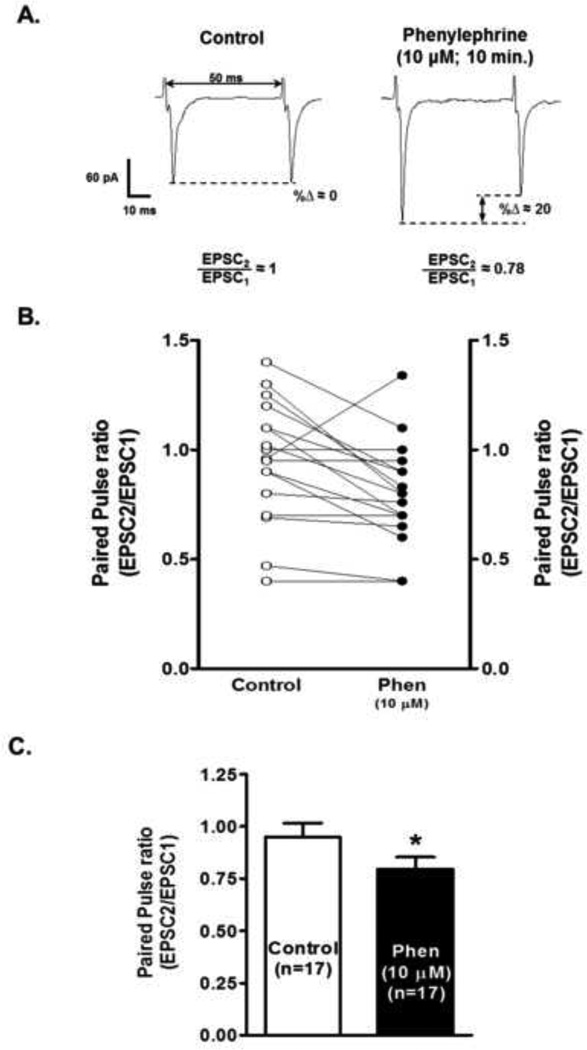
The α1-AR effect could be attributable to an increase in presynaptic glutamate release or to an upregulation of postsynaptic AMPA receptor function. In order to provide evidence that the observed increases in AMPA EPSC peak amplitude were presynaptic, we examined the EPSC peak amplitudes evoked by two closely spaced stimuli. The analysis of their ratio, the paired-pulse ratio (PPR=EPSC2/EPSC1), has been established as a sensitive measure of glutamate release probability (Manabe et al., 1993). Fig. 3 A shows the decrease in PPR sample recordings after 10 µM phenylephrine’s superfusion. The PPR decreased from 0.95 ± 0.06 to 0.8 ± 0.05 after 10 minutes phenylephrine’s application (n=17; paired t-test, p<0.05, Figs. 3 B and C). These results clearly supports that activation of α1-ARs evokes EPSCs through a presynaptic increase in the release probability of glutamate.
Figure 3.
Phenylephrine decreases paired-pulse ratio in putative VTA DA neurons. A. Representative recordings from a neuron illustrating that phenylephrine super fusion (10 µM, 10 min application), induces a significant decrease in paired pulse ratio (PPR = EPSC2/EPSC1) in a putative VTA DA cell voltage clamp at a-70 mV. Note that the time interval between consecutive EPSCs is 50 ms. B. Graph summarizing the change in PPR of 17 cells after 10 min phenylephrine (10 µM) bath application. C. Bar graph showing that phenylephrine-induced decrease in PPR is statistically significant. *p < 0.05, paired t-test.
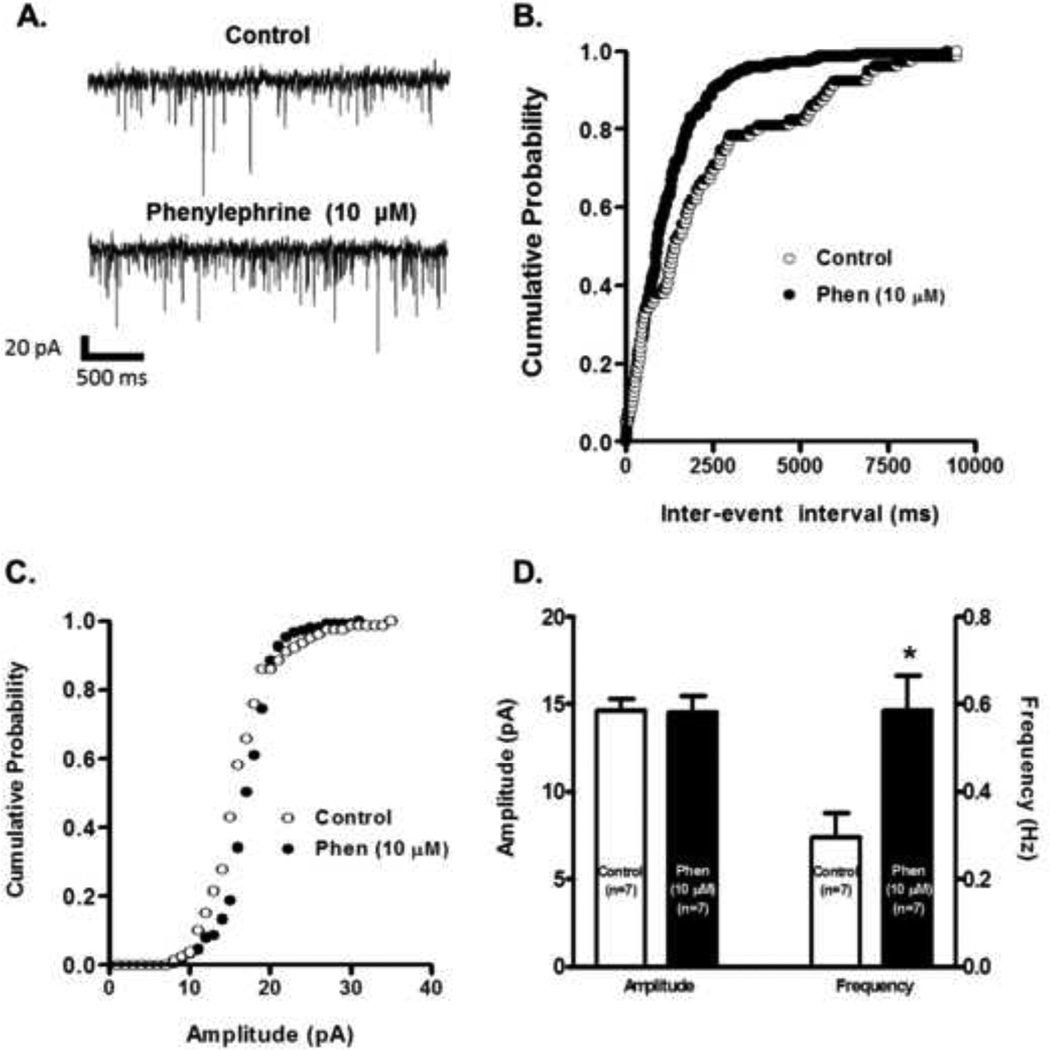
To confirm if the observed effects were mediated by a presynaptic mechanism we also recorded spontaneous EPSCs (sEPSCs). Sample recordings showed that phenylephrine’s superfusion increased the frequency of sEPSC 10 minutes after its application (Fig. 4 A). Fig. 4 B shows that after 10 minutes of phenylephrine’s administration there is an increase in the probability of shorter intervals between successive sEPSCs without changes in the amplitude distribution. Phenylephrine significantly increased the frequency distribution after 10 minutes bath application (from 0.30 ± 0.05 to 0.59 ± 0.08 Hz, n=7, paired t-test p<0.05, Fig. 4 D), but not the amplitude (from 14.65 ± 0.63 to 14.51 ± 0.95 pA, n=7, paired t-test p=0.76, Fig. 4D) compared to control recordings. This finding supports the notion that α1-ARs activation is enhancing glutamate’s presynaptic release onto VTA DA neurons.
Figure 4.
Phenylephrine increases the frequency but not the amplitude of sEPSCs. A. Representative recordings from a cell illustrating that phenylephrine’s application (10 µM) increases sEPSC frequency but not the amplitude. The cell was voltage clamped at −70 mV during the recordings. B. Phenylephrine’s superfusion (10 µM, 10 min application) results in a shift to the left of the inter-event interval cumulative distribution (K-S, p<0.05) implying an increase in sEPSCs frequency. The plot was constructed from the cell used in part A. C. Phenylephrine does not shift the sEPSCs amplitude cumulative distribution. The plot was constructed from the cell used in A. D. Summary graph showing that phenylephrine increased the mean frequency without affecting the mean amplitude of sEPSCs (n=7). *p < 0.05, paired t-test.
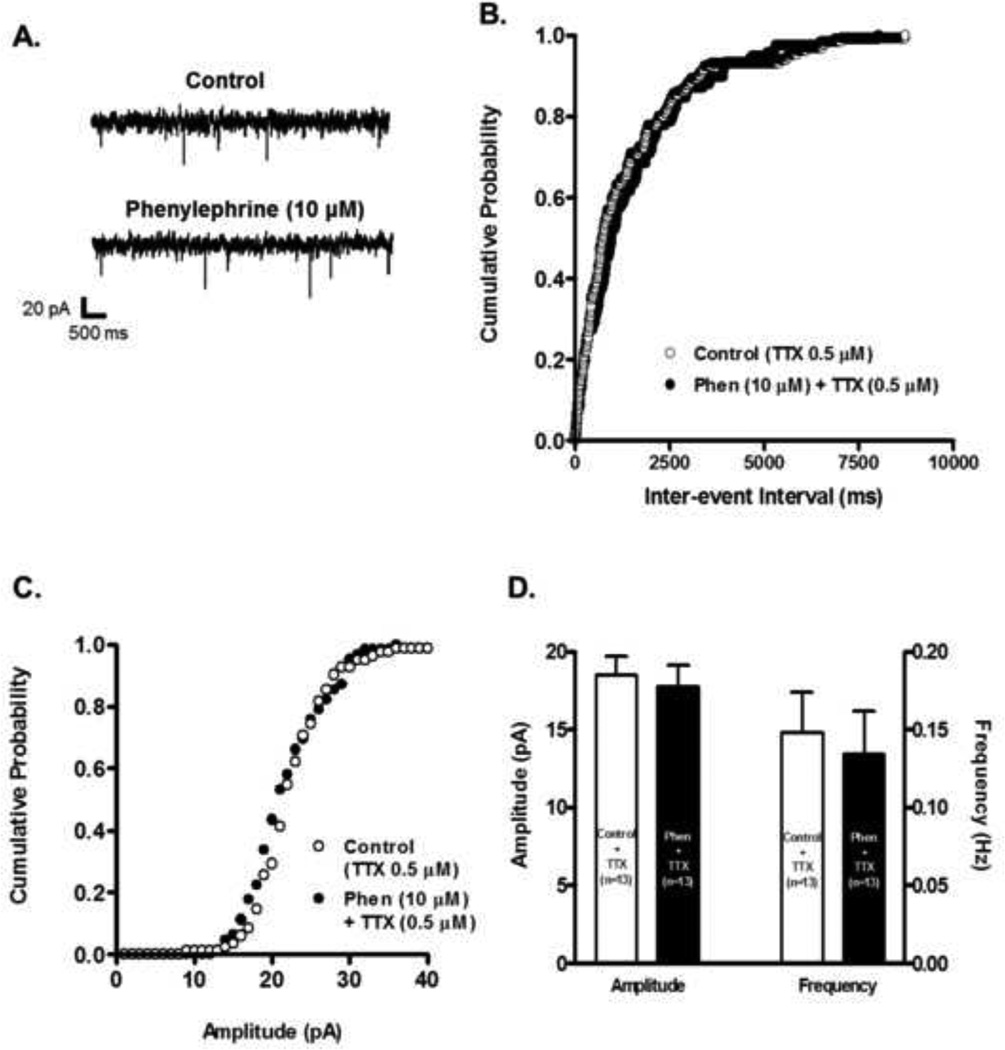
Another way in which we determined if modulation of VTA DA neurons’ electrical activity upon α1-AR activation depends on a presynaptic mechanism was to measure changes in miniature EPSCs (mEPSCs) in the presence of tetrodotoxin (TTX, 0.5 µM). Under these conditions, sample recordings before and after 10 minutes phenylephrine’s application showed that there was no change in mEPSC frequency or amplitude (Fig. 5 A). Estimates of inter-event intervals probability and amplitude distribution demonstrated nosignificant changes after 10 minutes phenylephrine’s administration (Figs. 5 B and C, respectively). Population analysis illustrate that phenylephrine did not produce significant changes in frequency (from 0.14 ± 0.02 to 0.13 ± 0.02 Hz, n=13, paired t-test p=0.53, Fig. 5 D), or amplitude (from 18.50 ± 1.16 to 17.74 ± 1.4 pA, n=13, paired t-test p=0.51, Fig. 5D) compared to control recordings. Altogether, these results support the notion that α1-AR effect on glutamate release onto VTA DA neurons is action potential-dependent.
Figure 5.
Phenylephrine had no effect on mEPSCs frequency or amplitude. A. Representative recordings from a neuron illustrating that in presence of TTX (0.5 µM) phenylephrine (10 µM) does not change the frequency or amplitude of mEPSCs. The neuron was voltage clamped at −70 mV during the recordings. B. Phenylephrine’s superfusion (10 µM, 10 min. application) does not shift the inter-event interval cumulative distribution of mEPSCs. The plot was constructed from the cell used in A. C. Phenylephrine does not shift the amplitude cumulative distribution of mEPSCs. The plot was constructed from the cell used in A. D. Summary graph showing that phenylephrine’s superfusion in the presence of TTX had no effect on frequency or amplitude mEPSCs (n=13).
α1-AR activation modulatory effect needs both: extracellular and intracellular calcium
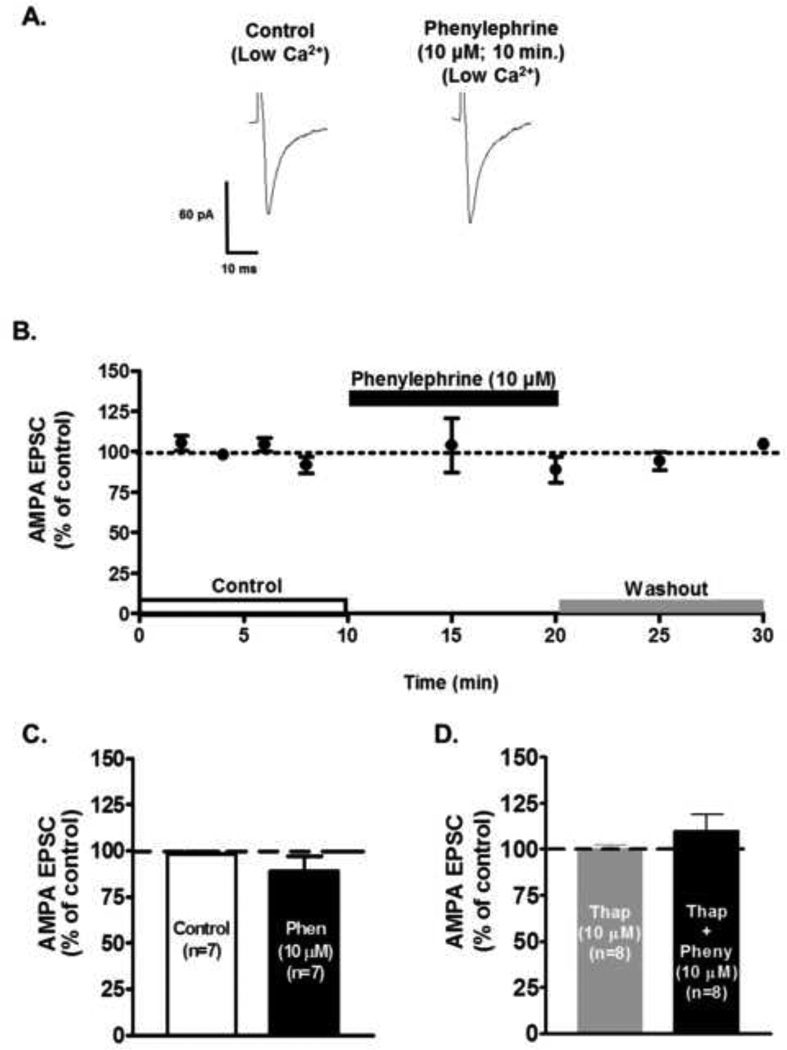
Activation of α1-AR increases the intracellular calcium concentration by liberation of calcium stores from the endoplasmic reticulum and through the phosphorylation of plasma membrane calcium channels (Tanaka and Nishizuka, 1994). To test if extracellular calcium was involved in the observed α1-ARs effects, we reduced the calcium concentration of the ACSF from 2.0 mM to 1.0 mM to limit the calcium influx to the presynaptic terminal. Fig. 6A shows sample traces of AMPA EPSCs before and after phenylephrine’s administration in the presence of a reduced calcium concentration. Phenylephrine in 1.0 mM calcium failed to increase the AMPA EPSC (88.76 ± 8.11% of control, n=6, ANOVA F2,15 = 0.51, p=0.60, Fig. 6 B), confirming the importance of extracellular calcium on the modulatory effect of α1-AR activation.
Figure 6.
The α1-AR mediated increase in AMPA EPSCs amplitude depends on extra- and intracellular calcium. A. Representative recordings from a neuron showing that low calcium ACSF (1 mM), completely prevents the phenylephrine-induced increase of AMPA EPSCs in VTA DA cells. B. Summary time course of 7 neurons illustrating the population effects. C. Bar graph showing a summary of the effects of low Ca2+ ACSF on phenylephrine-induce increase of AMPA EPSCs (10 µM, 10 min). D. Bar graph showing that bath application of phenylephrine (10 µM, 10 min) did not increase evoked AMPA EPSCs in the presence of thapsigargin (Thap, n=8). Slices were pre-incubated with 10 µM thapsigargin for 30 minutes before recordings were conducted.
We also, explored whether intracellular calcium stores participate in the α1-ARs mediated effect. Brain slices were pre-incubated in thapsigargin (10 µM; 30 min) which depletes intracellular Ca2+ stores by blocking the ATPase that mediates Ca2+ uptake (Thastrup et al., 1990, Mathew and Hablitz, 2008).Thapsigargin blocked phenylephrine’s effect on AMPA EPSC (109.84 ± 9.10% of control, n=8, ANOVA F2,21 = 0.85, p=0.43, Fig. 6 D). These results suggest that intracellular Ca2+ stores contribute to phenylephrine’s facilitation of evoked AMPA EPSCs.
α1-AR-mediated increase in glutamate release through PKC pathway
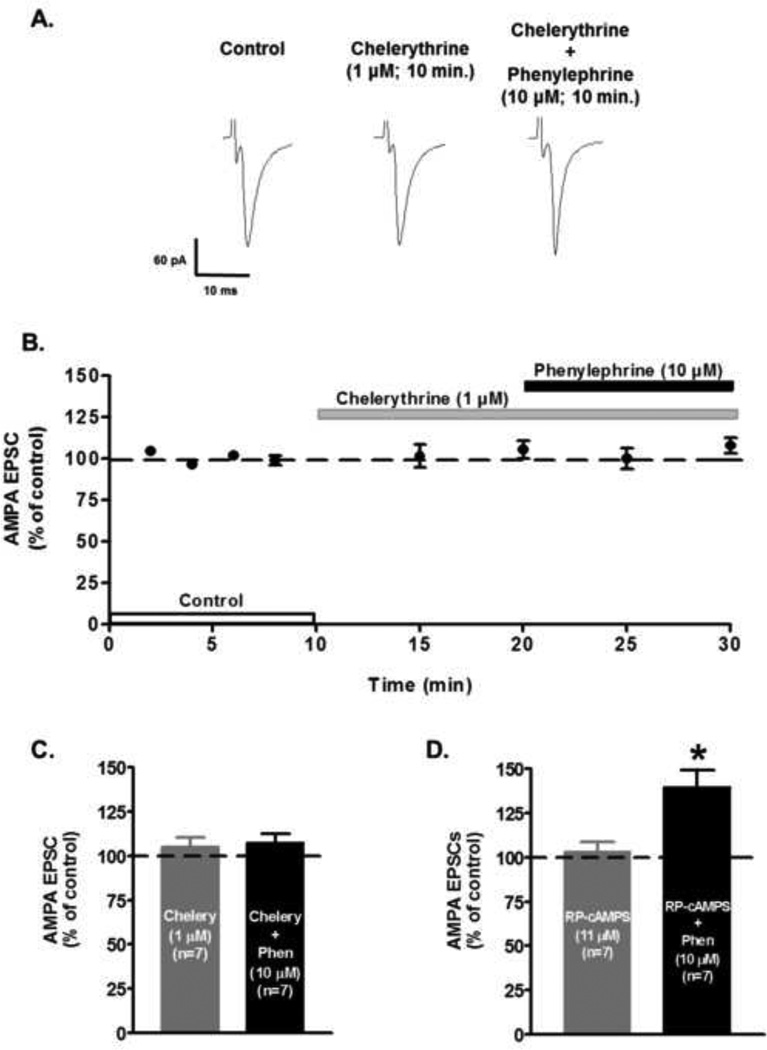
The effect of α1-AR on glutamate release could be mediated by direct coupling via protein kinase C (PKC). PKC has been shown to be a downstream element in the intracellular signaling pathway of α1-AR activation (Tamura et al., 1993). To explore whether PKC is required for the α1-AR mediated presynaptic glutamate release, slices were superfused with the membrane-permeable PKC inhibitor chelerythrine (1 µM). Phenylephrine’s application after 10 minutes of chelerythrine superfusion failed to induce changes on AMPA EPSCs peak amplitude (control: 100.6 ± 0.57%; chelerythrine 10 min: 110.0 ± 6.59%; phenylephrine 10 min: 10.9.6 ± 6.69%; n=7; ANOVA F4,30 = 0.37, p=0.79, Fig. 7). Moreover, superfusion of 11 µM Rp-cAMPS, an specific inhibitor of protein kinase A (PKA), did not block the action of the α1-AR agonist (control 103.1 ± 2.5; Rp-cAMPS 103.3 ± 5.4%; Rp-cAMPS + phenylephrine 139.6 ± 9.3%; n=7; ANOVA F4,30 = 7.10, p<0.01, Fig. 7D). These results confirm that α1-AR activation requires the involvement of a PKC pathway to increase glutamate release onto VTA DA neurons.
Figure 7.
Involvement of the intracellular PKC signaling in the α1-AR mediated increase in AMPA EPSCs amplitude. A. Representative recordings from a neuron showing that chelerythrine (1 µM), a selective PKC inhibitor, completely prevents the phenylephrine-induced increase of AMPA EPSCs in VTA DA neurons. B. Summary time course illustrating that PKC selective inhibitor chelerythrine (1 µM) blocks the phenylephrine-induced increase of AMPA EPSCs amplitude. Note that chelerythrine alone has no effect on EPSCs amplitude. Each point represents n=7 ± SEM. C. Bar graph showing that application of Chelerythrine (Chelery), completely abolished phenylephrine’s effect (10 µM; 10 min application) on AMPA EPSCs in VTA DA neurons. Note that Chelerythrine alone had no effect. D. Bar graph showing that application of Rp-adenosine 3’,5’-cyclic monophosphorothioate triethylammonium salt hydrate (Rp-cAMPS, 11 µM), did not block phenylephrine’s effect (10 µM) on AMPA EPSCs (n=7). *p < 0.05, One-way ANOVA, Newman-Keuls post-hoc.
DISCUSSION
To our knowledge, the present study is the first to confirm in brain slices using whole-cell recordings that activation of presynaptic α1-ARs modulates glutamatergic inputs which affect VTA DA neuron excitability. We found that phenylephrine and methoxamine applications significantly increased AMPA EPSCs amplitude, suggesting an enhancement of glutamate synaptic transmission. The fact that prazosin blocked phenylephrine’s effect indicates that the enhanced glutamate transmission is specifically mediated by an α1-AR. In addition, phenylephrine significantly decreased the PPR and increased the frequency but not the amplitude of sEPSC demonstrating that α1-ARs activation augmented the release probability of glutamate presynaptically. Moreover, mEPSC recordings showed no significant differences in frequency or amplitude after phenylephrine’s application, suggesting that the presynaptic α1-ARs effect is action potential dependent. Phenylephrine’s action depends on extracellular calcium since its administration in low Ca2+ ACSF (1 mM) failed to increase the AMPA EPSC amplitude. Also, depletion of intracellular calcium stores with thapsigargin blocked α1-ARs action on AMPA EPSCs. It was further established that α1-AR-mediated increase in glutamate release involves a selective activation of the PKC intracellular pathway.
The activity of α1-AR in VTA DA cells has been linked to neuronal excitation (Grenhoff et al., 1993, Grenhoff and Svensson, 1993). The application of an α1-AR agonist, for example, results in cell depolarization, augmentation of the firing rate and a facilitation of the transition from pacemaker firing to bursting activity (Grenhoff et al., 1995). In addition, the action of α1-AR stimulation on IPSPs were found in the presence of TTX and in the absence of Ca2+, suggesting a postsynaptic effect on dopamine neurons (Grenhoff et al., 1995, Paladini et al., 2001). In contrast, with these studies, the present results showed that α1-AR activation increase AMPA mediated EPSCs in VTA DA cells, specifically through presynaptic modulation of glutamate release. These discrepancies could be due to differences in electrophysiological methods used and the fact that two distinct neurophysiological parameters, namely IPSPs vs EPSCs, were recorded. Further research should be conducted to clarify possible differences in the α1-AR modulation of GABAergic and glutamatergic inputs onto VTA DA neurons.
It has been reported that the α1b-AR subtype is located in the VTA (Greene et al., 2005). Therefore, the effects found in the present study might be mediated by an α1b-AR subtype. Dysfunction of this type of metabotropic receptors could compromise DA cell excitability as has been shown in other brain areas (Mirnics et al., 2001, Arnsten, 2004).
Studies from several brain regions have shown diverse effects of α1-AR activation on glutamate release. For instance, phenylephrine’s superfusion onto layer V of pyramidal cortical tissue decreased AMPA EPSCs amplitude and produced no differences in PPR. Such results suggest, that α1-AR activation decreases glutamatergic-induced excitation by a postsynaptic modulation of synaptic transmission (Kobayashi et al., 2009). In addition, it has been reported that α1-AR activation leads to a depression of excitatory transmission that is long lasting in several structures such as visual cortex, hippocampus and BNST (Kirkwood et al., 1999, Scheiderer et al., 2004, McElligott and Winder, 2008, Scheiderer et al., 2008, McElligott and Winder, 2009). In these studies the presence of paired-pulse stimulation and NE, acting specifically via α1-AR, triggered an NMDA receptor-dependent homosynaptic long-term depression (LTD) in visual cortex (Kirkwood et al., 1999). Similarly, activation of an α1-AR induced an LTD at CA3-CA1 synapses in hippocampal and on the BNST slices (Scheiderer et al., 2004, Scheiderer et al., 2008, McElligott and Winder, 2009). Some of these results are in disagreement with ours; however, differences between recording sites, tissue preparation and recording conditions could be possible reasons for these discrepancies. Also, activation of noradrenergic receptors can produce different effects that depend on their localization at synapses and their receptor type.
Our studies found that α1-AR activation decreases paired pulse ratio (PPR), which is a measurement sensitive to changes in presynaptic glutamate release. Modulation in PPR is a widely accepted analysis to predict the mechanism underlying synaptic response to experimental treatments. Lack of changes in PPR are consistent with postsynaptic effects while decreases in PPR are associated to enhancements in transmitter release (Zucker, 1989, Manabe et al., 1993). Therefore, our findings strongly support that presynaptic activation of an α1-AR increase VTA DA neurons excitability through the modulation of glutamate release in this region. These functional findings are consistent with recent ultrastructural identification of α1-AR immunoreactivity at pre-synaptic elements (Rommelfanger et al., 2009).
The effects of α1-AR stimulation on spontaneous synaptic transmission have been previously assessed with contradictory results in cultured rat hippocampal neurons, and in brain slices of hypothalamus and cerebral cortex (Marek and Aghajanian, 1999, Aubert et al., 2001, Gordon and Bains, 2003, Dong et al., 2005, Gordon and Bains, 2005). Investigations on different nuclei of the hypothalamus and prelimbic cortex preparations have demonstrated that α1-AR activation increase mEPSC frequency. No data on sEPSCs were presented (Gordon and Bains, 2003; Dong et al., 2005, Gordon and Bains, 2005). In contrast, experiments made on neurons of layer V of the medial prefrontal cortex and in mature cultured hippocampal neurons showed that activation of α1-ARs increase the frequency but not the amplitude of sEPSC. No differences were found on mEPSC (Marek and Aghajanian, 1999, Aubert et al., 2001). In accordance with the latter data, our results showed that enhancement of EPSCs by α1-AR activation is dependent on presynaptic action potentials given that the increased frequency was not observed in the presence of TTX, an established inhibitor of voltage-gated sodium channels (Narahashi et al., 1967). Therefore, α1-AR actions on DA neuron excitability follow synaptic activation induced by action potentials from presynaptic neurons. In accordance with our results, Cucchiaroni et al.,(2011) reported that α1-AR activation increases glutamate release onto DA cells of mesencephalic-striatal co-cultures. The enhanced glutamate release was dependent on presynaptic action potentials and had no effect on the sensitivity of postsynaptic glutamate receptors in DA cells.
Protein kinase C (PKC) activation potentiates synaptic transmission through a mechanism which causes increases in glutamate release (Malenka et al., 1986, Lou et al., 2008). It has also been observed that PKC activation makes synapses potentiation-competent in hippocampal neurons (Wierda et al., 2007). Activation of the PKC pathway has also been claimed to increase the fusion probability of vesicles in the readily releasable pool on calyx of Held synapses (Lou et al., 2008). The latter effect could result from increases in the calcium sensitivity of vesicle fusion, which in turns enhances the spontaneous and evoked release necessary for the potentiation (Lou et al., 2005).
α1-AR are Gq-protein-coupled receptors, known to activate phospholipase C, and stimulate protein kinase C (PKC) through the increase in diacylglycerol levels (Tanaka and Nishizuka, 1994). Triggering of PKC, via α1-AR activation, has been reported to increase glutamate release from excitatory afferent fibers in hypothalamic nucleus (Gordon and Bains, 2003). This PKC effect, through α1-AR, has also been observed in pyramidal neurons from layers V-VI of the prelimbic cortex (Dong et al., 2005). Our data showed that in the presence of chelerythrine, a PKC inhibitor, the stimulation of evoked glutamate release by phenylephrine application was blocked. Thus, a PKC-dependent mechanism plays an important role in the α1-AR effect on glutamate release onto VTA DA neurons.
The cooperation between the influx of extracellular calcium and PKC activation has been described (Swartz, 1993, Sena et al., 1999). Our results demonstrate that α1-ARs activation could increase glutamate release by an interaction with extracellular calcium influx. This interaction can be related to the PKC facilitation of voltage-gated calcium channel activation. There are several possibilities to explain the facilitation of glutamate release, calcium-currents and synaptic transmission by the activation of PKC. At presynaptic terminals, voltage-gated calcium channels are coupled with protein involved in exocytosis of neurotransmitters (Llinas et al., 1992, Stanley, 1997). Presynaptic facilitation shown by PKC is related to the ability to up-regulate the peak current of some calcium-channels. Different calcium channels support the modulation of glutamate release by presynaptic receptors. PKC activation can facilitate presynaptic glutamate release by both, N type and P/Q type calcium channels (Stea et al., 1995, Vazquez and Sanchez-Prieto, 1997, Herlitze et al., 2001). In some neurons, specific types of voltage-gated calcium channels are closely located to a particular calcium-activated potassium channel, presumably for the effective regulation of cell membrane excitability (Marrion and Tavalin, 1998). These regulatory actions of calcium can be amplified, if calcium-induced calcium release increases calcium liberation from intracellular storages such as the endoplasmic reticulum (Endo, 1977, Kuba, 1994, Verkhratsky and Petersen, 1998).
The α1-AR role’s on the intracellular calcium stores of ventral midbrain neurons has been previously described (Paladini and Williams, 2004). α1-AR activation induced an intracellular calcium wave that appears to be regenerative in DA neurons. Also, increase in intracellular calcium via α1-AR and mGluRs interactions can have a positive effect on firing pattern of midbrain DA neurons (Paladini et al., 2001, Paladini and Williams, 2004). In our study, PKC inhibition and depletion of intracellular calcium stores were able to block the α1-AR effect on AMPA EPSCs. Therefore, it seems that PKC, together with the availability of intra- and extracellular calcium, are key mechanisms mediating the enhancement of presynaptic glutamate release onto VTA DA neurons (Fig 8).
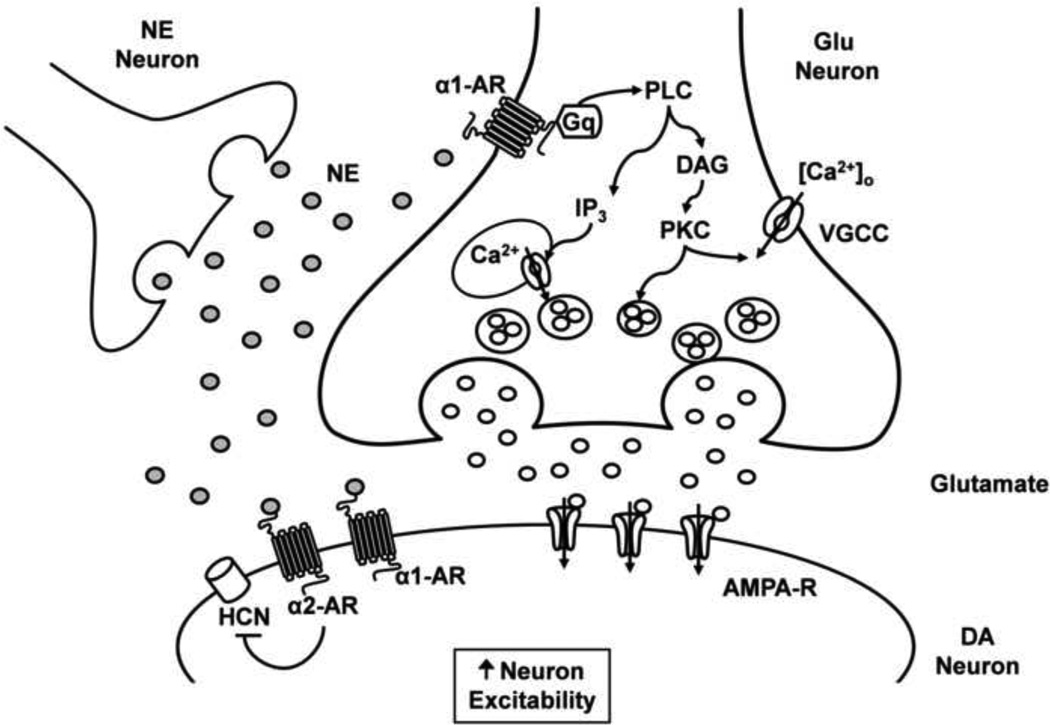
Figure 8. Possible mechanism underlying the increase in glutamate release onto VTA DA neurons via α1- AR activation.
Stimulation of presynaptic α1- AR at excitatory glutamatergic neurons that project onto VTA DA neurons causes activation of phospholipase C (PLC), probably via a Gq-mediated mechanism, resulting in diacylglycerol (DAG) formation and inositol (1,4,5) trisphosphate activation (IP3). The activated DAG stimulates a protein kinase C (PKC) phosphorylation, which may further increase calcium currents through voltage gated calcium channels (VGCC). On the other hand, IP3 activation increases calcium release from intracellular stores (Tanaka and Nishizuka, 1994). Our results indicate that PKC activation and an increase in intracellular calcium stimulate glutamate release onto VTA DA neurons. The enhancement of glutamate release and HCN current inhibition through α1- AR and α2- AR, respectively, could mediate an increase in VTA DA neuronal excitability (Inyushin et al., 2010). NE, norepineprhine; Glu, glutamate.
Glutamate release onto VTA cells contributes to changes in cognition, stress and reward, and is critical to the effects induced by drugs of abuse (Ungless et al., 2001, Saal et al., 2003, You et al., 2007). α1-AR could activate glutamatergic inputs into the VTA from cortical and subcortical structures (Carr and Sesack, 2000, Omelchenko and Sesack, 2007). BNST,and laterodorsal tegmentum have powerful glutamatergic excitatory influences onto VTA DA neurons (Georges and Aston-Jones, 2002, Omelchenko and Sesack, 2007). These glutamatergic connections have been linked to reward-directed behaviors such as cocaine self-administration and drug seeking (Ungless et al., 2001, Saal et al., 2003, You et al., 2007, Wise, 2009). Also, α1-ARs could be located on glutamatergic fibers impinging on VTA DA neurons projecting to prefrontal cortex and nucleus accumbens (Dobi et al., 2010). Consequently, the enhancement of glutamatergic transmission onto VTA DA neurons via α1-ARs can contribute to the facilitation of addiction development of different psychostimulants such as cocaine, amphetamine or morphine (Drouin et al., 2002, Shi et al., 2004, Jimenez-Rivera et al., 2006, Zhou et al., 2006).
A recent study showed that VTA DA cells related to reward and aversive stimuli project to nucleus accumbens (NAc) lateral shell and have a large Ih current (Lammel et al., 2011). In the present study, the VTA DA cells recorded had an Ih greater than 200 pA, as mentioned in the experimental procedures. Therefore, according to Lammel et al., (2011), the subpopulation of cells that we recorded could be modified by addiction and/or aversion. On the other hand, aversive stimuli can increase norepinephrine release onto the VTA, which can lead to α1-AR activation. Stimulation of α1-ARs can produce an increase in glutamate release on VTA DA cells that project to NAc lateral shell, suggesting that this modulation can encode occurrence of a salient stimulus independent of its valence. It is possible that VTA DA cells projecting into NAc medial shell, which have small Ih and could be modified only by addiction, are not taken into consideration in the present study. Other studies must be carried out focusing on this cell sub-population which seems very important in addiction processes.
CONCLUSION
The present results demonstrate that α1-ARs activation at the presynaptic site increases excitability of putative DA cells within the VTA. In addition, they suggest that α1-AR might be heterosynaptically localized at glutamatergic fibers terminating onto VTA DA neurons. This interaction may play a central role in the synaptic plasticity changes that are known to occur in the VTA after drug exposure. Clear understanding of the α1-AR mediated activation of VTA DA neurons could provide possible avenues for therapeutic pharmacological interventions.
Highlights.
Presynaptic α1-ARs activation enhances glutamate release onto VTA DA neurons.
This effect involves a selective activation of the PKC intracellular pathway.
Presynaptic α1-AR-mediated effect requires extra- and intracellular.
Acknowledgments
This work was funded by MBRS-SCORE (GM-08224) to C.A.J.R. M.C.V.M received economical support from Universidad Industrial de Santander, Colombia. The authors thank Dr. Priscilla Sanabria, Francisco Arencibia and Maria E. Velez for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnsten AF. Adrenergic targets for the treatment of cognitive deficits in schizophrenia. Psychopharmacology (Berl) 2004;174:25–31. doi: 10.1007/s00213-003-1724-3. [DOI] [PubMed] [Google Scholar]
- Aubert M, Guiramand J, Croce A, Roch G, Szafarczyk A, Vignes M. An endogenous adrenoceptor ligand potentiates excitatory synaptic transmission in cultured hippocampal neurons. Cereb Cortex. 2001;11:878–887. doi: 10.1093/cercor/11.9.878. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cucchiaroni ML, Freestone PS, Berretta N, Viscomi MT, Bisicchia E, Okano H, Molinari M, Bernardi G, Lipski J, Mercuri NB, Guatteo E. Properties of dopaminergic neurons in organotypic mesencephalic-striatal co-cultures--evidence for a facilitatory effect of dopamine on the glutamatergic input mediated by alpha-1 adrenergic receptors. Eur J Neurosci. 2011;33:1622–1636. doi: 10.1111/j.1460-9568.2011.07659.x. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Fu YM, Sun JL, Zhu YH, Sun FY, Zheng P. Neurosteroid enhances glutamate release in rat prelimbic cortex via activation of alpha1-adrenergic and sigma1 receptors. Cell Mol Life Sci. 2005;62:1003–1014. doi: 10.1007/s00018-005-5004-8. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Gao M, Liu CL, Yang S, Jin GZ, Bunney BS, Shi WX. Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci. 2007;27:5414–5421. doi: 10.1523/JNEUROSCI.5347-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Bains JS. Priming of excitatory synapses by alpha1 adrenoceptor-mediated inhibition of group III metabotropic glutamate receptors. J Neurosci. 2003;23:6223–6231. doi: 10.1523/JNEUROSCI.23-15-06223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Bains JS. Noradrenaline triggers multivesicular release at glutamatergic synapses in the hypothalamus. J Neurosci. 2005;25:11385–11395. doi: 10.1523/JNEUROSCI.2378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--3. Evidence for electrotonic coupling. Neuroscience. 1983;10:333–348. doi: 10.1016/0306-4522(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace CR, Cowsik SM, Shim JY, Welsh WJ, Howlett AC. Unique helical conformation of the fourth cytoplasmic loop of the CB1 cannabinoid receptor in a negatively charged environment. J Struct Biol. 2007;159:359–368. doi: 10.1016/j.jsb.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JG, Dingledine R, Greenamyre JT. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis. 2005;18:19–31. doi: 10.1016/j.nbd.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, North RA, Johnson SW. Alpha 1-adrenergic effects on dopamine neurons recorded intracellularly in the rat midbrain slice. Eur J Neurosci. 1995;7:1707–1713. doi: 10.1111/j.1460-9568.1995.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- Hague C, Chen Z, Uberti M, Minneman KP. Alpha(1)-adrenergic receptor subtypes: non-identical triplets with different dancing partners? Life Sci. 2003;74:411–418. doi: 10.1016/j.lfs.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Zhong H, Scheuer T, Catterall WA. Allosteric modulation of Ca2+ channels by G proteins, voltage-dependent facilitation, protein kinase C, and Ca(v)beta subunits. Proc Natl Acad Sci U S A. 2001;98:4699–4704. doi: 10.1073/pnas.051628998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inyushin MU, Arencibia-Albite F, Vazquez-Torres R, Velez-Hernandez ME, Jimenez-Rivera CA. Alpha-2 noradrenergic receptor activation inhibits the hyperpolarization-activated cation current (Ih) in neurons of the ventral tegmental area. Neuroscience. 2010;167:287–297. doi: 10.1016/j.neuroscience.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Rivera CA, Feliu-Mojer M, Vazquez-Torres R. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann N Y Acad Sci. 2006;1074:390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- Jones BE, Halaris AE, McIlhany M, Moore RY. Ascending projections of the locus coeruleus in the rat. I. Axonal transport in central noradrenaline neurons. Brain Res. 1977;127:1–21. doi: 10.1016/0006-8993(77)90377-8. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kojima M, Koyanagi Y, Adachi K, Imamura K, Koshikawa N. Presynaptic and postsynaptic modulation of glutamatergic synaptic transmission by activation of alpha(1)- and beta-adrenoceptors in layer V pyramidal neurons of rat cerebral cortex. Synapse. 2009;63:269–281. doi: 10.1002/syn.20604. [DOI] [PubMed] [Google Scholar]
- Kuba K. Ca(2+)-induced Ca2+ release in neurones. Jpn J Physiol. 1994;44:613–650. doi: 10.2170/jjphysiol.44.613. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse. 2004;52:233–244. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Korogod N, Brose N, Schneggenburger R. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28:8257–8267. doi: 10.1523/JNEUROSCI.0550-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986;321:175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Mathew SS, Hablitz JJ. Calcium release via activation of presynaptic IP3 receptors contributes to kainate-induced IPSC facilitation in rat neocortex. Neuropharmacology. 2008;55:106–116. doi: 10.1016/j.neuropharm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33:2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–1335. doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Moore JW, Poston RN. Tetrodotoxin derivatives: chemical structure and blockage of nerve membrane conductance. Science. 1967;156:976–979. doi: 10.1126/science.156.3777.976. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Mitrano DA, Smith Y, Weinshenker D. Light and electron microscopic localization of alpha-1 adrenergic receptor immunoreactivity in the rat striatum and ventral midbrain. Neuroscience. 2009;158:1530–1540. doi: 10.1016/j.neuroscience.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha 1 adrenergic receptors. J Neurophysiol. 2004;91:1071–1077. doi: 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Smith CC, McCutchen E, McCoy PA, Thacker EE, Kolasa K, Dobrunz LE, McMahon LL. Coactivation of M(1) muscarinic and alpha1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3-CA1 synapses in rat hippocampus. J Neurosci. 2008;28:5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz B, Schultz A. EEG monitoring improves quality and safety of TIVA. Anaesth Intensive Care. 2002;30:817–818. [PubMed] [Google Scholar]
- Sena CM, Santos RM, Boarder MR, Rosario LM. Regulation of Ca2+ influx by a protein kinase C activator in chromaffin cells: differential role of P/Q-and L-type Ca2+ channels. Eur J Pharmacol. 1999;366:281–292. doi: 10.1016/s0014-2999(98)00908-x. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 2004;29:2160–2167. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci. 1997;20:404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- Stea A, Soong TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Swartz KJ. Modulation of Ca2+ channels by protein kinase C in rat central and peripheral neurons: disruption of G protein-mediated inhibition. Neuron. 1993;11:305–320. doi: 10.1016/0896-6273(93)90186-u. [DOI] [PubMed] [Google Scholar]
- Tamura K, Manabe T, Kyogoku T, Andoh K, Ohshio G, Tobe T. Effect of postischemic reperfusion on the pancreas. Hepatogastroenterology. 1993;40:452–456. [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vazquez E, Sanchez-Prieto J. Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. Eur J Neurosci. 1997;9:2009–2018. doi: 10.1111/j.1460-9568.1997.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky AJ, Petersen OH. Neuronal calcium stores. Cell Calcium. 1998;24:333–343. doi: 10.1016/s0143-4160(98)90057-4. [DOI] [PubMed] [Google Scholar]
- Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wise RA. Ventral tegmental glutamate: a role in stress-, cue-, and cocaine-induced reinstatement of cocaine-seeking. Neuropharmacology 56 Suppl. 2009;1:174–176. doi: 10.1016/j.neuropharm.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bunney BS, Shi WX. Differential effects of cocaine on firing rate and pattern of dopamine neurons: role of alpha1 receptors and comparison with L-dopa and apomorphine. J Pharmacol Exp Ther. 2006;317:196–201. doi: 10.1124/jpet.105.094045. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]