Abstract
OBJECTIVES
To determine whether mildly impaired physical function (based on performance-based assessment) is associated with the development of dementia of the Alzheimer type (DAT) in cognitively normal older adults.
DESIGN
Longitudinal, observational study with yearly assessments of physical and cognitive function. Mean follow-up was 5 years.
SETTING
Knight Alzheimer’s Disease Research Center at Washington University, St. Louis, Missouri.
PARTICIPANTS
Four hundred thirty-five cognitively normal adults, age 60 years or older participating in longitudinal studies of aging.
MEASUREMENTS
Survival analyses were used to examine whether scores on the 9-item Physical Performance Test (PPT) predicted time to DAT diagnosis. Cox proportional hazards models were used to examine associations between the PPT total scores and time to cognitive impairment and DAT; as well as the association of time to these events while adjusting for, and simultaneously testing the effects of age, gender, education, and presence of at least one apolipoprotein (APOE) ε4 allele.
RESULTS
During the follow-up period, 81 participants developed DAT. Compared to those who remained cognitively normal, participants diagnosed with DAT were older (81 vs 74.2 years; p=.001) and had worse performance on the PPT (25.5 vs 28.1; p=.009). Time to DAT diagnosis was associated with total scores on the PPT (hazard ratio [HR] =.89, 95% CI=.86–.93, p<.001) such that time to a DAT diagnosis was slower for participants with higher physical performance scores. In the adjusted analysis, the PPT scores significantly predicted time to a DAT diagnosis (HR =.94, 95% CI=.89–.99, p<.022).
CONCLUSION
The presence of mild physical impairment in cognitively normal older adults is associated with subsequent development of DAT. Although the physical impairment may be sufficiently mild that it is recognized only with performance-based assessments, its presence may predate clinically detectable cognitive decline.
Keywords: Dementia of Alzheimer type, physical performance, predictors, frailty
INTRODUCTION
Dementia of the Alzheimer type (DAT) is the most common cause of cognitive impairment in older adults and is associated with significant disability requiring substantial use of health care resources.1,2 Although the clinical features of DAT are thought to be primarily cognitive, an emerging body of literature supports a relationship between DAT and decrements in physical function.3–5
Decline in physical performance has been well documented in moderate to severe dementia;1, 6 however, recent data suggests that physical impairment is also detectable in mild dementia. Several studies support the hypothesis that physical impairments may precede the diagnosis of dementia. A recent report found that physical frailty is associated with the risk of incident mild cognitive impairment (MCI), a prodromal syndrome of DAT.7 However, the construct of frailty contains subjective domains such as fatigue and may be conflated in older adults who are cognitively impaired.8 Additionally, many reports linking physical and cognitive impairment do not distinguish between DAT and dementia in general. This lack of distinction is important because several dementia etiologies such as Parkinson’s disease, Dementia with Lewy Bodies, and vascular dementia are often associated with physical impairment well before the onset of cognitive decline.
Although data supports a relationship between physical function and DAT, the temporal relationship between the two have not been well-characterized. It is therefore difficult to determine the causal relationship. It remains unclear whether preclinical pathophysiological changes in the brain contribute to physical impairment or whether physical function contributes to the development of cognitive decline. Nonetheless, if physical impairment can be detected prior to noticeable cognitive impairment, performance-based physical assessments may facilitate the identification of patients with preclinical DAT. Performance based physical assessments could be useful because the current tools used to diagnose DAT are primarily based on cognitive assessments, thus limiting the clinician’s ability to identify patients at increased risk for developing DAT.
We have previously reported that the onset of DAT is preceded by changes in body weight9 and impairment in visuospatial function10 by as much as three years before appearance of overt episodic memory impairment. Thus, we hypothesize that alterations in physical performance also may precede the onset of dementia. To investigate the relationship between physical performance and development of DAT, we longitudinally assessed objective measures of physical performance and cognitive function in a well-characterized cohort of cognitively normal older adults and tested whether physical performance predicts time to a DAT diagnosis.
METHODS
Participants
All participants were older adults involved in studies of cognitive and functional aging at the Knight Alzheimer’s Disease Research Center (ADRC) at Washington University between January 1998 and August 2007. The ADRC recruits both cognitively healthy and demented older adult volunteers from the greater metropolitan St. Louis area (population: 2.5 million). All participants of the study were enrolled in the ADRC longitudinal study and completed annual clinical assessments. Inclusion criteria for this study were cognitively normal at the initial clinical assessment, age over 60 years, ambulatory, able to complete all assessments, at least one follow-up assessment, and Physical Performance Test (PPT) score greater than 5 (range of possible PPT scores, from worst to best performance, is 0 to 36). A total of 510 individuals completed all assessments required for this study and 440 participants met the initial inclusion criteria. During follow-up, five participants were diagnosed with non-DAT dementia- two with dementia with Lewy Bodies, two with vascular dementia and one with dementia due to Parkinson’s disease. These individuals were excluded from analyses giving a final sample size of 435.
Clinical and Cognitive Assessments
The clinical evaluation at baseline and at each annual follow-up visit included obtaining past medical, social and family history from a reliable informant, usually a spouse or adult child. Information regarding possible cognitive change and functional loss was obtained by a clinician from semi-structured interviews with the informant and separately with the participant. Included in the clinical assessment protocol were the Mini Mental State Examination,11 the Short Blessed Test,12 an aphasia battery, a medication inventory, a detailed neurological examination and a depressive features battery.
Using all information from the clinical assessment protocol but without reference to the participant’s psychometric performance, the clinician determined the Clinical Dementia Rating (CDR) for the participant.13 In this study, cognitive normality was denoted by a CDR score of 0 and cognitive impairment by a CDR score > 0, where CDR 0.5, 1, 2 and 3 indicate very mild, mild, moderate and severe dementia. The diagnosis of DAT was made in accordance with standard criteria14 and, in our ADRC, is confirmed by histopathology in 93% of cases, even for individuals diagnosed in the CDR 0.5 stage15.
Physical Assessment
Following the general physical and neurological examination, the PPT was administered by a trained research nurse. We used a modification of the original PPT instrument,16 which uses the chair rise and Progressive Romberg test of standing balance to simulate stair climbing tasks. Performance on these two tasks, which have been included in other physical performance batteries,17,18 has been associated with self reported disability, nursing home placement and mortality.17 Specific tasks in our modified PPT are: 1) writing a sentence, 2) simulated eating (i.e., spooning beans into a container), 3) lifting a book, 4) simulated dressing (i.e., putting on and taking off a jacket), 5) picking up a penny from the floor, 6) turning in a complete circle, 7) walking 50 ft., 8) the chair rise (i.e., sitting in and rising from a chair five times), and 9) the Progressive Romberg test of standing balance (i.e., standing with feet in tandem, semi tandem and side by side positions). The tasks in our PPT were scored on a 5 pt scale, from worst to best performance: 0,1,2,3 and 4. The total PPT score, a simple summation of the individual item scores, is a composite measure of physical function. The maximum (i.e., best) total score was 36, with a decreasing score indicating increasing disability. Based on our prior work,19 we classified participants as functional (PPT scores > 28) or impaired function (PPT scores < 28).
Statistical Analyses
Analyses were performed using SAS (Cary, NC). Change from cognitively normal status to a diagnosis of DAT was the primary outcome measure. Survival analyses were used to examine whether scores on the PPT predicted time to receipt of a DAT diagnosis. Kaplan-Meier survival curves were used to test unadjusted associations between scoring at a functional vs. impaired function and DAT diagnosis. Because they are able to accommodate continuous predictor variables, Cox proportional hazards models were used to examine unadjusted associations between the PPT total scores and time to DAT; as well as the association of time to DAT with baseline PPT total scores and testing at the functional level on the PPT; while adjusting for, and simultaneously testing the effects of, age, gender, education, and presence of at least one apolipoprotein (APOE) ε4 allele. A p-value of less than .05 was taken to indicate statistical significance in all analyses.
RESULTS
Sample Characteristics
A total of 435 participants met the inclusion criteria for the study. During the follow-up period, 81 participants were diagnosed with DAT. The mean baseline PPT score was 27.6 (SD=5; range 19–34). Additional demographic information for these participants is shown in. Table 1.
Table 1.
Baseline Characteristics of Study Participants. (N = 435)
| Mean/N | SD/% | |
|---|---|---|
| Age, years | 75.9 | 8.8 |
| Education, years | 14.8 | 3.1 |
| Women | 265 | 60.9% |
| African American | 36 | 8.3% |
| MMSE | 28.8 | 1.3 |
| SBT | 1.5 | 2.2 |
| PPT, total score | 27.6 | 4.7 |
| Functional, total PPT score ≥28 | 258 | 59.3% |
Abbreviations: MMSE = Mini-Mental State Exam, range 0–30; SBT = Short Blessed Test, range 0–28; PPT = Physical Performance Test.
Participants had a mean age of 75.9 years at entry and were mostly women (60.9%). All participants were CDR 0 at baseline with mean MMSE score 28.8. The sample was evaluated over a mean of 5 years (range 2–9). Compared to those who remained cognitively normal, were older (81 vs 74.2 years; p=.001) and had worse performance on the PPT (25.5 vs 28.1; p=.009).(Table 2).
Table 2.
Comparison of Participants who Developed DAT and those who remained Nondemented. (N = 435)
| Characteristic | Diagnosed with DAT n= 81 | Cognitively Normal n= 354 | p value |
|---|---|---|---|
| Age, years | 81.0 (7) | 74.2 (9) | .001 |
| Education, years | 14.2 (3) | 14.9 (3) | .161 |
| PPT score | 25.5 (6) | 28.1 (4) | .009 |
| SBT | 1.8 (2) | 1.3 (2) | .735 |
| MMSE | 28.1 (2) | 28.9 (1) | .007 |
| Women | 59.3% | 61.4% | .437 |
| APOE ε4+ | 66.7% | 70.6% | .64 |
Abbreviations: MMSE = Mini-Mental State Exam, range 0–30; SBT = Short Blessed Test, range 0–28; DAT = Dementia of the Alzheimer Type; PPT = Physical Performance Test; APOE ε4+ = presence of at least one apolipoprotein ε4 allele.
Predicting future DAT
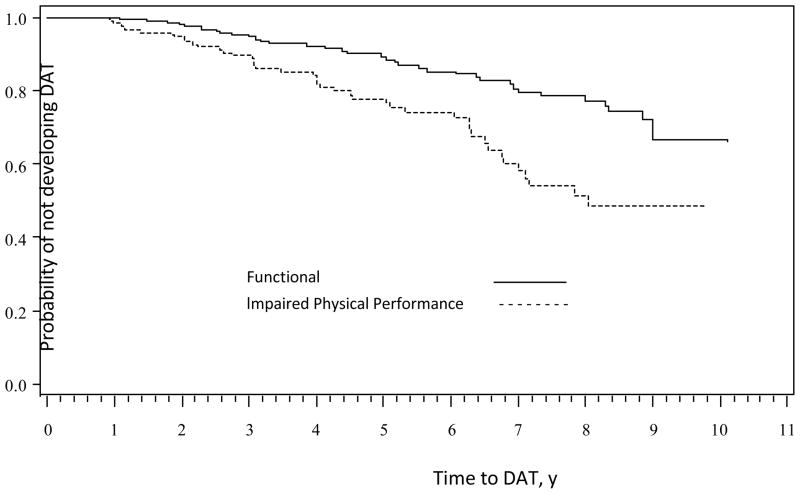
Based on prior work,19 participants were categorized using predetermined cut-off scores for the PPT to determine presence of physical impairment (PPT scores < 28) or functional (PPT scores >28). In this sample, 59.3% of participants had PPT scores in the functional category. In the unadjusted analyses, time to a DAT diagnosis was associated with total scores on the PPT (HR=.89, 95% CI=.86–.93, p<.001) such that time to a DAT diagnosis was slower for participants with better physical performance scores. Physical impairment predicted a faster time to a first DAT diagnosis (p<.001, Figure 1).
Figure 1.
Kaplan-Meier Curves Showing the Association between Functional Status on the Physical Performance Test at Baseline and Time to Diagnosis of Dementia of the Alzheimer Type (DAT).
We then applied Cox proportional hazards models to adjust for age, gender, education, and the presence of an APOE ε4 allele to predict time to a DAT diagnosis (Table 3). Impaired physical performance measured with the PPT significantly predicted time to a DAT diagnosis. Older baseline age and having one or more APOE ε4 alleles were also significantly associated with a faster time to DAT diagnosis.
Table 3.
Results of the Adjusted Cox Proportional Hazards Models
| Time to DAT diagnosis
|
||||
|---|---|---|---|---|
| HR | 95% CI | P-value | ||
| Lower | Upper | |||
| PPT | ||||
| Total score | .94 | .89 | .99 | .022 |
| Female gender | .88 | .56 | 1.39 | .585 |
| Age, y | 1.07 | 1.03 | 1.10 | <.001 |
| Education, y | .95 | .89 | 1.02 | .174 |
| APOE ε4+ | 2.10 | 1.29 | 3.42 | .003 |
Abbreviations: HR = Hazard Ratio; CI = Confidence Interval; PPT = Physical Performance Test; APOE ε4+ = presence of at least one apolipoprotein ε4 allele.
DISCUSSION
In this sample, cognitively normal participants who developed DAT had worse physical performance at baseline compared to those who did not develop DAT. Impaired physical performance was associated with a faster time to the diagnosis of DAT while participants whose physical performance status was functional (PPT score ≥28) had a longer time to diagnosis of DAT. These findings support the hypothesis that impairments in physical function can precede the cognitive symptoms of DAT.
Currently, the tools being used and studied for the detection of preclinical Alzheimer’s disease (AD) focus on cognitive measures and biological measures using cerebrospinal fluid (CSF) and neuroimaging. These tools may be difficult to utilize or access for many primary care physicians who will be the first contact for patients with DAT. A physical performance measure may be a more practical tool for primary care physicians and offer a warning of possible deleterious cognitive outcomes.
Several reports describe that frailty is a significant predictor of cognitive decline20 and that more rapid decline in cognitively impaired older adults may be due to increased physical impairment.21 However, there is no consensus on the definition of frailty, which may include physical measurement only or a more global assessment that includes comorbidities, disabilities and subjective complaints.22 We focused here on physical performance rather than the more subjective construct of frailty. Performance-based measures are more reproducible and have greater sensitivity to change, making them more reliable for clinical use than self-report measures.23, 24
Previous studies support a strong relationship between cognitive and physical performance and show that declines in one sphere may predict future decline in the other.25,26 In a cohort of 823 older adults followed longitudinally, baseline frailty increased the risk of AD (HR 2.44) and the annual rate of change in frailty was associated with the development of dementia (HR 3.3).27 In a study of 367 persons with AD,28 decline in physical performance was predicted by lower cognitive performance, while another study29 noted that poor physical function and decreased muscle strength often coexist with cognitive impairment. Likewise, improving physical activity may provide protective effects for the onset of dementia.30
A recent randomized trial31 reported that a 6-month program of physical activity provided improvement in cognition in older adults with subjective memory complaints. This group of individuals, although reported as nondemented, had a mean global CDR of 1, suggesting that more than subjective memory complaints were present. In this study, we report predictors of future DAT in nondemented older adults characterized by informant interviews. Likewise, a number of studies used composite measures of frailty including subjective complaints of fatigue, body composition and components of the Unified Parkinson Disease Rating scale22, 27, 32 for their physical assessments. While these measurements certainly capture aspects of physical limitations, it is unclear if they measure frailty (a multidimensional construct that represents age-dependent changes in physical reserve), extra pyramidal signs or somatic complaints.
This study supports and confirms prior reports and utilizes a clinically well-characterized sample of older adults who were cognitively healthy at enrollment. We report here results based on the PPT, a validated, performance-based measure of physical function, and show that individuals with impaired physical performance (PPT score <28) have a faster time to development of DAT than individuals with better physical performance. While this study includes a large sample of older adults and a representative sample of African Americans (8.2%), the participants are well-educated, and relatively healthy. Therefore, the population is not representative of all community-dwelling older adults. Although participants with higher PPT scores were slower to develop DAT may suggest that exercise and increased physical activity can delay DAT, we cannot confirm this because our study does not include data regarding physical activity and physical fitness. This report does support further investigation regarding the use of performance-based measures of physical function to identify older adults at risk for DAT.
Acknowledgments
We are indebted to the Clinical Core of the Alzheimer’s Disease Research Center at Washington University for providing the clinical and diagnostic data used in this report. We particularly thank the Core’s clinical nurse specialists for obtaining the Physical Performance Test data. This study was supported by grants from the National Institutes of Health: K23 AG026768 (CHW), P01 AG03991 (JCM), and P50 AG05681 (JCM), by the Postdoctoral Program of 1UL1RR024992-01 from the National Center for Research Resources (CMR), and by the Charles and Joanne Knight Alzheimer Research Initiative of Washington University’s Alzheimer’s Disease Research Center (JCM).
Sponsor’s Role: This study had no sponsor.
Footnotes
Conflict of Interest:
Dr. Wilkins has grants from the National Institutes of Health and the Barnes-Jewish Hospital Foundation.
Dr. Morris has participated or is currently participating in clinical trials of anti dementia drugs sponsored by the following companies: Janssen Immunotherapy, Eli Lilly and Company, and Pfizer.
Dr. Morris has served as a consultant or has received speaking honoraria for the following companies: Eisai, Janssen Alzheimer Immunotherapy Program/Elan, Glaxo-Smith-Kline, Novartis, Otsuka Pharmaceuticals, and Pfizer/Wyeth.
Dr. Galvin has grants from the National Institutes of Health, Michael J Fox Foundation, Morris and Alma Schapiro Fund and the State of New York. He holds the copyright for the AD8 dementia screening test and received licensing fees from Novartis, Pfizer and Eisai.
Author Contributions:
Dr. Wilkins participated in the study concept and design, acquisition of subjects and/or data, interpretation of data, and preparation of manuscript.
Dr. Roe participated in the study concept and design, analysis and interpretation of data, and preparation of manuscript.
Dr. Morris participated in the study concept and design, acquisition of subjects and/or data, interpretation of data, and preparation of manuscript.
Dr. Galvin participated in the study concept and design, acquisition of subjects and/or data, interpretation of data, and preparation of manuscript.
References
- 1.Hill JW, Futterman R, Duttagupta S, et al. Alzheimer’s disease and related dementias increase costs of comorbidities in managed medicare. Neurology. 2002;58:62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Ernst RL, Hay JW, Fenn C, et al. Cognitive function and the costs of Alzheimer disease: An exploratory study. Arch Neurol. 1997;54:687–693. doi: 10.1001/archneur.1997.00550180013006. [DOI] [PubMed] [Google Scholar]
- 3.Marquis S, Moore MM, Howieson D, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 4.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: Findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Larson EB, Bowen JD. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 6.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle PA, Buchman AS, Wilson RS, et al. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58:248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: An emerging research and clinical paradigm - Issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DK, Storandt M, Morris JC, et al. Longitudinal Study of the Transition From Healthy Aging to Alzheimer Disease. Arch Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Katzman R, Brown T, Fuld P, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, Edland S, Clark C, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43:2457–2465. doi: 10.1212/wnl.43.12.2457. [DOI] [PubMed] [Google Scholar]
- 15.Berg L, McKeel DW, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease - relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 16.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Biol Sci Med Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Belle Gv, Kukull WB, et al. Predictors of Functional Change: A Longitudinal Study of Nondemented People Aged 65 and Older. J Am Geriatr Soc. 2002;50:1525–1534. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins CH, Roe CM, Morris JC. A brief clinical tool to assess physical function: The mini-physical performance test. Arch Gerontol Geriatr. 2010;50:96–100. doi: 10.1016/j.archger.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camicioli R, Wang Y, Powell C, et al. Gait and posture impairment, parkinsonism and cognitive decline in older people. J Neural Transm. 2007;114:1355–1361. doi: 10.1007/s00702-007-0778-5. [DOI] [PubMed] [Google Scholar]
- 21.Dumont C, Voisin T, Nourhashemi F, et al. Predictive factors for rapid loss on the mini-mental state examination in Alzheimer’s disease. J Nutr Health Aging. 2005;9:163–167. [PubMed] [Google Scholar]
- 22.Abellan van Kan GRY, Houles M, Gillette-Guyonnet S, et al. The Assessment of Frailty in Older Adults. Clin Geriatr Med. 2010;26:275–286. doi: 10.1016/j.cger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Branch LG, Cummings SR, et al. Physical performance measures in aging research. J Gerontol. 1989;44:M141–146. doi: 10.1093/geronj/44.5.m141. [DOI] [PubMed] [Google Scholar]
- 24.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchman AS, Schneider JA, Leurgans S, et al. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panza F, D’Introno A, Colacicco AM, et al. Cognitive frailty: Predementia syndrome and vascular risk factors. Neurobiol Aging. 2006;27:933–940. doi: 10.1016/j.neurobiolaging.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Buchman AS, Boyle PA, Wilson RS, et al. Frailty is associated with incident alzheimer disease and cognitive decline in the elderly. Psychosom Med. 2007;69:483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 28.Hebert LE, Scherr PA, McCann JJ, et al. Change in direct measures of physical performance among persons with Alzheimer’s disease. Aging Ment Health. 2008;12:729–734. doi: 10.1080/13607860802154390. [DOI] [PubMed] [Google Scholar]
- 29.Auyeung TW, Kwok T, Lee J, et al. Functional Decline in Cognitive Impairment --The Relationship between Physical and Cognitive Function. Neuroepidemiology. 2008;31:167–173. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: The Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63:529–535. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 31.Pijpers E, Ferreira I, van de Laar RJJ, et al. Predicting mortality of psychogeriatric patients: a simple prognostic frailty risk score. Postgrad Med J. 2009;85:464–469. doi: 10.1136/pgmj.2008.073353. [DOI] [PubMed] [Google Scholar]
- 32.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal NT, Wilson RS, Beck TL, et al. Motor dysfunction in mild cognitive impairment and the risk of incident alzheimer disease. Arch Neurol. 2006;63:1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]