Abstract
Purpose
The combination of DNA methylation inhibitors and histone deacetylase inhibitors is synergistic in gene expression activation and may overcome platinum resistance. Sequential treatment with azacitidine and valproic acid (VPA) in combination with carboplatin may overcome resistance to platinum-based therapy, and we conducted a phase I trial to assess safety, maximum tolerated dose (MTD), and clinical correlates.
Experimental Design
Patients with advanced solid tumors refractory to standard therapy were eligible. In cohorts of escalating doses, patients received azacitidine for 5 days from days 1 to 5, VPA for 7 days from days 5 to 11, and carboplatin starting in the second cycle on days 3 and 10. Clinical correlates included evaluation of epigenetic changes, methylation patterns, and histone acetylation levels in peripheral blood mononuclear cells.
Results
Thirty-two patients were treated. The MTD was 75 mg/m2 azacitidine, 20 mg/kg VPA, and AUC 3.0 carboplatin. Minor responses or stable disease lasting ≥4 months were achieved by six patients (18.8%), including three with platinum-resistant or platinum-refractory ovarian cancer. The most common adverse events grade ≥3 were fatigue (81%) and neutropenia (69%). Dose-limiting toxicity occurred in six patients (18.8%), including four patients with grade 3 altered mental status. Death receptor 4 (DR4) methylation was shown to decrease in a subset of patients, but there was no relationship with tumor response or number of cycles received.
Conclusions
Combination of azacitidine, VPA, and carboplatin demonstrates decreased DR4 methylation and modest evidence of antitumor activity in patients with heavily treated advanced malignancies.
Keywords: azacitidine, valproic acid, carboplatin, methylation, histone deacetylase inhibition
INTRODUCTION
DNA methylation is a crucial epigenetic modification of the genome that is involved in regulating many cellular processes [1]. Genes involved in cell-cycle regulation, tumor cell invasion, DNA repair, chromatin remodeling, cell signaling, transcription, and apoptosis become abnormally hypermethylated and silenced in nearly every tumor type [2]. Histone deacetylases mediate complex changes in the histone structure resulting in the establishment of repressive chromatin structures [3]. Loss of lysine acetylation in histones is the first step in gene silencing and can lead to decreased DNA repair accelerating molecular events resulting in the development of cancer [3, 4].
Carboplatin is an effective chemotherapeutic agent for patients with epithelial ovarian cancer, but resistance to platinum therapy eventually develops [5–8]. Azacitidine inhibits methylation of replicating DNA by stoichiometric binding with DNA methyltransferase 1 thus inducing re-expression of epigenetically silenced genes [9]. Valproic acid (VPA), a histone deacetylase inhibitor, induces a hyper-acetylated state of histones resulting in a more open chromatin structure rendering cells more prone to apoptosis induced by chemotherapy [10]. Concurrent DNA demethylation and histone deacetylase inhibition are synergistic in gene expression activation, possibly because decreased methylation is a prerequisite for effective transcription following histone deacetylase inhibition [11]. This concept of gene reactivation overcoming platinum resistance has been demonstrated preclinically in which death receptor 4 (DR4) mRNA expression was increased in platinum-resistant ovarian cancer cell line 2008/C13 after azacitidine treatment, and such treatment increased the sensitivity at 2008/C13 to platinum [12].
In a phase 1b-2a trial of azacitidine and carboplatin, decreased DR4 methylation in peripheral blood mononuclear cells (PBMC) correlated with responders (75%) versus non-responders (38%) suggesting that reactivation of silenced genes might be associated with responses [13]. VPA in combination with cisplatin showed synergistic cytotoxicity in ovarian cancer cells resistant to cisplatin [14]. In a phase 1/2 study with leukemia patients, combination treatment with VPA and decitabine, a deoxy derivative of azacitidine, showed a transient reversal of aberrant epigenetic modifications with twelve (22%) objective responses and a 50% response rate in a subset of patients without prior therapy [15]. Since hypomethylation induced by a DNA methylation inhibitor is optimized between days 5 and 12, introducing a histone deacetylase inhibitor sequentially during this therapeutic window may maximize its effect [16].
We performed a phase I trial administering sequential azacitidine and VPA in combination with carboplatin based on our hypothesis that this combination may overcome resistance to platinum. The primary objectives of this study were a) to determine the acceptable dosages of these three agents used in combination in the treatment of patients with advanced solid malignancy; b) to determine whether DNA methylation and histone acetylation of tumor tissue and PBMCs occurred at tolerable dose levels of the combination; and c) to describe the toxicity profile of this drug combination.
MATERIALS AND METHODS
Patients
Patients were eligible if they had histologic proof of advanced cancer and progressed following standard therapy or for whom no standard effective therapy was available; measurable disease, as defined by Response Evaluation Criteria in Solid Tumors 1.0 (RECIST) [17]; Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less; adequate function of bone marrow (absolute neutrophil count ≥1,500/uL, platelet count ≥100,000/uL, hemoglobin ≥9.0 g/dL), liver (albumin ≥3.0 g/dL, bilirubin ≤2.0 mg/dL, alanine aminotransferase ≤3x upper limit of normal), and kidney (creatinine ≤2.0 mg/dL). Patients must have been off all previous chemotherapy or radiotherapy for 3 weeks (within two weeks if given weekly, six weeks for nitrosoureas or mitomycin C, and 30 days if previously treated with an investigational drug). All patients signed consent in accordance with the guidelines of the M.D. Anderson Cancer Center Institutional Review Board.
Procedures
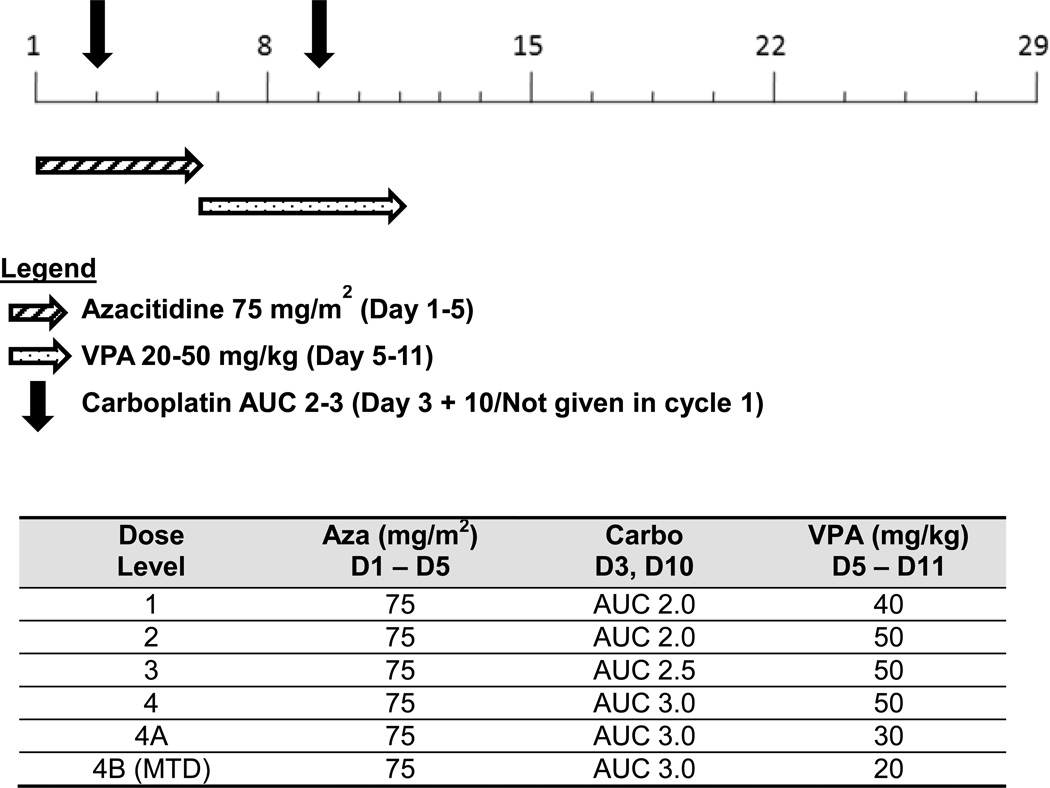
A modified 3+3 design was used for this study (Supplementary Material). Patients started with subcutaneous injection of azacitidine 75 mg/m2 daily for 5 days (days 1 to 5) followed by oral intake of VPA at the dosage escalated from 40 mg/kg daily to the target dosage at 50 mg/kg daily for 7 days from days 5 to 11 (Fig. 1). If the patient was not able to tolerate subcutaneous injection of azacitidine, intravenous infusion of azacitidine at the same dosage was allowed. Starting in cycle 2, patients also received intravenous infusion of carboplatin at the dosage escalated from AUC 2.0 to AUC 2.5 and then 3.0 on day 3 and day 10 in addition to azacitidine and VPA. Intra-patient dose escalation was not allowed. The treatment repeated once every 28 days until prohibitive toxicity, tumor progression, or patient withdrawal. Prophylactic pegfilgastrim was permitted on day 12 at the discretion of treating physician.
Fig. 1.
Dose schema for azacitidine, carboplatin, and valproic acid (VPA). Abbreviations: AUC, area under the curve; D, day; MTD, maximum tolerated dose
Toxicities were graded using the Common Terminology Criteria for Adverse Events (version 3.0) [18]. Treatment on subsequent cycles required adequate bone marrow function (absolute neutrophil count greater than 1,500/uL, platelet count greater than 75,000/uL) and resolution of toxicity to less than grade 2 or pre-therapy baseline. Dose escalation proceeded if no more than one of three or four patients experienced dose-limiting toxicity (DLT). Hematological DLT was defined as platelets less than 25,000/uL or bleeding associated with platelets less than 50,000/uL, absolute neutrophil count less than 500/uL for more than 7 days, neutropenic fever, or more than 14 days of delay in initiation of subsequent treatment because of inadequate hematological parameters. Non-hematological DLT was defined as grade 3 or greater nonhematological toxicity other than nausea, vomiting, or fatigue occurring during the first cycle.
Tumor response was assessed with RECIST (version 1.0) [17] and serum CA125 (Supplementary Material). Baseline radiological assessments were done within 28 days before initiation of treatment. The first restaging evaluation was performed after the third cycle of treatment and every two cycles thereafter or earlier if clinically indicated.
DNA Extraction and Methylation Analysis
Blood samples from patients were collected into EDTA-vials at enrollment and before each cycle of treatment. White blood cells were separated on Ficoll-Hypaque gradients and stored at –20°C until analyzed.
DNA was extracted from PBMCs obtained from patients before and after carboplatin, azacitidine, and VPA treatment using standard phenol-chloroform extraction, representing DNA from lysed normal blood cells as well as circulating tumor cells. Methylation analysis was done using a methylation kit (EZ-96 gold; Zymo Research, Orange, CA). MethPrimer software was used for the prediction of CpG island of DR4 (ACCESSION EF064713; GI: 117606477) and design of methylation specific primers. The sequence of primers for methylated DR4 promoter was forward, TTGGAGCGTAATGGTTTTATTTC; reverse, AATACCTATAATCCCAACCACTCG, and that for unmethylated DR4 promoter was forward, GTTGGAGTGTAATGGTTTTATTTTG; reverse, AATACCTATAATCCCAACCACTCAA. The methylation-specific PCR conditions were 94°C for 5 minutes with hot start, then 94°C for 30 seconds, 58°C or 60°C for 30 seconds, and 72°C for 1 minute repeated for 40 cycles. Universal methylated and unmethylated control DNAs were used for the positive control (Chemicon International). All methylation-specific PCRs were repeated twice separately. Methylated DR4 was normalized by both unmethylated DR4. Image analysis (Scion Image for Windows) was used for semi-quantitative measurement of methylated and unmethylated DR4. We defined at least 10% of DR4 methylation changes as a cut-off value.
Western Blot Analysis of Histone H3 Acetylation
Histone H3 acetylation was determined by Western blot analysis. Briefly, acid extracts from PBMCs obtained from patients before and after treatment were separated on a 10% gel by SDS-PAGE and transferred to nitrocellulose paper, blocked with 5% milk for 2 hours at room temperature (RT) and incubated with polyclonal antibody against acetyl-Histon H3 (Millipore, Billerica, MA) overnight at 4°C followed by incubation with horseradish peroxidase-conjugated second antibody for 1 hours at RT. Blots were developed with the ECL Western Blotting Detection Kit (GE Healthcare). GAPDH was used for loading control.
Statistical Analysis
The association between DR4 methylation in responders versus non-responders and high versus low dose of VPA was analyzed using an unpaired t-test. The difference in DR4 methylation between cycle 1 and cycle 2 was analyzed using a paired t-test.
RESULTS
Patient Characteristics
Thirty-two patients who met the inclusion and exclusion criteria were enrolled in this study (Table 1). Most patients were heavily pretreated, with a median of five prior systemic treatments; 53% had previously been treated on a phase 1 trial. Two patients (6%) had brain metastasis at the time of enrollment. The majority of patients had ECOG performance status of 1. The most common tumor type enrolled was ovarian (n=10), and all patients with ovarian cancer were platinum-refractory or resistant.
Table 1.
Patient characteristics
| Characteristic | Total |
|---|---|
| Number of patients | 32 |
| Median age | 62 yrs (Range 38–78) |
| Sex | |
| Male | 12 (37.5%) |
| Female | 20 (62.5%) |
| ECOG Performance Status | |
| 0 | 8 (25.0%) |
| 1 | 23 (71.9%) |
| 2 | 1 (3.1%) |
| Prior treatment | |
| Surgery | 29 (90.6%) |
| Radiation | 19 (59.4%) |
| Chemotherapy | 32 (100.0%) |
| Phase I trial | 17 (53.1%) |
| Median number of prior systemic treatments | 5 (Range 1–10) |
| Diagnosis | |
| Ovarian | 10 |
| Melanoma | 4 |
| Colorectal | 3 |
| Breast | 2 |
| Endometrial | 2 |
| Nasopharyngeal | 2 |
| Cervical | 1 |
| Cholangiocarcinoma | 1 |
| Esophageal | 1 |
| Non-small cell lung carcinoma | 1 |
| Pancreatic | 1 |
| Prostate | 1 |
| Sebaceous Cell | 1 |
| Thymic | 1 |
| Squamous cell carcinoma larynx | 1 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group
Toxicity and Recommended Dose
All patients treated were evaluable for toxicity (Table 2). The most common (≥20% incidence) drug-related toxicities observed included fatigue (81%), neutropenia (69%), anemia (50%), thrombocytopenia (47%), nausea (47%), drowsiness (31%), and altered mental status (25%). Twenty-five patients (78%) experienced drug-related toxicities ≥ grade 3.
Table 2.
Treatment-related grade 3–4 adverse events
| Dose Level | 1 n=3 |
2 n=3 |
3 n=8 |
4 n=7 |
4A n=8 |
4B† n=3 |
Total n=32 |
|
|---|---|---|---|---|---|---|---|---|
|
Azacitidine Dose, mg/m2 SC D1-D5 |
75 | 75 | 75 | 75 | 75 | 75 | ||
|
Valproic Acid Dose, mg/kg PO D5-D11 |
40 | 50 | 50 | 50 | 30 | 20 | ||
|
Carboplatin Dose, AUC IV D3, D10 |
2.0 | 2.0 | 2.5 | 3.0 | 3.0 | 3.0 | ||
| Altered Mental Status | ||||||||
| Grade 3 | 1 | 0 | 1 | 2 (2 DLTs) | 2 (2 DLTs) | 0 | 6 (19%) | |
| Anemia | ||||||||
| Grade 3 | 1 | 0 | 3 (1 DLT) | 1 | 0 | 0 | 5 (16%) | |
| Grade 4 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (3%) | |
| Dizziness | ||||||||
| Grade 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (3%) | |
| Drowsiness | ||||||||
| Grade 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (3%) | |
| Fatigue | ||||||||
| Grade 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 (3%) | |
| Fever | ||||||||
| Grade 3 | 0 | 0 | 1 (1 DLT) | 0 | 0 | 0 | 1 (3%) | |
| Hypotension | ||||||||
| Grade 3 | 1 | 0 | 0 | 0 | 0 | 0 | 1 (3%) | |
| Infection | ||||||||
| Grade 3 | 1 | 0 | 0 | 0 | 0 | 0 | 1 (3%) | |
| Nausea | ||||||||
| Grade 3 | 1 | 2 | 0 | 1 | 0 | 0 | 4 (13%) | |
| Neutropenia | ||||||||
| Grade 3 | 2 | 3 | 1 | 1 | 0 | 1 | 8 (25%) | |
| Grade 4 | 1 | 0 | 4 (1 DLT) | 2 | 3 (1 DLT) | 2 | 12 (38%) | |
| Thrombocytopenia | ||||||||
| Grade 3 | 0 | 0 | 1 | 1 | 1 | 1 | 4 (13%) | |
| Grade 4 | 1 | 0 | 1 | 1 | 0 | 0 | 3 (9%) | |
| Vomiting | ||||||||
| Grade 3 | 1 | 2 | 0 | 1 | 0 | 0 | 4 (13%) | |
Abbreviations: DLT, dose-limiting toxicity; IV, intravenous; PO, per oral; SC, subcutaneous; D1-D5, day 1 thru day 5; D5-D11, day 5 thru day 11; D3, day 3; D10, day 10
Maximum Tolerated Dose
No DLTs were observed at the first two dose levels. At dose level 3 (75 mg/m2 azacitidine, AUC 2.5 carboplatin, and 50 mg/kg VPA), one out of eight patients experienced grade 4 neutropenia and grade 3 fever and anemia. At dose level 4 (75 mg/m2 azacitidine, AUC 3.0 carboplatin, and 50 mg/kg VPA), two out of seven patients experienced grade 3 altered mental status which was attributed to VPA. A lower dose of VPA was introduced in dose level 4A (75 mg/m2 azacitidine, AUC 3.0 carboplatin, and 30 mg/kg VPA), and two out of eight patients experienced grade 3 altered mental status and one out of eight patients experienced grade 4 neutropenia. The dose of VPA was reduced further in dose level 4B (75 mg/m2 azacitidine, AUC 3.0 carboplatin, and 20 mg/kg VPA), and no dose-limiting toxic effects were observed.
Adverse events that needed a reduction in dose were noted in 12 out of 32 patients. The most common cause of dose reduction was neutropenia (n=5). Two patients died while on treatment, however, no deaths resulted from treatment-related adverse events. There are no other serious adverse events to report.
Antitumor Activity
Six out of 32 patients (19%) received more than 4 cycles (1 cycle = 4 weeks) of treatment. The median number of cycles received was 3 (range 1–11). Twelve out of 32 patients had a follow-up restaging with measurable disease. Among the remaining 20 patients, three were evaluable but not measurable and 17 were not evaluable for first restaging because treatment was discontinued before the first restaging evaluation (10 for clinical progressive disease, 4 for toxicity, and 3 withdrew consent), and all of these patients are considered treatment failures.
Among the ten patients with ovarian cancer, three (30%) achieved minor responses or stable disease per RECIST lasting ≥ 4 months (Table 3). CA125 reductions were seen in some patients, with one individual demonstrating a reduction of more than 50% who received treatment for seven months. This patient showed a tumor decrease of 26%.
Table 3.
Responses in ovarian cancer patients (n=10)
| Pt # | Dose Level |
# of Prior Regimens |
% Maximum Change in DR4 Methylation |
Best Response (RECIST) |
Best CA-125 Response |
# Months Treated |
|---|---|---|---|---|---|---|
| 4 | 2 | 5 | −64% | −4% SD |
2110.5 → 1314.6 (Decreased 37.7%) |
9 |
| 6 | 2 | 9 | −11% | +19% SD |
8593.4 → 5478.9 (Decreased 36.2%) |
5 |
| 7 | 3 | 5 | −43% | −26% SD |
5196.3 → 929.9 (Decreased 82.1%) |
7 |
| 8 | 3 | 10 | −49% | +6% PD* |
171.3 → 90.9 (Decreased 46.9%) |
3 |
| 9 | 3 | 7 | −32% | Not Measurable PD |
73.0 → 72.5 (Decreased 0.68%) |
3 |
| 10 | 3 | 10 | −55% | +12% PD* |
1322.5 -→ 5122.7 (Increased 287.3%) |
3 |
| 13 | 3 | 3 | N/A | +11% SD |
579.0 → 982.6 (Increased 69.7%) |
1 |
| 14 | 3 | 6 | N/A | +43% PD |
41.3 → 89.3 (Increased 116.2%) |
3 |
| 19 | 4 | 6 | −32% | +35% PD |
182.0 → 170.0 (Decreased 6.5%) |
3 |
| 31 | 5 | 7 | N/A | Not Restaged | N/A | 1 |
Abbreviations: DR4, death receptor 4; N/A, not available; PD, progressive disease; SD; stable disease
new lesions present
One patient with prostate cancer (3%) completed 11 cycles of treatment and achieved stable disease per RECIST lasting ≥9 months. Two other patients (6%), one with cervical cancer and another with colorectal cancer, achieved stable disease lasting ≥4 months.
Death Receptor 4 (DR4) Methylation and Histone Acetylation in PBMCs
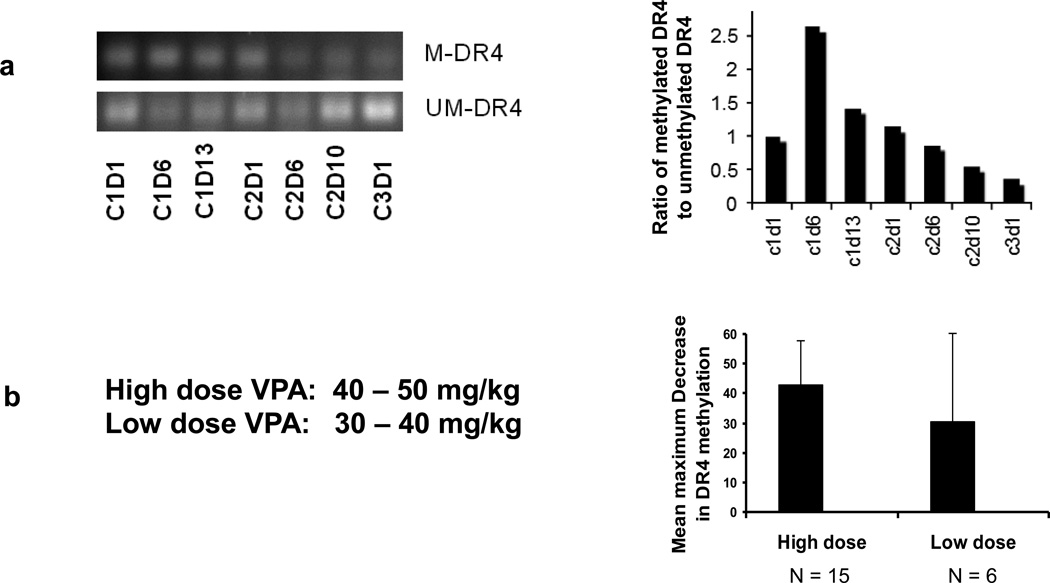
To define changes in DNA methylation, DR4 methylation levels were determined using methylation-specific PCR of DNA extracted from PBMC sampled from patients before and after carboplatin, azacitidine, and VPA treatment. When the dynamic changes of DR4 methylation in PBMC were analyzed in patient #4 with ovarian cancer, who had stable disease and received 9 months of treatment (Table 3), we found that DR4 methylation decreased slightly during the second cycle and reached a maximum decrease on cycle 3 day 1 by about 64% (Fig. 2a). Of the 19 patients with evaluable serial PBMCs, 18 (95%) achieved at least a 10% maximum decrease of DR4 methylation while on treatment, and 15 of 19 patients (79%) achieved at least a maximum decrease of 30%. There was no statistically significant association between the decrease in DR4 methylation and the dose of VPA (Fig. 2b), clinical response, or number of cycles received.
Fig. 2.
Dynamic changes in DR4 methylation in peripheral blood mononuclear cell (PBMC) DNA from patient #4, with ovarian cancer, who had stable disease and received 9 months of treatment (A). No correlation between valproic acid (VPA) dose and decrease in DR4 methylation in PBMC DNA (p = 0.3) (B). Abbreviations: M-DR4, Methylated DR4; UM-DR4, Unmethylated DR4
The mean maximum decrease in DR4 methylation was 7% in cycle 1 versus 24% in cycle 2 (p = 0.08). The changes in DR4 methylation between patients who achieved prolonged stable disease (≥4 months) versus those who had progressive disease on treatment were not significant (maximum decrease 37% versus 47% respectively, p = 0.21).
Histone acetylation analysis was limited to a subset of only six patients because of inadequate remaining DNA after DR4 methylation analysis. Qualitative assessment of histone acetylation in the six patients by Western blot indicated no clear trend of histone acetylation changes while on treatment.
DISCUSSION
Our study demonstrates the first attempt to reverse platinum resistance with combination methylation and histone deacetylase inhibition. Three out of ten patients (30%) with platinum-refractory or resistant ovarian cancer achieved stable disease per RECIST, including one patient with a 26% decrease in tumor size (82% decrease in CA-125) who received treatment for seven months and one patient on treatment for 9 months (30% decrease in CA-125). These three patients were heavily pretreated, with 5, 5, and 9 prior treatment regimens received in the past. Importantly, these three patients achieved disease stability for longer duration on this clinical trial than on each patient’s previous treatment, including 9 months vs. 2 months (topotecan), 5 months vs. 3 months (docetaxel), and 7 months vs. 2 months (letrozole), respectively. Of note, the patient with the largest decrease in methylation received treatment longer than all other patients with ovarian cancer. In a similar study that combined azacitidine and carboplatin, 3% of platinum-refractory or resistant ovarian cancer patients achieved complete response, 10% achieved partial response, and 33% achieved stable disease per RECIST, but these patients were less heavily pretreated [13]. VPA was added sequentially in our study in an effort to further overcome platinum resistance and maximize response because VPA has been shown to synergistically improve cytotoxicity in combination with cisplatin in platinum resistant ovarian cancer cell lines [14]. In combination with a DNA methylation inhibitor, the addition of a histone deacetylase inhibitor has shown robust re-expression of silenced genes [11].
A maximum decrease of at least 30% in DR4 methylation was seen in 79% of patients, suggesting pharmacodynamic response to the treatment regimen. However, the changes in DR4 methylation were not significantly different between patients with stable disease ≥4 months compared to those with progressive disease. Considering that this was a phase 1 study with variable dose levels and a heterogeneous heavily pretreated population, assessment of the correlation between methylation status and response is not possible; such assessment will require a prospective study in a larger and homogenous population. Similar challenges have been encountered in patients with advanced cancers on combination azacitidine and VPA therapy in another phase 1 study [19]. An initial spike in DR4 methylation was observed in week one of the first cycle, as seen in Fig. 2a and in other studies [20] which has been attributed to a stress response or another unclear etiology upon starting the treatment regimen.
The delayed initiation of carboplatin therapy until cycle 2 is the primary limitation of this study. Azacitidine and VPA were administered sequentially in order to maximize the therapeutic window. However, the cytotoxic agent wasn’t introduced until the second cycle in order to determine the methylation changes induced by azacitidine and VPA without interference from the platinum therapy. Nonetheless, the delay of platinum treatment may have contributed to the modest antitumor response seen. Indeed, 10 patients progressed clinically before the first restaging. Future trials testing similar concepts should consider initiating all three study drugs in the first cycle in order to maximize response.
The combination of azacitidine, VPA, and carboplatin was poorly tolerated. The drugs were given sequentially in an effort to maximize their therapeutic effects based on previous work [16], and we anticipated that the staggered administration might also result in less toxicity. Unfortunately, 78% of patients still experienced adverse events of grade 3 or higher, most commonly fatigue (81%) and neutropenia (69%). Confusion and somnolence occurred in 25% of patients, probably secondary to VPA treatment. Therefore, the last two dose levels required de-escalation of the VPA dose. The recommended phase 2 doses are azacitidine 75 mg/m2 (days 1–5), VPA 20 mg/kg (days 5–11), and carboplatin AUC 3.0 (days 3 and 10, not given in cycle 1).
Genetic mutations have also been shown to influence platinum resistance. Mutation of the BRCA1/BRCA2 gene in ovarian cancer patients is correlated with positive responses to platinum treatment [21], and reversion mutation of BRCA2 has led to platinum resistance [22]. Other mechanisms aside from epigenetic modification can lead to platinum resistance and should be explored further.
Methylation of hMLH1 and DAPK have been shown to result in chemoresistance [23–25], whereas methylation of BRCA1 and FANCF result in increased platinum sensitivity and positive response [26, 27]. Hypomethylation can also activate rare oncogenes typically silenced in cancer, such as EGFR and COX2 [28]. Because methylation is associated with both sensitivity and resistance, hypomethylation treatment may need to be tailored according to each patient’s individual methylation profile.
At higher doses of VPA, a trend of greater demethylation was observed, but did not reach statistical significance and may suggest that VPA is not a potent histone deacetylase inhibitor, as has been observed in other studies of VPA [29–31]. With the increased incidence and severity of somnolence and altered mental status requiring dose reduction of VPA at FDA-approved doses, it may not be possible to achieve the dose level necessary for clinical benefit. Future studies should examine the use of other, possibly more potent, histone deacetylase inhibitors such as belinostat [32] or vorinostat [33].
Dose delays and dose reductions due to toxicity made it difficult to maintain consistent delivery of treatment with this drug combination. The high incidence of toxicities observed in our study may prevent the duration of treatment necessary in order to achieve clinical response [20]. Development of resistance may also explain the diminished treatment response seen [34]. The frequent dose interruptions may have caused drug resistance to develop rapidly. Long-term use of a DNA methyltransferase 1 (DNMT1) inhibitor may be compensated for by surrounding DNMTs ultimately resulting in methylation and re-silencing of the gene [35]. Upon failure of azacitidine, subsequent treatment with decitabine has demonstrated an overall response rate of 28% without significant toxicity [36]. Resistance should be considered in future studies and use of decitabine as second line treatment further explored.
Future studies should consider preselecting patients based on known methylation status in order to optimize treatment response. CpG-island methylation patterns and targets have been identified with tumor-type specificity [37, 38], and a genomic screen for colorectal cancer has been used to identify which genes should be targeted with demethylating therapy [39]. Lower, more continuous doses of treatment that avoid dose interruptions might also be more successful. This strategy results in methylation and acetylation changes in the clinic setting [19]. The combination of methylation and histone deacetylase inhibition merits further examination in a defined subgroup of patients.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Adrienne Howard and Alambardar Khuwaja (Department of Investigational Cancer Therapeutics) for their contributions to this study’s operation and DeYu Shen (Department of Gynecologic Oncology and Reproductive Medicine) for technical assistance.
GRANT SUPPORT
Financial support for this study was provided by Celgene Corporation and UT MD Anderson SPORE in Ovarian (P50 CA083639) and Uterine (P50 CA098258) cancer.
Footnotes
Financial Support: Celgene
ETHICAL STANDARDS
The research performed complies with the current laws of the country in which it was performed. All patients signed consent in accordance with the guidelines of the M.D. Anderson Cancer Center Institutional Review Board.
CONFLICT OF INTEREST
Dr. Falchook received research funding from Celgene. The other authors have no conflict of interest to disclose.
REFERENCES
- 1.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 3.Mutskov V, Felsenfeld G. Silencing of transgene transcription precedes methylation of promoter DNA and histone H3 lysine 9. EMBO J. 2004;23:138–149. doi: 10.1038/sj.emboj.7600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell-cycleregulated histone H3 lysine 56 acetylation in the DNA damage response. Nature. 2005;436:294–298. doi: 10.1038/nature03714. [DOI] [PubMed] [Google Scholar]
- 5.Markman M. Pharmaceutical management of ovarian cancer : current status. Drugs. 2008;68:771–789. doi: 10.2165/00003495-200868060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RJ, Jr, Alvarez RD, Armstrong DK, Boston B, Chen LM, Copeland L, Fowler J, Gaffney DK, Gershenson D, Greer BE, Grigsby PW, Havrilesky LJ, Johnston C, Lancaster JM, Lele S, Matulonis U, O'Malley D, Ozols RF, Remmenga SW, Sabbatini P, Schink J, Teng N. Ovarian cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2008;6:766–794. doi: 10.6004/jnccn.2008.0058. [DOI] [PubMed] [Google Scholar]
- 7.Markman M. Second-line therapy for ovarian cancer. Clin Adv Hematol Oncol. 2008;6:421–422. [PubMed] [Google Scholar]
- 8.Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34:S1–S15. doi: 10.1053/j.seminoncol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Creusot F, Acs G, Christman JK. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982;257:2041–2048. [PubMed] [Google Scholar]
- 10.Sato T, Suzuki M, Sato Y, Echigo S, Rikiishi H. Sequence-dependent interaction between cisplatin and histone deacetylase inhibitors in human oral squamous cell carcinoma cells. Int J Oncol. 2006;28:1233–1241. [PubMed] [Google Scholar]
- 11.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Hu W, Shen DY, Kavanagh JJ, Fu S. Azacitidine enhances sensitivity of platinum-resistant ovarian cancer cells to carboplatin through induction of apoptosis. Am J Obstet Gynecol. 2009;200:177 e171–179. doi: 10.1016/j.ajog.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Fu S, Hu W, Iyer R, Kavanagh JJ, Coleman RL, Levenback CF, Sood AK, Wolf JK, Gershenson DM, Markman M, Hennessy BT, Kurzrock R, Bast RC., Jr Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer. 2011;117:1661–1669. doi: 10.1002/cncr.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CT, Lai HC, Lee HY, Lin WH, Chang CC, Chu TY, Lin YW, Lee KD, Yu MH. Valproic acid resensitizes cisplatin-resistant ovarian cancer cells. Cancer Sci. 2008;99:1218–1226. doi: 10.1111/j.1349-7006.2008.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, Rytting M, Wierda WG, Ravandi F, Koller C, Xiao L, Faderl S, Estrov Z, Cortes J, O'Brien S, Estey E, Bueso-Ramos C, Fiorentino J, Jabbour E, Issa JP. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S, Talpaz M, Garcia-Manero G, Kantarjian HM. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 19.Braiteh F, Soriano AO, Garcia-Manero G, Hong D, Johnson MM, Silva Lde P, Yang H, Alexander S, Wolff J, Kurzrock R. Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res. 2008;14:6296–6301. doi: 10.1158/1078-0432.CCR-08-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang F, Balch C, Schilder J, Breen T, Zhang S, Shen C, Li L, Kulesavage C, Snyder AJ, Nephew KP, Matei DE. A phase 1 and pharmacodynamic study of decitabine in combination with carboplatin in patients with recurrent, platinum-resistant, epithelial ovarian cancer. Cancer. 2010;116:4043–4053. doi: 10.1002/cncr.25204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 22.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 23.Strathdee G, MacKean MJ, Illand M, Brown R. A role for methylation of the hMLH1 promoter in loss of hMLH1 expression and drug resistance in ovarian cancer. Oncogene. 1999;18:2335–2341. doi: 10.1038/sj.onc.1202540. [DOI] [PubMed] [Google Scholar]
- 24.Gifford G, Paul J, Vasey PA, Kaye SB, Brown R. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin Cancer Res. 2004;10:4420–4426. doi: 10.1158/1078-0432.CCR-03-0732. [DOI] [PubMed] [Google Scholar]
- 25.Tang X, Wu W, Sun SY, Wistuba II, Hong WK, Mao L. Hypermethylation of the death-associated protein kinase promoter attenuates the sensitivity to TRAILinduced apoptosis in human non-small cell lung cancer cells. Mol Cancer Res. 2004;2:685–691. [PubMed] [Google Scholar]
- 26.Teodoridis JM, Hall J, Marsh S, Kannall HD, Smyth C, Curto J, Siddiqui N, Gabra H, McLeod HL, Strathdee G, Brown R. CpG island methylation of DNA damage response genes in advanced ovarian cancer. Cancer Res. 2005;65:8961–8967. doi: 10.1158/0008-5472.CAN-05-1187. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi T, Tischkowitz M, Ameziane N, Hodgson SV, Mathew CG, Joenje H, Mok SC, D'Andrea AD. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9:568–574. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 28.Issa JP, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009;15:3938–3946. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths EA, Gore SD. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45:23–30. doi: 10.1053/j.seminhematol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F, Cortes J, Wierda WG, Ouzounian S, Quezada A, Pierce S, Estey EH, Issa JP, Kantarjian HM, Garcia-Manero G. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 31.Blum W, Klisovic RB, Hackanson B, Liu Z, Liu S, Devine H, Vukosavljevic T, Huynh L, Lozanski G, Kefauver C, Plass C, Devine SM, Heerema NA, Murgo A, Chan KK, Grever MR, Byrd JC, Marcucci G. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25:3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 32.Steele N, Finn P, Brown R, Plumb JA. Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br J Cancer. 2009;100:758–763. doi: 10.1038/sj.bjc.6604932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaser KB. HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2'-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachman KE, Park BH, Rhee I, Rajagopalan H, Herman JG, Baylin SB, Kinzler KW, Vogelstein B. Histone modifications and silencing prior to DNA methylation of a tumor suppressor gene. Cancer Cell. 2003;3:89–95. doi: 10.1016/s1535-6108(02)00234-9. [DOI] [PubMed] [Google Scholar]
- 36.Borthakur G, Ahdab SE, Ravandi F, Faderl S, Ferrajoli A, Newman B, Issa JP, Kantarjian H. Activity of decitabine in patients with myelodysplastic syndrome previously treated with azacitidine. Leuk Lymphoma. 2008;49:690–695. doi: 10.1080/10428190701882146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomaki P, Lang JC, Schuller DE, Yu L, Bloomfield CD, Caligiuri MA, Yates A, Nishikawa R, Su Huang H, Petrelli NJ, Zhang X, O'Dorisio MS, Held WA, Cavenee WK, Plass C. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 38.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.