Abstract
Using an olfactory conditioning procedure, brain stimulation reward threshold measurements, and functional magnetic resonance imaging (fMRI), we investigated brain stimulation reward threshold change and fMRI neural activation in response to a cocaine-associated odor cue. In the first brain stimulation experiment, over 10 days of rate–frequency curve-shift testing, rats were administered intravenous cocaine (1.0 mg/kg) paired with a contextual cue of peppermint odor previously placed in the operant chamber or they were given vehicle treatment (no cocaine) in the presence of no olfactory cue. Following a 14-day drug-free rest period, rats were again given the rate–frequency curve-shift threshold test with or without the odor cue. In a second experiment, rats were similarly conditioned with a peppermint odor but with intraperitoneally delivered cocaine (10 mg/kg). After a 14 day rest period, rats were imaged on a 7-T MRI for their blood oxygen level dependent (BOLD) in response to the cocaine-paired peppermint odor versus an unpaired neutral lemon odor. In the brain stimulation experiment, expected significant reward threshold shifts were produced by cocaine and, importantly, about half that level of shift was produced by the paired contextual olfactory cue. In the fMRI experiment, the insular cortex showed a significantly greater BOLD activation in cocaine-treated versus saline-treated animals to the olfactory cue, but not with the unpaired lemon scent. These data are in agreement with previous studies suggesting a role of the insular cortex in attributing reward value (positive or negative) to conditioned odor stimuli.
Keywords: Cocaine, Cue, Olfactory, Odor, Addiction, Reward, Brain stimulation, fMRI, Blood oxygen level dependent signal, Insular cortex
1. Introduction
Drug-paired stimuli, or cues, can elicit relapse to cocaine seeking [19,34,35,40] and can act as an incentive in the absence of the drug [8]. Previously neutral stimuli repeatedly associated with self-administered cocaine can provoke heightened levels of drug seeking, even after periods of abstinence from drug use [5,19]. Experiments in humans [14,27,41], non-human primates [1,2], and laboratory rodents [17,23,34] are providing insight on the neural circuits involved in drug–cue associations.
Site-specific lesions have supported a role of basolateral and central amygdala, prefrontal cortices, and ventral striatal regions in distinct stages of reinstatement of intravenous self-administration [35,38]. Human functional MRI studies provide evidence of neural processing in prefrontal cortex and striatum during presentation of drug-paired stimuli [14,40,41]. In many studies the conditioned stimulus modality is visual and seldom is the cue olfactory. However, the proximity of olfactory input to neural cortices involved in reward suggests a unique and intimate relationship of olfactory stimuli with reward-seeking behavior. Olfactory stimuli paired with cocaine have been shown to reinstate active operant lever responses for cocaine [9], modulate cocaine seeking behavior [37] and contribute to cocaine conditioned locomotor effects [30].
Functional MRI has been used to investigate cocaine stimulated brain activation in both anesthetized and awake rats [4,11,20,22]. Febo et al. [11] used pharmacological MRI in awake rats to investigate areas of the brain showing increased blood oxygen level dependent (BOLD) signal in response to cocaine. They observed blunted BOLD signal responses to cocaine with repeated exposure compared with acutely administered cocaine. More recently, a study was carried out in rats trained to self-administer cocaine for 20 days and imaged for their response to intravenous cocaine following 3 day abstinence period [20]. Cerebral blood volume changes in the prefrontal cortex correlated with history of drug intake. The present study used methods similar to those cited above for awake rats in order to investigate the neural processing of a cocaine-associated odor cue. Brain stimulation reward has long been investigated using the rate–frequency reward-shift method to get quantitative estimates of reward threshold shifts under cocaine or under cues associated with cocaine [10,21,36]. These two methods were combined in separate experiments in this report.
2. Materials and methods
Adult male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) used for intracranial self-stimulation (ICSS) studies weighed between 350 and 550 g and were individually housed. For functional MRI, male Sprague–Dawley rats (Charles River) weighing 200–225 g at the start of the experiment were pair housed. All rats received ad libitum water and food and were kept on a 12 h light–dark cycle (lights on at 07:00 h) in a temperature-and humidity-controlled room. All experiments were performed during the light phase of the cycle. Rats were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (8th Edition, 2011) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The Institutional Animal Care and Use Committee at Northeastern University approved the protocols used for this study.
2.1. Intracranial self-stimulation
Nine rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and implanted with a monopolar, stainless steel electrode (Plastics One, Roanoke, VA) aimed at the lateral hypothalamus according to previous procedures [10]. Bregma-based stereotaxic coordinates [28] were: A.P. = +3.0 mm, M.L. = ±1.7 mm, D.V. = +7.5 mm. The electrode’s ground wire was attached to the skull screws serving as a return path for the current. The entire electrode assembly was secured to the skull with dental acrylic. In a separate surgery, rats were implanted with intravenous catheters for cocaine administration.
One week following surgery, behavioral testing was conducted in a 25 cm3 operant Plexiglas chamber with a house light mounted on the ceiling and a reinforcement light mounted adjacent to the metal lever positioned 5 cm above the chamber’s floor [10]. Animals were trained on a variable interval (VI) schedule of reinforcement to lever press for 0.25 s bursts of 0.1 ms square-wave monophasic cathodal pulses of 100 Hz. During the initial phase of training, schedule of reinforcement was progressively switched from VI.1 s to VI.3 s. Animals were then given extinction/reacquisition training, where the pulse frequency alternated between 10 Hz and 158 Hz every 90 s. At the completion of training, subjects were given a chance to respond on a rate–frequency curve for six descending stimulation frequencies (158–16 Hz in 0.2 log unit steps). For the next three weeks of stabilization, rats were tested on the rate–frequency curve under baseline conditions until daily variations in locus of rise (LOR) were not greater than 0.1 log units for at least 10 consecutive days.
Cocaine solutions (Sigma–Aldrich Co., St. Louis, MO) were prepared daily in 0.9% bacteriostatic sterilized saline and administered by a single IV injection of 1.0 mg/kg 5 min prior to brain stimulation testing. IV injections were administered in the same room where the animals were tested and where the operant chambers were perfumed with five drops of peppermint mouth freshener (Walgreens Co.) on slips of Kimwipe®. For 10 days an alternating daily pattern of training with cocaine + odor versus no cocaine + no odor was followed with a brain stimulation test administered on each day. Then 14 days of rest was given. On two final test sessions, rats were placed in the operant chamber once a day for 2 days and either the cocaine-associated peppermint odor was presented or no peppermint odor was presented. The order of presentation of these two conditions was randomized across subjects.
LOR values were derived from each rate–frequency curve were determined by the broken line method [3]. Subtracting the LOR value for each test measured under saline and cocaine condition from the previously established average baseline LOR value (no drug) yielded the reported “difference from baseline” values for LOR. LOR difference data across training days 1 through 5 were analyzed using a two-way ANOVA with repeated measures. Post hoc testing was done using Sidak’s multiple comparison test (α = 0.05). Data on day 14 (challenge day) were analyzed with two-tailed unpaired t-test.
2.2. Functional MRI of a cocaine associated odor cue
Cocaine HCl (Sigma–Aldrich, St. Louis, MO) was dissolved in 0.9% sterile saline and injected at a dose of 10 mg/kg IP every other day over 10 days. On alternating days, rats received an IP injection of 0.9% sterile saline alone. Therefore, over the 10-day treatment period, cocaine treated animals received 5 days of cocaine alternated by 5 days of saline vehicle. Control animals only received 0.9% sterile saline every day over 10 days (10 saline injections). All injections were made in a separate room from the olfactory cue conditioning room. The olfactory cue was provided on a slip of Kimwipes® with five drops of peppermint mouth freshener (Walgreens Co.), which was placed in the middle of the perforated lid of a locomotor activity cage (clear Plexiglas cages, dimensions: 40 cm3). The peppermint odor was presented only on cocaine treatment days and no odor was present on alternating saline injection days. Control rats received the same odor as cocaine subjects but without the cocaine injection. A 3-min delay between injections and odor conditioning ensured that the odor was ultimately associated with the emergent peripheral and hedonic effects of the cocaine and not pain associated with the injection procedure. Rats were kept in the locomotion box for 30 min with the room lights turned off. After the 10-day period, subjects were left in their home cages and received no drug or vehicle administration for 14 days.
Rats were acclimated to head and body restraint and MRI pulse sequence noise [18]. Animals were imaged once every day with a different olfactory stimulus presentation. The order of odor presentation was no scent (‘mock’ trial), lemonene (an unassociated odor; Sigma, St. Louis, MO), and finally peppermint (cocaine cue). Odor delivery during scanning was achieved by tubing connected at one end to an air pump and at the other end placed near the rats’ nose inside the bore of the magnet. Just outside of the magnet, an easily detachable plastic connector linked the clean tubing to the outlet tubing towards the rats’ nose. During odor delivery the connector was switched with a connector containing the odor on a strip of absorbent cotton swab with five drops of either the peppermint or lemonene scent.
Experiments were conducted in a 300 MHz Bruker USR 7T/20 cm horizontal magnet (Bruker, Germany) equipped with a Paravision 5.0 console (Bruker, Billerica, MA, USA). Functional imaging was performed using a multi-segmented T2-weighted fast spin echo pulse sequence (TE = 45 ms; TR = 2.5 s). Anatomical scans were obtained for each subject at a resolution of 2562 × 20 slices and a field of view of 30 mm with a slice thickness of 1.0 mm. Subsequent functional imaging is performed at a resolution of 642 × 20 slices with the same FOV and slice thickness. Full details for the MRI data analysis using Medical Image Visualization and Analysis (MIVA; ccni.wpi.edu) and in house software has been previously reported [12]. Scans were pre-screened and corrected for drift and minor head motion. The initial number of animals per each treatment group was 10 (10 saline and 10 cocaine). However, following screening for motion artifact, 4 rats per each group were deemed to have excessive, uncorrectable motion artifact. Each subject was registered to a fully segmented electronic rat brain atlas [28]. Statistical t tests were performed on each subject with the baseline period used as 26 repetitions immediately preceding odor and a stimulation window of 26 repetitions following odor. A conservative estimate of significance was employed by using a false-positive detection-controlling algorithm [15]. Activated voxel numbers and percent signal changes were exported to Graphpad Prism 6.0 (La Jolla, CA) for between groups analysis (two way ANOVA with Fisher’s LSD post hoc test and odor × drug as independent variables; α = 0.05). The groups were Saline-Lemon (n = 6), Saline-Peppermint (n = 6), Cocaine-Lemon (n = 10) and Cocaine-Peppermint (n = 6).
3. Results
In Experiment 1, cocaine significantly reduced LOR (increased reward) in the 0.2–0.3 log unit range compared with saline treatment (drug effect across all days F1,7 = 90, p < 0.0001). Peppermint odor alone reduced percent change in LOR below those observed without the odor (two tailed unpaired t-test t7 = 2.5, p = 0.04) (Fig. 1). Note that the olfactory cue alone produced about half the LOR shift of the cocaine treatment.
Fig. 1.
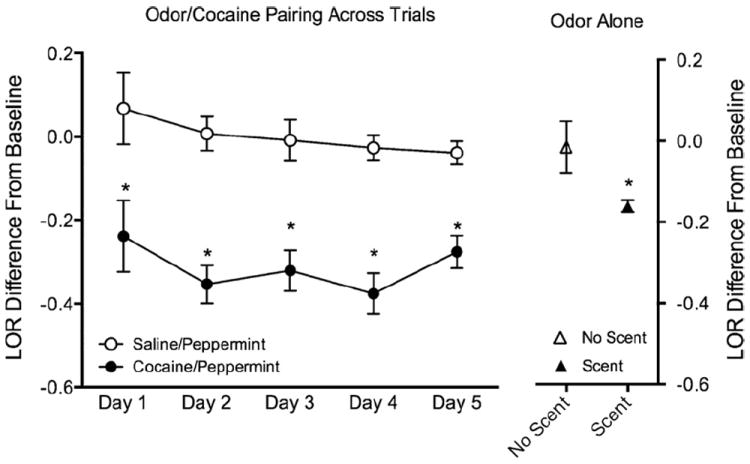
Effect of cocaine and a cocaine paired odor on brain stimulation reward. (Left) ICSS following treatment with saline or cocaine (paired with peppermint). (Right) ICSS in the absence and presence of a previously paired cocaine odor cue. Data presented as locus of rise (LOR) difference from baseline (±standard error). *p < 0.05 (repeated measures ANOVA for data on the right and t-test for data on the left).
In Experiment 2, we analyzed a total of 30 regions of interest (ROI) involved in reward processing, cognition-memory, sensorimotor functions, and olfactory processing. Fig. 2 shows composite coronal maps for peppermint-saline, peppermint-cocaine, lemonsaline and lemon-cocaine rats. A two way ANOVA revealed significant effects of odor stimulus (lemon versus peppermint; two way ANOVA F3,600 = 11.2) on the number of activated voxels in the insular cortex (p = 0.0006), piriform cortex (p = 0.01), dorsolateral and ventrolateral divisions of the dorsal striatum (p = 0.0001 and p = 0.0005, respectively). In these areas, lemon odor elicited a greater number of activated voxels than peppermint. Out of the 30 ROI studied only the insular cortex showed a selective effect of the cocaine-paired cue (Figs. 2 and 3). Peppermint odor elicited a greater activation in cocaine treated than in saline treated animals (F1,22 = 7.5, p = 0.01 Fisher’s LSD; Fig. 3). Percent change in BOLD signal in insular cortex was also greater in cocaine versus saline treated rats presented with peppermint but not lemon odor (Fig. 3).
Fig. 2.
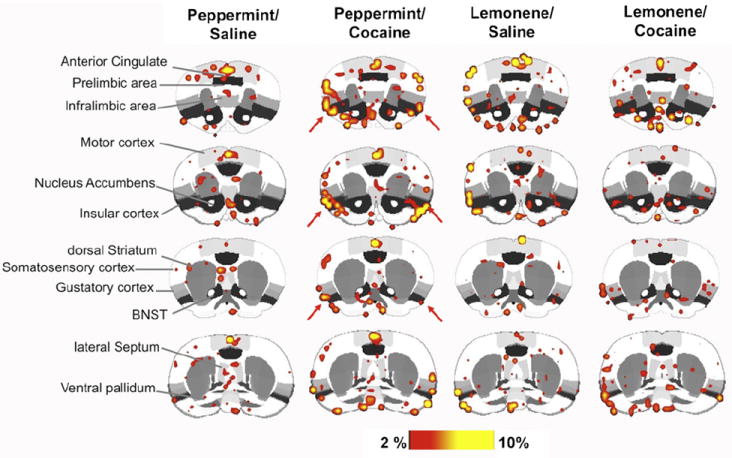
Composite functional magnetic resonance activation maps in rats treated with saline or cocaine in the presence of an olfactory stimulus. Images correspond to cocaine-paired (peppermint) and neutral odor (lemon). Scale bar hue indicates percent change value of voxels. These correspond to % changes but also to p value <0.05, FDR corrected. Red arrows highlight areas of the insular cortex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 3.
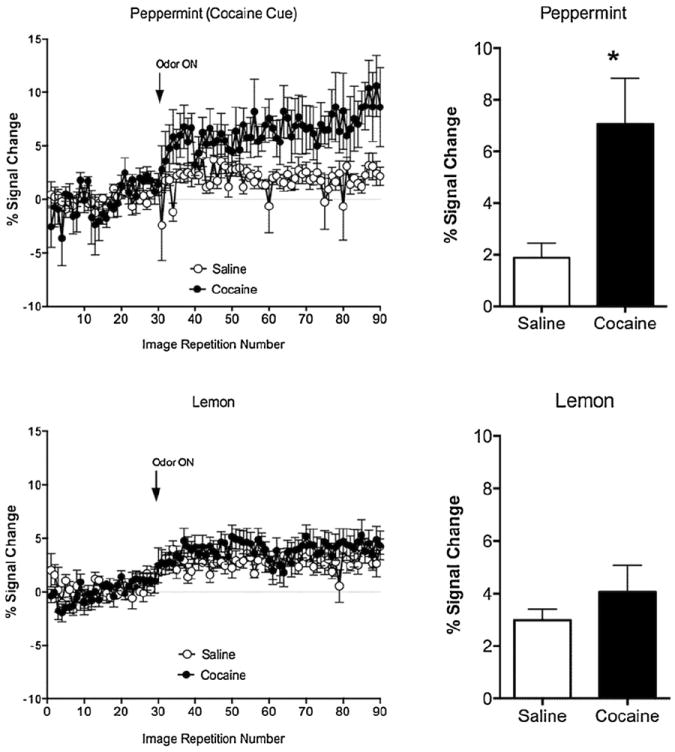
Percent change in blood oxygen level dependent signal of the insular cortex in response to a cocaine paired (peppermint) or a neutral unpaired (lemon) odor. Graphs on the left show data for BOLD signal over time (mean ± standard error) for peppermint (top left) and lemon odor (bottom left). Arrows show the time at which odor presentation was initiated. Bar graphs on the right show data averaged during the odor presentation epoch. *p < 0.05 (two way ANOVA).
4. Discussion
Results from the brain stimulation reward experiment demonstrate the expected reward-increasing effect of cocaine treatment in training, with cocaine-treated animals showing lowered stimulation threshold (LOR decrease) than saline-treated animals. Under testing with the cocaine-paired peppermint odor alone, the shift to a decreased reward threshold was also seen and to a surprisingly strong extent with LOR shifts under the odor alone condition being about half of what was seen with the cocaine itself. The initial behavioral results led us to hypothesize that the reward system of the rat would develop a greater reactivity to a cocaine-paired olfactory cue under fMRI testing. We observed instead that insular cortex showed a greater BOLD response to the cocaine-associated cue than in saline treated animals. This was observed in the volume of activated voxels in the insular area, particularly in the agranular regions.
Insular cortex has been previously shown to support ICSS in rats [32]. Increased spiking activity of neurons within this region has also been shown during ICSS in the rat [31]. Neurons within insular cortex may encode reward value [31].
Naqvi et al. showed that stroke patients sustaining damage specifically in the insula not only stopped smoking but also had no cravings [25]. Contreras et al. showed that reversible lidocaine-inactivation of the insular cortex of rats reduced amphetamine-induced conditioned place preference (CPP) [7]. Rats with inactivated insula preferred the non-conditioned side, suggesting a switch from reward to negative or aversive effects. Another study showed that lesions of the insula, but not of the orbital frontal cortex (OFC), disrupts CPP testing for nicotine [33]. Recent work in non-human primates demonstrates increased activity in the insula in response to cues signaling certain and uncertain reward, but not to cues that would indicate an unrewarded task [24]. An interesting functional relationship between insular cortex and other prefrontal regions has been reported [9]. Rats sustaining lesions of the insular cortex will show reductions in cue-induced reinstatement when the cue is olfactory and not auditory. Conversely, prelimbic lesions interfere with cue-evoked reinstatement of cocaine self-administration if the cue is auditory but not odor [9]. Thus, the present evidence suggests a role for the insular cortex in drug–odor associations, which seems highly important when subjects are re-exposed following an abstinence period to the drug cue alone.
In the rat, odor input is delivered from the olfactory bulbs directly to the olfactory peduncle, piriform cortex, olfactory tubercle, cortical amygdala, entorhinal cortex, orbital prefrontal cortex and agranular insula [26,29]. These areas share connectivity with limbic prefrontal regions, the hypothalamus, thalamus and hippocampal formation [16,39], which in turn may provide descending modulatory inputs [13]. Direct and indirect projections from the olfactory system to the mPFC and agranular insula have been reported in rats [26]. Neuroanatomical studies done by Vertes using anterograde tracers show that mPFC projects profusely to subcortical areas such as the amygdala, accumbens, VTA, PAG, raphe and also demonstrates high levels of corticocortical connectivity within the medial and lateral PFC [39]. Physiological interactions between olfactory inputs and insular and limbic prefrontal cortical areas are particularly interesting [7]. Recently it has been shown that neuronal activity in the insular cortex is closely tied to expecting negative or aversive outcomes and thus may be important in interpreting sensory stimuli as aversive [6]. This observation contrasts with the medial prefrontal cortex, which in turn may be thought of as having a role in expecting rewarding or pleasurable stimuli and determining beneficial strategies [6]. An anatomical and functional interaction between these two cortical sites may therefore control behavioral responses contingent upon whether the stimuli are anticipated to be aversive, rewarding or neutral. In conclusion, results from the present work provide evidence of a role of the insular cortex in processing cocaine-paired odor stimuli. The findings, although not all-encompassing, provide support for a functional role of the insular cortex in ICSS and odor-driven processing of rewarding stimuli.
HIGHLIGHTS.
-
►
Examines shifts in brain reward thresholds in presence of cocaine cue.
-
►
Shows that odor cue in absence of cocaine lowers threshold for reward.
-
►
Investigates neural activity in response to cocaine-associated cue.
-
►
fMRI in awake rats shows insular cortex is highly responsive to cocaine cue.
Acknowledgments
The work was supported by a grant from the Whitehall Foundation to JRS and NIH grant DA019946 to MF. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. TRJ was supported by a Provost award from Northeastern University.
References
- 1.Baeg EH, Jackson ME, Jedema HP, Bradberry CW. Orbitofrontal and anterior cingulate cortex neurons selectively process cocaine-associated environmental cues in the rhesus monkey. Journal of Neuroscience. 2009;29:11619–11627. doi: 10.1523/JNEUROSCI.3206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. Journal of Neuroscience. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell KA, Evans G, Gallistel CR. A microcomputer-based method for physiologically interpretable measurement of the rewarding efficacy of brain stimulation. Physiology and Behavior. 1985;35:395–403. doi: 10.1016/0031-9384(85)90315-4. [DOI] [PubMed] [Google Scholar]
- 4.Chen YC, Mandeville JB, Nguyen TV, Talele A, Cavagna F, Jenkins BG. Improved mapping of pharmacologically induced neuronal activation using the IRON technique with superparamagnetic blood pool agents. Journal of Magnetic Resonance Imaging. 2001;14:517–524. doi: 10.1002/jmri.1215. [DOI] [PubMed] [Google Scholar]
- 5.Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. British Journal of Addiction. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 8.Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. European Journal of Neuroscience. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- 9.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. European Journal of Neuroscience. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 10.Dobrovitsky V, Pimentel P, Duarte A, Froestl W, Stellar JR, Trzcinska M. CGP 44532, a GABAB receptor agonist, is hedonically neutral and reduces cocaine-induced enhancement of reward. Neuropharmacology. 2002;42:626–632. doi: 10.1016/s0028-3908(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 11.Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30:936–943. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neuroscience. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. Journal of Comparative Neurology. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- 14.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 15.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 16.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 17.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 18.King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. Journal of Neuroscience Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2012;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Chefer S, Kurup PK, Guillem K, Vaupel DB, Ross TJ, Moore A, Yang Y, Peoples LL, Stein EA. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. Neuroimage. 2012;62:1857–1866. doi: 10.1016/j.neuroimage.2012.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maldonado-Irizarry CS, Stellar JR, Kelley AE. Effects of cocaine and GBR-12909 on brain stimulation reward. Pharmacology Biochemistry and Behavior. 1994;48:915–920. doi: 10.1016/0091-3057(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 22.Mandeville JB, Jenkins BG, Kosofsky BE, Moskowitz MA, Rosen BR, Marota JJ. Regional sensitivity and coupling of BOLD and CBV changes during stimulation of rat brain. Magnetic Resonance in Medicine. 2001;45:443–447. doi: 10.1002/1522-2594(200103)45:3<443::aid-mrm1058>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 24.Mizuhiki T, Richmond BJ, Shidara M. Encoding of reward expectation by monkey anterior insular neurons. Journal of Neurophysiology. 2012;107:2996–3007. doi: 10.1152/jn.00282.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neville KR, Haberly LB. Olfactory cortex. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford University Press; New York: 2004. pp. 415–454. [Google Scholar]
- 27.Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ. Neuroimaging for drug addiction and related behaviors. Reviews in the Neurosciences. 2012;22:609–624. doi: 10.1515/RNS.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Vol. 1. Academic Press; Boston: 1997. [Google Scholar]
- 29.Price JL. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. Journal of Comparative Neurology. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Borrero E, Bernardo Colon A, Burgos-Martir MA, Alvarez Carillo JE, del Campo YE, Abella-Ramirez C, Maldonado-Vlaar CS. NMDA antagonist AP-5 increase environmentally induced cocaine-conditioned locomotion within the nucleus accumbens. Pharmacology Biochemistry and Behavior. 2006;85:178–184. doi: 10.1016/j.pbb.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 31.Rolls ET, Cooper SJ. Activation of neurones in the prefrontal cortex by brain-stimulation reward in the rat. Brain Research. 1973;60:351–368. doi: 10.1016/0006-8993(73)90795-6. [DOI] [PubMed] [Google Scholar]
- 32.Routtenberg A, Sloan M. Self-stimulation in the frontal cortex of Rattus norvegicus. Behavioral Biology. 1972;7:567–572. doi: 10.1016/s0091-6773(72)80218-9. [DOI] [PubMed] [Google Scholar]
- 33.Scott D, Hiroi N. Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biological Psychiatry. 2012;69:1052–1059. doi: 10.1016/j.biopsych.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.See RE. Neural substrates of cocaine–cue associations that trigger relapse. European Journal of Pharmacology. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 35.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacology Biochemistry and Behavior. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 36.Stellar JR, Rice MB. Pharmacological basis of intracranial self-stimulation. In: Liebman JM, Copper SJ, editors. The pharmacological basis of reward. Clarendon Press; Oxford, UK: 1989. pp. 14–65. [Google Scholar]
- 37.Su ZI, Kichaev G, Wenzel J, Ben-Shahar O, Ettenberg A. Weakening of negative relative to positive associations with cocaine-paired cues contributes to cue-induced responding after drug removal. Pharmacology Biochemistry and Behavior. 2012;100:458–463. doi: 10.1016/j.pbb.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. Journal of Neuroscience. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]


