Abstract
Problem Addressed
Electrical stimulation has been shown effective in restoring basic lower extremity motor function in individuals with paralysis. We tested the hypothesis that a Flat Interface Nerve Electrode (FINE) placed around the human tibial or common peroneal nerve above the knee can selectively activate each of the most important muscles these nerves innervate for use in a neuroprosthesis to control ankle motion.
Methodology
During intraoperative trials involving three subjects, an 8-contact FINE was placed around the tibial and/or common peroneal nerve, proximal to the popliteal fossa. The FINE’s ability to selectively recruit muscles innervated by these nerves was assessed. Data were used to estimate the potential to restore active plantarflexion or dorsiflexion while balancing inversion and eversion using a biomechanical simulation.
Results, Significance, and Potential Impact
With minimal spillover to non-targets, at least three of the four targets in the tibial nerve, including two of the three muscles constituting the triceps surae were independently and selectively recruited in all subjects. As acceptable levels of spillover increased, recruitment of the target muscles increased. Selective activation of muscles innervated by the peroneal nerve was more challenging. Estimated joint moments suggests that plantarflexion sufficient for propulsion during stance phase of gait and dorsiflexion sufficient to prevent foot drop during swing can be achieved, accompanied by a small but tolerable inversion or eversion moment.
Keywords: Functional Electrical Stimulation (FES), Flat Interface Nerve Electrode (FINE), Human, Tibial, Fibular, Peroneal, Plantarflexion, Dorsiflexion
1. Introduction
Stroke, spinal cord injury (SCI) and multiple sclerosis (MS) can result in partial to complete loss of motor and sensory function, resulting in high healthcare costs, greatly affecting patient independence, and having profound changes on quality of life [1–5]. In the United States alone, there are approximately 270,000 people living with spinal cord injury, which affects approximately 12,000 people per year [6]. Stroke is the primary cause of severe, chronic disability in the US [7], with over 7 million survivors [8,9] and approximately 795,000 new cases annually [9,10]. Electrical stimulation can improve or restore function in individuals with neurological compromise and has been shown effective in restoring basic lower extremity motor function in individuals with paralysis from SCI [11–14] or stroke [15–17]. Exercise with electrical stimulation has been demonstrated to increase muscle mass and cardiopulmonary capacity and acutely decrease spasticity in individuals with SCI [18]. It has also been shown to decrease spasticity as well as improve gait and independence with gains significantly better than conventional therapies in survivors of stroke [16,19,20]. Neuroprostheses employing electrical stimulation have also been used to control the actions of muscles acting at the ankle to offer improvements in standing balance and walking for persons with paraplegia [21]. In addition, electrical activation of the paralyzed ankle dorsiflexors has been shown to improve strength and gait speed in stroke survivors [22–24].
Many neuroprostheses employing electrical stimulation rely on surface, epimysial, or intramuscular electrodes. These systems require placement of an electrode near the motor point for each muscle and can require a stimulating current approaching 20 mA [25]. Alternatives to locating electrodes in the muscles include placing electrodes in or around the nerve, which brings the stimulating contact in closer proximity to the target axons and reduces the current required to achieve threshold. These include cuff electrodes located outside the perineurium and arrays of penetrating electrodes located between or within fascicles. Penetrating electrode arrays have been implanted chronically for selective stimulation of the sciatic nerve in cats [26] and have been selective enough to stimulate functionally synergistic muscles to reduce onset of fatigue [27]. However, they are inherently invasive, as they require penetrating the protective and structural layers of the nerve, and the long-term stability near joints and in regions where there is significant movement of the nerve and surrounding tissues has not yet been fully established.
Like penetrating electrodes, nerve cuff electrodes require lower stimulation amplitudes than epimysial or intramuscular electrodes [28] and can selectively recruit multiple muscles or independent motor unit pools with a single cuff [29]. Nerve cuff electrodes such as the Case Western Reserve University (CWRU) spiral have already been successfully used in many chronic clinical applications [29–38]. In fact, implanted spiral nerve cuff electrodes have proven to produce higher joint moment at the knee than epimysial electrodes [39] with long term stability [40,41]. Recognizing that many nerves are not round in cross section, the FINE improves upon the design of the spiral nerve cuff by maintaining a nerve’s oblong cross section. The FINE typically recruits muscles more selectively than a spiral nerve cuff while remaining exterior to the nerve. Selective recruitment is exceptionally important when the function being restored requires coordinated contractions of several muscles innervated by a common nerve trunk, such as during the different phases of gait. While the spiral nerve cuff electrode can selectively generate moments from functionally synergistic groups of muscles [42], the FINE is likely to provide even greater control via increased selectivity of individual muscles [43]. In acute human trials, a FINE placed on the femoral nerve was consistently able to achieve selective and independent activation of multiple synergistic muscles [43]. This represented a large improvement in selectivity over intraoperative tests of spiral cuffs placed at the same location on the femoral nerve, which consistently activated only a single muscle or group of synergists [31]. In chronic animal trials, the FINE has been able to maintain ovoid geometry around the nerve while avoiding nerve damage and producing stable stimulation of targeted muscles [44,45].
In general, nerve cuffs must be able to selectively target axons innervating muscles of interest while avoiding muscles that elicit undesired motion. While control of some ankle function has been noted in humans with stimulation via nerve cuff electrodes located as proximal as the spinal nerve roots, the selectivity of such systems was insufficient for control of standing balance and walking [46]. The fascicular anatomy of the human distal sciatic nerve and its main branches have been mapped to aid in the design of nerve cuff electrodes [47]. Computer models suggests that a FINE on the sciatic nerve will require many contacts for selective restoration of active ankle plantarflexion or dorsiflexion with balanced inversion and eversion [48]. However, simulations suggest that locating cuffs distal to the sciatic bifurcation – on the tibial and common peroneal nerves – will reduce the required number of contacts [49]. Thus, we have explored the efficacy of placing FINEs on the tibial and common peroneal nerves, which innervate the muscles acting at the ankle and foot. The common peroneal nerve primarily innervates ankle dorsiflexors, such as tibialis anterior, extensor digitorum longus, extensor hallucis longus, and ankle evertors, such as peroneus longus and brevis. The tibial nerve primarily innervates ankle plantarflexors, such as the medial and lateral gastrocnemius and soleus, as well as ankle inverters, such as tibialis posterior. Placing FINEs just distal to the sciatic nerve bifurcation, should allow for increased selectivity of both plantar and dorsiflexors while still allowing placement of nerve cuffs proximal to the popliteal fossa. Locations proximal to the popliteal fossa avoid having the electrode leads cross the knee joint, thereby reducing the risk that torque will be developed on the lead and transmitted to the cuff and nerve and minimizing the potential for bending fatigue induced lead failure. The purpose of this study was to determine whether an 8-contact FINE placed on the human tibial and common peroneal nerves without a priori knowledge of the underlying neuroanatomy and fascicular distribution will selectively active target muscles required for plantarflexion and dorsiflexion, respectively, without inducing excess inversion or eversion from a single surgical location above the knee.
2. Methods
2.1 Subject Recruitment
Subjects were recruited from a pool of patients scheduled for a lower extremity vascular surgery that exposed the tibial and common peroneal nerve. Surgeries were conducted at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center (LSCDVAMC) in Cleveland, OH. Subjects undergoing surgical implantation of a lower extremity neuroprosthesis for standing balance that involved exposure of the tibial and peroneal nerves for installation of spiral nerve cuffs were also included. Implant surgeries were conducted at MetroHealth Medical Center (MHMC) in Cleveland, OH. The Institutional Review Board (IRB) of each institution approved the study and the subjects provided consent prior to participation.
2.2 FINE Design
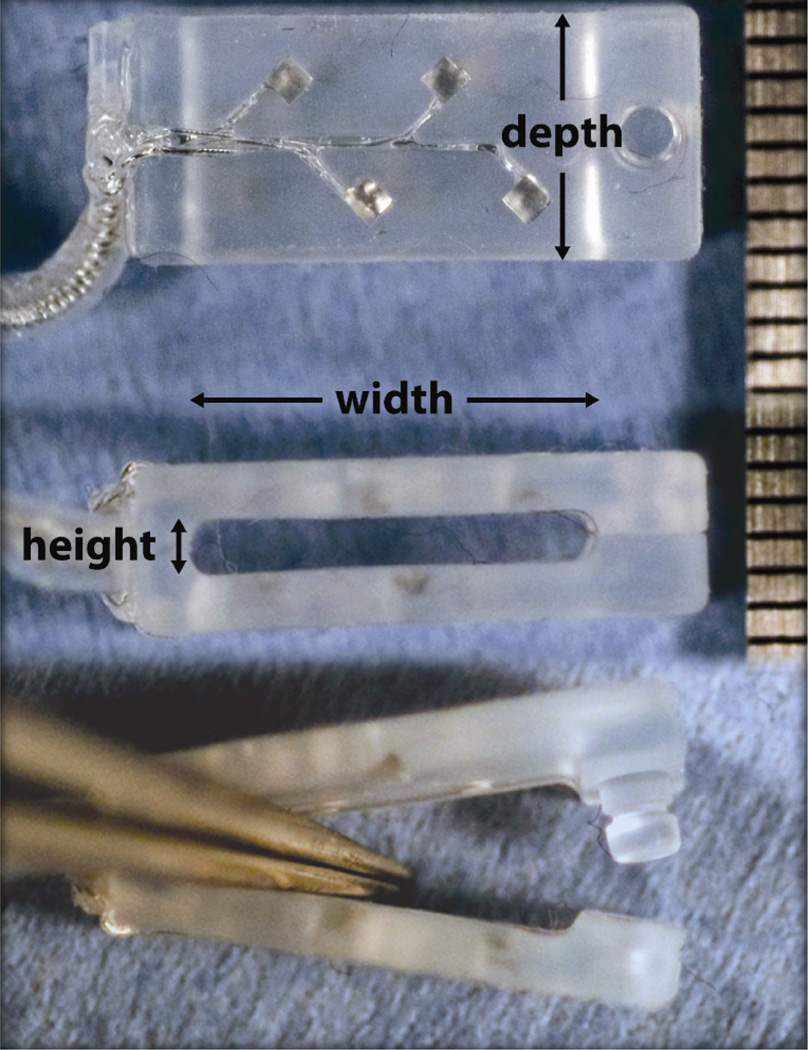
The FINE had eight platinum-iridium (PtIr) contacts: four on the upper inner surface (visible in Figure 1) and four on the lower inner surface (not visible in Figure 1). The aperture exposing each embedded contact was 0.5 mm in diameter. The inter-contact distance was approximately 2 mm. Upper contacts were offset from lower contacts by half the inter-contact distance. Each contact was laser cut (Norman Noble, Cleveland OH) and secured to an independent multistranded stainless steel Teflon-insulated lead wire (Ardiem, Indiana PA). Four lead wires were helically coiled in tandem and enclosed in a separate elastomer tube for the upper contacts and lower contacts. Contacts and leads were molded into a silicone housing with integral strain relief and snap closure mechanism (Point Medical Corporation, Crown Point IN). Three FINE luminal dimensions were available (width × height): 15 mm × 1.5 mm, 10 mm × 1.5 mm, 10 mm × 1.0 mm. All FINEs were 7 mm deep along the length of the nerve. Assembled FINEs were packaged, sealed, and sterilized in ethylene oxide offsite (Ethox International, Buffalo NY).
Figure 1.
A Flat Interface Nerve Electrode (FINE) similar to the one pictured here was used in intraoperative experiments. Contacts were offset to maximize the spatial volume that was stimulated. Top view (top) shows the offset contacts. Side view (middle) shows the lumen through which the nerve passed. Side view (bottom) shows the open FINE and the button designed to keep the FINE closed. Scale on right is in mm. Adapted from [43], with permission.
2.3 FINE Implant and Stimulation Procedure
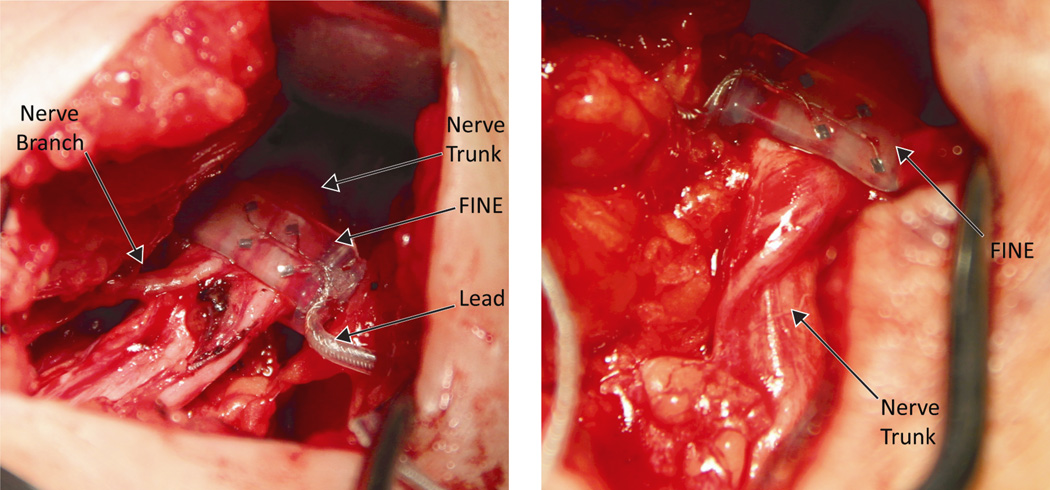
Prior to implantation, a FINE of each size was submerged in heated sterile saline in an ultrasonic cleaner and sonicated for 60–120 seconds to ensure that no air bubbles occluded the recessed contacts. To implant the FINE, a 2 cm section of the tibial or common peroneal nerve was exposed distal to the sciatic bifurcation and proximal to the popliteal fossa (Figure 2). The width and height of the nerve were measured with a sterile tape by the surgeon. The FINE closest to but not smaller than the target nerve was selected and positioned the FINE around the target nerve. A 13 mm needle electrode was inserted subcutaneously near the incision to serve as the return electrode (Figure 3). The FINE leads were connected to a custom-designed current-controlled stimulator, the Universal External Control Unit (UECU), developed at CWRU. The programmable UECU delivered monopolar, charge-balanced, biphasic, cathodic-phase first, square pulses. Pulse amplitude ranged from 0.1 to 5.0 mA with a resolution of 0.1 mA at or below 2.0 mA or 1 mA above 2.0 mA. Pulse width ranged from 1 to 255 µs with a resolution of 1 µs.
Figure 2.
A 10 mm × 1.0 mm FINE placed around the left peroneal nerve (left) and a 10 mm × 1.5 mm FINE placed around the right tibial nerve (right) of Subject 2.
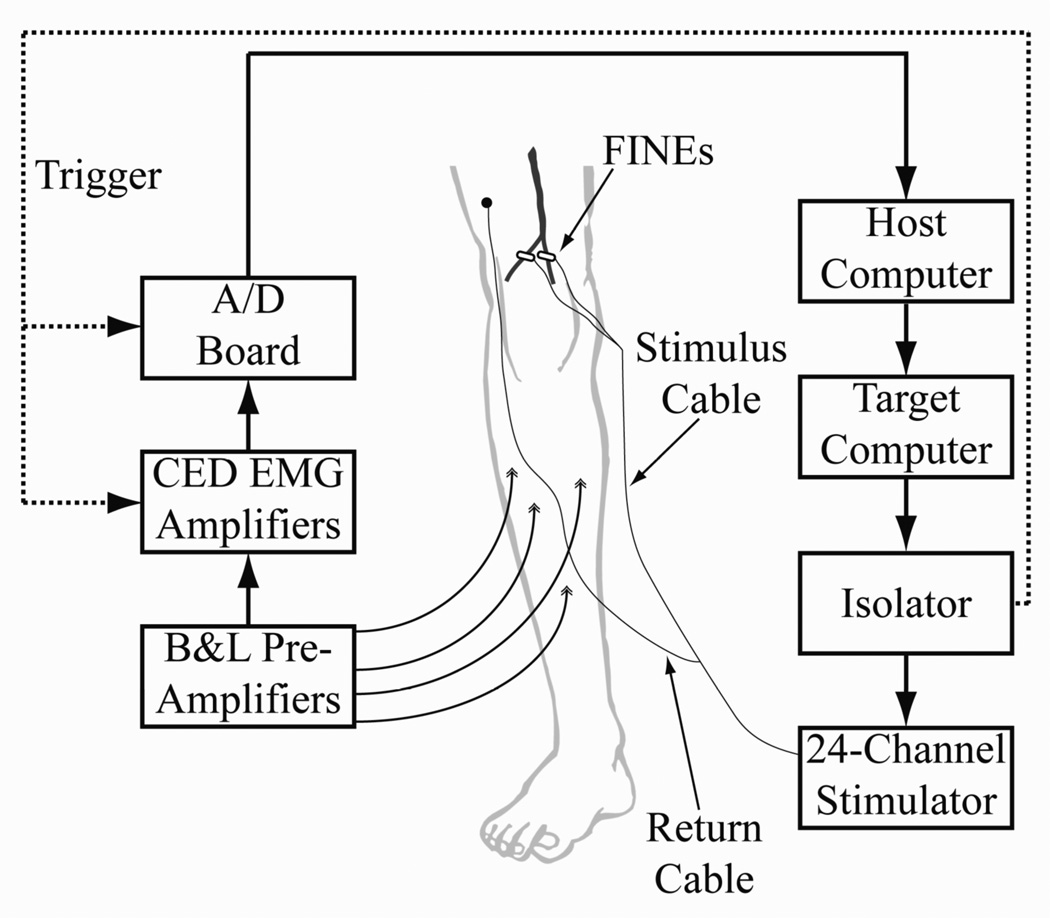
Figure 3.
Experimental setup for testing the FINE on the tibial or common peroneal nerve. A custom, current-controlled stimulator delivered stimulus pulses to the FINE. Differential EMG was collected from each of four muscles innervated by the target nerve. EMG was referenced to a ground patch (not shown), amplified, filtered, and collected.
2.4 EMG Recording Procedures
During pre-operative preparation in the operating room, a surface reference electrode (2 in × 4 in, Nicolet-VIASYS, Madison, WI) was placed over a stationary bony location. For surgeries at the LSCDVAMC, the reference was placed over the anterior superior iliac spine (ASIS). For surgeries at MHMC, the reference was placed over the lumbar spine. During surgery, pairs of EMG needle electrodes (27 gauge, Axon Systems, Hauppauge, NY) were placed 1 cm apart into four muscles innervated by the tibial or common peroneal nerve [50,51] (Table 1). The response to stimulation with a hand-held stimulator attached to each pair of electrodes verified electrode placement.
Table 1.
| Nerve | Muscle | EMG Needle Placement |
|---|---|---|
| Tibial | Soleus (S) | Distal to the gastrocnemius muscle belly; medial and anterior to the Achilles tendon |
| Medial Gastrocnemius (MG) | One handbreadth below the popliteal crease on the medial mass of the calf | |
| Lateral Gastrocnemius (LG) | One handbreadth below the popliteal crease on the lateral mass of the calf | |
| Tibialis Posterior (TP) | One handbreadth distal to the tibial tuberosity just off the medial edge of the tibia. | |
| Common Peroneal |
Tibialis Anterior (TA) | Approximately 1.5 cm lateral to the tibial crest; at the junction of the upper and middle third of the leg. |
| Peroneus Longus (PL) | Three fingerbreadths below the fibular head; directed toward the lateral aspect of the fibula. | |
| Peroneus Brevis (PB) | One handbreadth proximal to the lateral malleolus and anterior to the peroneus longus tendon. | |
| Extensor Digitorum Longus (EDL) | At the middle third of the leg between the anterior border of the tibia and the lateral border of the fibula. |
Each pair of EMG electrodes was connected to a differential pre-amplifier (B&L Engineering, Tustin, CA) with a gain of 325 and a bandwidth of 12 to 2975 Hz (Figure 3). Input impedance of the pre-amplifiers was 1 GΩ. Programmable amplifiers (1902, Cambridge Electronic Design (CED), Cambridge U.K.) served to further amplify and filter responses. The CED amplifiers had a variable programmable gain allowing for overall signal gain of 1,155 to 1,155,000. Gain was selected such that the EMG response to a maximal stimulus was full-scale without saturating the amplifiers. CED amplifiers clamped the EMG signal for 3 ms starting at the onset of stimulation to prevent amplifier saturation and remove stimulus artifact. AC coupling removed DC drift from the electrode- tissue interface. Signals were low pass filtered at 1 kHz. A laptop computer running a custom MATLAB software suite was used to interface with the amplifiers and stimulator. Data were sampled at 2.5 kHz using a National Instruments A/D DAQ board (BNC-6259, National Instruments, Webster TX).
2.5 Pulse Space Modulation
A muscle’s response to nerve stimulation was quantified as the normalized, rectified and integrated EMG signal [43]. The window of integration was specific for each muscle and set to capture the m-wave. The largest rectified and integrated value for a muscle, defined as the maximum activation of that muscle, was used for normalization. Thus, a muscle’s response to stimulation ranged from 0% to 100%.
Extending the technique of pulse width (PWM) and pulse amplitude-modulated (PAM) recruitment curve generation reported in similar studies [31,43], a hybrid simultaneous PWM and PAM technique – pulse space modulation (PSM) – was used to characterize the response of a muscle to electrical nerve stimulation. PSM recruitment surfaces were acquired from each muscle and for each contact of the FINE. During PSM, the pulse width was varied between 1 and 255 µs and the amplitude was varied between 0.1 and 5 mA. Modulation proceeded according to an adaptive gradient search in which an algorithm compared the EMG responses at spatially nearby stimuli [52]. If the difference in EMG responses was greater than a set threshold, a new stimulus was added at the midpoint between the two stimulus levels. EMG responses were calculated using the average of the response to three identical stimuli.
2.6 Selectivity
The normalized, rectified, and integrated EMG response of a muscle ranged from 0% to 100%, where a value of 100% indicated that the stimulus parameters resulted in full activation of the target muscle. Based on prior work, the activation threshold of a non-target muscle was limited to 10% of the maximum activation observed for that non-target muscle [30,33,43,53], which has been shown to correspond to the first visible or palpable muscle twitch [30]. Additionally, the data were analyzed with a non-target activation cutoff of 20% to assess the effect of increased allowable spillover on target activation efficacy. In addition to muscular selectivity, functional selectivity was assessed with respect to motion at the ankle. When assessing function, recruitment of any of the muscles contributing to the function was allowed. Similar to the threshold in muscular selectivity, non-target moment was limited to 10%. Agonists were considered those muscles that contribute to plantarflexion (soleus and the two heads of the gastrocnemius) or those muscles that contribute to dorsiflexion (tibialis anterior and extensor digitorum longus).
2.7 Estimated Joint Moments
Measurement of muscle moment was not possible during intraoperative trials. Therefore, moments and the resulting function were estimated using an OpenSim (National Center for Simulation in Rehabilitation Research, Stanford University, Stanford CA) biomechanical model [54–58]. To estimate the joint moment generated as a result of stimulation, the maximum static moment produced by each muscle in the model was scaled by its activation level [43]. Each muscle’s moment was then summed to estimate the resulting joint moments at the ankle. Each muscle’s moment was adjusted to 50% of able-bodied values to account for muscle weakness and other long-term effects of paralysis [59]. This is an estimate of the expected functional response to an implanted FINE.
3. Results
A total of four tibial and four common peroneal nerves were evaluated (Table 2). Because the cuff closest in size without being smaller than the nerve was always chosen, the tibial nerve was greater in size than the common peroneal nerve in all cases. Full characterization of the muscles’ responses to monopolar electrical stimulation across all eight contacts of the FINE required 22±5 minutes, or approximately 2.75 minutes per contact. Following nerve exposure, FINE implantation and removal was accomplished using standard surgical tools and was accomplished in less than one minute.
Table 2.
Evaluated FINE sizes in intraoperative 734 experiments, in mm.
| Tibial Cuff | Common Peroneal Cuff | |||
|---|---|---|---|---|
| Subject | Left | Right | Left | Right |
| 1 | - | 10.0 × 1.5 | - | 10.0 × 1.0 |
| 2 | 10.0 × 1.5 | 10.0 × 1.5 | 10.0 × 1.0 | - |
| 3 | 15.0 × 1.5 | - | 10.0 × 1.5 | 10.0 × 1.5 |
3.1 Muscle Recruitment and Selectivity
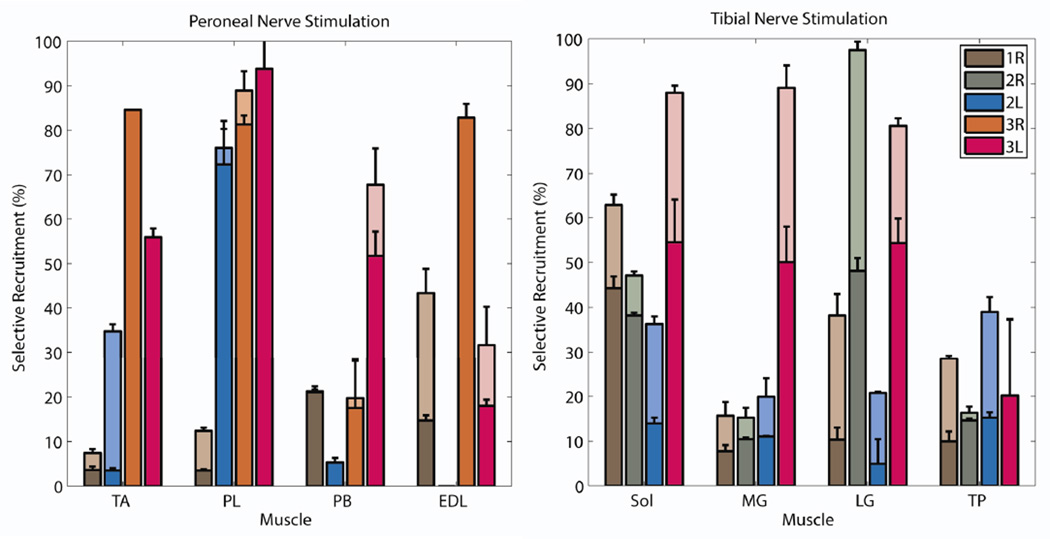
EMG acquired during pulse space modulation (PSM) is shown for a typical recruitment (Figure 4). The signal to noise ratio of EMG for all supra-threshold stimuli was approximately 30. The maximum selectivity obtained for each muscle in each subject was plotted when spillover threshold was set to 10% (lower bars) or 20% (higher bars) for the tibial and common peroneal nerves (Figure 5). Of the 16 recorded muscles, 14 and at least three in each subject were selectively recruited above threshold during tibial nerve stimulation. At least two of the three muscles comprising the triceps surae, the primary target for restoring propulsive plantarflexion, and always the soleus could be selectively activated. Additionally, the tibialis posterior was selectively recruited in all subjects. Three to six of the eight FINE contacts were found to be selective for the individual muscles of the triceps surae. Relaxation of the threshold constraint from 10% to 20% resulted in an increase in the number of muscles that were activated and the level to which they were activated. On average, a muscle’s activation increased from 26%±19% to 45%±29% by increasing the threshold level.
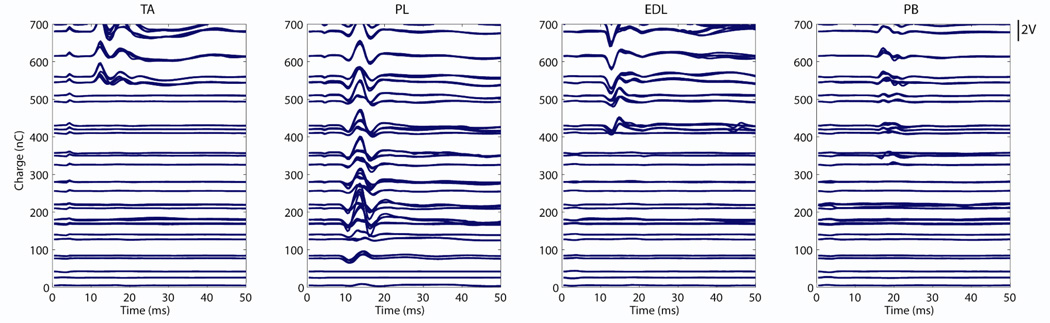
Figure 4.
The EMGs acquired during peroneal nerve stimulation with contact 3 in Subject 1. While a range of pulsewidths (µs) and amplitudes (mA) were explored in stimulus space, they have been represented here on the y-axis as their charge in nC. The EMG signals themselves are in volts and have been scaled for visualization (scale bar at right). The traces illustrate that the peroneus longus was recruited selectively at lower stimulus levels, followed by onset of the peroneus brevis, extensor digitorum longus, and then tibialis anterior. Stimulus was at time=0 ms.
Figure 5.
The maximized selectivity when threshold was set at 10% (lower of stacked bars) or 20% (higher of stacked bars) for each muscle within each subject is shown for common peroneal nerve stimulation (left) and tibial nerve stimulation (right). Legend: 1, 2, or 3 is Subject 1, 2, or 3; R or L is Right or Left. Muscles are: soleus (SOL), medial gastrocnemius (MG), lateral gastrocnemius (LG), tibialis posterior (TP), tibialis anterior (TA), peroneus longus (PL), peroneus brevis (PB), and extensor digitorum longus (EDL). A reliable signal from EDL was not achieved in Subject 2.
During peroneal nerve stimulation, 11 of the 15 recorded muscles (the EDL was not recorded for Subject 2) were independently and selectively recruited when the spillover threshold was limited to 10%. The tibialis anterior, a primary target for restoring dorsiflexion, was selectively recruited in two of four nerves. As many as three contacts were found to be selective for the tibialis anterior. However, three to seven contacts were found to be selective for the peroneus brevis and longus muscles, which are not primary targets because they produce eversion and antagonistic plantarflexion. As with tibial nerve stimulation, relaxation of the threshold constraint from 10% to 20% resulted in increased target activation from 41%±34% to 48%±31. Additionally, allowing 20% spillover facilitated tibialis anterior recruitment in Subject 2L.
3.2 Functional Selectivity and Estimated Joint Moments
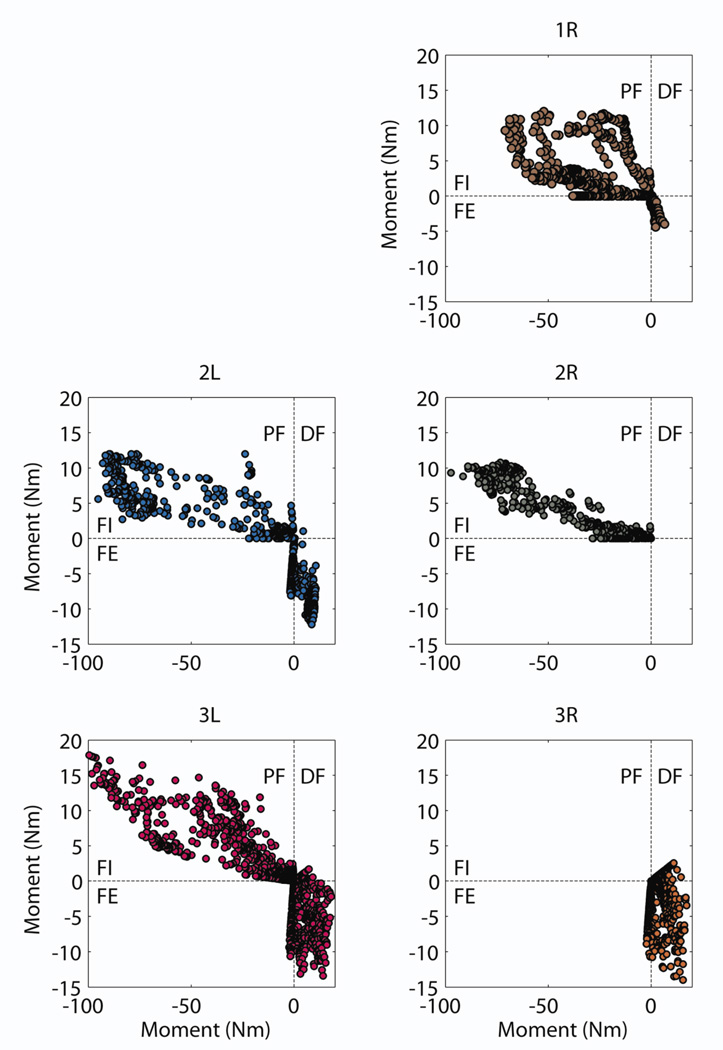
The normalized activation levels of each muscle in response to a stimulus applied to a contact were used to estimate the cumulative moments at the ankle. The estimated plantarflexion-dorsiflexion and inversion-eversion moments were found across all stimuli applied to each contact (Figure 6). Based on ankle moments acquired during walking [60], a target of 90 Nm of plantarflexion and 8 Nm of dorsiflexion for gait restoration was set. Estimated moments for Subject 1 approached but did not exceed these two thresholds whereas they did for Subjects 2 and 3.
Figure 6.
Estimated ankle moments for each subject based on recorded EMG during single channel stimulation after accounting for a 50% reduction in muscle strength. PF: plantarflexion, DF: dorsiflexion, FI: foot inversion, FE: foot eversion.
During tibial nerve stimulation, 4±1 contacts (range: 2–5) were found to be selective for plantarflexors without exceeding 1.8 Nm of inversion, which is 10% of the model-derived maximum inversion moment. When the constraint on inversion moment was removed, 7±1 contacts (range: 6–8) were found to be selective for restoring plantarflexion. During peroneal nerve stimulation, 2±1 contacts (range: 1–3) were found to be selective for restoring dorsiflexion without exceeding 1.7 Nm of eversion, which is 10% of the model-derived maximum eversion moment. Similar to tibial nerve stimulation, removal of this constraint increased the number of effective contacts to 3±2 (range: 2–7).
4 Discussion
This study marks the first time FINEs have been tested on the human tibial and common peroneal nerves. The data support the hypothesis that an 8-contact FINE can selectively activate muscles innervated by these nerves from locations just distal to the sciatic bifurcation above the knee, and that the electrodes can be expected to produce functional and rehabilitative ankle moments. Under the strictest spillover criterion in which non-target muscles could not be activated above a 10% threshold, at least three of the four muscles were independently and selectively activated for tibial nerve stimulation. Additionally, at least two heads of the triceps surae could be selectively activated.
Results from stimulation of the common peroneal nerve, however, were less selective but still can be considered effective for providing dorsiflexion. When targeting the common peroneal nerve, the tibialis anterior, the strongest target dorsiflexor, was not selectively activated in Subject 1, even when spillover was allowed to approach 20%, and only with the higher acceptable spillover was the tibialis anterior recruited to a sufficient level in Subject 2. Only in Subject 3 was the tibialis anterior selectively recruited while limiting spillover to 10%. This suggests that an 8-contact FINE on the common peroneal nerve will be effective, however multiple muscles instead of a single muscle are likely to be activated to restore dorsiflexion, which may be accompanied by small competing plantarflexion and eversion moments.
The charge threshold to elicit muscle contraction was 41±36 nC, which was comparable to although slightly greater than the thresholds found in similar studies: 25±17 nC during chronic studies with a spiral nerve cuff implanted on upper extremity nerves in humans; 34±16 nC during intraoperative evaluation of the spiral nerve cuff on the human femoral nerve; 21±18 nC during intraoperative evaluation of the FINE on the human femoral nerve; 23±8 and 29±17 nC during chronic studies with a spiral nerve cuff implanted on the distal femoral nerve in human [30,31,39,43]. As expected, the threshold found in this study was greater than the 0.24 nC to 4.1 nC reported for intrafascicular electrodes [27,61,62].
Some muscles innervated by the nerve are synergists; for example, the three muscles of the triceps surae. While not all muscles could be selectively stimulated with an 8-contact FINE, groups of synergists could be selectively recruited. By allowing spillover to synergists, plantarflexors and dorsiflexors were recruited above threshold in all subjects. Allowing up to 10% spillover to non-synergists, plantarflexors were recruited to 23±4% and dorsiflexors were recruited to 51±29%. Not surprisingly, when restrictions on spillover to all muscles other than those considered antagonists were removed, plantarflexors could be recruited to 89±11% and dorsiflexors could be recruited to 66±33%.
When targeting the tibial nerve, two to five contacts were found to be selective for plantarflexors, two to four contacts were found to be selective for inverters, and as many as two contacts were found to not be selective. When targeting the common peroneal nerve, one to three contacts were found to be selective for dorsiflexors, two to six contacts were found to be selective for evertors, and as many as two contacts were found to not be selective. In 60% of the trials, one contact did not recruit any muscles. In these cases, the contact was always on the edge of the cuff (contact 1, 4, 5, or 8) and was likely not in contact with the nerve or located directly over sensory axons without a motor output. Instead, current was most likely shunted through extraneural fluid to the return electrode. Lack of EMG responses in these cases were not due to lead breakage or stimulator failure.
While selectivity was reported on a scale of 0%–100%, achieving a selectivity of 100% was very unlikely. This was due to the normalization procedure in which the largest EMG response for the muscle was used to normalize all responses for the muscle. Typically, the largest muscle response was elicited during multi-contact stimulation (data not shown). Indeed, Subject 1 did not achieve the estimated 90 Nm of plantarflexion because the maximum responses for the triceps surae muscles were obtained when using multiple contacts. When selectivity with a single contact approached 100%, it suggested that a single contact was in close proximity to the entire population of axons innervating a muscle. Alternatively, when selectivity using a monopole remained lower, it suggested that the fascicle(s) containing axons innervating the muscle were spatially distributed, constituting a larger area of the nerve, or distributed between contacts rather than adjacent to them. Although Sunderland [63] suggested that nerves were typically plexiform in nature, with axons traversing from fascicle to fascicle, there is sufficient evidence to suggest that, at least at the target locations along these nerves, the axons and fascicles within the nerve are more organized. While histological studies of the tibial and common peroneal nerve did not resolve to the axon level, they do not support the plexiform model within these nerves [47]. They do, however, suggest that there can be a large amount of variation in the location and number of fascicles between nerves.
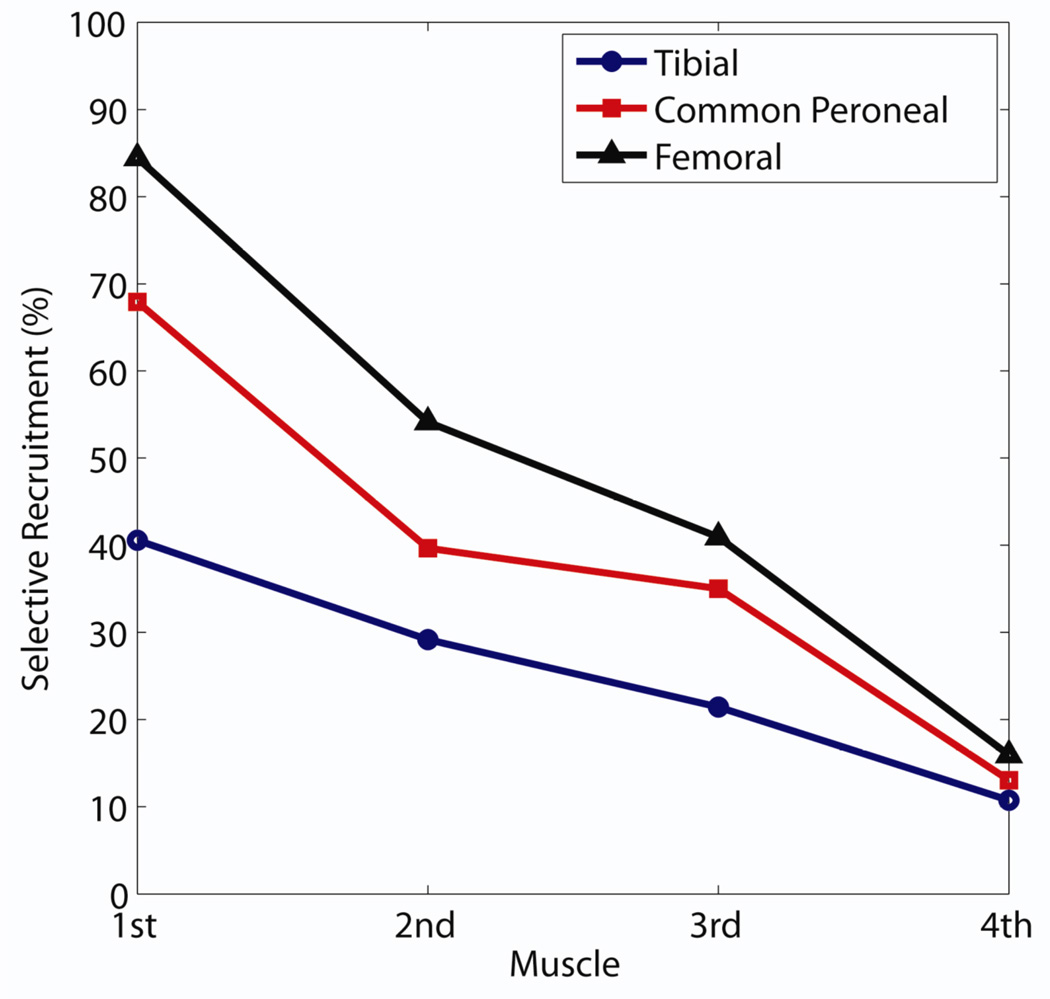
This study provides the first opportunity to compare the efficacy of an 8-contact FINE across different human nerves. During a series of intraoperative experiments, the FINE was previously shown to be selective on the human femoral nerve [43]. The trend in selectivity obtained during those experiments was similar to that obtained in this study (Figure 7). When selectivity values were sorted in descending order, the selectivity obtained on these three nerves converged toward threshold for the least selective muscle and exhibited no significant difference. The selectivity obtained on the tibial nerve was significantly less than that obtained on the femoral nerve for the first (one-tailed t-test, p=0.001) and second (p=.035) muscles and nearly for the third muscle (p=0.054). It should be noted that the femoral nerve study was attempting to selectively activate six muscles rather than four. Thus, selectivity values for the femoral nerve would be expected to be higher had only three muscles contributed to spillover costs.
Figure 7.
Sorted mean selectivity, from highest to lowest, obtained for the tibial, common peroneal, and femoral nerves.
There are notable differences between the human femoral, tibial, and common peroneal nerves which may contribute to the observed differences in selectivity (Table 3). As the number of fascicles innervating a target muscle increases, the probability of selectively recruiting that muscle increases, which may explain why the femoral selectivity was greater than that of the tibial or common peroneal nerves. Additionally, the distance between the cuff and the terminal nerve branches to target muscles (“Cuff-Branch Distance”) was much shorter in the femoral nerve. This is likely to affect selectivity because the nerve’s fascicles containing axons that innervate a specific muscle must be clustered together to form the terminal branch. The closer that the branch points are to the cuff, the higher the probability that the distal, post-branch organization is maintained in the proximal region of the nerve where the cuff is located.
Table 3.
Anatomical characteristics of the tibial, common peroneal, and femoral nerves at the level where the FINE 739 was tested [47,64].
| Tibial | Common Peroneal | Femoral | |
|---|---|---|---|
| Size | 10.8 mm × 5.1 mm | 8.3 mm × 3.8 mm | 10.5 mm × 2.3 mm |
| Area | 55.1 mm2 | 31.5 mm2 | 25.2 mm2 |
| Cuff-Branch Distance | 9 cm | 17 cm | 2 cm |
| Muscles Innervated | 18 | 7 | 5 |
| Fascicles | 26±3 | 9±2 | 33±14 |
| Fascicles/mm2 | 0.5 | 0.3 | 1.4 |
| Fascicles:Muscles | 1.4 | 1.3 | 6.6 |
While the fascicle per muscle ratio and distance to terminal branches may explain why the femoral nerve tended to have higher selectivity values, the variability observed between and within subjects for selective stimulation of a specific nerve is most likely due to the underlying variability of the fascicular distribution within the nerve. As can be observed in Figure 5, there were significant differences in the selectivity obtained for the left and right soleus and lateral gastrocnemius of Subject 2 and all of the muscles innervated by the peroneal nerve in Subject 3. Additionally, for any given muscle except the tibialis posterior, the selectivity obtained across subjects varied widely. This amount of variability is similar to that observed in the femoral nerve study [43]. While the fascicles of the tibial and common peroneal nerves [47] have not been traced in as much detail as they were in the femoral nerve [64], femoral fascicular tracings show that the number of fascicles innervating a specific muscle and the location of those fascicles can be very different between and within subjects.
It is possible that the axons innervating a target muscle were located toward the center of the nerve while the axons innervating non-target muscles were situated between the contact and the target axons. In this case, the fascicles wouldn’t be considered to be between contacts, but rather, too deep within the nerve. This is more likely to be observed with a cylindrical or spiral extraneural electrode than with the FINE. The FINE was designed to minimize the likelihood that this non-optimal distribution of fascicles will occur, but only if the FINE is sized to fit the specific nerve and accounts for the distribution of fascicle diameters within the nerve. The FINEs used in this study were designed with the sciatic, tibial, and common peroneal nerves in mind and the cuffs fit the nerve well. Nevertheless, if the nerve fell between the three available sizes it was likely that one or more edge contacts would not be in contact with the nerve.
With limited time to collect data in the operating room, a restricted area of the stimulus space was searched. This study used an efficient PSM algorithm instead of relying exclusively on PWM or PAM. As a result, nearly four times more data were collected in the same amount of time as earlier intraoperative studies [31,43]. Nonetheless, the recruitment characteristics of a muscle tended to be very steep. That is, at low levels of stimulation, there was negligible recruitment of a muscle, but within a small increase in pulse width and/or amplitude, the normalized recruitment rapidly approached 100%, followed by a plateau at higher stimulus levels. Although PSM proved more effective than the previously tested PWM and PAM algorithms, and while the algorithm tended to cluster points at areas where muscle recruitment grew the fastest, the resolution between neighboring data points in the pulse space was low. For this reason, it is unlikely that absolute maximum selectivity values were obtained. Further, recruitment was achieved with monopolar stimulation. Field shaping through multi-contact stimulation should produce higher selectivity [48,49,65,66].
Spillover costs to non-target muscles were assumed to be of equal weight and sensory fiber activation was not considered during the calculation of selectivity. For the target group of users with a spinal cord injury, activation of sensory fibers is unlikely to produce discomfort or interfere with use, unless a reflex is activated. On one occasion, a reflex was activated during stimulation, causing active ipsilateral hip flexion and accompanying knee flexion, although the latter may have been passive and due to gravity. This response was observed only at stimulus levels in the upper pulse width and amplitude ranges, well beyond levels that would considered selective.
The data suggest that plantarflexion can be restored with an 8-contact FINE applied to the tibial nerve. The maximum estimated moment met or approached the 90 Nm threshold to restore propulsion, even after reducing muscle strength by 50%. The accompanying inversion moment was estimated at 10 Nm (5–13 Nm). This moment arose because the muscles of the triceps surae were not selectively recruited without recruitment of the tibialis posterior with the 8-contact FINE. Similarly, the data suggest that an accompanying eversion moment of up to 5 Nm will arise when restoring dorsiflexion. This is supported by findings in feline studies, in which dorsiflexion and plantarflexion could be restored, but usually with the accompaniment of medial or lateral rotation [29,32,35,38].
A concurrent inversion moment has been observed during gait in healthy individuals as well as those using an ankle-foot orthosis [67–70]. Although a goal of this on-going research is to restore plantarflexion or dorsiflexion with absent or balanced inversion and eversion, physiological data suggest that it may not be necessary. In fact, the tibialis anterior – the primary dorsiflexor – produces simultaneous foot inversion. Nonetheless, a neutral ankle position with regard to inversion and eversion remains an objective because a percentage of the population that would benefit from a lower extremity neuroprosthesis, particularly those with a spinal cord injury, will have impaired sensory feedback. Absent sensory feedback, the risks of ankle sprains and stumbles that might lead to falls due to an inverted or everted ankle become exaggerated. Based on the data from this and an accompanying modeling study [49], restoration of propulsive plantarflexion without inversion will require additional contacts within the FINE, which will improve selective activation of the triceps surae muscles and minimize spillover to the tibialis posterior. Even with the inversion moment that the tibialis anterior creates, the data also suggest that dorsiflexion absent eversion is not likely unless spillover to the peroneus longus, peroneus brevis, and, to a lesser extent, extensor digitorum longus is minimized.
It was assumed that normalized EMG, which reflects the maximum isometric force during a maximum voluntary contraction (MVC), is a reasonable metric to estimate moment [71]. However, the MVC was not directly obtained from the subjects. Instead, the MVC for each muscle was based on validated OpenSim simulations. This and similar models have been shown to accurately represent physiological data measured during most normal operations with the possible exception being accuracy in dynamic acceleration [72–85]. The musculoskeletal models used to calculate moments in this study were quasi-static making them less sensitive to error. Therefore, they are expected to maintain an acceptable level of accuracy. To account for muscle atrophy and weakness, the MVC of each muscle in the simulation was reduced by 50% [59]. However, due to the low number of samples, the results of the biomechanical model may not accurately represent what would be expected from the population at large. In spite of the inability to generalize the results, this observation offers a compelling preliminary indication of the potential utility of the FINE on the chosen neural targets.
Additionally, it was assumed that focal EMG obtained from needle electrodes accurately represented the activity throughout the entire muscle. Normalizing the EMG signals to their maximal values should, in large part, account for any artifacts due to the spatial sapling inherent in recording with needle and wire electrodes. In fact, normalized EMG obtained from the soleus, one of the larger muscles of the lower leg, using microwires has been shown to represent whole-muscle activity [86]. Therefore, it is likely that the EMG obtained with needle electrodes in this study reasonably represented the activity throughout the muscle. Surface EMG recordings were not an option since they are prone to cross-talk between muscles which would have obscured the evaluation of selectivity, the primary outcome measure of the study. Future studies can improve upon these methods by eliminating EMG recording altogether and removing the conversion from normalized EMG to moment. Instead, isometric moment could be measured about the ankle in all three planes via a light weight and sterilizable ankle moment transducer that fits all subjects and does not impede surgery.
While recording from the muscles discussed in this study proved relatively straight forward, other muscles were also considered that ultimately proved very difficult and impractical to instrument with needle electrodes. For tibial nerve stimulation, this included the flexor digitorum longus and flexor hallucis longus. For common peroneal nerve stimulation, this included the extensor hallucis longus and peroneus tertius. While recording EMG from these muscles would present a more complete picture of FINE selectivity, to do so accurately and consistently throughout the duration of an experiment would require the use of fine wires instead of needles. However, fine wires, by their very nature, require more time to implant and attach to amplifiers, which is impractical for an experimental procedure of this complexity in the operating room.
Whether the data from otherwise healthy subjects undergoing vascular surgery and individuals paralyzed by spinal cord injuries can be pooled together depends on the precise nature of the study. In this case, each subject and each muscle within each subject were treated as his/its own control. We sought to evaluate the efficacy of the FINE to selectively stimulate specific muscles. Comparing the activation of a specific muscle within or between subjects should not present problems because the activations were normalized to their own maximum values. There is no evidence that the selectivity obtained for individuals undergoing vascular surgery differs from that observed in subjects with a spinal cord injury, nor are we aware of changes to the fascicular distribution within a nerve due to spinal cord injury that would affect selectivity.
Since the signals were normalized to their maximum values for each nerve and subject, and because there is no evidence that fascicles merge or divide after spinal cord injury, the only factor that might affect selectivity would be a change in the distribution of axon diameters within the nerve in such a manner that the population of axons change in different ways for each muscle (e.g., axons innervating muscle 1 tend toward a larger diameter while those innervating muscle 2 tend toward a smaller diameter). As this also seems very unlikely, especially since fiber types tend to transform to fast-twitch after spinal cord injury, it is unlikely to be necessary to analyze the data from the two groups separately.
There is a growing body of evidence that suggests that the FINE provides a safe option when, like any cuff electrode, the cuff is appropriately sized and positioned in mechanically stable locations. Evidence includes the pre- and post-operative manual muscle tests from an intraoperative study of the FINE applied to the human femoral nerve [43] and on-going chronic study in which multiple FINEs have been placed around upper-extremity nerves since May 2012. This body of evidence supplements multiple animal studies in which the FINE has also been shown to produce selective stimulation without motor or sensory impairment or tissue damage [44,45,53,87,88]. Additionally, feasibility studies of chronic human implantation of the FINE are approved under an Investigational Device Exemption (IDE) from the FDA.
In order to achieve finer control over the musculature, the data from this study, as well as other animal [44,53,88–91], intraoperative [31,43], and modeling studies [48,66,92], suggest that the next generation of FINEs will likely need a higher density of contacts and a stimulator that can control multiple contacts simultaneously. Currently, a genetic algorithm (GA) is being evaluated in non-human primates to explore these issues [93]. The GA is designed for cuffs that have a high channel density and identifies the best channels to activate either with cathodic or anodic current to selectively stimulate one or more user-specified muscles.
5 Conclusion
The combination of intraoperative and modeling results from this study indicate that an 8-contact FINE placed on the tibial or common peroneal nerve will be sufficiently selective to restore plantarflexion or dorsiflexion, respectively. Data also suggest that the anticipated moment during selective stimulation of these nerves will be adequate to nearly or fully restore propulsive plantarflexion and ground-clearing dorsiflexion. The data indicate that an 8-contact FINE has functional but not muscular redundancy. Not all muscles are anticipated to be selectively recruited above threshold and stimulated plantar- and dorsiflexion moments are expected to be accompanied by inversion or eversion. Muscular redundancy, minimized undesired moment, overall larger muscular selectivity, and smaller variance in muscular selectivity would likely be obtained with a cuff housing a higher channel density.
Acknowledgments
This research was funded by R01-EB001889 from the National Institute of Biomedical Imaging and Bioengineering of the NIH and the NIH Musculoskeletal Training grant T32AR007505. The authors are grateful to Jeanne Marlow, RN, the study coordinator, and Lisa Lombardo, PT,for their pre- and post-surgical assistance. Thanks also to Dr. Lee Fisher, Dr. Thomas Bulea, Natalie Brill, MS, Sarah Chang, BS, and Matthew Stone, BS, for help during experiments. Finally, the authors wish to thank the LSCDVAMC and MHMC for access to the OR to conduct these experiments.
References
- 1.Di Carlo A. Human and economic burden of stroke. Age and Aging. 2008;38:4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]
- 2.Brown DL, Boden-Albala B, Langa KM, Lisabeth LD, Fair M, Smith MA, Sacco RL, Morgenstem LB. Projected costs of ischemic stroke in the United States. Neurology2. 2006;67:1390–1395. doi: 10.1212/01.wnl.0000237024.16438.20. [DOI] [PubMed] [Google Scholar]
- 3.Taylor TN, Davis PH, Tomer JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 4.Boakye M, Leigh B, Skelly A. Quality of life in persons with spinal cord injury: comparisons with other populations. Journal of Neurosurgery: Spine. 2012;17:29–37. doi: 10.3171/2012.6.AOSPINE1252. [DOI] [PubMed] [Google Scholar]
- 5.Creasey G, Dahlberg J. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Archives of Physical Medicine and Rehabilitation. 2001;82:1520–1525. doi: 10.1053/apmr.2001.25912. [DOI] [PubMed] [Google Scholar]
- 6.National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. 2012
- 7.Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. National Vital Statistical Reports. 2011;59:1–126. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Adult Awareness of Tobacco Advertising, Promotion, and Sponsorship—14 Countries. Morbidity and Mortality Weekly Report. 2012;61:2006–2010. [PubMed] [Google Scholar]
- 9.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM. Heart Disease and Stroke Statistics - 2012 Update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams GR. Incidence and characteristics of total stroke in the United States. BMC Neurology. 2001;1:2. doi: 10.1186/1471-2377-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kralj A, Bajd T. Functional electrical stimulation: standing and walking after spinal cord injury. CRC Press; 1989. [Google Scholar]
- 12.Creasey GHG, Ho CH, Triolo RRJ, Gater DR, DiMarco AF, Bogie KM, Keith MW. Clinical applications of electrical stimulation after spinal cord injury. The journal of spinal cord medicine. 2004;27:365–375. doi: 10.1080/10790268.2004.11753774. [DOI] [PubMed] [Google Scholar]
- 13.Bertoti D. Electrical stimulation: a reflection on current clinical practices. Assistive Technology. 2000;12:21–32. doi: 10.1080/10400435.2000.10132007. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal S, Triolo RRJ, Kobetic R, Miller M, Bieri C, Kukke S, Rohde L, Davis JA. Long-term user perceptions of an implanted neuroprosthesis for exercise, standing, and transfers after spinal cord injury. Journal Of Rehabilitation Research And Development. 2003;40:241–252. [PubMed] [Google Scholar]
- 15.Marsolais EB, Kobetic R. Functional walking in paralyzed patients by means of electrical stimulation. Clin Orthop Relat Res. 1983;175:30–36. [PubMed] [Google Scholar]
- 16.Yan T, Hui-Chan CWY, Li LSW. Functional electrical stimulation improves motor recovery of the lower extermity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. 2005;36:80–85. doi: 10.1161/01.STR.0000149623.24906.63. [DOI] [PubMed] [Google Scholar]
- 17.Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clinical Rehabilitaiton. 1997;11:201–210. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- 18.Peng C, Chen S, Lai C, Chen C. Review: Clinical Benefits of Functional Electrical Stimulation Cycling Exercise for Subjects with Central Neurological Impairments. Journal of Medical and Biological Engineering. 2011;31:1–11. [Google Scholar]
- 19.Bogataj U, Gros N, Kljajić M, Aćimović R, Maležič M. The Rehabilitation of Gait in Patients With Hemiplegia: A Comparison Between Conventional Therapy and Multichannel Functional Electrical Stimulation Therapy. Physical therapy. 1995;75:490–502. doi: 10.1093/ptj/75.6.490. [DOI] [PubMed] [Google Scholar]
- 20.Bogataj U, Gros N, Maležič M, Kelih B, Kljajić MRAčimović. Restoration of Gait During Two to Three Weeks of Therapy with Multichannel Electrical Stimulation. Physical therapy. 1989;69:319–327. doi: 10.1093/ptj/69.5.319. [DOI] [PubMed] [Google Scholar]
- 21.Jaime R-PR-P, Matjacic Z, Hunt HJKJ, Matjacić Z. Paraplegic standing supported by FES-controlled ankle stiffness. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2002;10:239–248. doi: 10.1109/TNSRE.2002.806830. [DOI] [PubMed] [Google Scholar]
- 22.Sabut S, Sikdar C, Kumar R, Mahadevappa M. Functional electrical stimulation of dorsiflexor muscle: effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation. 2011;29:393–400. doi: 10.3233/NRE-2011-0717. [DOI] [PubMed] [Google Scholar]
- 23.Sabut S, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disability and Rehabilitation. 2010;32:1594–1603. doi: 10.3109/09638281003599596. [DOI] [PubMed] [Google Scholar]
- 24.Bailey S, Hardin E. Neurotherapeutic and neuroprosthetic effects of implanted functional electrical stimulation for ambulation after incomplete spinal cord injury. J Rehabil Res. 2010;47:7. doi: 10.1682/jrrd.2009.03.0034. [DOI] [PubMed] [Google Scholar]
- 25.Uhlir JP, Triolo RJ, Davis Ja, Bieri C. Performance of epimysial stimulating electrodes in the lower extremities of individuals with spinal cord injury. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2004;12:279–287. doi: 10.1109/TNSRE.2004.827224. [DOI] [PubMed] [Google Scholar]
- 26.Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann RA. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng. 2004;51:146–157. doi: 10.1109/TBME.2003.820321. [DOI] [PubMed] [Google Scholar]
- 27.McDonnall D, Clark Ga, Normann RA. Interleaved, multisite electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses. IEEE Trans Neural Syst Rehabil Eng. 2004;12:208–215. doi: 10.1109/TNSRE.2004.828425. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez FJ, Ceballos D, Schuttler M, Valero A, Valderrama E, Stieglitz T, Navarro X. Polyimide cuff electrodes for peripheral nerve stimulation. Journal of neuroscience methods. 2000;98:105–118. doi: 10.1016/s0165-0270(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 29.Tarler MD, Mortimer JT. Linear summation of torque produced by selective activation of two motor fascicles. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2007;15:104–110. doi: 10.1109/TNSRE.2007.891377. [DOI] [PubMed] [Google Scholar]
- 30.Polasek KH, Hoyen HA, Keith MW, Tyler DJ. Human nerve stimulation thresholds and selectivity using a multi-contact nerve cuff electrode. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2007;15:76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- 31.Polasek KH, Schiefer Ma, Pinault GCJ, Triolo RJ, Tyler DJ. Intraoperative evaluation of the spiral nerve cuff electrode on the femoral nerve trunk. J Neural Eng. 2009;6:66005. doi: 10.1088/1741-2560/6/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2004;12:251–257. doi: 10.1109/tnsre.2004.828415. [DOI] [PubMed] [Google Scholar]
- 33.Tarler MD, Mortimer JT. Comparison of joint torque evoked with monopolar and tripolar-cuff electrodes. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2003;11:227–235. doi: 10.1109/TNSRE.2003.816867. [DOI] [PubMed] [Google Scholar]
- 34.Richardson AG, McIntyre CC, Grill WM. Modelling the effects of electric fields on nerve fibres: influence of the myelin sheath. Med Biol Eng Comput. 2000;38:438–446. doi: 10.1007/BF02345014. [DOI] [PubMed] [Google Scholar]
- 35.Grill WM, Mortimer JT. Non-invasive measurement of the input-output properties of peripheral nerve stimulating electrodes. Journal of neuroscience methods. 1996;65:43–50. doi: 10.1016/0165-0270(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 36.Veraart C, Grill WM, Mortimer JT. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Transactions on Biomedical Engineering. 1993;40:640–653. doi: 10.1109/10.237694. [DOI] [PubMed] [Google Scholar]
- 37.Grill W, Mortimer J. Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Transactions on Rehabilitation Engineering. 1998;6:364–373. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- 38.Grill WM, Mortimer JT, Grill WM., Jr Quantification of recruitment properties of multiple contact cuff electrodes. IEEE Transactions on Rehabilitation Engineering. 1996;4:49–62. doi: 10.1109/86.506402. [DOI] [PubMed] [Google Scholar]
- 39.Fisher LE, Miller ME, Bailey SN, Davis JA, Anderson JS, Rhode L, Tyler DJ, Triolo RJ. Standing after spinal cord injury with four-contact nerve-cuff electrodes for quadriceps stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2008;16:473–478. doi: 10.1109/TNSRE.2008.2003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polasek KH, Hoyen HA, Keith MW, Kirsch RF, Tyler DJ. Stimulation stability and selectivity of chronically implanted multicontact nerve cuff electrodes in the human upper extremity. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2009;17:428–437. doi: 10.1109/TNSRE.2009.2032603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher LE, Tyler DJ, Anderson JS, Triolo RJ. Chronic stability and selectivity of four-contact spiral nerve-cuff electrodes in stimulating the human femoral nerve. J Neural Eng. 2009;6:46010. doi: 10.1088/1741-2560/6/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher LE, Anderson JS, Tyler DJ, Triolo RJ. Optimization of stimulus parameters for selective peripheral nerve stimulation with multi-contact electrodes; Conference proceedings of the IEEE Engineering in Medicine and Biology Society; 2011. pp. 3039–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiefer MA, Polasek KH, Triolo RJ, Pinault GCJ, Tyler DJ. Selective stimulation of the human femoral nerve with a flat interface nerve electrode. Journal of neural engineering. 2010;7:26006. doi: 10.1088/1741-2560/7/2/026006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leventhal DK, Durand DM. Chronic measurement of the stimulation selectivity of the flat interface nerve electrode. IEEE Transactions on Biomedical Engineering. 2004;51:1649–1658. doi: 10.1109/TBME.2004.827535. [DOI] [PubMed] [Google Scholar]
- 45.Leventhal DK, Cohen M, Durand DM. Chronic histological effects of the flat interface nerve electrode. Journal of neural engineering. 2006;3:102–113. doi: 10.1088/1741-2560/3/2/004. [DOI] [PubMed] [Google Scholar]
- 46.Perkins TA, Donaldson NN. Control of leg-powered paraplegic cycling using stimulation of the lumbo-sacral anterior spinal nerve roots. Neural Systems and …. 2002;10:158–164. doi: 10.1109/TNSRE.2002.802860. [DOI] [PubMed] [Google Scholar]
- 47.Gustafson KJ, Grinberg Y, Joseph S, Triolo RJ. Human distal sciatic nerve fascicular anatomy: Implications for ankle control using nerve-cuff electrodes. Journal of rehabilitation research and development. 2012;49:309–322. doi: 10.1682/jrrd.2010.10.0201. [DOI] [PubMed] [Google Scholar]
- 48.Schiefer M, Tyler D, Triolo R. Probabilistic modeling of selective stimulation of the human sciatic nerve with a flat interface nerve electrode. Journal of computational neuroscience. 2012;33:179–190. doi: 10.1007/s10827-011-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiefer MA, Tyler DJ, Triolo RJ. Neural Interfances Conference. Salt Lake City: 2012. Design of nerve cuff electrodes for the sciatic, tibial, and common peroneal nerves using probabilistic models. [Google Scholar]
- 50.Lee HJ, DeLisa JA. Surface Anatomy for Clinical Needle Electromyography. New York: Demos Medical; 2000. [Google Scholar]
- 51.Perotto AO, Delagi EF. Anatomical Guide for the Electromyographer: The Limbs and Trunk. Springfield; Charles C. Thomas: 2005. [Google Scholar]
- 52.Freeberg M, Schiefer M, Triolo R. Conference proceedings of the IEEE Engineering in Medicine and Biology Society. Boston: 2011. Efficient serach and fit methods to find nerve stimulation parameters for multi-contact electrodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. Neural Systems and Rehabilitation. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 54.Wickiewicz T, Roy R. Muscle architecture of the human lower limb. Clinical orthopaedics and related research. 1983 [PubMed] [Google Scholar]
- 55.Brand RA, Pedersen DR. The sensitivity of muscle force predictions to changes in physiologic cross-sectional area. Journal of biomechanics. 1986;19:589–596. doi: 10.1016/0021-9290(86)90164-8. [DOI] [PubMed] [Google Scholar]
- 56.Delp SL. Surgery Simulation: A computer graphics system to analyze and design musculoskeletal reconstructions of the lower extermity. Stanford University; 1990. [Google Scholar]
- 57.Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- 58.Friederich JA, Brand RA. Muscle fiber architecture in the human lower limb. Journal of biomechanics. 1990;23:91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- 59.Acosta AM. Musculoskeletal modeling of the shoulder and elbow in cervical spinal cord injury. Cleveland: Case Western Reserve University; 2002. [Google Scholar]
- 60.Fatone S, Gard SA, Malas BS. Effect of ankle-foot orthosis alignment and foot-plate length on the gait of adults with poststroke hemiplegia. Archives of Physical Medicine and Rehabilitation. 2009;90:810–818. doi: 10.1016/j.apmr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 61.McDonnall D, Clark GA, Normann RA. Selective motor unit recruitment via intrafascicular multielectrode stimulation. Canadian Journal of Physiology and Pharmacology. 2004;82:599–609. doi: 10.1139/y04-047. [DOI] [PubMed] [Google Scholar]
- 62.Badia J, Boretius T, Andreu D, Azevedo-Coste C, Stieglitz T, Navarro X. Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. Journal of neural engineering. 2011;8:036023. doi: 10.1088/1741-2560/8/3/036023. [DOI] [PubMed] [Google Scholar]
- 63.Sunderland S. Nerves and Nerve Injuries. Churchill Livingstone; 1978. [Google Scholar]
- 64.Gustafson KJ, Pinault GC, Neville JJ, Syed I, Davis JA, Jr, Jean-Claude J, Triolo RJ. Fascicular anatomy of human femoral nerve: implications for neural prostheses using nerve cuff electrodes. J Rehabil Res Dev. 2009;46:973–984. doi: 10.1682/jrrd.2008.08.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grill WM, Veraart C. Selective activation of peripheral nerve fascicles: use of field steering currents; 13th International Conference of the IEEE Engineering in Medicine and Biology Society; 1991. [Google Scholar]
- 66.Schiefer MA, Triolo RJ, Tyler DJ. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2008;16:195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamaguchi S, Tanaka Y, Banks S, Kosugi S, Sasho T, Takahashi K, Takakura Y. In vivo kinematics and articular surface congruency of total ankle arthroplasty during gait. Journal of biomechanics. 2012;45:2103–2108. doi: 10.1016/j.jbiomech.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 68.Willems T, Witvrouw E, Delbaere K, De Cock a, De Clercq D. Relationship between gait biomechanics and inversion sprains: a prospective study of risk factors. Gait & posture. 2005;21:379–387. doi: 10.1016/j.gaitpost.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Butler RJ, Barrios Ja, Royer T, Davis IS. Effect of laterally wedged foot orthoses on rearfoot and hip mechanics in patients with medial knee osteoarthritis. Prosthetics and orthotics international. 2009;33:107–116. doi: 10.1080/03093640802613237. [DOI] [PubMed] [Google Scholar]
- 70.Chen C-C, Hong W-H, Wang C-M, Chen C-K, Wu KP-H, Kang C-F, Tang SF. Kinematic features of rear-foot motion using anterior and posterior ankle-foot orthoses in stroke patients with hemiplegic gait. Archives of Physical Medicine and Rehabilitation. 2010;91:1862–1868. doi: 10.1016/j.apmr.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Bogey RA, Perry J, Gitter AJ. An EMG-to-force processing approach for determining ankle muscle forces during normal human gait. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005;13:302–310. doi: 10.1109/TNSRE.2005.851768. [DOI] [PubMed] [Google Scholar]
- 72.Hayashibe M, Guiraud D, Poignet P. EMG-based neuromuscular modeling with full physiological dynamics and its comparison with modified Hill model. Conference proceedings of the IEEE Engineering in Medicine and Biology Society. 2009;vol 2009:6530–6533. doi: 10.1109/IEMBS.2009.5333147. [DOI] [PubMed] [Google Scholar]
- 73.Elias JJ, Bratton DR, Weinstein DM, Cosgarea AJ. Comparing two estimations of the quadriceps force distribution for use during patellofemoral simulation. Journal of biomechanics. 2006;39:865–872. doi: 10.1016/j.jbiomech.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 74.Kesar TM, Perumal R, Reisman DS, Jancosko A, Rudolph KS, Higginson JS, Binder-Macleod Sa. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: effects on poststroke gait. Stroke; a journal of cerebral circulation. 2009;40:3821–3827. doi: 10.1161/STROKEAHA.109.560375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kesar TM, Ding J, Wexler AS, Perumal R, Maladen R, Binder-Macleod Sa. Predicting muscle forces of individuals with hemiparesis following stroke. Journal of neuroengineering and rehabilitation. 2008;5:7. doi: 10.1186/1743-0003-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hunter BV, Thelen DG, Dhaher YY. A three-dimensional biomechanical evaluation of quadriceps and hamstrings function using electrical stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2009;17:167–175. doi: 10.1109/TNSRE.2009.2014235. [DOI] [PubMed] [Google Scholar]
- 77.Piazza S, Delp S. The influence of muscles on knee flexion during the swing phase of gait. Journal of Biomechanics. 1996 doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 78.Sinclair PJ, Davis GM, Smith RM. Musculo-skeletal modelling of NMES-evoked knee extension in spinal cord injury. Journal of biomechanics. 2006;39:483–492. doi: 10.1016/j.jbiomech.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 79.Wilkenfeld AJ, Audu ML, Triolo RJ. Feasibility of functional electrical stimulation for control of seated posture after spinal cord injury: A simulation study. The Journal of Rehabilitation Research and Development. 2006;43:139. doi: 10.1682/jrrd.2005.06.0101. [DOI] [PubMed] [Google Scholar]
- 80.Audu ML, Nataraj R, Gartman SJ, Triolo RJ. Posture shifting after spinal cord injury using functional neuromuscular stimulation – a computer simulation study. J Biomech. 2011;44:1639–1645. doi: 10.1016/j.jbiomech.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nataraj R, Audu ML, Kirsch RF, Triolo RJ. Center of Mass Acceleration Feedback Control for Standing by Functional Neuromuscular Stimulation – a Simulation Study. J Rehabil Res Dev. 2012;49:279–296. doi: 10.1682/jrrd.2010.12.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gartman SJ, Audu ML, Kirsch RF, Triolo RJ. Selection of optimal muscle set for 16-channel standing neuroprosthesis. J Rehabil Res Dev. 2006;43:216–231. doi: 10.1682/jrrd.2007.10.0164. [DOI] [PubMed] [Google Scholar]
- 83.Nataraj R, Audu ML, Triolo RJ. Comparing joint kinematics and center of mass acceleration as feedback for control of standing balance by functional neuromuscular stimulation. Journal of neuroengineering and rehabilitation. 2012;9:25. doi: 10.1186/1743-0003-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nataraj R, Audu ML, Kirsch RF, Triolo RJ. Comprehensive Joint Feedback Control for Standing by Functional Neuromuscular Stimulation – a Simulation Study. IEEE Trans Neural Syst Rehabil Eng. 2010;18:646–657. doi: 10.1109/TNSRE.2010.2083693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambrecht J, Audu ML, Triolo RJ, Kirsch RF. Musculoskeletal model of trunk and hips for development of seated-posture-control neuroprosthesis. J Rehabil Res Dev. 2009;46:515–528. doi: 10.1682/jrrd.2007.08.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bogey RA, Perry J, Bontrager EL, Gronley JK. Comparison of across-subject EMG profiles using surface and multiple indwelling wire electrodes during gait. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2000;10:255–259. doi: 10.1016/s1050-6411(00)00015-8. [DOI] [PubMed] [Google Scholar]
- 87.Tyler DJ, Durand DM. Chronic response of the rat sciatic nerve to the flat interface nerve electrode. Annals of Biomedical Engineering. 2003;31:633–642. doi: 10.1114/1.1569263. [DOI] [PubMed] [Google Scholar]
- 88.Yoo PB, Sahin M, Durand DM. Selective stimulation of the canine hypoglossal nerve using a multi-contact cuff electrode. Annals of Biomedical Engineering. 2004;32:511–519. doi: 10.1023/b:abme.0000019170.74375.fb. [DOI] [PubMed] [Google Scholar]
- 89.Leventhal DK, Durand DM. Subfascicle Stimulation Selectivity with the Flat Interface Nerve Electrode. Annals of Biomedical Engineering. 2003;31:643–652. doi: 10.1114/1.1569266. [DOI] [PubMed] [Google Scholar]
- 90.Yoo PB, Durand DM. Effects of selective hypoglossal nerve stimulation on canine upper airway mechanics. Journal of applied physiology (Bethesda, Md.: 1985) 2005;99:937–943. doi: 10.1152/japplphysiol.00652.2004. [DOI] [PubMed] [Google Scholar]
- 91.Brill N, Polasek KH, Oby E, Ethier C, Miller L, Tyler DJ. Nerve cuff stimulation and the effect of fascicular organization for hand grasp in nonhuman primates. Conference proceedings of the IEEE Engineering in Medicine and Biology Society. 2009;vol 2009:1557–1560. doi: 10.1109/IEMBS.2009.5332395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brill N, Tyler DJ. Optimizing nerve cuff stimulation of targeted regions through use of genetic algorithms. Conference proceedings of the IEEE Engineering in Medicine and Biology Society. 2011;vol 2011:5811–5814. doi: 10.1109/IEMBS.2011.6091438. [DOI] [PubMed] [Google Scholar]
- 93.Brill N, Hess A, Miller L, Ethier C, Tyler DJ. Neural Interfances Conference. Salt Lake City: 2012. Selective activation of upper extremity muscles using high density nerve cuff electrodes in nonhuman primates. [Google Scholar]