Abstract
Modulators of unconditioned fear are potential targets for developing treatments for anxiety disorders. We used blood oxygen level dependent (BOLD) MRI to investigate the pattern of brain activity during the presentation of a predator odor (cat fur) and a repulsive novel odor, butyric acid (BA), to awake rats. We further tested whether odor-evoked BOLD activation involved oxytocin (OT) and vasopressin V1a receptors. Animals were subdivided into groups either administered an intracerebroventricular injection of artificial cerebrospinal fluid (CSF), an OT receptor antagonist or a V1a antagonist (125 ng/10 μL each) 90 min before studies. BA odor evoked robust brain activation across olfactory, sensory, memory and limbic regions. The magnitude of BOLD activation across these regions was greater for BA than with cat fur. However, blockade of OT and V1a receptors differentially modulated odor evoked neural activity, particularly in the amygdala. OT and V1a antagonism preferentially modulated BOLD responding to BA in the cortical amygdala. While, OT and V1a antagonisms preferentially modulated BOLD responding to cat fur in the central amygdala. The data suggest that although OT receptors modulate BOLD activation in response to a novel and repulsive odor such as BA, vasopressin V1a receptors exert a modulatory influence on the neural response to a predator odor.
Keywords: Vasopressin, Oxytocin, V1a receptor, Cat, BOLD, fMRI, Rat, Predator, Anxiety, Butyric acid
1. Introduction
Rodents exposed to predator odors show elevated anxiety, including fear-associated freezing behavior, a heightened physiological stress response and defensive reactions (Blanchard et al., 2003; Dielenberg et al., 2001; Li et al., 2004; McGregor et al., 2002; Staples et al., 2008). For example, freezing behavior is increased in the presence of a chemical extract of fox feces, 2,4,5-trimethylthiazoline (TMT) (Fendt et al., 2003). TMT triggers behavioral and autonomic signatures of fear and anxiety that are not produced by other repulsive odors. It has also been reported to elevate plasma corticosterone levels and increase prefrontal cortical dopamine metabolism, which are considered signs of intense stress reactivity (Morrow et al., 2000a, 2000b; Thomas et al., 2006; Vendruscolo et al., 2006). The behavioral responses to TMT seem to be mediated through specific neural circuits. Lesions of the ventral bed nucleus of stria terminalis (BNST) and lateral septum have been reported to significantly attenuate TMT-induced freezing (Endres and Fendt, 2008; Fendt et al., 2003). Expression of c-fos in response to TMT has been observed in the central amygdala (Staples et al., 2008), a structure that controls autonomic output (Huber et al., 2005; Viviani et al., 2011). Increased c-fos expression has also been observed in the olfactory system, orbital cortex and anterior cortical amygdala (Staples et al., 2008). However, the latter areas were not unique to TMT and other brain areas are activated by cat odors but not by TMT (Staples et al., 2008). Moreover, unconditioned fear measured by performance on an elevated plus maze (EPM) is enhanced by cat fur odor but not TMT (McGregor et al., 2002). TMT does not lead to conditioned fear, whereas exposure to cats produces contextual fear learning in rats (Blanchard et al., 2003). There is concern that rodents do not recognize TMT as an actual predator threat, but instead it constitutes a naturally repulsive odor that is capable of reproducing only partial activation of fear circuitry (Endres and Fendt, 2008). On the other hand, odors derived from cat fur elicit consistent freezing behavior, specific defensive reactions and lasting effects on anxiety levels (Blanchard et al., 2003; McGregor et al., 2002). The use of cat odors instead of TMT may therefore improve research on the neural circuitry of unconditioned fear, its neurobiological control and pharmacological modulation.
Brain regions associated with unconditioned fear overlap to a certain degree with receptors for the neuropeptides oxytocin (OT) and vasopressin (AVP). The central distribution of OT and AVP suggest they play a role in modulating olfactory processing, social behavior, as well as cognitive, emotion-related and goal-directed behavioral functions (Huber et al., 2005; Ostrowski et al., 1994; Szot et al., 1994; Tribollet et al., 1988a; Tribollet et al., 1988b; Veinante and Freund-Mercier, 1997; Yoshimura et al., 1993). The distribution of the receptor sites for OT and AVP support their role in neuromodulation. There is growing evidence that OT-mediated effects have a role in anxiolysis, while AVP actions through the V1a receptor promote aggression and heightened anxiety (Appenrodt et al., 1998; Knobloch et al., 2012; Murgatroyd et al., 2004; Waldherr and Neumann, 2007). Not only do these neuropeptides control anxiety, but also their presence in amygdala and hippocampus suggests a prominent role in cognitive mechanisms, such as conditioned fear learning processes (de Wied et al., 1984a; De Wied et al., 1984b). Based on their location within the components of the olfactory system, such as the anterior olfactory nucleus and the olfactory tubercles, as well as a differential expression of their messenger RNA in regions such as the central amygdala and BNST, these peptides may control olfactory perception and the association between smell and emotionality (Ferris et al., 2008). Given the spatial distribution of the relevant neuroanatomy, we used functional MRI to investigate the role of OT and V1a receptors in modulating odor evoked brain activity in the awake rat. A novel repulsive odor for the rats, butyric acid (BA), and unknown chemical constituents present in cat fur were chosen in order to examine unconditioned fear and anxiety responses. Our data show a role of OT receptors in modulating the neural response to BA, while V1a receptors appear to preferentially modulate the neural response to cat fur.
2. Results
2.1. OT blockade reduces avoidance-like behavior in the presence of cat fur and butyrate
A modified version of a place preference test cage was used to examine place aversion in the presence of BA and cat fur. Using these methods, we examined the natural and unconditioned tendency of rats to avoid a novel repulsive odor and to avoid odorants present in cat fur. Results are presented in Fig. 1 and Supporting Online Fig. S2. Supporting Online Fig. S2 shows an example video frame with one rat on the far left approaching the odor tube and another located away from the odor compartment inside the no odor part of the cage. Analysis of variance revealed a significant main effect of time spent in each compartment (F2,81=5.8, p=0.004) and interaction with ICV drug treatments (F4,81=3.2, p=0.02). In CSF treated rats, we observed a significant reduction in time spent inside the cat odor-paired side of the test cage compared to no odor condition (Kruskall–Wallis H3=9.9, p=0.007; Dunn’s multiple posthoc comparison test p <0.05). Interestingly, CSF rats also spent less time in the non-odor paired side of the cage (Kruskall–Wallis H3=6.9, p=0.03; Dunn’s multiple posthoc comparison test p <0.05) and much of its time was spent inside the middle adjacent compartment (Kruskall–Wallis H3 =6.7, p=0.03; Dunn’s multiple posthoc comparison test p <0.05). This was not observed with BA presentation. Thus, in CSF treated rats cat fur but not BA elicited avoidance behavior and rats spent most of the time inside the middle (smaller) chamber of the test cage and avoided the larger, open ends of the cage. Blockade of OT receptors resulted in indiscriminate transitions between all three sub-compartments of the test cages, regardless of the presence or absence of odors. Thus, OT blockade reduced, and it could perhaps even be said that it eliminated avoidance in response to cat fur odor (Fig. 1). Blockade of V1a receptors did not alter the pattern of behavioral responses when no odor was present. V1a-A treated rats showed less time spent in the odor-paired side of the test cage when cat fur was present than when no odor was present (Kruskall–Wallis H3=9.6, p=0.008; Dunn’s multiple posthoc comparison test p <0.05). There was a tendency for greater time spent in the middle or adjacent compartment, which is consistent with the observations of CSF-treated rats. Supporting Online Fig. S2 summarizes the same data from a different perspective, which shows the sharp shift in time spent from side compartments to the middle (adjacent) compartment with cat odor in CSF and V1a-A treated animals but not OT-A treated rats.
Fig. 1.
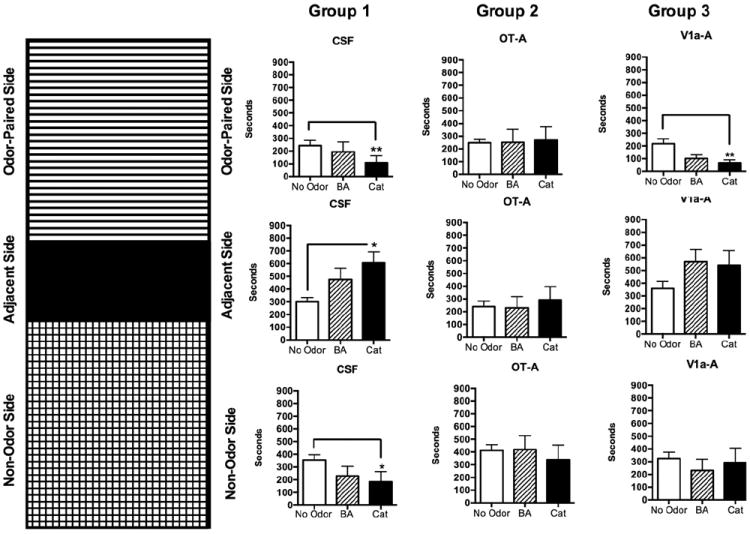
Time spent in one of three compartments of a test chamber during no odor, butyric acid (BA) and cat fur exposure. Each odor was presented during a 15-min (900 s) test during which an empty vial or a vial containing an odor was present in one compartment. Ninety minutes before behavioral testing, rats received a 10 uL intracerebroventricular injection of CSF, an oxytocin antagonist (OT-A) or a vasopressin V1a receptor antagonist. Figure on the left depicts the three-compartment test cage with odor-paired, adjacent, and non-odor paired sides. Data are presented to the right in that same order from top to bottom. Columns represent the three different groups, CSF-treated, OT-A and V1a-A treated male rats. All data presented as mean±standard error. Asterisks denote significant differences from no odor condition (open columns; two way ANOVA *p<0.05;**p<0.01).
2.2. OT and AVP V1a receptor antagonists modulate butyrate and cat fur odor evoked neural activity
We analyzed stimulus evoked signal changes across 69 major and minor ROI’s. Fig. S1 shows an example of an anatomical scan well aligned with a digital atlas of the rat brain. Signal increases and decreases in response to both odors were analyzed. Fig. 2 shows composite statistical maps of positive BOLD activity in response to BA odor and Fig. 3 summarizes the results for cat fur. As an olfactory stimulus, BA exerts a robust effect on the extent of activation across the rat brain, including multiple olfactory, limbic and cortical structures. This is much less so for cat fur. BA odor increases BOLD activity in areas of the medial prefrontal cortex (mPFC), dorsal and ventral striatum, olfactory tubercles, piriform cortex, amygdala, hypothalamus, insular and gustatory cortices, and the anterior and medial thalamic areas, among others (Fig. 2). The volume activation was also more limited with cat fur (Fig. 3). Although some significant activation (red–yellow pixels in Fig. 3) is observed in mPFC areas, gustatory and insular cortices, hypothalamic structures, the extent or volume is much smaller when compared to BA. Since there appeared to be a greater response to BA versus cat fur, we compared the responses to an innocuous odor that would also not be appetitive to rats, cinnamon (Supporting Online Fig. S3). BA was still observed to evoke greater BOLD activation than this other novel, but innocuous scent, regardless of presentation order (Supporting Online Fig. S4).
Fig. 2.
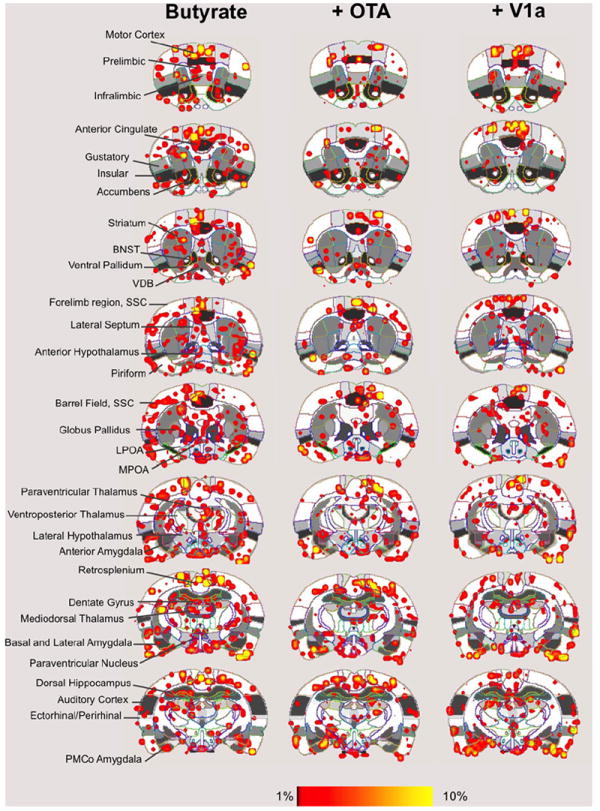
Butyric acid induced neural activity in the awake rat. Shown are composite maps for significant increases in signal intensity during presentation of air containing 5 uL 170 mM butyrate (airflow rate 1200 cc/min). Ninety minutes before behavioral testing, rats received a 10 uL intracerebroventricular injection of CSF (left column images), an oxytocin antagonist (+OT-A) or a vasopressin V1a receptor antagonist (+V1a). Overlay coloring threshold is set between 1% and 10% signal changes. Left column indicates specific regions of interest. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
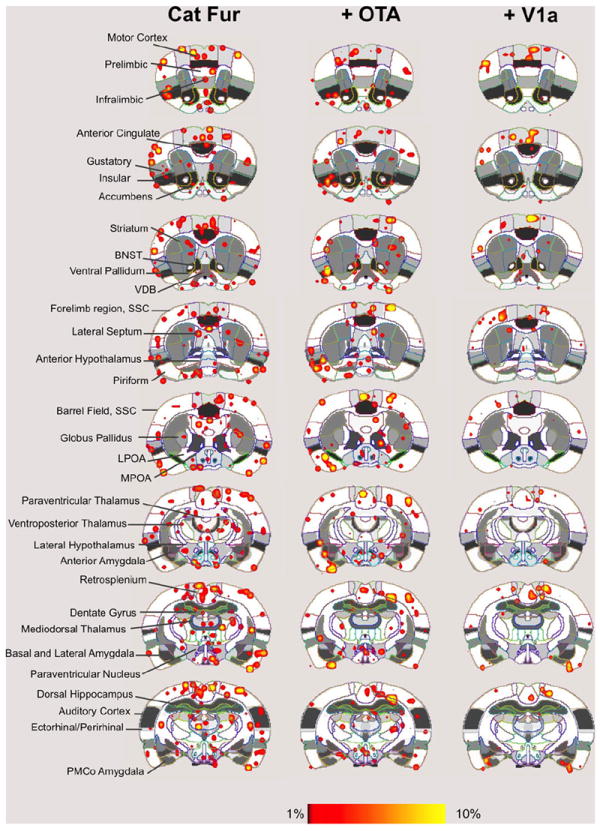
Cat fur induced neural activity in the awake rat. Shown are composite maps for significant increases in signal intensity during presentation of air flowing through a connector vial containing humidified cat fur (airflow rate 1200 cc/min). Ninety minutes before behavioral testing, rats received a 10 uL intracerebroventricular injection of CSF (left column images), an oxytocin antagonist (+OT-A) or a vasopressin V1a receptor antagonist (+V1a). Overlay coloring threshold is set between 1% and 10% signal changes. Left column indicates specific regions of interest. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
ANOVA showed a main effect of smell (BA versus cat fur) in the anterior thalamic nucleus (F2,47=5.8, p=0.02), BNST (F2,47=18.5, p <0.0001), ventral CA3 (F2,47=4.7, p=0.03), cortical amygdala (F2,47=7.8, p=0.007), lateral amygdala (F2,47=11.4, p=0.001), medial amygdala (F2,47=7.5, p=0.008), olfactory tubercle (F2,47=3.9, p=0.05), posterior amygdala (F2,47=4.2, p=0.04), PAG (F2,47=5.1, p=0.03), posterior hypothalamus (F2,47=11.4, p=0.009), dorsomedial, dorsolateral and ventromedial regions of the striatum (ventromedial F2,47=7.4, p=0.02), nucleus accumbens core (F2,47=4.2, p=0.04), subiculum (F2,47=11.3, p=0.001), and ventral tegmental area (F2,47=8.7, p=0.005). In all cases, BA-induced percent BOLD activation was greater than that observed with cat fur exposure.
We investigated the effect of blocking OT and V1a receptors on the BOLD response to BA and cat fur. Significant effects of treatment (CSF, OT-A and V1a-A) were observed in the agranular insular cortex (F2,47=3.2, p=0.05), cortical amygdala (F2,47=7.7, p=0.001), olfactory tubercles (F2,47=4.8, p=0.01), periaqueductal grey (PAG; F2,47=6.7, p=0.002), and entorhinal cortex (F2,47=3.2, p=0.04). Bonferroni’s multiple comparisons posthoc test indicated that out of these regions, the cortical amygdala, lateral amygdala, and olfactory tubercle were significantly different between the treatment conditions (p <0.05). In all 3 regions, the V1a blockade group showed a lower BOLD signal response to BA odor than CSF treated animals (p <0.05). However, the cortical amygdala was the only ROI showing a significant interaction between odor and treatment (F2,47=5.0, p=0.01; Fig. 4). While OT-A rats showed a lower BOLD signal response to BA, V1a-A treated animals showed a significantly greater response compared to CSF treatment. The temporal profile for the cortical amygdala is shown in Fig. 5B. A similar pattern of BOLD activation was not observed in the olfactory tubercle (Fig. 5A). Here, V1a blockade showed a lower BOLD signal response than CSF treated animals and OT-A treatment showed a similar but non-significant trend. Indeed, the multiway ANOVA did not reveal any significant effects of OT and V1a receptor blockade on the BOLD response to cat fur. However, given the magnitude of the differences between the odor conditions (Figs. 2 and 3) and the nature of the stimuli (a known odorant chemical such as BA versus cat fur, which has unknown chemical or set of chemicals), we performed one way ANOVA to examine the effects of drug treatment (independent variable) on BOLD response to cat fur separate from that of BA. Using this strategy, we observed that several brain regions showed a significantly attenuated BOLD signal response to cat odor with V1a blockade (Fig. 6). These included the dorsal CA1 (F2,28=3.3, p=0.05), ventral CA3 (F2,28=3.2, p=0.05), olfactory tubercle (F2,28=4.0, p=0.02), PAG (F2,28= 10.0, p=0.0006), central amygdala (F2,28=7.8, p=0.002), and the subiculum (F2,28=5.1, p=0.01). However, upon evaluation of the temporal profile of the BOLD signal (Supporting Online Fig. S5), it was still apparent that the BOLD signal magnitude in response to cat fur was tenuous compared with that produced by BA (Fig. 5). Moreover, the increase in BOLD evoked by BA appears immediate, whereas that of cat fur builds up over time (comparison between Fig. 5A and Supporting Online Fig. S5). Signal decreases (or negative BOLD signal changes) mostly followed a similar pattern as that observed for positive signal changes. This is consistent with ‘vascular steal’ effects and does not support selective inhibition. However, there were some specific effects associated with signal decreases in the presence of BA versus cat fur. Negative BOLD signal changes and statistical comparisons are reported as online supporting material (Supporting Online Fig. S6).
Fig. 4.
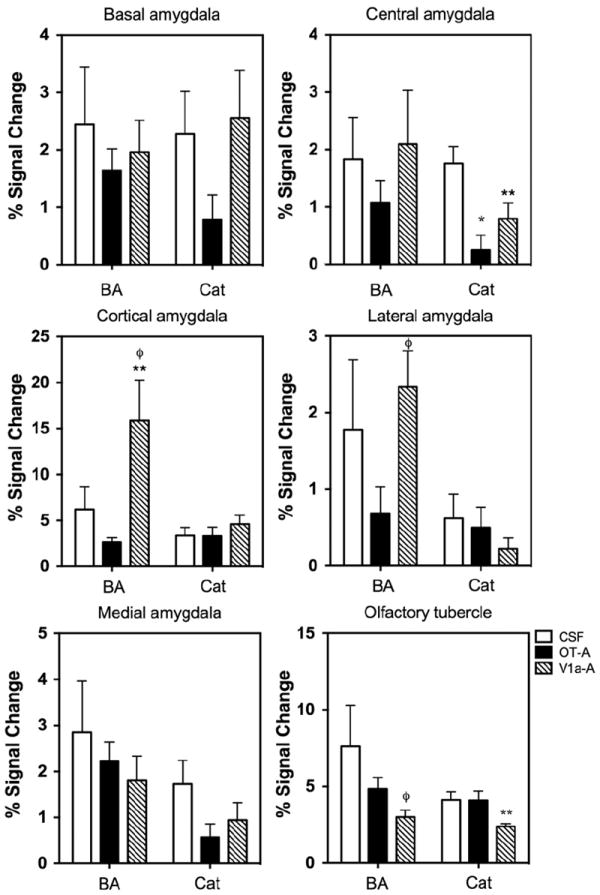
BOLD signal changes in response to a novel butyric acid odor and cat fur odor. Shown are subnuclei of the amygdala and the lower right panel shows data for the olfactory tubercle. All data presented as mean±standard error. Each panel shows the mean percent change in BOLD signal in response to butyric acid and cat fur for CSF, OT-A and V1a-A treated animals. *OT-A significantly different from CSF, **V1a-A significantly different from CSF and φV1a-A significantly different from OT-A (Two way ANOVA p<0.05).
Fig. 5.
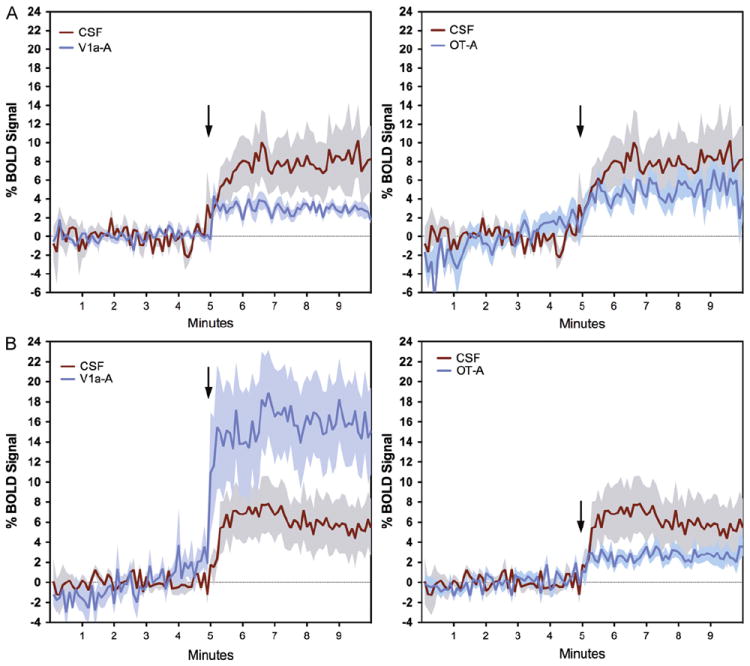
Temporal profile of the BOLD signal change in response to butyric acid odor. Data are shown for the olfactory tubercle (A) and the cortical amygdala (B). Shaded areas surrounding plot lines indicate±standard error. Arrows show the time of onset of odor delivery.
Fig. 6.
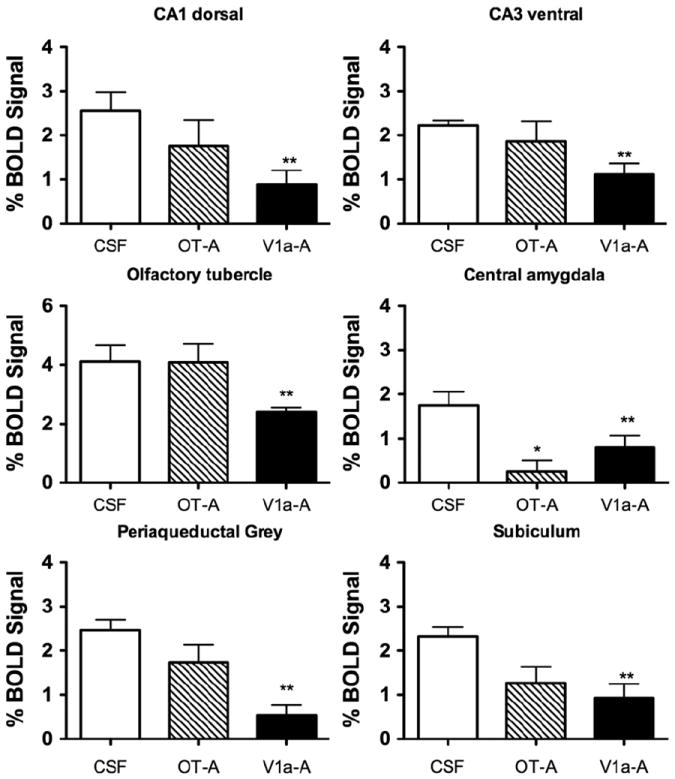
BOLD signal changes in response to cat fur odor. All data presented as mean±standard error. Each panel shows the mean percent change in BOLD signal in response to cat fur for CSF, OT-A and V1a-A treated animals. *OT-A significantly different from CSF and **V1a-A significantly different from CSF (One way ANOVA p<0.05).
3. Discussion
This is the first study to investigate the neural circuitry of unconditioned fear by presenting cat fur odor as a stimulus to awake rats undergoing functional MRI. Our results indicate that BA-induced BOLD signal increases were significantly greater than with cat fur. While BA activated several olfactory system structures and areas of the brain involved in the neural processing of smell, cat fur odor only caused modest activation. We did observe a significant effect of cat fur in the present study across numerous regions, including the anterior thalamic nucleus, BNST, ventral CA3, cortical amygdala, lateral amygdala, medial amygdala, olfactory tubercle, posterior amygdala, PAG, posterior hypothalamus, dorsomedial, dorsolateral and ventromedial regions of the striatum, nucleus accumbens core, subiculum, and ventral tegmental area. Across all of the regions, BA induced greater BOLD signal changes than cat fur. Behavioral tests done in a separate group of animals provided support for the presence of a volatile odorant chemical in the cat fur. Therefore, we expected cat fur would have elicited greater BOLD activation. However, although it is assumed here that cat fur contains such an odorant chemical, or a set of chemicals, that drive activity through stimulation of main olfactory receptors, it is possible that a putative odorant chemical(s) present in cat fur may drive activation through the accessory olfactory system instead. Thus, the pattern of activation produced by the cat fur odor could have involved a different mechanism than that of BA, perhaps through the vomeronasal system (Papes et al., 2012). Alternatively, a full neural response to cat fur may require activation through other sensory modalities in addition to olfactory stimulation. In spite of the weaker activity generated by cat fur, an important finding was that OT and V1a receptors differentially modulated BA- and cat fur-induced BOLD signal responses in several brain areas. BA-induced BOLD signal changes were curtailed in the cortical amygdala by OT-A. AVP V1a receptor blockade exerted an opposite effect. Interestingly, the V1a receptor antagonist reduced cat fur-induced BOLD signal changes in the amygdala, hippocampus and PAG. OT blockade had no effect on the response to cat fur in these and other regions of the brain, only in the central amygdala where both OT-A and V1a-A both curtailed the BOLD response to cat fur.
Overall, our findings suggest that OT-mediated neuro-transmission is important in responding to a novel and repulsive odor such as BA. Since this is the first exposure to BA, then OT receptor activation might be involved in an olfactory learning process during the initial experience of the odor (habituation). On the other hand, AVP V1a mediated neurotransmission appeared more closely associated with cat fur induced BOLD activation across several brain regions. This could indicate an association between AVP V1a receptors and unconditioned fear rather than a habituation process. Our behavioral data provide only partial support for this. We used a very simple measure of avoidance (less time spent in an odor-paired side of a test cage) that did not truly represent fear, as would be the case with the measurement of freezing behavior, performance on an EPM, light-dark transitions and startle response. The avoidance may have been influenced by the repulsive and/or novel nature of the odors presented and not by the triggering of an unconditioned fear response. Notwithstanding, OT blockade increased time spent near the odor-paired side (for both cat and BA) and V1a receptor blockade had no effect compared to the CSF vehicle group. Thus, OT blockade reduced avoidance whereas V1a blockade was indistinct from vehicle effects. Overall, the present data support actions via OT receptors, which modulate the neural response to odors. However the results only partly direct our attention towards a possible role of these neuropeptide receptors on innate fear circuitry.
We initially expected that regions such as the ventral BNST, amygdala, ventral hippocampus, lateral septum, and other regions previously identified with cellular markers of neuronal activation would distinguish the effects of cat odor from BA. Using c-fos immunolabelling, Staples et al. (2008) identified regions of the rat brain that responded selectively to cat and TMT over no odor and formalin odor conditions. They reported selective cat-induced activation of c-fos labeling in VTA, striatum (dorsal and ventral), basal and medial amygdaloid nuclei, subareas of the anterior olfactory nucleus, medial prefrontal cortex, anterior and ventromedial hypothalamic nuclei, BNST and dorsal premammilary nucleus (Staples et al., 2008). TMT had only modest effects on cellular activation, increasing activity in the cortical amygdala (anterior portion), piriform and ventral orbital cortices. Rosen et al. (2005) reported increased mRNA expression of egr-1 in the hypothalamic paraventricular nucleus (PVN) of rats following exposure to a cat (Rosen et al., 2005). This is interesting since this region contains OT and V1a receptors that control the central release of their corresponding neuropeptides via magnocellular neurons (Knobloch et al., 2012). Several lesion studies have provided evidence of a role for the ventral hippocampus and BNST in unlearned fear reactivity. Inactivation of the ventral BNST or blockade of norepinephrine α2 receptors reduces freezing in response to TMT (Fendt et al., 2003, 2005). Similar effects are observed with lateral septum inactivation (Endres and Fendt, 2008). These areas express V1a receptor mRNA (Veinante and Freund-Mercier, 1997). In the case of the ventral hippocampus, lesions to this site result in significantly more time spent in the open arms of an EPM (Kjelstrup et al., 2002). The ventral hippocampus also shows significant V1a receptor mRNA expression (Ostrowski et al., 1994). Here, we found significant effects of V1a blockade on cat but not BA induced BOLD activation (Fig. 6), which is consistent with some of the aforementioned studies.
The fact that both BA and cat fur odors were both novel odors for the tested rats could help explain the lower BOLD response to cat versus BA. The saturation of olfactory epithelia with BA odor before the presentation of cat fur may have obscured the effects of the latter on neural activity. This argument would assume, however, an overlap between the odorant receptors that are activated by cat odor and BA. It is possible that the lower BOLD response is due to the inability to calibrate the concentration of the fear-inducing component of cat fur. This scenario still presents potentially interesting future directions. Rats exposed to BA and cat fur showed avoidance-like responses (during behavioral tests), which may have arisen from novelty effects and mild stress during this first exposure. It appears that only OT blockade was able to reduce this effect by increasing the amount of time spent near the odor source (for BA and cat). Thus, instead of anxiety produced by OT blockade, the perception of the novel and repugnant odors may have been reduced. One possible mechanism could be through the modulatory effects of OT on the olfactory system and amygdala, which was observed in the present study. V1a blockade did not have a similar effect and given that our behavioral measurements may have been insensitive to fear associated behaviors, one might argue that only the novelty associated and not the fear inducing effects of the odors were examined in our behavioral assay. However, the V1a antagonist clearly modulated the neural response to cat fur odor, thereby providing some evidence that it does exert central effects on brain activity. Our BOLD MRI findings for the cat odor should be viewed with some caution, as we did not (or could not) counterbalance the order of odor presentation (see Section 4.5.2, MRI procedures). All rats received a receptor antagonist/CSF, then BA, then the cat odor. As the cat odor was always presented after BA, it is not possible to separate out any potential effects of the BA pre-treatment on the BOLD MRI response to the cat odor. It is possible that exposure to cat odor alone would not have elicited the changes we report here.
We did not find any cat odor-specific effects or any effects of AVP and OT antagonists in the lateral amygdala and prefrontal cortex, although these regions play key roles in fear conditioning. TMT increases dopamine metabolism in the prelimbic prefrontal cortex and disrupts its cognition-related functions such as working memory (Morrow et al., 2000a, 2000b). Prelimbic dopamine increases with TMT exposure appears to adapt over time, possibly indicating changes in stress reactivity (Morrow et al., 2000b). A tetrodotoxin-inactivation study found, however, that the mPFC plays an active role in the expression of conditioned fear but not predator induced fear responses (Corcoran and Quirk, 2007). Similarly, lesions of the lateral amygdala, which is important in context dependent fear conditioning, does not reduce the expression of unconditioned fear response to a predator (Wallace and Rosen, 2001). We also expected an outcome partly consistent with previous fMRI work using TMT as the predator stimulus (Febo and Pira, 2011). In the previous study, several brain areas showed greater BOLD percent changes with TMT than BA. These included the ACC, retrosplenial cortex, subiculum, and the SSC (Febo and Pira, 2011). Most of the areas were cortical and regions such as the BNST, amygdala, septum and prefrontal cortex did not distinguish between BA and TMT. This differs somewhat from Chen et al. (2009), which measured the BOLD signal response to TMT in female rats and reported increased in BOLD in BNST, septum, hypothalamic nuclei, hippocampus, in addition to similar regions reported in the present study and in Febo and Pira (2011). However, Chen et al. (2009) did not include a control odor during scanning which makes it difficult to compare the studies and to discern whether the pattern of BOLD signal changes corresponds to innate fear circuitry or the neural response to an aversive odor.
It is important to note that the rats in the present experiment, as well as those in other neuroimaging experiments, are restrained and consequently unable to elicit full freezing responses, defensive reactions, or escape. Ferris et al. (2008) cleverly measured piloerection in aggressive male rats before and after neuroimaging and therefore provided a behavioral index for their aggression studies (Ferris et al., 2008). We failed to take such measures here. Importantly, the pattern of BOLD activation (the magnitude signal changes and the set of regions that are active during the initial exposure to the cat fur and BA) may correspond to neuronal processing before the triggering of behavioral responses. In other words, the pattern of activation observed with fMRI may be different from c-fos studies due to the mere fact that animals in immunolabelling experiments are able to express the full set of behavioral responses. However, markers of cellular activity cannot provide real-time neural activity and many events can trigger cellular activation of c-fos during 1–2 h before tissue harvesting. Collectively, fMRI studies and ex vivo work could provide a complementary picture of the neural control of unconditioned fear that would otherwise be inaccessible using one or the other method alone.
AVP V1a receptors are important mediators of anxiety in rats. Intra-septal infusion of the V1 receptor antisense oligonucleotide sequence dramatically increased time spent in open arms of an elevated plus maze (Landgraf et al., 1995). AVP V1a receptor knockout mice show significantly reduced anxiety as demonstrated by increased dark–light box transitions, increased time spent in open arms of an elevated plus maze and time spent in center of an open field (Bielsky et al., 2004; Egashira et al., 2007). This was observed in the absence of differences in acoustic startle response or paired pulse inhibition, suggesting that inhibitory control is unaffected in these mice (Bielsky et al., 2004). Rats inbred for high anxiety behavior trait show greater basal levels of AVP synthesis in the PVN but not brain V1a receptor density or OT levels (Murgatroyd et al., 2004). Single nucleotide polymorphism of an AVP gene promoter sequence that prevents the binding of a transcriptional repressor protein may underlie the higher AVP levels in these animals (Murgatroyd et al., 2004). Our findings using fMRI may arise from the release of AVP in some brain regions during predator odor presentation, an effect that is blocked by the V1a receptor antagonist. Opposite modulatory effects of OT on anxiety, stress and/or fear have been reported, which could arise from cell populations in the central amygdala (Huber et al., 2005). AVP is reduced in the bloodstream and OT is increased in rats during unconditioned responses to footshock (Yagi and Onaka, 1996). We did not observe a large effect of the OT receptor antagonist on BOLD response to cat fur. However, an interesting interaction between drug treatment and smell was observed in the central and cortical amygdala and the olfactory tubercle. In the central amygdala, both OT and V1a antagonists curtailed the BOLD response to cat fur but not BA. OT and V1a receptors are expressed within the central amygdala and inputs to this region have recently been proven to arise from the PVN and accessory magnocellular nucleus (Knobloch et al., 2012). OT and AVP mediated responses in the central amygdala gate the output of separate populations of neurons that perhaps lead to behavioral and autonomic responses associated with fear (Huber et al., 2005; Viviani et al., 2011). The reduced BOLD in the central amygdala is consistent with blockade of receptors found within this region, and would also be consistent with its role in fear and anxiety. Both OT and V1a antagonists reduced BOLD activity in the olfactory tubercle, an effect that is consistent with the activation of the main olfactory system. This would also suggest that both neuropeptides exert effects on olfactory perception. The effect upon the cortical amygdala is interesting in light of the opposite effects of these antagonists on the BOLD response to BA (Fig. 5). The cortical amygdala shows moderate to high levels of OT receptors and almost no V1a expression (Ostrowski et al., 1994; Veinante and Freund-Mercier, 1997; Yoshimura et al., 1993). This site may be involved in the reduced behavioral avoidance we observe with OT-A treatment.
Finally, we observed signal decreases (negative BOLD signal changes) across several of the same regions and in the same magnitude as with the positive BOLD signal changes. Negative signal changes in fMRI studies are still a subject of research and are difficult to interpret. Shmuel et al. (2006) reported evidence that BOLD signal decreases in the visual cortex of anesthetized Rhesus macacques correlates well with local field potentials over individual spike activity (as reported for BOLD signal increases in Logothetis et al. (2001)). In addition, they noted that the signal decreases could be largely explained by reductions in synaptic activity in localized regions of the cortex (Shmuel et al., 2006). Based on their results, it is tempting to infer that the aforementioned set of regions is inhibited or inactive during the presentation of the predator stimulus odor. However, a ‘vascular steal’ effect in these regions is also a mechanism to consider and could account for the observed changes in negative BOLD (Harel et al., 2002). The presence of the V1a receptor in arteriolar smooth muscle cells may have partial effects on vasoconstriction, which may reduce the BOLD response to cat odor. However, given the low-to-moderate dose used here, and the findings with OT-A and the reduction in the BOLD response to BA, this is unlikely to contribute significantly to our present findings.
4. Conclusion
In summary, our data provide an initial account of the regions of the rat brain that respond to the first 5 min of exposure to a predator odor and a novel odor. Our behavioral results show that cat fur elicits a greater avoidance behavior than BA. Blocking OT receptors reduces avoidance behavior, whereas blocking V1a receptors was without any significant effect when compared to CSF treated rats. This counterintuitive finding, which suggests that OT receptors mediate avoidance behaviors and blocking these receptors eliminates this effect, may be in part due to the presence of OT receptors in the olfactory system. Indeed, our fMRI data show that OT receptor blockade reduces BOLD activation in olfactory-related structures. Interestingly, our fMRI data also show that V1a receptor blockade exerted actions that were specific for cat fur odor and not BA. Thus, even though our behavioral findings using a place avoidance assay do not support a role for V1a receptors in avoidance, fMRI findings do show reduced BOLD activity that may underscore the role of this neuropeptide receptor within innate fear circuitry. Recent data show the presence of vasopressin within the anterior olfactory nucleus and olfactory bulb and it appears that this AVP system regulates social interactions in rodents (Tobin et al., 2010; Wacker et al., 2010). Thus, it is possible that our present data reflect mechanisms involving AVP and OT in controlling social olfactory processing.
5. Experimental procedures
5.1. Subjects
Male Long Evans rats weighing 300–400 g were purchased from Charles River laboratories (Wilmington, MA). Animals were housed in pairs under a 12 h light:dark cycle (lights off at 19:00 h). Rats were singly housed following intracranial surgeries (see below). Water and Purina rat chow were provided ad libitum. Rats were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (8th Edition, 2011) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The Institutional Animal Care and Use Committee at Northeastern University approved the protocols used for this study. Two separate experiments were carried out. The first evaluated the behavioral effects of cat odor and butyric acid (BA) exposure on place avoidance and the second study examined neural activity in response to the same odors using fMRI.
5.2. Chemicals
The OT receptor antagonist (OT-A) (d(CH2)5-[Tyr(Me)2,Thr4, ]Ornithine vasotocin, 125 ng/10 μL), the V1a receptor antagonist (V1a-A) [β-Mercapto-β,β-cyclopentamethylenepropionyl1, O-me-Tyr2,Arg8]-Vasopressin were purchased from Sigma-Aldrich (St. Louis, MO). Sterile artificial cerebrospinal fluid (CSF) was purchased from Harvard Apparatus (Holliston, MA). Butyric acid (BA) was purchased from Sigma-Aldrich and diluted to 170 mM. 5 μL was on a piece of absorbant paper. Cat fur was obtained from a local veterinary hospital and was collected by a staff member as part of standard grooming procedures. 1.5 mL microcentrifuge tubes were filled with cat fur and replaced between test sessions.
5.3. Surgical procedures for behavior and MRI studies
Before behavior experiments, animals were fitted with stainless steel injection cannulas (Plastics One, Roanoke, VA) in one of the lateral ventricles. Surgical implants were performed 7 days before behavioral testing. The inner cannula guide penetrated 2 mm into the brain to just above the lateral ventricle. Three to four steel screws and dental cement were used for securing the cannula to the skull. Injector tips extended 1.5 mm beyond the tip of the cannula guide into the lateral cerebral ventricle (Bregma coordinates: DV −3.5 mm, ML ±1.5 mm, AP −0.3 mm). Ninety minutes before studies rats received a 10-μl bolus injection containing 125 ng OT-A, 125 ng V1a-A or artificial CSF.
For MRI studies, rats were fitted with custom-designed MR compatible injection cannulas (Plastics One, Roanoke, VA). Surgeries were done 3–5 days prior to MR experiments. The outside pedestal of the cannula guide extended 2 mm above the surface of the skull to allow the animal to be positioned inside the radiofrequency coil system without compressing the cannula. The inner plastic cannula guide penetrated 2 mm into the brain to just above the ventricle (Supporting Online Fig. S1A). Three to four plastic screws, along with a thin layer of acrylic dental cement, were used for securing the cannula to the bone. Injectors extended 1.5 mm beyond the tip of the cannula guide into the lateral cerebral ventricle (Bregma coordinates: DV −3.5 mm, ML ±1.5 mm, AP −0.3 mm). Fig. S1A shows an example of cannula placement. Only scans with good cannula placements were used. Ninety minutes before MRI studies rats received a 10 μl bolus injection containing 125 ng OT-A, 125 ng V1a-A or artificial CSF.
5.4. Experiment 1: Behavior
5.4.1. Place avoidance following OT receptor and AVP V1a receptor blockade
Thirty animals were tested inside a 3-compartment place preference chamber (Med-Associates, St. Albans, VT). The test chambers are typically used for conditioned place preference or conditioned avoidance; however, we modified their use for the present study in order to examine several unconditioned avoidance-like behaviors in the presence of cat fur and BA odors. Each test chamber contains two equal-sized compartments, each measuring 8.25 in. W × 8.24 in. H × 11 in. L. One compartment has black walls and floor made from 0.1875 in. (4.8 mm) stainless steel rods, placed on 0.625 in. (16 mm) centers, and the other compartment has white walls and a 0.5 in. × 0.5 in. (1.25 cm × 1.25 cm) stainless steel mesh floor. A third and smaller compartment located in between the other two measures 8.25 in. W × 8.24 in. H × 4.75 in. L and has grey walls and a smooth PVC floor. All three chambers have hinged clear polycarbonate lids. The chambers were located in a test room with a digital video camera located above the chambers. Videos were recorded during test sessions and imported to a PC for further analysis of various behaviors using OdLog software (Macropod Inc.). Animals were habituated to the boxes and an odor presentation tube for 1 h on the day before testing.
On the day of testing, animals were given an ICV injection 90 min before being placed into the chambers. Animals were first placed into the middle (smaller) compartment and allowed to freely move within the 3 compartments inside the test chamber. Four animals were tested per session, with at least one animal from each of injection group (CSF, OT-A, and V1a-A; 10 rats per each group). Odors were always handled inside a fume hood in an adjacent laboratory and were stored inside a refrigerator at 4 °C in the same laboratory. Presentation of odors inside the boxes was achieved by adhering a 1.5 mL plastic eppendorf tube to the wall of one of the lateral compartments. The height of the tube was just below the cage lids and the rats could stand and extend to sniff and approach the tube (Fig. S2). Animals were also habituated to the odor tube and test cages on the previous day. Three consecutive 15-min test sessions were carried out in which the rats inside the chambers were exposed to an empty tube first, followed by BA or cat fur. The placement of the tube inside the chamber was counterbalanced across groups between the white and dark side compartments (Fig. S2). The chamber was cleansed with 70% ethanol solution between test sessions and the chambers and the room were ventilated for 20–30 min before the subsequent sessions. A researcher blind to the treatment scored the videos. The purpose was to examine place avoidance. Time spent in each of the 3 subcompartments (odor-side, non-odor side and in the middle or adjacent compartment) was scored. Raw values were analyzed using a two way analysis of variance with odor (No Odor × Cat × BA) and ICV treatment (CSF × OT-A × V1a-A) as independent variables (significant p <0.05). Posthoc tests were done using Bonferroni’s multiple comparisons test.
5.5. Experiment 2: Functional MRI
5.5.1. Acclimation to restraint for MRI experiments
A group of rats were acclimated for 5 days to MRI restraint before surgical procedures and MRI experiments using procedures outlined previously (King et al., 2005). Animals were anesthetized with 2–4% isoflurane gas anesthesia and prepared for a ‘mock’ imaging session by placing them inside of a replica of a radiofrequency coil system (insightMRI, Shrewsbury, MA). On each acclimatization day, rats were placed inside a chamber that simulated the spectrometer bore environment. They were then exposed to pre-recorded MR pulse sequence sound. The acclimatization procedures were repeated over 5 days, with daily increments in the amount of time spent under restraint (20 min on the 1st day to 60 min the last day).
5.5.2. MRI procedures
We examined odor-evoked brain activation using fMRI in awake rats. Animals were presented with air that continuously flowed through vials containing cat fur (unconditioned fear odor stimulus) or a small patch of absorbent paper containing 5 uL of 170 mM butyrate (BA) solution (repulsive odor). Both of these were novel odors for the experimental subjects. The hair from various male cats was redistributed into equal amounts in airtight Ziplock bags and stored at 4 °C until used. All open vial/bag odors were handled inside a fume hood to minimize dispersion of odors. Dedicated plastic tubing lines for each scent were used and thoroughly washed or discarded after each imaging session. Before imaging, rats were given an ICV injection of CSF, OT-A or V1a-A in order to investigate the effects of blocking OT or AVP V1a receptors on odor stimulated BOLD activation. Animals were anesthetized with 4% isoflurane before MR scanning and setup with the radiofrequency coil system. Imaging sessions included an anatomical scan lasting 6 min followed by two 10-min functional scans. Each functional scan had a 5-min baseline epoch followed by a 5-min stimulus period. The stimulus for the first functional scan was BA. The same animal was then exposed to cat fur following a subsequent baseline epoch. Functional scans were carried out in immediate succession. Air was continuously delivered to animals during baseline and stimulus periods; however, odors were presented during the latter 5 min. A pump (Aqua Culture Air Pump MK 1504) was used to deliver odors (up to 1200 cc/min). The entire imaging session was over before an hour to minimize prolonged exposure to restraint. It is important to note here that although a counterbalancing of smells is important across many studies, we avoided utilizing such a design in face of possible conditioning effects of the cat fur odor. Cat before BA is likely to impact the normal neural response to BA within the 5-min of MRI data acquisition (more than vice versa). Cat fur could serve as an unconditioned fear stimulus that leads to processing of BA as a conditioned fear stimulus. In the present design we attempted to avoid such effects. Counterbalancing is important across many studies, for this imaging experiment specifically it is not favorable due to the lasting effects of cat fur.
Experiments were conducted in a Bruker 7T/20 cm horizontal magnet controlled by a Paravision 5.0 (Bruker, Billerica, MA USA). Studies were performed with a quadrature (transmit/receive) radiofrequency (rf) coil (InsightMRI, Shrewsbury, MA). Functional imaging was performed using a T2-weighted fast spin echo pulse sequence with the following parameters: repetition time TR=1562 ms, echo time TE=7.5, effective echo time TEeff=45 ms and an echo train length ETL=16. Geometry was setup as follows: 12 slices, field of view of 28 mm, 1.0 mm thick slices with no gaps, data matrix of 642 for functional scans and 2562 for anatomical scans (The in plane 2D pixel resolution was 438 μm2 for functional and 117 μm2 for anatomical scans). A full set of 12 coronal slices across the brain was collected at each effective repetition time and was completed every 6 s.
5.5.3. Statistical analysis
Full details for the MRI data analysis using Medical Image Analysis and Visualization software (MIVA; ccni.wpi.edu) has been previously reported (Ferris et al., 2008). Scans were pre-screened for motion and corrected for linear drift using previously described criteria and methods (Ferris et al., 2008). Scans exceeding an average displacement threshold of 25% (110 mm along X–Y direction) were removed from the study (Supporting Online Fig. S1B and C). Original groups sizes were 10–13 rats per group. Final group sizes were Cat/CSF=9, Cat/V1a-A=10, Cat/OT-A=10, BA/CSF=7, BA/V1a-A=8, and BA/OT-A=9. Each subject was registered to an electronic rat brain atlas (Supporting Online Fig. S1A). Statistical t tests were performed on each subject within the original coordinate system. The baseline period used was 48 repetitions immediately preceding odor and the stimulation window was 48 repetitions. Statistical t tests used a 95% confidence level, two-tailed distribution, and heteroscedastic variance assumptions. In order to provide a conservative estimate of significance, a false-positive detection-controlling algorithm was introduced into the analysis (Genovese et al., 2002). This ensures that the false-positive detection rate is below our confidence level of 5% (Ferris et al., 2005). Statistically significant pixels were assigned their percentage change values (stimulus mean minus control mean). Percent signal change values were exported for statistical comparisons between groups. Percent change values were statistically evaluated between 6 scan groups (cat/csf, cat/V1a, cat/OT, BA/csf, BA/V1a, BA/OT) using a two by three analysis of variance with odor (Cat × BA) and ICV treatment (CSF × OT-A × V1a-A) as independent variables (significant p <0.05). Posthoc tests were done using Bonferroni multiple comparisons test.
Supplementary Material
Acknowledgments
Support was provided by NIH grant DA019946 and startup funds from Northeastern University. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. The NIH and NIDA had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.brainres.2012.11.045
References
- Appenrodt E, Schnabel R, Schwarzberg H. Vasopressin administration modulates anxiety-related behavior in rats. Physiol Behav. 1998;64:543–547. doi: 10.1016/s0031-9384(98)00119-x. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- Chen W, Shields J, Huang W, King JA. Female fear: influence of estrus cycle on behavioral response and neuronal activation. Behav Brain Res. 2009;201:8–13. doi: 10.1016/j.bbr.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wied D, Gaffori O, van Ree JM, de Jong W. Central target for the behavioural effects of vasopressin neuropeptides. Nature. 1984a;308:276–278. doi: 10.1038/308276a0. [DOI] [PubMed] [Google Scholar]
- De Wied D, Gaffori O, Van Ree JM, De Jong W. Vaso-pressin antagonists block peripheral as well as central vasopressin receptors. Pharmacol Biochem Behav. 1984b;21:393–400. doi: 10.1016/s0091-3057(84)80101-x. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Endres T, Fendt M. Inactivation of the lateral septum blocks fox odor-induced fear behavior. Neuroreport. 2008;19:667–670. doi: 10.1097/WNR.0b013e3282fb78d9. [DOI] [PubMed] [Google Scholar]
- Febo M, Pira M. Increased BOLD activation to predator stressor in subiculum and midbrain of amphetamine-sensitized maternal rats. Brain Res. 2011;1382:118–127. doi: 10.1016/j.brainres.2010.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000a;864:146–151. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Elsworth JD. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull. 2000b;52:519–523. doi: 10.1016/s0361-9230(00)00290-2. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Wigger A, Frank E, Singewald N, Bunck M, Holsboer F, Landgraf R, Spengler D. Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J Neurosci. 2004;24:7762–7770. doi: 10.1523/JNEUROSCI.1614-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Young WS., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2012;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Adamec RE, Thompson BL. Expression of egr-1 (zif268) mRNA in select fear-related brain regions following exposure to a predator. Behav Brain Res. 2005;162:279–288. doi: 10.1016/j.bbr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Brain Res Mol Brain Res. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol. 2006;201:308–315. doi: 10.1016/j.expneurol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Dreifuss JJ, Jard S. Autoradiographic localization of vasopressin and oxytocin binding sites in rat kidney. Kidney Int. 1988a;33:959–965. doi: 10.1038/ki.1988.94. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988b;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Vendruscolo JC, Terenina-Rigaldie E, Raba F, Ramos P, Takahashi RN, Mormede P. Genetic influences on behavioral and neuroendocrine responses to predator-odor stress in rats. Neurosci Lett. 2006;409:89–94. doi: 10.1016/j.neulet.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Wacker DW, Tobin VA, Noack J, Bishop VR, Duszkiewicz AJ, Engelmann M, Meddle SL, Ludwig M. Expression of early growth response protein 1 in vasopressin neurones of the rat anterior olfactory nucleus following social odour exposure. J Physiol. 2010;588:4705–4717. doi: 10.1113/jphysiol.2010.196139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21:3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K, Onaka T. Chlordiazepoxide discriminates between the neural circuits mediating neuroendocrine responses to fear- and anxiety-producing stimuli in the rat. Neurosci Res. 1996;24:151–158. doi: 10.1016/0168-0102(95)00988-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


