Abstract
Microbiological contamination is an important water-quality problem worldwide. Human impact on this category of contamination is significant and several human-related activities, and also the population explosion, have affected and are still affecting dramatically the aquatic environment. Extensive industrialization and agriculture have led to increased pollution and hydromorphological changes in many river basins. The Danube river is one of the most affected by these changes where human involvement is undeniable, and subsequently, the Danube Delta Biosphere Reserve became one of the most vulnerable ecosystems. This review is an attempt to analyse the microbiological contamination and to identify the major role human activities play in altering the water quality of the rivers.
Introduction
Some of the key rivers in Europe are affected by pollution, alteration in the river basin and hydraulic engineering (Schiemer et al., 1999; Besemer et al., 2005). Free suspended bacteria in the water and bacteria associated with suspended materials are quoted amongst pollutants (Noble et al., 1997).
Pathogenic organisms are normal components of all ecosystems, but microbiological contamination with faecal bacteria subsequent to anthropogenic activity is considered to be a crucial issue throughout the rivers and especially in the Danube basin (Bayoumi Hamuda & Patko, 2012). Assessment of surface-water and groundwater quality continues to be of main public interest in the developed world. There is a strong demand for monitoring water quality (Pekárová et al., 2009), therefore the assessment of the presence of pathogenic bacteria in water represents a major concern for human- and animal-health protection (Fey et al., 2004; Straub & Chandler, 2003; WHO, 2002).
The Danube is one of the most important rivers in Europe and in the world. Its delta-like estuary at the Black Sea, the Danube Delta Biosphere Reserve, a paradise for various bird and fish species, was included in 1991 in the United Nations Educational Scientific and Cultural Organization (UNESCO) World Heritage List.
Human and animal pathogens of enteric origin are considered important contaminants of the environment, with transmission through the soil, agriculture, water and sediment (Bonetta et al., 2011). In order to monitor the water quality of the Danube river, riparian countries currently use different methods for microbiological analysis. Bacteria are ideal markers of microbial pollution of surface waters because of their quick response to environmental changes. Faecal coliforms and intestinal enterococci are good indicators for assessing faecal pollution and the potential presence of pathogenic agents, which are mainly caused by untreated sewage originating from agricultural land and pastures.
Aquatic ecosystems are currently threatened by human population growth, accompanied by the increased growth of agricultural and industrial activities. Therefore, detection of microbial and genotoxic pollution sources (Fig. 1) is essential for proper watershed management to maintain water traits according to quality goals (Farnleitner et al., 2001; Kolarević et al., 2011).
Fig. 1.
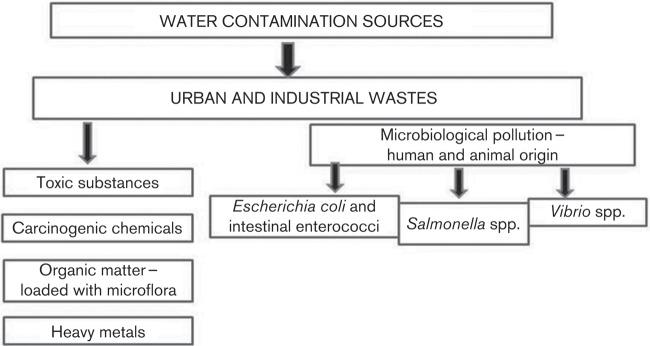
Primary sources of water contamination.
General description
Located in central Europe, the Danube basin is ‘the most important non-oceanic body of water’ and flows into the Black Sea through a large delta (Mladenović-Ranisavljević et al., 2012). With a total length of 2857 km from its source at a height of 1078 m in the Black Forest, Germany, to form a delta before the Black Sea in Romania (Helmer & Hespanhol, 1997), the river flows through nine countries and five capitals with 0.5–2 500 000 inhabitants (Winter et al., 2007; Bayoumi Hamuda & Patko, 2012). The watershed of the Danube covers 817 000 km2 and drains all or significant parts of Germany, Austria, the Czech Republic, the Slovak Republic, Hungary, Croatia, Slovenia, Bulgaria, Romania, Moldova, Ukraine and parts of the Federal Republics of Yugoslavia, Bosnia and Herzegovina (Helmer & Hespanhol, 1997). Its coastline is almost 240 km long, of which about 75 km represents the coastline of the Kilia distributary delta (Ukraine) and 165 km comprising the Sulina section, the Sfântu Gheorghe distributary delta and the lagoon complex Razim–Sinoie (Romanian territory) (DeltaNet; www.deltanet-project.eu/).
Sources of pollution
Due to transiting several countries and industrialized urban centres (Kolarević et al., 2011), the waters of the river are exposed to human activities and factories, which contribute to extensive use of the water and therefore pollution. Urban and industrial wastes are often characterized by numerous toxic and carcinogenic chemicals, such as heavy metals, and also organic matter, loaded with microflora, which can contaminate water and enter the food chain, posing considerable danger to public health. A large number of known point-source emissions of municipal, industrial and agro-industrial waste enter the Danube and its tributaries, and are listed in the EMIS emission database 2002 (Kirschner et al., 2009).
The contamination of water resources by faecal pollutants poses significant risks to human and animal health since numerous pathogens are often associated with faeces (Reischer et al., 2008). Thorough investigation of catchment hydrology and pollution dynamics is a prerequisite for successful quantitative microbial evaluation. Long-term monitoring of water quality and seasonal changes in the dynamics of microbial sources is a very important requirement (Reischer et al., 2008). Standard tests based on microbial indicator concentrations are used to protect the environment and prevent consumer exposure to pathogens (Lemarchand & Lebaron 2003; Sugumar et al., 2008; Duris et al., 2009).
Subsequent to the research conducted within ‘The Joint Danube Survey’, the microbiological water quality of the Danube river and its tributaries has been classified on the basis of standard parameters (faecal pollution and organic pollution) in five quality classes: little pollution (class I), moderate pollution (class II), critical pollution (class III), strong pollution (class IV), excessive pollution (class V). Based on samples harvested from Romania (river Danube and Danube delta) strongly faecal (classes III–IV) and moderate to critical organic pollution (classes II–III) were observed. The river Arges is amongst the most contaminated tributaries, which has a significant role in altering the water quality. Similarly, rivers Siret (class III) and Prut (class IV) were significant pollution factors for the Danube (Kavka et al., 2006).
Faecal indicator bacteria such as total coliforms, faecal coliforms (thermotolerant coliforms), Escherichia coli and intestinal enterococci (faecal streptococci) are excreted by humans and warm-blooded animals, pass sewage treatment plants in large amounts, and survive, preserving their pathogenicity for a certain time.
Microbiological pollution
Salmonella spp., enteropathogenic E. coli and Vibrio spp., represent important pathogens. The genus Salmonella comprises more than 2400 serotypes, most of which are considered as an endemic public-health concern worldwide (Baker et al., 1983, Baggesen et al., 2000; Soto et al., 2006). Salmonella enterica serovar Typhimurium, diagnosed by random amplified polymorphic DNA typing, antimicrobial resistance, and plasmid and integron profiles, represents a common cause of enteric disease in many countries (Soto et al., 2006), and has been involved in water-borne disease of humans and animals (Dondero et al., 1977; Davies, 2001; Kariuki et al., 1999; Mmolawa et al., 2002; Martinez-Urtaza et al., 2004).
Salmonellosis is more commonly associated with contaminated foods and feeds than with waters; nevertheless, salmonellae have frequently been found in effluents from sewage treatment plants, in industrial wastes, and in streams that receive a variety of sewages and industrial wastes (Kampelmacher & Van Noorle Jansen, 1970; Dondero et al., 1977). Frequent isolation of salmonellae from the surface waters of an area gives rise to questions as to the origins of the organisms, their survival or persistence, and their relevance to public health (Kampelmacher & Van Noorle Jansen, 1970). In the Danube river, an increase in the incidence of antibiotic-resistant Salmonella strains was identified (Janousková et al., 1975). In order to detect these pathogens it is necessary to assess the virulence factors that have been shown to be relevant to the pathogenesis of S. enterica serovar Typhimurium, and endonucleases (XbaI and BlnI). Detection and mapping of the V genes macrorestiction profiles and their comparison with available data for other strains of Salmonella is an established strategy (Soto et al., 2006).
E. coli is one of the specific indicators of faecal contamination in tropical and temperate regions. Investigation of the bacterial density of water could provide an approach to assess the reliability of monitoring data (Bayoumi Hamuda & Patkó, 2011). Enterohaemorrhagic E. coli have emerged as a serious gastrointestinal pathogen in many countries. Although the mode of transmission is mainly through the consumption of contaminated meat (Mead et al., 1999), outbreaks associated with water-borne enterohaemorrhagic E. coli have also been described.
In the Danube river basin total coliforms, faecal coliforms and E. coli indicate persistent contamination, with lower values of total coliforms in July and the highest value in August. Variations in these parameters could be spatio-temporarily linked to the number of visitors in this ecosystem (Ajeagah et al., 2012).
Vibrio cholerae is a Gram-negative bacterium, native to brackish and estuarine environments, and associated with zooplankton, mainly copepods (Colwell et al., 1977; Huq & Colwell, 1996), and aquatic birds (Ogg et al., 1989). It is a major endemic (Rivera et al., 2003; WHO, 2006) pathogen, responsible for cholera, which mainly affects third world populations, and may cause high morbidity and mortality rates. Ecological studies of cholera and V. cholerae have revealed the occurrence of cholera to be correlated with sea surface temperature and sea surface height in endemic areas (Colwell, 1996; Lobitz et al., 2000; Louis et al., 2003). The incidence and severity of epidemics have been linked to salinity, water temperature, turbidity and plankton blooms (Huq et al., 2001; Louis et al., 2003).
V. cholerae is a heterogeneous species, with 206 serotypes identified to date, based on thermostable somatic O antigens. Only two serotypes, V. cholerae O1 and O139, with two main regions related to pathogenicity – the CTX genetic element and the VC pathogenicity island (VPI) (Karaolis & Kaper, 1999) – have been characterized as toxigenic and identified as the aetiological agent of epidemics. They are less frequently isolated in the aquatic environment than non-O1/non-O139 strains (Colwell et al., 1977; Faruque et al., 1998; Rashed et al., 2012).
In the Danube water, two species dominate: V. cholerae and Vibrio metschnikovii (Seman et al., 2012). Over 624 strains of V. cholerae O1 were isolated in Romania in 1977–1995, the highest number (64 %) originating in the Danube delta area (Israil et al., 1998). In recent years, cholera was positively diagnosed in many patients in Romania, the highest frequency being reported in the Danube delta and its neighbouring districts (Damian et al., 1998; Seman et al., 2012). The major sources of cholera contamination were linked mainly to the drinking of surface water directly from the Danube, followed by delta fish consumption and water consumption from fountains infected with cholera vibrios (Israil et al., 1998; Seman et al., 2012).
Routine isolation from water is not achieved by the conventional culture method, V. cholerae is present in natural aquatic environments (Huq & Colwell, 1994) and the role of water in the transmission of V. cholerae is well established (Colwell, 1993, 2000; Islam et al., 1994; Chomvarin et al., 2007). Recent molecular biology techniques, including the PCR technique, are used for rapid detection of toxigenic V. cholerae in the aquatic environment (Chakraborty et al., 1999; Kapley & Purohit, 2001; Chomvarin et al., 2007). ctxAB and tcpA genes are known to play key roles in the virulence of V. cholerae, especially of serogroups O1 and O139 (Colombo et al., 1997; Faruque et al., 1998; Kovach et al., 1996; Shears, 2001; Singh et al., 2001; Radu et al., 2002; Chomvarin et al., 2007). Fast and precise detection of V. cholerae in the drinking water and aquatic environment is imperative for disease management and public-health protection (Huq et al., 1995; Kapley & Purohit, 2001).
Assessment and identification of faecal-origin-specific pathogens is a problem of utmost importance in terms of the safety and protection of drinking-water sources. The isolation of water-borne pathogens is difficult due to methodological limitations. The low bacterial concentration in surface water, high costs and protracted detection technology constitute a major problem (Thompson et al., 2006). PCR, showing a very high specificity and sensitivity (Feder et al., 2001), has become the usual method for the detection of pathogens from various sources, because this method can enhance small amounts of DNA (Touron et al., 2005; Moganedi et al., 2007).
Conclusion
Many of the world's leading environmental agencies have long centred the focus of their attention on the continued pollution of the Danube river. The main problems that affect the water quality of the river are the high pollution following different human activities and also the population explosion. Today, it is important to improve the water quality of the river ecosystem in order to meet the demand from different sectors and to improve the capacity of the water supply for domestic, agriculture, industry, energy and other uses.
In order to protect this precious natural resource, the Romanian government founded an organization, the Danube Delta Biosphere Reserve, but unfortunately this organization can do little to protect water against pollution and eutrophication. Therefore, multiple measures implemented by all responsible authorities are needed to protect this resource and to avoid damage. The fact that in a remarkable number of sampling sites microbiological parameters alone indicated anthropogenic impacts supports and vindicates the application of microbiological parameters in monitoring programs. Knowledge on microbial pollution in lotic aquatic environments appears essential for decision makers in order to take appropriate measures that result in acceptable river water quality and compliance with national and international quality standards and directives.
In order to improve water quality, it is important to set up a management strategy and plans that provide implementing strategies to address water quality. Similarly, for maintaining overall ecosystem health, it is very important to raise global awareness on the problems associated with faecal pollution of water resources.
Acknowledgements
This study was supported by the National Council of Scientific University Research of Romania, grant PNII/PCCA 61/2012.
References
- Ajeagah G., Cioroi M., Praisler M., Constantin O., Palela M., Bahrim G. (2012). Bacteriological and environmental characterisation of the water quality in the Danube River Basin in the Galati area of Romania. African Journal of Microbiology Research 6, 292–301 [Google Scholar]
- Baggesen D. L., Sandvang D., Aarestrup F. M. (2000). Characterization of Salmonella enterica serovar Typhimurium DT104 isolated from Denmark and comparison with isolates from Europe and the United States. J Clin Microbiol 38, 1581–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A., Smitherman R. O., McCaskey T. A. (1983). Longevity of Salmonella typhimurium in Tilapia aurea and water from pools fertilized with swine waste. Appl Environ Microbiol 45, 1548–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumi Hamuda H. E. A. F., Patkó I. (2011). Variations in water quality of Danube river at Budapest City. Óbuda University e-Bulletin 2, 1 [Google Scholar]
- Bayoumi Hamuda H. E. A. F., Patko I. (2012). Ecological monitoring of Danube water quality in Budapest region. Am J Environ Sci 8, 202–211 10.3844/ajessp.2012.202.211 [DOI] [Google Scholar]
- Besemer K., Moeseneder M. M., Arrieta J. M., Herndl G. J., Peduzzi P. (2005). Complexity of bacterial communities in a river-floodplain system (Danube, Austria). Appl Environ Microbiol 71, 609–620 10.1128/AEM.71.2.609-620.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetta S., Borelli E., Bonetta S., Conio O., Palumbo F., Carraro E. (2011). Development of a PCR protocol for the detection of Escherichia coli O157:H7 and Salmonella spp. in surface water. Environ Monit Assess 177, 493–503 10.1007/s10661-010-1650-x [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Khanam J., Takeda Y., Nair G. B. (1999). Application of PCR for detection of toxigenic Vibrio cholerae O1 in water samples during an outbreak of cholera. Trans R Soc Trop Med Hyg 93, 527–528 10.1016/S0035-9203(99)90366-8 [DOI] [PubMed] [Google Scholar]
- Chomvarin C., Namwat W., Wongwajana S., Alam M., Thaew-Nonngiew K., Sinchaturus A., Engchanil C. (2007). Application of duplex-PCR in rapid and reliable detection of toxigenic Vibrio cholerae in water samples in Thailand. J Gen Appl Microbiol 53, 229–237 10.2323/jgam.53.229 [DOI] [PubMed] [Google Scholar]
- Colombo M. M., Mastrandrea S., Leite F., Santona A., Uzzau S., Rappelli P., Pisano M., Rubino S., Cappuccinelli P. (1997). Tracking of clinical and environmental Vibrio cholerae O1 strains by combined analysis of the presence of toxin cassette, plasmid content and ERIC PCR. FEMS Immunol Med Microbiol 19, 33–45 10.1111/j.1574-695X.1997.tb01070.x [DOI] [PubMed] [Google Scholar]
- Colwell R. R. (1993). Nonculturable but still viable and potentially pathogenic. Zentralbl Bakteriol 279, 154–156 10.1016/S0934-8840(11)80392-0 [DOI] [PubMed] [Google Scholar]
- Colwell R. R. (1996). Global climate and infectious disease: the cholera paradigm. Science 274, 2025–2031 10.1126/science.274.5295.2025 [DOI] [PubMed] [Google Scholar]
- Colwell R. R. (2000). Viable but nonculturable bacteria: a survival strategy. J Infect Chemother 6, 121–125 10.1007/PL00012151 [DOI] [PubMed] [Google Scholar]
- Colwell R. R., Kaper J., Joseph S. W. (1977). Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198, 394–396 [PubMed] [Google Scholar]
- Damian M., Koblavi S., Carle I., Nacescu N., Grimont F., Ciufecu C., Grimont P. A. D. (1998). Molecular characterization of Vibrio cholerae O1 strains isolated in Romania. Res Microbiol 149, 745–755 10.1016/S0923-2508(99)80021-7 [DOI] [PubMed] [Google Scholar]
- Davies R. H. (2001). Salmonella typhimurium DT104: has it had its day? In Pract 23, 342–351 10.1136/inpract.23.6.342 [DOI] [Google Scholar]
- Dondero N. C., Thomas C. T., Khare M., Timoney J. F., Fukui G. M. (1977). Salmonella in surface waters of central New York state. Appl Environ Microbiol 33, 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duris J. W., Haack S. K., Fogarty L. R. (2009). Gene and antigen markers of shiga-toxin producing E. coli from Michigan and Indiana river water: occurrence and relation to recreational water quality criteria. J Environ Qual 38, 1878–1886 10.2134/jeq2008.0225 [DOI] [PubMed] [Google Scholar]
- Farnleitner A. H., Kirschner A. K. T., Zechmeister G., Kavka T. C., Mach R. L. (2001). Untersuchungstechniken in der mikrobiologischen Analyse von Wasser und Gewassern: Staus Quo und Perspektiven. ÖWAV Schriftenreihe Heft 150, 125–154 [Google Scholar]
- Faruque S. M., Albert M. J., Mekalanos J. J. (1998). Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev 62, 1301–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder I., Nietfeld J. C., Galland J., Yeary T., Sargeant J. M., Oberst R., Tamplin M. L., Luchansky J. B. (2001). Comparison of cultivation and PCR-hybridization for detection of Salmonella in porcine fecal and water samples. J Clin Microbiol 39, 2477–2484 10.1128/JCM.39.7.2477-2484.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey A., Eichler S., Flavier S., Christen R., Höfle M. G., Guzmán C. A. (2004). Establishment of a real-time PCR-based approach for accurate quantification of bacterial RNA targets in water, using Salmonella as a model organism. Appl Environ Microbiol 70, 3618–3623 10.1128/AEM.70.6.3618-3623.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer R., Hespanhol I. (1997). Water Pollution Control - a Guide to the Use of Water Quality Management Principles Published on behalf of the United Nations Environment Programme, the Water Supply & Sanitation Collaborative Council and the World Health Organization by E & F Spon. London: UNESCO, WHO & UNEP [Google Scholar]
- Huq A., Colwell R. R. (1994). Vibrios in the environment: viable but nonculturable Vibrio cholerae. In Vibrio Cholerae and Cholera: Molecular to Global Perspectives. Edited by Wachsmuth P. A., Blake P. A., Olsvik O. Washington, DC: American Society for Microbiology [Google Scholar]
- Huq A., Colwell R. R. (1996). Vibrios in the marine and estuarine environment: tracking Vibrio cholerae. Ecosyst Health 2, 198–214 [Google Scholar]
- Huq A., Colwell R. R., Chowdhury M. A., Xu B., Moniruzzaman S. M., Islam M. S., Yunus M., Albert M. J. (1995). Coexistence of Vibrio cholerae O1 and O139 Bengal in plankton in Bangladesh. Lancet 345, 1249 10.1016/S0140-6736(95)92038-2 [DOI] [PubMed] [Google Scholar]
- Huq A., Sack R. B., Colwell R. R. (2001). Cholera and global ecosystems. In Ecosystems Change and Public Health: a Global Perspective. Edited by Aron J. L., Patz J. A. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Islam M. S., Drasar B. S., Sack R. B. (1994). The aquatic flora and fauna as reservoirs of Vibrio cholerae: a review. J Diarrhoeal Dis Res 12, 87–96 [PubMed] [Google Scholar]
- Israil A., Nacescu N., Cedru C. L., Ciufecu C., Damian M. (1998). Changes in Vibrio cholerae O1 strains isolated in Romania during 1977-95. Epidemiol Infect 121, 253–258 10.1017/S0950268896001100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janousková J., Krcméry V., Kadlecová O. (1975). Salmonellae with antibiotic resistance and R plasmids in Danube River. Zentralbl Bakteriol [Orig A] 233, 495–504 [PubMed] [Google Scholar]
- Kampelmacher E., Van Noorle Jansen L. M. (1970). Salmonella – its presence in and removal from a wastewater system. J Water Pollut Control Fed 42, 2069–2073 [PubMed] [Google Scholar]
- Kapley A., Purohit H. J. (2001). Detection of etiological agent for cholera by PCR protocol. Med Sci Monit 7, 242–245 [PubMed] [Google Scholar]
- Karaolis D. K. R., Kaper J. B. (1999). Pathogenicity islands and other mobile virulence elements of Vibrio cholerae. In Pathogenicity Islands and Other Mobile Virulence Elements. Edited by Kaper J. B., Hacker J. Washington, DC: American Society for Microbiology [Google Scholar]
- Kariuki S., Gilks C., Kimari J., Muyodi J., Waiyaki P., Hart C. A. (1999). Analysis of Salmonella enterica serotype Typhimurium by phage typing, antimicrobial susceptibility and pulsed-field gel electrophoresis. J Med Microbiol 48, 1037–1042 10.1099/00222615-48-11-1037 [DOI] [PubMed] [Google Scholar]
- Kavka G. G., Kasimir D., Farnleitner A. H. (2006). Microbiological water quality of the River Danube (km 2581 – km 15): longitudinal variation of pollution as determined by standard parameters. In Proceedings of the 36th International Conference of the IAD, pp. 415–421 International Association of Danube Research. [Google Scholar]
- Kirschner A. K., Kavka G. G., Velimirov B., Mach R. L., Sommer R., Farnleitner A. H. (2009). Microbiological water quality along the Danube River: integrating data from two whole-river surveys and a transnational monitoring network. Water Res 43, 3673–3684 10.1016/j.watres.2009.05.034 [DOI] [PubMed] [Google Scholar]
- Kolarević S., Knežević-Vukčević J., Paunović M., Tomović J., Gačić Z., Vuković-Gačić B. (2011). The anthropogenic impact on water quality of the river Danube in Serbia: microbiological analysis and genotoxicity monitoring. Arch Biol Sci 63, 1209–1217 10.2298/ABS1104209K [DOI] [Google Scholar]
- Kovach M. E., Shaffer M. D., Peterson K. M. (1996). A putative integrase gene defines the distal end of a large cluster of ToxR-regulated colonization genes in Vibrio cholerae. Microbiology 142, 2165–2174 10.1099/13500872-142-8-2165 [DOI] [PubMed] [Google Scholar]
- Lemarchand K., Lebaron P. (2003). Occurrence of Salmonella spp. and Cryptosporidium spp. in a French coastal watershed: relationship with fecal indicators. FEMS Microbiol Lett 218, 203–209 10.1111/j.1574-6968.2003.tb11519.x [DOI] [PubMed] [Google Scholar]
- Lobitz B., Beck L., Huq A., Wood B., Fuchs G., Faruque A. S. G., Colwell R. R. (2000). Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci U S A 97, 1438–1443 10.1073/pnas.97.4.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis V. R., Russek-Cohen E., Choopun N., Rivera I. N. G., Gangle B., Jiang S. C., Rubin A., Patz J. A., Huq A., Colwell R. R. (2003). Predictability of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol 69, 2773–2785 10.1128/AEM.69.5.2773-2785.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Urtaza J., Liebana E., Garcia-Migura L., Perez-Piñeiro P., Saco M. (2004). Characterization of Salmonella enterica serovar Typhimurium from marine environments in coastal waters of Galicia (Spain). Appl Environ Microbiol 70, 4030–4034 10.1128/AEM.70.7.4030-4034.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead P. S., Slutsker L., Dietz V., McCaig L. F., Bresee J. S., Shapiro C., Griffin P. M., Tauxe R. V. (1999). Food-related illness and death in the United States. Emerg Infect Dis 5, 607–625 10.3201/eid0505.990502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenović-Ranisavljević I., Takić L., Vuković M., Nikolić Đ., Nenad Ž., Milosavljević P. (2012). Multi-criteria ranking of the Danube water quality, on its course through Serbia. Serbian Journal of Management 7, 299–307 10.5937/sjm7-2549 [DOI] [Google Scholar]
- Mmolawa P. T., Willmore R., Thomas C. J., Heuzenroeder M. W. (2002). Temperate phages in Salmonella enterica serovar Typhimurium: implications for epidemiology. Int J Med Microbiol 291, 633–644 10.1078/1438-4221-00178 [DOI] [PubMed] [Google Scholar]
- Moganedi K. L. M., Goyvaerts E. M. A., Venter S. N., Sibara M. M. (2007). Optimization of the PCR-invA primers for the detection of Salmonella in drinking and surface waters following a pre-cultivation step. Water SA 33, 195–202 [Google Scholar]
- Noble P. A., Bidle K. D., Fletcher M. (1997). Natural microbial community compositions compared by a back-propagating neural network and cluster analysis of 5S rRNA. Appl Environ Microbiol 63, 1762–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg J. E., Ryder R. A., Smith H. L., Jr (1989). Isolation of Vibrio cholerae from aquatic birds in Colorado and Utah. Appl Environ Microbiol 55, 95–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekárová P., Onderka M., Pekár J., Rončák P., Miklánek P. (2009). Prediction of water quality in the Danube River under extreme hydrological and temperature conditions. J Hydrol Hydromech 57, 3–15 10.2478/v10098-009-0001-5 [DOI] [Google Scholar]
- Radu S., Vincent M., Apun K., Abdul Rahim R., Benjamin P. G., Yuherman, Rusul G. (2002). Molecular characterization of Vibrio cholerae O1 outbreak strains in Miri, Sarawak (Malaysia). Acta Trop 83, 169–176 10.1016/S0001-706X(02)00110-9 [DOI] [PubMed] [Google Scholar]
- Rashed S. M., Mannan S. B., Johura F. T., Islam M. T., Sadique A., Watanabe H., Sack R. B., Huq A., Colwell R. R. & other authors (2012). Genetic characteristics of drug-resistant Vibrio cholerae O1 causing endemic cholera in Dhaka, 2006-2011. J Med Microbiol 61, 1736–1745 10.1099/jmm.0.049635-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischer G. H., Haider J. M., Sommer R., Stadler H., Keiblinger K. M., Hornek R. W., Zerobin W., Mach R. L., Farnleitner A. H. (2008). Quantitative microbial faecal source tracking with sampling guided by hydrological catchment dynamics. Environ Microbiol 10, 2598–2608 10.1111/j.1462-2920.2008.01682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera I. N. G., Lipp E. K., Gil A., Choopun N., Huq A., Colwell R. R. (2003). Method of DNA extraction and application of multiplex polymerase chain reaction to detect toxigenic Vibrio cholerae O1 and O139 from aquatic ecosystems. Environ Microbiol 5, 599–606 10.1046/j.1462-2920.2003.00443.x [DOI] [PubMed] [Google Scholar]
- Schiemer F., Baumgartner C., Tockner K. (1999). Restoration of floodplain rivers: the ‘Danube restoration project’. Regul Rivers: Res Manage 15, 231–244 [DOI] [Google Scholar]
- Seman M., Prokšová M., Rosinský J., Ferianc P. (2012). Isolation, identification, and characterization of Vibrio cholerae from the Danube River in Slovakia. Folia Microbiol (Praha) 57, 191–197 10.1007/s12223-012-0116-7 [DOI] [PubMed] [Google Scholar]
- Shears P. (2001). Recent developments in cholera. Curr Opin Infect Dis 14, 553–558 10.1097/00001432-200110000-00008 [DOI] [PubMed] [Google Scholar]
- Singh D. V., Matte M. H., Matte G. R., Jiang S., Sabeena F., Shukla B. N., Sanyal S. C., Huq A., Colwell R. R. (2001). Molecular analysis of Vibrio cholerae O1, O139, non-O1, and non-O139 strains: clonal relationships between clinical and environmental isolates. Appl Environ Microbiol 67, 910–921 10.1128/AEM.67.2.910-921.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto S. M., Rodríguez I., Rodicio M. R., Vila J., Mendoza M. C. (2006). Detection of virulence determinants in clinical strains of Salmonella enterica serovar Enteritidis and mapping on macrorestriction profiles. J Med Microbiol 55, 365–373 10.1099/jmm.0.46257-0 [DOI] [PubMed] [Google Scholar]
- Straub T. M., Chandler D. P. (2003). Towards a unified system for detecting waterborne pathogens. J Microbiol Methods 53, 185–197 10.1016/S0167-7012(03)00023-X [DOI] [PubMed] [Google Scholar]
- Sugumar G., Chrisolite B., Velayutham P., Selvan A., Ramesh U. (2008). Occurrence and seasonal variation of bacterial indicators of faecal pollution along Thoothukudi Coast, Tamil Nadu. J Environ Biol 29, 387–391 [PubMed] [Google Scholar]
- Thompson D. E., Rajal V. B., De Batz S., Wuertz S. (2006). Detection of Salmonella spp. in water using magnetic capture hybridization combined with PCR or real-time PCR. J Water Health 4, 67–75 [PubMed] [Google Scholar]
- Touron A., Berthe T., Pawlak B., Petit F. (2005). Detection of Salmonella in environmental water and sediment by a nested-multiplex polymerase chain reaction assay. Res Microbiol 156, 541–553 10.1016/j.resmic.2005.01.001 [DOI] [PubMed] [Google Scholar]
- WHO (2002). The World Health Report 2002 – Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization; [DOI] [PubMed] [Google Scholar]
- WHO (2006). Cholera 2005. Wkly Epidemiol Rec 81, 297–307 [PubMed] [Google Scholar]
- Winter C., Hein T., Kavka G., Mach R. L., Farnleitner A. H. (2007). Longitudinal changes in the bacterial community composition of the Danube River: a whole-river approach. Appl Environ Microbiol 73, 421–431 www.deltanet-project.eu 10.1128/AEM.01849-06 [DOI] [PMC free article] [PubMed] [Google Scholar]


