Abstract
Objectives
To determine the prevalence of HPV and cervical neoplasia among HIV-infected women in southwestern China.
Methods
Cervical cytology, HPV detection by Hybrid Capture-2™ assay, and diagnostic colposcopy were followed by cervical biopsy if indicated. Logistic regression analysis was used to analyze associations between HPV co-infection and cervical intraepithelial neoplasia (CIN), and HIV-related clinical and laboratory parameters.
Results
Colposcopic-histopathologically proven CIN2+ lesions were present in 7/83 (8.4%) HIV-infected women. Nearly half (41/83, 43%) were co-infected with carcinogenic HPV genotypes. HPV co-infection was higher in women with colposcopic-histopathologically proven CIN2+ lesions than women with <CIN1 after adjusting for age (OR: 8.3, 95% CI: 0.9, 73.4). Women with CD4+ cell counts less than 350 cells/μL had higher CIN2+ prevalence after adjusting for current ART status and age (adjusted OR: 6.3, 95% CI: 1.1, 36.5).
Conclusions
HIV/AIDS care and treatment programs should integrate effective cervical cancer prevention services to mitigate the risk of invasive cervical cancer among HIV-infected women in China.
Keywords: China, HIV/AIDS, HPV, cervical cancer, screening, prevention
Introduction
Women infected with the human immunodeficiency virus (HIV) have a higher risk of incidence and persistence of human papillomavirus (HPV)-associated cervical intraepithelial neoplasia (CIN) and cervical cancer than women without HIV (Palefsky and Holly, 2003; Strickler et al., 2005). The HIV/acquired immunodeficiency syndrome (AIDS) epidemic first began in China among injection drug users (IDUs) in the Dehong Prefecture of Yunnan Province, in southwestern China (Zheng, 1991; Zheng et al., 1994; Yu et al., 1996). Heroin use and needle sharing were spurred by Dehong’s proximity to the “Golden Triangle” of drug trafficking where Myanmar, Thailand, and Laos converge (Zhang et al., 2006; Jia et al., 2008). It has been estimated that the HIV-1 prevalence rate reached over 80% among some groups of IDUs in Yunnan, making it the most severely affected Chinese province (Lu et al., 2004; Zhang et al., 2004). More than half (54.4%) of the approximately 15,000 IDUs in Dehong prefecture are infected with HIV (Jia et al., 2008). The problems in Yunnan are worrisome for the rest of China, as the country is estimated to have the world’s largest population of IDUs (approximately 2.4 million), and 6.7–13.4% of these drug users are currently living with HIV (Joint United Nations Programme on HIV/AIDS & World Health Organization, 2010).
Despite the known increased risk for cervical cancer among this population, the burden of HPV and cervical neoplasia among HIV-infected women in China has not been reported. The advent of antiretroviral therapy (ART) against HIV and its increasing availability worldwide has lengthened the life expectancy of HIV-infected women (Franceschi and Jaffe, 2007). Yet in the absence of appropriate prevention services, the lengthened life span makes HIV-infected women vulnerable to persistence of HPV infection and its development to invasive cervical cancer (Franceschi and Jaffe, 2007). Cervical cytology, with its low to moderate sensitivity, may underestimate the true prevalence of cervical cancer precursors in HIV-infected women (Nanda et al., 2000; Sahasrabuddhe et al., 2011). Studies which use diagnostic colpolscopy and, if indicated, histopathology, as well as HPV DNA testing, are needed to investigate the true prevalence of CIN in this high-risk cohort of women to better quantify risk, and our knowledge of HIV-HPV co-infection. We undertook a descriptive epidemiology study in Mangshi, Dehong Prefecture, China to determine the prevalence and predictors of HPV infection and colposcopic-histopathologically confirmed CIN among HIV-infected women.
Materials and Methods
Study Setting and Participants
The Women and Children’s Hospital of Luxi County in Mangshi, Dehong Prefecture, was chosen as the site of this study. Participants were recruited through outreach efforts by hospital personnel familiar to the local HIV-infected population. Eligibility criteria included having documented serologic evidence of HIV infection, no evidence of pregnancy, absence of debilitating illness that might have precluded a pelvic examination, no prior history of screening or treatment for cervical neoplasia, and no prior hysterectomy. Participants were recruited regardless of their CD4+ counts or current ART status. The study was approved by the institutional review boards of Vanderbilt University and the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CICAMS).
Study Procedures
After detailed explanation of study procedures, a chance for participants to ask questions, and obtaining written informed consent, trained nurses administered a questionnaire to collect sociodemographic information, sexual and reproductive history, and medical history relevant to HIV/AIDS and cervical cancer. A blood sample was obtained for CD4+ T-cell count estimation, and tested at the Center for Disease Control and Prevention, Dehong, China. All women underwent a pelvic examination by a trained gynecologist. Specimens were taken from the ectocervix and endocervix for Pap smears (cytology) using a plastic Ayers spatula and a cytobrush, respectively. Cervical samples were also collected from the endocervix to test for the presence of high-risk HPV DNA using the Digene Hybrid Capture 2™ (HC2) assay (Qiagen, Inc., Gaithersburg, MD, USA) in a certified laboratory as per manufacturer’s instructions. (Terry et al., 2001) On all participants, visualization of the cervix was performed with acetic acid and Lugol’s iodine followed by colposcopy. Cervical punch biopsies were advised and performed on consenting participants with clinical evidence of cervical abnormalities and a trained pathologist analyzed the histopathology samples (Dr Pu Ping, Pathology Department, Kunming Medical University, China). The final diagnosis for each woman was based on either the histopathology results whenever available, or on the initial colposcopy impression if no biopsy was performed or the histopathology results were inconclusive. Results were reported as per the Bethesda CIN staging system by increasing disease severity: normal/no neoplastic abnormalities, CIN1, CIN2, CIN3, or invasive cervical cancer (ICC) (Richart, 1973).
Statistical Analysis
The statistical analysis was conducted using STATA 11.1 (StataCorp LP, College Station, TX, USA). Women HC2 test positive or with CD4+ counts lower than 350 cells/μL were stratified by cytology and colposcopic-histopathological diagnosis; data were analyzed with bivariate logistic regression to identify associations between these variables. Crude and adjusted odds ratios and confidence intervals were also calculated to identify sociodemographic and clinical characteristics predicting diagnoses of CIN and high-risk HPV DNA positivity (Harrell et al., 1985). Sensitivity and specificity estimates were calculated for the HC2 HPV DNA and Pap smear tests.
Results
Sociodemographic Information
In April 2009, 96 HIV-infected women were recruited for this study. One patient who consented did not eventually participate in the clinical protocol, and thus data from 95 women were included in subsequent analyses. The mean age of participants was 33.5 years, with a range from 19–68 years. The median CD4+ count was 441 cells/μL and slightly more than half (55/95, 57.9%) of the participants were concurrently taking anti-retroviral therapy for their HIV infection (Table 1).
Table 1.
Sociodemographic and Laboratory Results of the Study Participants
| Information | All participants (N=95) |
|---|---|
| Mean age, years (range) | 33.5 (19–68) |
| Nationality, N (%) | |
| Han | 43 (45.3) |
| Dai | 32 (33.7) |
| Jing Bo | 11 (11.6) |
| Othera | 9 (9.5) |
| Median CD4+ counts, cells/μL (range) | 441 (89–6789) |
| ART treatment, N (%) | 55 (57.9) |
| Mean duration of ART use, months (range)b | 23.6 (3–59) |
| Mean number of sexual partners (range) | 1.91 (1–30) |
| Number of smokers, N (%) | 9 (9.5) |
| Median parity (range) | 2 (0–6) |
| HPV DNA positivityc, N (%) | 41 (43.1) |
| Women with normal cytology | 25 (33.3) |
| Cytology, N (%) | |
| Negative | 75 (79.0) |
| ASC-US | 7 (7.4) |
| LSIL | 12 (12.6) |
| HSIL | 1 (1.1) |
| Coloposcopic-histopathological diagnosisd, N (%) | |
| No evidence of CIN | 67 (70.5) |
| CIN1 | 9 (9.5) |
| CIN2 | 5 (5.3) |
| CIN3 | 2 (2.1) |
| Unsatisfactory/Missinge | 12 (12.6) |
Other nationalities: De An (5 women), Hui (1), Wei Wu Er (1), Bu Yi (1), and Ar Chan (1);
Mean duration of ART use for those taking ART;
HPV DNA Positive if Hybrid Capture 2™ result ≥1.00 relative light units;
Colposcopic-Histopathological diagnosis: Biopsy results if available or colposcopy results if no biopsy;
Unsatisfactory/missing colposcopic-histopathological diagnoses: 9 unsatisfactory and 3 missing; ART, Anti-retroviral HIV therapy; CIN, Cervical intraepithelial neoplasia; ASC-US, Aytpical squamous cells of undetermined significance; LSIL, Low-grade squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion
Colposcopy and Histopathology
Colposcopy was performed on all but three participants, one of which experienced heavy cervical bleeding post-sample collection and two refused consent for the colposcopy portion of the exam. Of the remaining 92 women, 12 had unsatisfactory colposcopy examinations but refused endocervical curettage (ECC) at that time. Three of these women later tested positive for both cervical intraepithelial lesions by Pap smear and HPV high-risk DNA by HC2 test and were called back to the clinic for ECC and cervical biopsies. As a result, a total of 83 women were given a final colposcopic-histopathological diagnosis from this study. Fifty-five women (66.3%) had no evidence of CIN on colposcopy, 16 women (19.3%) showed evidence of CIN1 and 9 women (10.8%) showed evidence of CIN2+ lesions.
The final composite colposcopic-histopathological diagnoses revealed no evidence of CIN in 67 out of 83 women (80.7%). CIN1 was reported in 9 women (9.5%), CIN2 in 5 women (5.3%), and CIN3 in 2 women (2.1%). Invasive cervical cancer was not reported in any women (Table 1). Thus, of women with a final diagnosis, 16/83 (19.3%) had evidence of CIN1+ lesions and 7/83 (8.4%) had evidence of CIN2+ lesions.
Cytology and HPV DNA Testing
Specimens for cytology and high-risk HPV DNA testing were collected from every participant before colposcopy. Seventy-five women (79%) tested negative for pre-cancerous lesions, seven showed atypical squamous cells of undetermined significance (ASC-US), 12 showed low-grade intraepithelial lesions (LSIL) and one showed high-grade intraepithelial lesions (HSIL). Of women who tested negative on cytology, 48 women (75%) were negative by colposcopy as well, but 13 women (20.3%) had evidence of CIN1 on colposcopy, and three women (4.7%) had CIN2+. Of women who tested ASC-US/LSIL on cytology, six (40%) had CIN2+. Of women who tested negative on the HC2 DNA test, six (14.0%) had evidence of CIN1 and two (4.7%) had evidence of CIN2+ (Table 2). Forty-one HIV-infected women (43.2%) tested positive for high-risk HPV DNA per the HC2 test. Using the colposcopic-histopathological diagnosis as the reference standard, the sensitivity of the HC2 test for CIN2+ in this study was 85.7% (95% confidence interval (CI): 42.0–99.2) while the specificity was 55.3% (95%CI: 43.5–66.5). The sensitivity of cytology for a comparable level of cervical lesion (HSIL) detection was lower at 6.3% (95% CI: 0.0–47.1), but the specificity was higher at 98.1% (95% CI: 91.1–99.8) (Figure 1). Compared to women with normal cytology results, women with ASC-US, LSIL, and HSIL cytology had progressively higher rates of HPV infection (Table 3).
Table 2.
Comparison of Colposcopy with Cytology and HPV DNA Results among HIV-infected Women
| Colposcopy result | N | % | |
|---|---|---|---|
| Cytology | |||
| Negative | Negative | 48 | 75 |
| CIN1 | 13 | 20.3 | |
| CIN 2+ | 3 | 4.7 | |
| ASC-US/LSIL | Negative | 6 | 40 |
| CIN1 | 3 | 20 | |
| CIN 2+ | 6 | 40 | |
| HSIL | Negative | 1 | 100 |
| CIN1 | 0 | 0 | |
| CIN 2+ | 0 | 0 | |
| HPV Testing | |||
| Negative | Negative | 35 | 81.4 |
| CIN1 | 6 | 14 | |
| CIN 2+ | 2 | 4.7 | |
| Positivea | Negative | 20 | 66.7 |
| CIN1 | 10 | 33.3 | |
| CIN 2+ | 0 | 0 | |
HPV positive: HC2 ≥ 1.00 relative light units (RLU); CIN, Cervical intraepithelial neoplasia; ASC-US, Aytpical squamous cells of undetermined significance; LSIL, Low-grade squamous intraepithelial lesion; HSIL, High-grade squamous intraepithelial lesion
Figure 1.
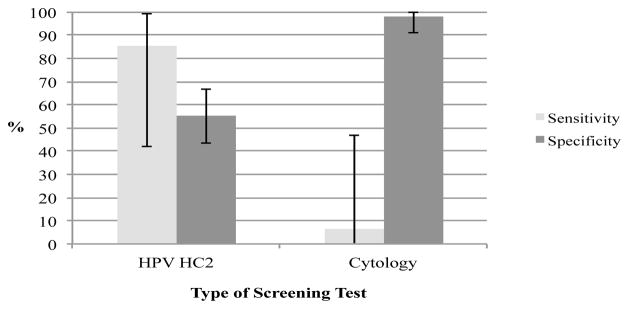
Sensitivity and Specificity for CIN2+ of HPV DNA (Hybrid Capture 2) Testing vs. Cytology among HIV-infected women in Yunnan, China
Table 3.
Proportion of HIV-infected Women Detected with HPV DNA Positivity and Low CD4+ Counts, Stratified by Cytology and Colposcopic-histopathological Diagnosis
| N | a HPV positive, N (%) | b Adjusted OR (95% CI) | CD4+ <350 cells/μL, % | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|
| Cytology | |||||
| Normal | 75 | 25 (33.3) | 1 | 18 (24.0) | 1 |
| ASC-US+ | 20 | 16 (80.0) | 8.0 (2.4–26.5) | 8 (40.0) | 2.2 (0.8–6.2) |
| ASC-US | 7 | 5 (71.4) | 7.6 (2.3–25.4) | 3 (42.9) | 2.3 (0.8–6.7) |
| LSIL | 12 | 10 (83.3) | 5 (41.7) | ||
| HSIL | 1 | 1 (100) | --- | 0 (0.0) | --- |
| Colposcopic-histopathological diagnosis | |||||
| Normal | 67 | 26 (38.8) | 1 | 15 (22.4) | 1 |
| CIN1+ | 16 | 14 (87.5) | 11.4 (2.4–54.7) | 9 (56.3) | 5.4 (1.6–17.8) |
| CIN1 | 9 | 8 (88.9) | 12.3 (1.5–105.0) | 4 (44.4) | 4.0 (0.9–19.0) |
| CIN2+ | 7 | 6 (85.7) | 10.3 (1.1–90.8) | 5 (71.4) | 8.2 (1.4–47.2) |
HPV positive: HC2 ≥ 1.00 relative light units (RLU);
Adjusted for age ≥ 30 years; CIN, Cervical intraepithelial neoplasia; ASC-US, Aytpical squamous cells of undetermined significance; LSIL, Low-grade squamous intraepithelial lesion; HSIL, High-grade squamous intraepithelial lesion; OR, Odds ratio; CI, Confidence interval
Predictors of Increasing Severity of CIN and Positivity for High-Risk HPV DNA
In analysis adjusted only for age, participants whose CD4+ counts were lower than 350 cells/μL had greater odds of CIN2+ as opposed to ≤ CIN1 (OR: 6.8, 95% CI: 1.2, 38.3) (Table 4). Although the odds decreased slightly when current ART status was added as a variable, the relationship maintained significance (OR: 6.3, 95% CI: 1.1, 36.5). Women with both CIN1+ and CIN2+ had higher HPV DNA prevalence than <CIN1 and <CIN2 respectively, although the difference reached the statistically significant level only in CIN1+ women due to the small sample size. Women with two or more lifetime sexual partners were more likely to be high-risk HPV DNA positive than women with less than two sexual partners, a difference which approached statistical significance.
Table 4.
Sociodemographic and Clinical Factors Associated with CIN2+, CIN1+, and HPV DNA Positivity among HIV-infected Women in Yunnan, China
| CIN2+ threshold | CIN1+ threshold | b HPV DNA positivity threshold | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a CIN2+, N(%) | ≤CIN1,) N(%) | c Adjusted OR, (95% CI) | a CIN1+, N(%) | Normal, N(%) | Adjusted OR, (95% CI) | HC2≥1.00 RLU, N (%) | HC2<1.00 RLU, N (%) | Adjusted OR, (95% CI) | |
| Age ≥ 30 years | 6 (10.9) | 49 (89.1) | --- | 11 (20.0) | 44 (80.0) | --- | 26 (39.4) | 40 (60.6) | --- |
| CD4+ <350/μL | 5 (20.8) | 19 (79.2) | 6.8 (1.2–38.3) | 9 (37.5) | 15 (62.5) | 5.4 (1.6–17.8) | 13 (50.0) | 13 (50.0) | 1.4 (0.5–3.7) |
| Currently taking ART | 5 (10.6) | 42 (89.4) | 2.1 (0.4–11.4) | 11 (23.4) | 36 (76.6) | 1.9 (0.6–6.1) | 24 (43.6) | 31 (56.4) | 1.1 (0.4–2.6) |
| ART Duration <6 months | 2 (5.3) | 36 (94.7) | 0.4 (0.1–2.5) | 6 (15.8) | 32 (84.2) | 0.7 (0.2–2.0) | 19 (44.2) | 24 (55.8) | 1.1 (0.5–2.7) |
| >2 Sexual partners | 0 (0) | 14 (100) | --- | 3 (21.4) | 11 (78.6) | 1.2 (0.3–5.2) | 10 (66.7) | 5 (33.3) | 3.2 (0.9–11.5) |
| Currently smoker | 1 (11.1) | 8 (88.9) | 1.3 (0.1–12.1) | 1 (11.1) | 8 (88.9) | 0.5 (0.1–4.2) | 5 (55.6) | 4 (44.4) | 1.5 (0.4–5.9) |
| Gravidity ≥4 children | 2 (11.8) | 15 (88.2) | 1.1 (0.2–6.7) | 4 (23.5) | 13 (76.5) | 1.6 (0.4–6.6) | 6 (31.6) | 13 (68.4) | 0.5 (0.2–1.7) |
| d HPV DNA Positive | 6 (15.0) | 34 (85.0) | 8.3 (0.9–73.4) | 14 (35.0) | 26 (65.0) | 11.4 (2.4–54.7) | --- | --- | --- |
Using data from women with colposcopic-histopathological diagnoses;
Using data from all 95 participants;
Adjusted for age ≥ 30 years;
HPV DNA Positive if Hybrid Capture 2™ result ≥1.00 relative light units; CIN, Cervical intraepithelial neoplasia; ART, Anti-retroviral HIV therapy; OR, Odds ratio; CI, Confidence interval
Discussion
Our study is the first report to date on prevalence and factors associated with HPV and cervical precancerous lesions among HIV-infected women in China. This study showed that HIV-infected women have a high burden of high-risk HPV DNA and CIN disease, and increasing risk with further immunosuppression as measured by lower CD4 counts. Contemporaneous CIN1+ prevalence rates from the general population in China have ranged from 7.3 to 10% (Belinson et al., 2003; Pan et al., 2003; Ting et al., 2010) as compared to over 19% women in this study with evidence of CIN1+. A recent review of cervical cancer and HPV burden in China estimated the prevalence of high-risk HPV DNA to be 17.2% (Shi et al., 2011), a figure much lower than the 43.1% found in our population of HIV-infected women. While not as high as the 70 to 80% or higher prevalence estimates from HIV-infected women in sub-Saharan African countries with generalized HIV epidemics (e.g., Zambia (Sahasrabuddhe et al., 2007), South Africa (Denny et al., 2008) and Kenya (Luchters et al., 2010)), these rates are similar to those reported from studies reporting HPV DNA prevalence among HIV-infected women from countries with low-level or concentrated HIV epidemics like India (Sahasrabuddhe et al., 2010), Brazil (de Andrade et al., 2011), and elsewhere (Tornesello et al., 2008; Videla et al., 2009).
The use of combined histopathology following colposcopy for women with clinical evidence of CIN as the diagnostic standard in this study avoided unnecessary invasive procedures while increasing the accuracy of diagnosis over that of cytology, or even that of colposcopy alone (Dexeus et al., 2002; Kosinski and Barnhart, 2003). It also enabled us to compare colposcopy results with cytological results in the same cohort of HIV-infected women. Twenty-five percent of women in our study who tested negative by cytology actually had some form of colposcopic evidence of CIN, and 40% of women with ASC-US or LSIL by cytology had CIN2+ as determined by colposcopy (Table 2). These data also suggest that cytology testing for cervical lesions is even less accurate in HIV-infected women than for the general population, as confirmed in other studies (Akinwuntan et al., 2008; Sahasrabuddhe et al., 2011). On the other hand, while the sensitivity of HPV testing was higher than cytology, it came at a significant loss of specificity (Figure 1). Given the high prevalence of HPV DNA among HIV-infected women, and the possibility of significant numbers testing positive using HPV-based screening, further research is needed to improve specificity (and reduce false positive referrals) by the use of molecular biomarkers (such as p16ink4a or E6/E7 mRNA) in conjunction with HPV testing.
Our study demonstrates that the presence of carcinogenic HPV DNA is an independent predictor of CIN and also shows that HIV-infected women with low CD4+ counts are at higher risk for pre-cancerous cervical lesions than women with higher CD4+ counts. These risk factors and the high prevalence of CIN in this population compared to the general population demand targeted screening and treatment for HIV-infected women to prevent high mortality from cervical cancer. China is investing new resources for nationwide expansion of the cervical cancer screening program to tackle the historically inadequate population coverage common to most developing countries. (Gakidou et al., 2008) Efforts are focusing on the development of low-cost, rapid HPV-based diagnostics, which should be effectively integrated with HIV/AIDS care and treatment programs (Mwanahamuntu et al., 2008; Qiao et al., 2008).
Our study had several limitations. We were only able to obtain CD4+ counts at the time of enrollment (as opposed to nadir CD4+ counts), which could incompletely reflect immune status. Also, colposcopic and histopathologic interpretations are subjective and are rater-dependent (Massad et al., 2001), raising the possibility of systematic misclassification of cervical lesions. Importantly, the cross-sectional study design precludes any evidence of causality and the small sample size does not allow adequate power for many statistical comparisons. We are currently conducting a prospective study in a larger cohort in the same area of Yunnan Province, China to better study the natural history of HPV co-infection and cervical neoplasia in HIV-infected women and the effect of ART in this high-risk population.
Our study is the first to demonstrate that HIV-infected women in southwest China have a high prevalence of carcinogenic HPV and precancerous lesions relative to the general population. In the context of constantly improving ART access and life expectancies among HIV-infected people, the importance of instituting early and effective detection and treatment services to protect this high-risk population from developing invasive cervical cancer cannot be understated.
Acknowledgments
We would like to thank pathologist Dr. Pu Ping and cytologist Dr Liu Wenli from Mangshi. We thank all women who participated in this study. This work was partially supported by the U.S. National Institutes of Health grants R24TW007988, D43TW001035, and P30AI054999 and the American Relief and Recovery Act.
References
- Akinwuntan AL, Adesina OA, Okolo CA, et al. Correlation of cervical cytology and visual inspection with acetic acid in HIV-positive women. J Obstet Gynaecol. 2008;28:638–41. doi: 10.1080/01443610802259977. [DOI] [PubMed] [Google Scholar]
- Belinson JL, Qiao YL, Pretorius RG, et al. Shanxi Province cervical cancer screening study II: self-sampling for high-risk human papillomavirus compared to direct sampling for human papillomavirus and liquid based cervical cytology. Int J Gynecol Cancer. 2003;13:819–26. doi: 10.1111/j.1525-1438.2003.13611.x. [DOI] [PubMed] [Google Scholar]
- de Andrade AC, Luz PM, Velasque L, et al. Factors associated with colposcopy-histopathology confirmed cervical intraepithelial neoplasia among HIV-infected women from Rio De Janeiro, Brazil. PLoS One. 2011;6:e18297. doi: 10.1371/journal.pone.0018297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny L, Boa R, Williamson AL, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol. 2008;111:1380–7. doi: 10.1097/AOG.0b013e3181743327. [DOI] [PubMed] [Google Scholar]
- Dexeus S, Cararach M, Dexeus D. The role of colposcopy in modern gynecology. Eur J Gynaecol Oncol. 2002;23:269–77. [PubMed] [Google Scholar]
- Franceschi S, Jaffe H. Cervical cancer screening of women living with HIV infection: a must in the era of antiretroviral therapy. Clin Infect Dis. 2007;45:510–3. doi: 10.1086/520022. [DOI] [PubMed] [Google Scholar]
- Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Matchar DB, Reichert TA. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer Treat Rep. 1985;69:1071–7. [PubMed] [Google Scholar]
- Jia Y, Sun J, Fan L, et al. Estimates of HIV prevalence in a highly endemic area of China: Dehong Prefecture, Yunnan Province. Int J Epidemiol. 2008;37:1287–96. doi: 10.1093/ije/dyn196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS and World Health Organization. AIDS epidemic update. 2010. [Google Scholar]
- Kosinski AS, Barnhart HX. Accounting for nonignorable verification bias in assessment of diagnostic tests. Biometrics. 2003;59:163–71. doi: 10.1111/1541-0420.00019. [DOI] [PubMed] [Google Scholar]
- Lu L, Jia MH, Zhang XB, et al. Analysis for epidemic trend of acquired immunodeficiency syndrome in Yunnan Province of China. Zhonghua Yu Fang Yi Xue Za Zhi. 2004;38:309–12. [PubMed] [Google Scholar]
- Luchters SM, Vanden Broeck D, Chersich MF, et al. Association of HIV infection with distribution and viral load of HPV types in Kenya: a survey with 820 female sex workers. BMC Infect Dis. 2010;10:18. doi: 10.1186/1471-2334-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad LS, Schneider M, Watts H, et al. Correlating papanicolaou smear, colposcopic impression, and biopsy: results from the women’s interagency HIV study. J Low Genit Tract Dis. 2001;5:212–8. [PubMed] [Google Scholar]
- Mwanahamuntu MH, Sahasrabuddhe VV, Stringer JS, Parham GP. Integrating cervical cancer prevention in HIV/AIDS treatment and care programmes. Bull World Health Organ. 2008;86 doi: 10.2471/BLT.08.056275. D–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–9. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- Palefsky JM, Holly EA. Chapter 6: Immunosuppression and co-infection with HIV. J Natl Cancer Inst Monogr. 2003;31:41–6. doi: 10.1093/oxfordjournals.jncimonographs.a003481. [DOI] [PubMed] [Google Scholar]
- Pan Q, Belinson JL, Li L, et al. A thin-layer, liquid-based pap test for mass screening in an area of China with a high incidence of cervical carcinoma. A cross-sectional, comparative study. Acta Cytol. 2003;47:45–50. doi: 10.1159/000326474. [DOI] [PubMed] [Google Scholar]
- Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- Richart RM. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301–28. [PubMed] [Google Scholar]
- Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96:1480–3. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabuddhe VV, Bhosale RA, Joshi SN, et al. Prevalence and predictors of colposcopic-histopathologically confirmed cervical intraepithelial neoplasia in HIV-infected women in India. PLoS One. 2010;5:e8634. doi: 10.1371/journal.pone.0008634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabuddhe VV, Bhosale RA, Kavatkar AN, et al. Comparison of visual inspection with acetic acid (VIA) and cervical cytology to detect high grade cervical neoplasia among HIV-infected women in India. Int J Cancer. 2011;130:234–40. doi: 10.1002/ijc.25971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi JF, Canfell K, Lew JB, Qiao YL. The burden of cervical cancer in China: Synthesis of the evidence. Int J Cancer. 2012;130:641–52. doi: 10.1002/ijc.26042. [DOI] [PubMed] [Google Scholar]
- Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- Terry G, Ho L, Londesborough P, et al. Detection of high-risk HPV types by the hybrid capture 2 test. J Med Virol. 2001;65:155–62. [PubMed] [Google Scholar]
- Ting J, Kruzikas DT, Smith JS. A global review of age-specific and overall prevalence of cervical lesions. Int J Gynecol Cancer. 2010;20:1244–9. doi: 10.1111/igc.0b013e3181f16c5f. [DOI] [PubMed] [Google Scholar]
- Tornesello ML, Duraturo ML, Giorgi-Rossi P, et al. Human papillomavirus (HPV) genotypes and HPV16 variants in human immunodeficiency virus-positive Italian women. J Gen Virol. 2008;89:1380–9. doi: 10.1099/vir.0.83553-0. [DOI] [PubMed] [Google Scholar]
- Videla S, Darwich L, Canadas MP, et al. Epidemiological data of different human papillomavirus genotypes in cervical specimens of HIV-1-infected women without history of cervical pathology. J Acquir Immune Defic Syndr. 2009;50:168–75. doi: 10.1097/QAI.0b013e3181938e63. [DOI] [PubMed] [Google Scholar]
- Yu ES, Xie Q, Zhang K, Lu P, Chan LL. HIV infection and AIDS in China, 1985 through 1994. Am J Public Health. 1996;86:1116–22. doi: 10.2105/ajph.86.8_pt_1.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen Z, Cao Y, et al. Molecular characterization of human immunodeficiency virus type 1 and hepatitis C virus in paid blood donors and injection drug users in china. J Virol. 2004;78(24):13591–9. doi: 10.1128/JVI.78.24.13591-13599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu L, Ba L, et al. Dominance of HIV-1 subtype CRF01_AE in sexually acquired cases leads to a new epidemic in Yunnan province of China. PLoS Med. 2006;3:e443. doi: 10.1371/journal.pmed.0030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Tian C, Choi KH, et al. Injecting drug use and HIV infection in southwest China. AIDS. 1994;8:1141–7. doi: 10.1097/00002030-199408000-00017. [DOI] [PubMed] [Google Scholar]
- Zheng XW. A preliminary study on the behavior of 225 drug abusers and the risk factors of HIV infection in Ruili county Yunnan Province. Zhonghua Liu Xing Bing Xue Za Zhi. 1991;12:12–4. (in Chinese) [PubMed] [Google Scholar]


