Abstract
A novel method for the facile production of gas-containing liposomes with simultaneous drug encapsulation is described. Liposomes of phospholipid and cholesterol were prepared by conventional procedures of hydrating the lipid film, sonicating, freezing and thawing. A single, but critical modification of this procedure generates liposomes that contain gas (air, perfluorocarbon, argon); after sonication, the lipid is placed under pressure with the gas of interest. After equilibration, the sample is frozen. The pressure is then reduced to atmospheric and the suspension thawed. This procedure leads to entrapment of air in amounts up to 10% by volume by lipid dispersions at moderate (10mg/ml) concentrations of lipids. The amount of gas encapsulated increases with gas pressure and lipid concentration. Utilizing 0.32 M mannitol to provide an aqueous phase with physiological osmolarity, 1, 3, 6 or 9 atm of pressure was applied to 4 mg of lipid. This led to encapsulation of 10, 15, 20, and 30 μl of gas in a total of 400 μl of liposome dispersion (10mg lipids/ml), respectively. The mechanism for gas encapsulation presumably depends upon the fact that air (predominantly nitrogen and oxygen), like most solutes, dissolves poorly in ice and is excluded from the ice that forms during freezing. The excluded air then comes out of solution as air pockets that are stabilized in some form by a lipid coating. The presence of air in these preparations sensitizes them to ultrasound (1MHz, 8 W/cm2,10 second) such that up to half of their aqueous contents (which could be a water soluble drug) can be released by short (10 s) applications of ultrasound. Both diagnostic and therapeutic applications of the method are conceivable.
Keywords: Phospholipid freeze-thawing, controlled release, drug encapsulation, ultrasound
INTRODUCTION
Liposomes have been developed as non-toxic, biodegradable and non-immunogenic drug delivery vehicles. They are suitable for encapsulating and delivering a variety of therapeutic agents, including hydrophilic and lipophilic drugs (Barenholz et al 2003), oligonucleotides (Leonetti et al 2001; Semple et al 2001) and proteins and peptides (Ye et al 2000). Lipids of the types used for liposomes are also capable of entrapping ultrasound-reflective gases and a number of lipid-based contrast agents are commercially available for diagnostic applications (Goertz DE et al 2007; Gorce JM, et al 2000). Recently, liposome preparations have been described that contain both a gas and an aqueous solute. These are sensitive to ultrasound in that the solute, which may be a water-soluble drug, can be released by brief, low-intensity insonation (Huang et al 2004).
Liposomes are characterized by clearly separated hydrophilic and hydrophobic regions. Hydrophilic portions of bilayer lipids are directed towards aqueous phases (external and internal), whereas hydrophobic portions of both lipid layers are directed towards one another, forming the internal core of a membrane. A special quality of liposomes for drug delivery is that they enable water-soluble and water-insoluble materials to be used together. Water-soluble materials are entrapped in the aqueous core, while water-insoluble and oil-soluble hydrophobic drugs or other agents reside within the bilayer (Nii et al 2005). When a gas is encapsulated, for energetic reasons, it can be presumed to behave similarly to hydrophobic drugs, in that air resides between the two monolayers of the liposome bilayer (or perhaps as a monolayer-covered air bubble within the aqueous compartment) of liposomes (Huang et al, 2004).
When gas-carrying liposomes also carry drugs within the aqueous compartment, they are sensitive to ultrasound-triggered release. We hypothesize that the rarefaction phase leads to expansion of the air pocket, that is, when the negative ultrasound wave impinges upon the liposomes, the air pocket expands, stressing the monolayers bounding it as well as those in the adjacent bilayer (surrounding bilayer in the case of an isolated air bubble within the liposome). If the pressure drop is large enough, the stress will exceed the elastic limit of the weakest surface and at some point, either the bilayer or the monolayers must rend. When the integrity of the vesicle is lost, some or all of the contents (depending upon how long resealing takes) will be released.
Ultrasound has previously been used to accelerate release from several different drug delivery preparations (Huang et al 2004, Rapoport et al 2003; Unger et al 1998). In some of these situations, drugs are associated with the outside surface of the particles and can potentially be exposed to the circulation such that they may lose their activity or cause general toxicity. Other investigators developed lipospheres that consist of gas cores coated with a layer of oil, which can be used to load hydrophobic drugs. The general procedure for making these gas-containing lipospheres involves agitating the lipid in the aqueous phase with simultaneous exposure to the gas phase (Gorce et al 2000; Shortencarier et al 2004; Unger et al 1998). Recently, we have developed a different method to generate gas and drug co-encapsulated liposomes that involves freezing in the presence of mannitol followed by lyophilization (Huang et al 2004). An important advantage of this method is efficient co-encapsulation of both hydrophilic gases and hydrophilic drugs. When gas-containing liposomes also carry drugs, they can be effective for both ultrasound-controlled/enhanced drug delivery and imaging.
The present communication describes a unique, simple and highly efficient method for liposome co-encapsulation of variety gases and hydrophilic solutes. Compared with the lyophilization method, it has the advantage of facile encapsulation of the gas phase. This method should allow development of liposomes different types of gas, such as the MRI enhancer, hyperpolarized xenon or the blood vessel relaxing factor, nitric oxide, for both diagnostic and therapeutic applications.
MATERIALS & METHODS
1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol (CH) and all other lipids were obtained from Avanti Polar Lipids (Alabaster, AL) and stored at −20 °C in chloroform. Argon was obtained from Airgas Inc.(Chicago, IL) and octafluorocyclobutane was from specialty gases of America, Inc. (Toledo, OH). Calcein (2′,7′-[(bis[carboxymethyl]-amino)methyl]-fluorescein) and cobalt chloride (CoCl2) were obtained from Sigma Chemical Co.(St. Louis, MO). Stock solutions of calcein (80 mM) were made by rapidly dissolving solid calcein at pH 9.0 and then adjusting the pH to 7.5 with NaOH. Triton X-100 and 3-morpholinepropanesulphonic acid (MOPS) were also from Sigma. The sonoporation unit was a Sonitron2000 with 1.2cm diameter soundhead, 1 MHz frequency, continuous and pulsed output, and 0–2.0 W/cm2 adjustable power from RichMar (Inola, OK). Costar transwell insert with a 0.4 μm pore polyester membrane was from Corning Incorporated Life Sciences (Lowell, MA).
Gas-containing liposome preparation
Lipid mixtures (5 mg total weight) of the desired composition were prepared from chloroform solutions by combining the appropriate molar amounts of the component lipids in a 4 ml (15×45mm) borosilicate glass test tube. The organic solvent was removed by evaporation under argon in a 50 °C water bath with constant rotation until a thin film of lipids was formed on the vial wall. The resulting lipid film was then placed under high vacuum (<100 mtorr) for 4–6 hours for complete removal of solvent. The dried lipid film was hydrated with 0.32M mannitol (mannitol is used in part as an osmoticant to provide physiologic osmolarity, but it also has beneficial effects for incorporation of air and entrapment of water soluble agents such as pharmaceutics) or other solutions of choice. The resultant liposomes (10mg/ml final concentration) were sonicated for 5 min in a water bath. A total of 500μl of the liposomal dispersion was transferred to 1.8 ml screw-cap borosilicate glass vials (12×32 mm) that were then capped with open screw caps containing Teflon-covered silicon rubber septa. The desired gas was introduced into the vial through the septum with a 10 ml syringe fitted with a 27G×1/2″ needle. The pressure created by the injected gas volume was calculated from Boyle’s Law and the volumes of the vial and syringe. The septa used were tested for leakage at the highest pressure required in our investigation and found not to release detectable amounts of any of the three gases investigated for at least 24 hours. The pressurized-gas/liposome dispersion was incubated for 1/2 hour at room temperature and then frozen by cooling to −78°C in dry ice for at least 1/2 hour. The pressure was released by unscrewing the caps immediately after their removal from dry ice. The depressured frozen liposomes were then thawed by exposure to room air, during which time the temperature of the dispersion changed from −78°C to 24°C within 10 minutes. Gas and calcein encapsulation were measured after the sample had thawed and warmed to room temperature.
Liposome preparation for co-encapsulation of gas and calcein
Calcein, a fluorescent dye used as a marker of the liposome internal volume, was encapsulated by a procedure similar to that for preparing gas-containing liposomes. To load the liposomes with calcein, 0.1 mM calcein was included in the 0.32 M mannitol normally used to hydrate the dried lipid film.
Measurement of the amount of gas into lipid dispersions
The amount of incorporated gas was determined using a previously described method (Huang et al 2002). Briefly, a 0.8–1 ml sample containing 5 mg lipid/ml is put into a 10-mL disposable syringe. A two-way Luerlock stopcock is attached to the syringe and the air is displaced from the syringe and stopcock by depressing the plunger. The stopcock is closed, and a 250-μL syringe, without a plunger, but containing a 20 ul volume of water is attached. The plunger of the large syringe is withdrawn to create a vacuum that releases air from the liposomes. After turning the stopcock to connect the large and small syringe bodies, and holding the large syringe so that the liposomes are at the bottom (away from the stopcock). The plunger is depressed to transfer the released air into the small syringe where its volume at ambient pressure is measured according to the displacement of the 20 ul bolus of water.
Determination of calcein encapsulation efficiency
Aqueous phase encapsulation efficiency was determined fluorometrically using a previously developed method (Huang et al 2004). In the embodiment used here, a 25 μl aliquot of resuspended liposomes (10 mg lipid/ml) was diluted to 500 μl with 50 mM MOPS buffer containing 110 mM NaCl. Then, 5 μl of 10 mM CoCl2 was added to quench the fluorescence of the external calcein so that the residual fluorescence represents the entrapped calcein. Then the liposomes were lysed with detergent (25 μl 10% Triton X-100) to determine the background fluorescence at zero encapsulated volume. Fluorescence intensity was measured before (Fb) and after (Fa) addition of CoCl2, and again after addition of X-100 (Ftotq) at 490 nm Ex, 520 nm Em. The % entrapment was calculated as:
| (1) |
Ultrasound imaging
To demonstrate ultrasound image enhancement (echogenicity), gas-containing liposomes, with or without encapsulated calcein, were diluted to a concentration of 25 μg/mL and placed in 12×16 mm glass vials. They were then imaged with a 20-MHz high-frequency intravascular ultrasound image catheter, as previously described (Huang et al 2002). Images were recorded onto videotape, subsequently digitalized and computer analyzed in terms of a 0–256 mean gray scale value (MGSV) of the entire image.
Ultrasound triggered release
Ultrasound-triggered release experiments were performed in a chamber as illustrated in Fig 1. This chamber consisted of a Costar transwell insert with a 0.4 μm pore polyester membrane resting on a sheet of Rho-C rubber. A thin layer of water was placed between the membrane and the rubber to exclude air and air bubbles. The open top of the transwell insert allowed for introduction of the liposome dispersion (400 μl) and placement of the ultrasound probe (1MHz, 8W/cm2,100% duty cycle, 10 sceond. Probe size: 1.2 cm diameter).
Fig. 1.
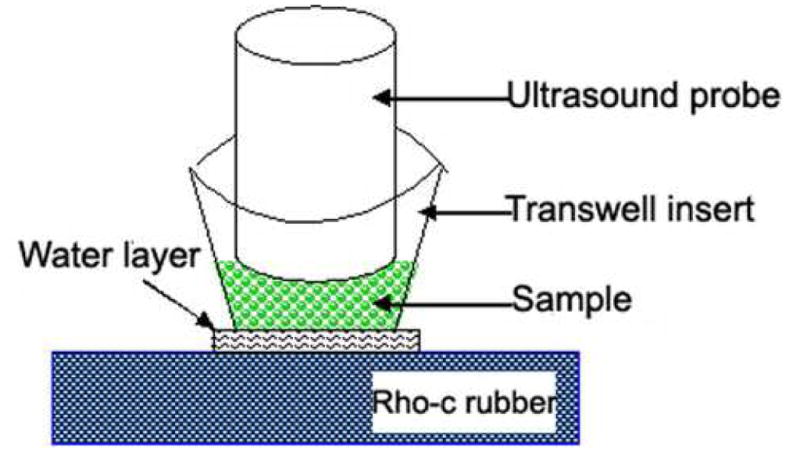
Experimental apparatus for ultrasound-triggered release (see text for details). This apparatus consists of a Costar transwell insert with 0.4 μm pore polyester membrane resting on a sheet of Rho-C rubber. Polyester membrane has a 100% of transmission ultrasound wave and Rho-C rubber eliminated the reflection of ultrasound wave. A thin layer of water was put between the membrane and the rubber to exclude air and air bubbles. The open top of transwell insert allowed for introduction of the liposome dispersion (400 μl) and placement of the ultrasound probe.
The release of calcein was determined using a modified version of the encapsulation measurement procedure. 100μl of calcein-containing, acoustically-active liposomes (10 mg lipid/mL), were diluted to 500 μl with 50 mM MOPS buffer containing 110 mM NaCl (to maintain isosmolality with liposome contents of 320 mM mannitol and 0.1 mM calcein). The fluorescence intensity (Fin) of the suspension was measured after addition of 5 μl of 40 mM CoCl2. Ultrasound was then applied for 10s and the resulting fluorescent intensity (Fultrasound) was measured. Finally, 25 μl of 10% Triton X-100 was added and the fluorescent intensity (Ftotq) was remeasured. Calcein release was calculated as:
| (2) |
Statistical Analysis
Data are presented as mean±SD. Comparisons between groups were made by ANOVA with significance taken to be P<0.05.
RESULTS
Preparation of gas-containing liposomes
As shown in Fig. 2, freezing the liposomal dispersion in the presence of elevated gas pressure led to gas entrapment in an amount that was related (essentially linearly over the range of pressures examined), to the pressure of the gas. With 0.32 M mannitol as the aqueous phase, 1, 3, 6 or 9 atm pressure applied to 4 mg of lipid led to incorporation of 10, 15, 20, and 30 μl air, respectively, into the dispersion. Given the dependence of air incorporation on the pressure, it appears that higher pressures would lead to even larger volumes of gas being incorporated into the lipid dispersion.
Fig. 2.
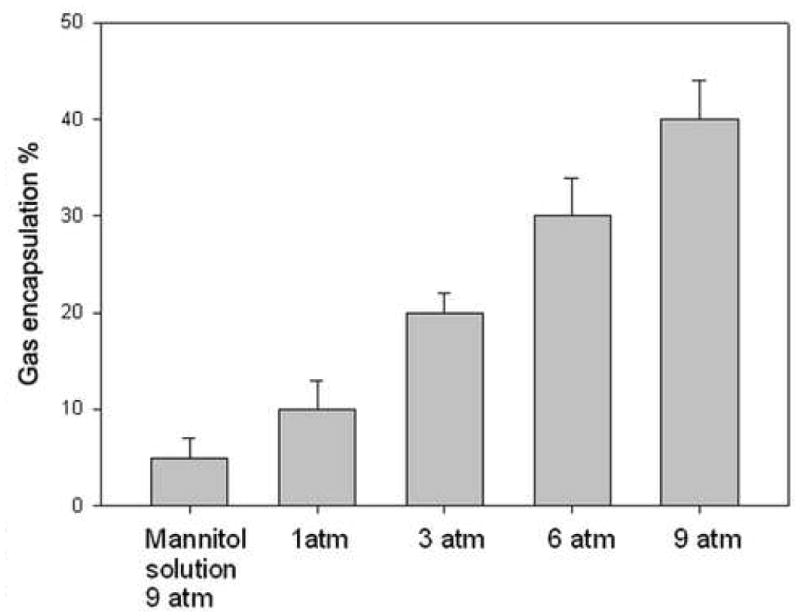
Effect of pressure on air encapsulation into liposomal formulation (DPPC:DOPC:CH at 60:30:10, total 5mg lipids). A total of 4 mg of liposomal dispersion at a concentration of 10mg/ml was pressured in the presence of 0.32 M mannitol. The gas encapsulation was measured after releasing te pressure and thrawing. The increased pressure led to linearly increasing gas entrapment. 1, 3, 6 or 9 atm pressure applied to 4 mg of lipid led to the incorporation of 10, 15, 20, and 30 μl of air, respectively, into the liposomal dispersion. Mean +/− SD, n=3. *p<0.05 ** p<0.001 vs mannitol solution only;
Freezing the dispersion is an essential step in this procedure because a pressured liposomal dispersion without freezing did not incorporate adequate amounts of air to provide ultrasound contrast enhancement. We therefore sought to determine whether the pressure effect occurred before freezing, during freezing, or during thawing. For this test, liposomes were made by a procedure analogous to that described for gas-containing liposomes except that the pressure was released either before freezing or after thawing. As shown in Fig 3, only a small amount of gas (10 μl) was taken up by the liposomal suspension when the pressure was released before the freezing step or after thawing step. The osmoticant mannitol solution without liposomes took up about 5–10 μl of gas. In contrast, when the pressure was released after freezing and before thawing, a much higher gas incorporation was measured (30 μl/4mg lipid). These results indicate that the air is entrapped when the liposomes are frozen under applied pressure and thawed without pressure.
Fig. 3.
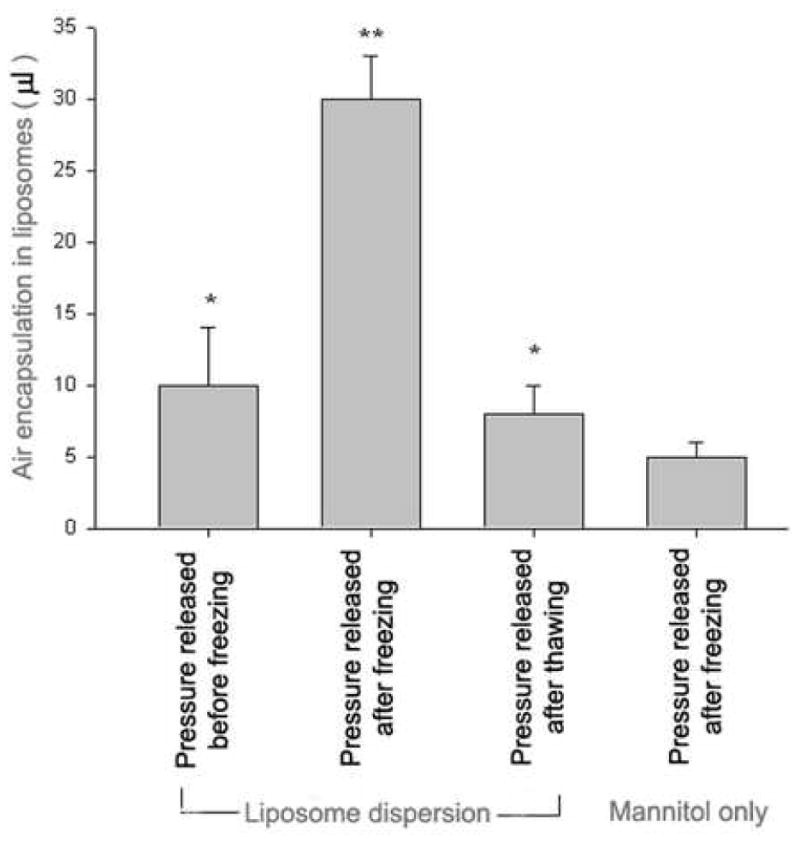
Effect on gas encapsulation efficiency of depressurization at different stages of preparation. Liposomes were made by a procedure analogous to that described for gas-containing liposomes except that the pressure was released either before freezing or after thawing. Only a small amount of gas (10 μl) was taken up by the liposomal suspension when the pressure was released before the freezing step or after thawing step. The osmoticant mannitol solution without liposomes took up about 5–10 μl of gas. In contrast, when the pressure was released after freezing and before thawing, a much higher gas incorporation was measured (30 μl/4mg lipid). * p< 0.05 vs depressed mannitol solution ** p<0.001 vs all other groups.
Effect of solutes other than mannitol on gas entrapment
Mannitol was used in the hydrating phase because it had been found to be the best osmoticant (provides physiological osmolarity) for preparing echogenic liposomal dispersions by the previously described lyophilization-based procedure. As shown in Fig. 4a, under same conditions, mannitol provided the best gas encapsulation -- about 40 μl gas in 5 mg liposomes. However, other solutions can also be used with reasonably good effect; in water, 20 μl of air was captured by 5 mg liposomes under 6 atm pressure. Non-cyroprotectant solutions like glycine and phosphate buffer (pH 7.4) also supported high air uptake. In the present of trehalose, air incorporation was low.
Fig. 4.
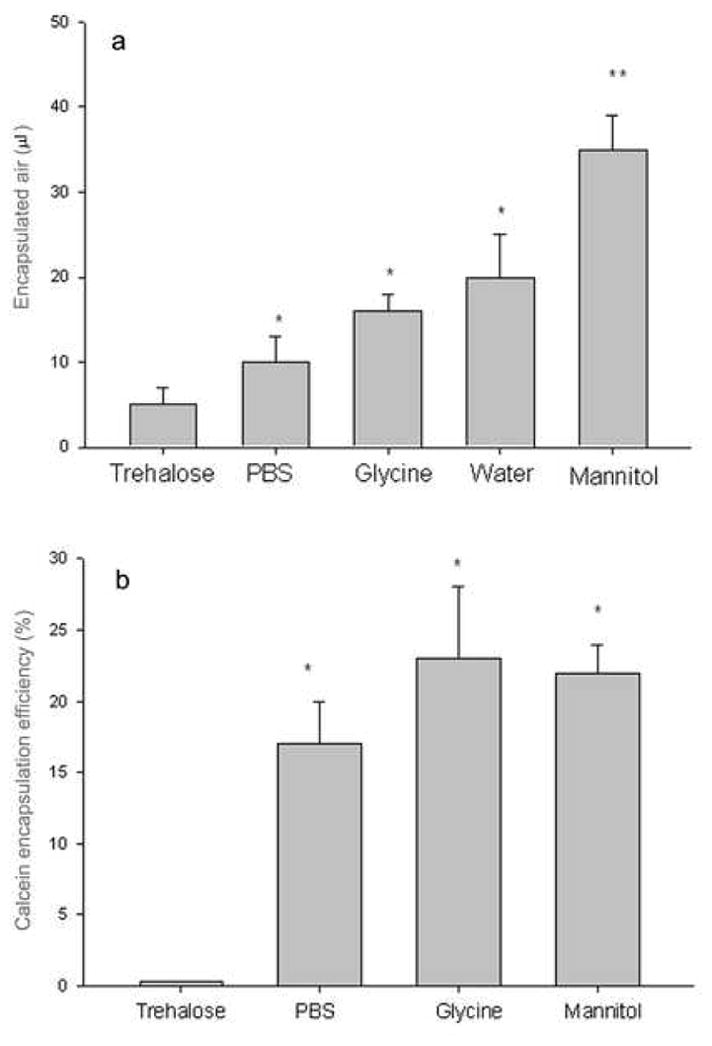
Effect on air(4a) and calcein(4b) encapsulation of various solutes present during the freezing step, Liposomes were made by a procedure analogous to that described for gas-containing liposomes except that calcein was included (at 0.1 mM) in the each of the solutions of mannitol or glycine or PBS and added in the lipid hydration step. Liposome composition: DPPC:DOPC:CH 35:35:30. Under same treatment and pressure, mannitol, glycine and PBS solutions provided 38, 18 and 10 μl of gas encapsulation by 5 mg lipid and 22%, 23% and 17% calcein encapsulation efficency, respectively. Mean +/− SD, n=4. *p<0.05 vs trehalose group, ** p<0.01 vs all other groups.
Encapsulation of an aqueous phase marker; potential for encapsulation of pharmaceutical agents within air-containing liposomes
As the air became incorporated after hydration of the lipid, we hypothesized that the air could be entrapped between the two constitutent monolayers of the lipid bilayers. Such a structure should allow co-encapsulation of drugs and gases in the same liposomes. Of particular interest were water-soluble drugs whose retention would be dependent upon the integrity of the vesicle, which, in turn, might be compromised by the pressure variation of an ultrasound wave. We sought to determine whether molecules in the aqueous phase could be encapsulated simultaneously with air or other gases. Liposomes were made by a procedure analogous to that described for gas-containing liposomes except that calcein, a fluorescent dye that is a convenient marker for encapsulation of the aqueous phase, was included (at 0.1 mM) in the mannitol solution and added in the lipid hydration step.
These aqueous marker encapsulation experiments demonstrated that this method leads to the production of true liposomes, i.e., particles characterized by an aqueous compartment separated from the bulk phase by a low-permeability membrane. As shown in Fig. 4b, three different osmoticants supported good calein encapsulation. Mannitol, glycine and PBS solutions gave 22%, 23% and 17% calcein encapsulation efficency, respectively. Trehalose was again unsatisfactory as an osmoticant, giving very low calcein encapsulation.
As can be seen in Fig 5, adequate echogenicity for good imaging was obtained with both gas encapsulation alone and with gas and calcein co-encapsulation.
Fig. 5.
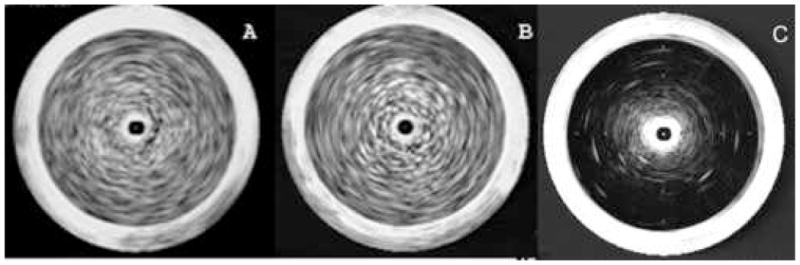
Echogenicity of gas-containing liposomes without (A) or with (B) calcein encapsulation and non-gas containing liposomes (C). Liposome composition: DPPC:DOPC:CH 35:35:30. Mean +/− SD,. Liposomes were imaged by 20 MHz intravascular catheter. A, liposomes prepared with air pressure at 9 atm in the absence of calcein. This MGSV for this image is 124+/−10. B, liposomes prepared so as to encapsulate both gas and calcein. The MGSV for this image is 118+/−12, C, liposomes preparaed by degassing under vacuum. The MGSV for this image is 12+/−3, for all images, n=4.
Ultrasound triggered release of aqueous contents from liposomes containing various gases
The potential for ultrasound-assisted release was tested using an 8 W/cm2 therapeutic probe to insonate liposome samples encapsulating calcein and containing one of three different gases. To investigate the hypothesis that different gases may affect the sensitivity of liposomes to ultrasound triggered-release, air (representing gas mixture), perfluorocyclobutane, and argon (representing a single gas) were loaded into liposomes by freezing under the same gas pressure in the present of mannitol. Ultrasound caused release of contents from all three types of gas-containing liposomes, but, as shown in Fig. 6, the liposomes containing air were the least sensitive. Although encapsulation of calcein was very similar for all three sets of liposomes, the amount of gas incorporated was larger in the case of the cycloperfluorobutane- and argon-containing samples than for the air-containing samples. Ultrasound effected more release of contents from the latter samples than from the air-containing sample. As argon is much less expensive than the fluorocarbon but gave similar results, subsequent experiments were done on argon-containing preparations.
Fig. 6.
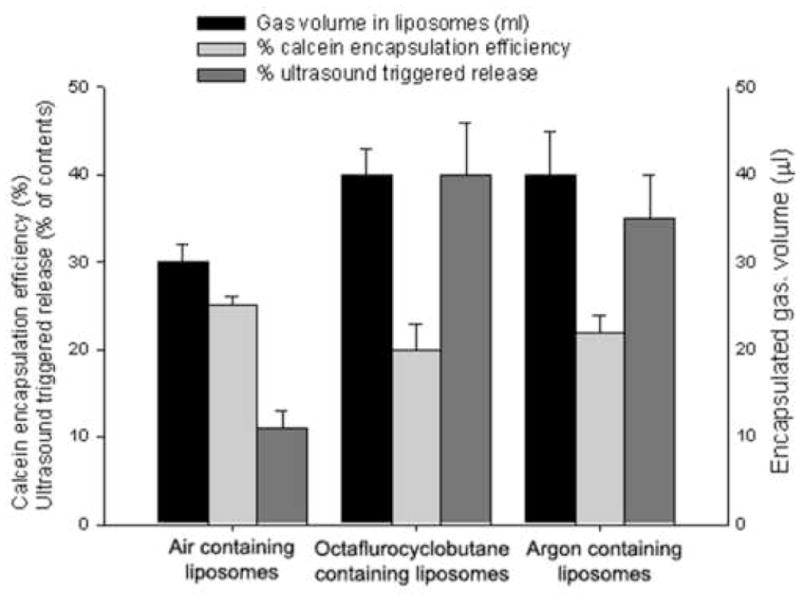
Effect of different gases on calcein encapsulation and sensitivity of liposomes to ultrasound triggered release. Air, perfluorocyclobutane, and argon were loaded into liposomes by freezing under the same pressure and in the present of mannitol. Ultrasound at 1 MHz, 8W/cm2, 100% duty cycle was applied for 10 seconds. Although encapsulation of calcein was very similar for all three sets of liposomes, the amount of gas incorporated was larger in the case of the cycloperfluorobutane- and argon-containing samples than for the air-containing samples. Mean+/− SD, n=4. *p<0.05 vs ultrasound triggered release from air containing liposomes.
Since echogenic, like conventional liposomes are susceptible to loss of contents spontaneously, it was important to determine how much extra susceptibility to lysis was conferred by the application of ultrasound. A comparison of calcein release from argon-containing liposomes without (dot), with one (triangle), or multiple (open circle) of applications of ultrasound is shown in Fig. 7. Ultrasound-triggered release from non-echogenic liposomes (prepared without pressurization) was used as control (open square). Less than 8% of the calcein was released from non-echogenic liposomes by ultrasound application. Calcein release was measured at time 0, 60, 180, 360 minutes and at 24 hours. In the case of one application of ultrasound, the ultrasound (1 MHz, 8 W/cm2, 100% duty cycle, 10 sceond) was applied at time 0. In the case of multiple applications of ultrasound, the same power was applied at times of 60, 180, and 360 minutes. The first application of ultrasound triggered the release of 23% of the entrapped calcein. Subsequent applications caused additional release and the combined effect of six applications was the release of a total of 42% of the initial contents.
Fig. 7.
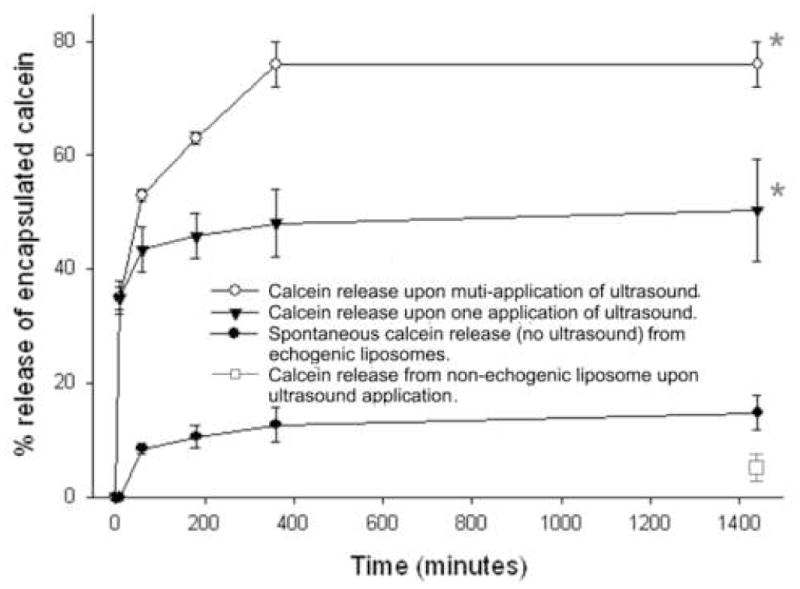
Ultrasound triggered release of calcein from echogenic liposomes. Ultrasound frequency, intensity and exposure time: 1 MHz, 8W/cm2, 10 s. Liposome composition: DPPC:DOPC:CH 60:30:10. Mean +/− SD. N=3. *p<0.01 vs no ultrasound treatment.
DISCUSSION
We have developed a procedure to introduce gas into liposomes such that they not only reflect ultrasound but also release of their contents when exposed to ultrasound. Of practical importance is that the method, which uses elevated-pressure in combination with freezing, is simple and allows ready encapsulation of solutes along with incorporation of a gas of choice. The method appears to have good potential for the preparation of both an ultrasound contrast agent and an ultrasound-controlled drug delivery system.
Conventional procedures for preparing liposomes do not allow for incorporation of a gas because the solubility of gas in water is low. According to Henry’s Law, however, the solubility of a gas in a liquid is directly proportional to the pressure of that gas over the liquid. Thus, if the pressure is increased, the gas molecule concentration in solution is increased, and when the pressure is lowered, the excess gas is released as vapor.
The pressure-freeze technology described here is based on this principle, An essential role of freezing is to concentrate both gas and solute molecules so as to favor their encapsulation. Indeed, it has long been known that during freezing, air is released and often trapped as bubbles in the resultant ice. This phenomenon has important biological consequences in that bubble formation contributes to freezing damage in long-term preservation of cells and tissues (Carte et al 1961; Karlsson et al 1993; King et al 1974; Korber 1988; Kruuv 1985; Lipp et al 1987; Lipp et al 1994; Morris et al 1981; Steponkus et al 1981; Tao et al 2002; Toner et al 1990).
We will now consider the individual steps in somewhat more detail. Gas incorporation in liposomes was observed to be proportional to pressure. This is expected (Henry’s Law) since gas uptake by the liposomes should be proportional to the amount in solution at the first step. The subsequent freezing step probably serves two purposes, increasing the local concentration of dissolved gas and nucleating formation of small pockets of bulk gas phase. Gases, like other solutes, are more soluble in liquid water than in solid ice. Thus, as the ice crystals grow, dissolved gas is progressively displaced from ice to unfrozen solution, with the result that the dissolved gas becomes increasingly concentrated in the ever-diminishing volume of liquid solution. When the dissolved gas concentration becomes sufficiently high, a gas bubble may nucleate and grow (Zhang et al 2006).
The gas might be expected to come out of solution at areas of contact with the hydrophobic interior of the lipid bilayer, which has a relatively low surface tension against air; however, the effect of trehalose on air incorporation suggests a more complex process. Trehalose, which functions as a cryoprotectant by favoring glass formation rather than crystallization (either of itself or of the water) supported much less echogenicity than did mannitol. Mannitol is rather distinctive among sugars in readily crystallizing out of solution upon cooling and we previously found that freezing a mannitol solution inflicts damage upon liposomes (Huang et al 2002). It may be that such sites of damage provide gas bubble nucleation loci; this would be consistent with a hypothesis that similar interfaces are the preferred site of nucleation of nitrogen bubbles in decompression sickness which can affect divers (Craig et al 1996)
Why is echogencity low when samples are thawed prior to reducing the pressure back to ambient pressure? Under these conditions, the ice melts and the water produced is essentially degassed, so air associated with lipid (in all forms) will diffuse into this water. The procedure we describe precludes such diffusive loss because, when the pressure is lowered first and the sample thawed second, the air concentration in the solution that initially melts is high because it contains most of the air that dissolved in the suspension upon pressurization. Because of its high solute (mannitol) content, the ice in the environment of the liposomes will melt first and immediately expose the lipid to ambient (1 atm) pressure. This initially melting phase is not only highly supersaturated with air, but it also contains air pockets that will grow when exposed to ambient pressure. Hence, air will come out of solution, expanding the gas nuclei that formed during freezing. The result is the formation of air pockets that are stabilized by a monolayer of lipid.
The procedure developed leads to both air and aqueous phase encapsulation (calcein solution) and, evidently air and calcein are entrapped in the same particles because 95% of the FITC-labeled liposomes floated on the top of the sample after mixing echogenic lipid dispersions with 0.32 M mannitol. The latter solution is essentially what is inside the liposomes, so they would have the same density and would not float unless they contained air. Accordingly it appears that 95% of the liposomes in the preparation contain air.
The liposome contents, according to the increase in quenchable and hence no longer internal, calcein fluorescence, can be largely released by ultrasound application. This is consistent with our conclusion from a prior investigation in which we evaluated a different procedure for preparing echogenic liposomes. At that time, we concluded that two compartments are present, one being the internal aqueous compartment of a bilayer vesicle and the other being an air pocket within the lipid bilayer such that the rarefaction phase of the sound wave triggers contents release by stretching the liposome membrane beyond its elastic limit. (An alternative configuration is a monolayer-stabilized air bubble within a conventional liposome). When the integrity of the vesicle is lost, some or all of the contents will be released. This interpretation is supported by preliminary experiments on the effect of lowering the external osmolarity by 20%. This effectively “prestretches” the bilayer so that the liposome becomes more sensitive to the ultrasound rarefaction pressure.
Co-encapsulation of air and an aqueous solute has potential advantages in drug delivery as it allows for release of liposomal contents by application of ultrasound. Since acoustically active liposomes also reflect ultrasound, it should be possible to not only to localize the release of the drug according to the site of application of ultrasound, but also to image the therapeutic agent while it is being activated for delivery. Moreover, targeting of the liposomes themselves is possible. We previously showed that echogenic liposomes conjugated to anti-ICAM-1 can be seen accumulating at early atheroma by ultrasound imaging, (Demos et al 1997; Demos et al 1999)
In addition to releasing liposomal contents and providing an image of the process, ultrasound itself can have a therapeutically beneficial influence, namely facilitating access of the drug to its target through cavitation effects. For example, Porter et al. found site-specific drug delivery can be achieved by destroying drug-filled, contrast microbubbles in the target area with high-intensity US (Porter et al 1996).. In addition, Shohet (Shohet et al 2000) found that albumin-coated microbubbles could be used to effectively deliver an adenoviral transgene to rat myocardium via US-mediated microbubble destruction. We have also found enhanced uptake of plasmid DNA in the presence of acoustically active liposomes and with the simultaneous application of ultrasound (Huang et al 2003)
The sensitivity of echogenic liposomes to ultrasound stimulation can probably be improved further by varying the liposomal composition, the encapsulated gas and/or the ultrasound application parameters. The lipid bilayer has self-sealing properties, so it is likely that changing the rigidity of the lipid membrane will affect its response to ultrasound. The choice of the optimal gas will involve both high volume in the liposomes and low rate of release in the blood stream. The most effective ultrasound pulses would seem to be a small number at the highest intensity that the tissue can withstand. Moreover, it can confidently be predicted that increasing the gas pressure beyond the level we used will increase the amount of gas captured by the lipid All of these parameters are presently being investigated.
We believe that the development of a novel and simple method to produce multifunctional liposomes for ultrasound imaging and controlled delivery may lead to a practical in vivo drug delivery system applicable to many pathologic situations.
Acknowledgments
This work was supported by an American Heart Association Award 0535512Z (SL.Huang) and NIH grants NIH 5R01 HL 74002-3 (D.D. McPherson). We thank Susan Liaing, Melvin Klegerman, Patrick Kee, and Hyunggun Kim for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barenholz Y. Relevancy of drug loading to liposomal formulation therapeutic efficacy. J Liposome Res. 2003;13(1):1–8. doi: 10.1081/lpr-120017482. [DOI] [PubMed] [Google Scholar]
- Carte AE. Air Bubbles in Ice. Proceedings of the Physical Society of London. 1961;77:757. [Google Scholar]
- Craig VSJ. Formation of micronuclei responsible for decompression sickness. Journal of Colloid and Interface Science. 1996;183:260–8. [Google Scholar]
- Demos SM, Onyuksel H, Gilbert J, Roth SI, Kane B, Jungblut P. In vitro targeting of antibody-conjugated echogenic liposomes for site-specific ultrasonic image enhancement. J Pharm Sci. 1997;86(2):167–71. doi: 10.1021/js9603515. [DOI] [PubMed] [Google Scholar]
- Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol. 1999;33(3):867–75. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- Gorce JM, Arditi M, Schneider M. Influence of bubble size distribution on the echogenicity of ultrasound contrast agents: a study of SonoVue. Invest Radiol. 2000;35(11):661–71. doi: 10.1097/00004424-200011000-00003. [DOI] [PubMed] [Google Scholar]
- Goertz DE, de Jong N, van der Steen AF. Attenuation and size distribution measurements of definity and manipulated definity populations. Ultrasound Med Biol. 2007;33 (9):1376–88. doi: 10.1016/j.ultrasmedbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Huang SL, Hamilton AJ, Pozharski E, Nagaraj A, Klegerman ME, McPherson DD, MacDonald RC. Physical correlates of the ultrasonic reflectivity of lipid dispersions suitable as diagnostic contrast agents. Ultrasound Med Biol. 2002;28(3):339–48. doi: 10.1016/s0301-5629(01)00512-9. [DOI] [PubMed] [Google Scholar]
- Huang SL, Tiukinhoy S, Wang L, MacDonald R, Nagaraj A, McPherson D. Acoustically-active liposomes of novel cationic-anionic composition in conjunction with ultrasound for gene delivery into vascular smooth muscle cells. MOL THER. 2003;7(5):422. Part 2. [Google Scholar]
- Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim Biophys Acta. 2004;1665(1–2):134–41. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Karlsson JOM, Cravalho EG, Toner M. Intracellular Ice Formation - Causes and Consequences. Cryo-Letters. 1993;14:323–36. [Google Scholar]
- King CJ. Pressurized freezing for retarding shrinkage after drying: a quantitative interpretation. Cryobiology. 1974;11:121–26. doi: 10.1016/0011-2240(74)90301-0. [DOI] [PubMed] [Google Scholar]
- Korber C. Phenomena at the Advancing Ice Liquid Interface - Solutes, Particles and Biological Cells. Quarterly Reviews of Biophysics. 1988;21:229–98. doi: 10.1017/s0033583500004303. [DOI] [PubMed] [Google Scholar]
- Kruuv J, Brailsford LL, Glofcheski DJ, Lepock JR. Effect of Dissolved-Gases on Freeze-Thaw Survival of Mammalian-Cells. Cryo-Letters. 1985;6:233–38. [Google Scholar]
- Leonetti C, Biroccio A, Benassi B, Stringaro A, Stoppacciaro A, Semple SC. Encapsulation of c-myc antisense oligodeoxynucleotides in lipid particles improves antitumoral efficacy in vivo in a human melanoma line. Cancer Gene Ther. 2001;8(6):459–68. doi: 10.1038/sj.cgt.7700326. [DOI] [PubMed] [Google Scholar]
- Lipp G, Korber C, Englich S, Hartmann U, Rau G. Investigation of the Behavior of Dissolved-Gases During Freezing. Cryobiology. 1987;24:489–503. [Google Scholar]
- Lipp G, Galow S, Korber C, Rau G. Encapsulation of human erythrocytes by growing ice crystals. Cryobiology. 1994;31(3):305–12. doi: 10.1006/cryo.1994.1036. [DOI] [PubMed] [Google Scholar]
- Morris GJ, Mcgrath JJ. Intracellular Ice Nucleation and Gas Bubble Formation in Spirogyra. Cryo-Letters. 1981;2:341–47. [Google Scholar]
- Nii T, Ishii F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int J Pharm. 2005;298 (1):198–205. doi: 10.1016/j.ijpharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Porter TR, Iversen PL, Li S, Xie F. Interaction of diagnostic ultrasound with synthetic oligonucleotide-labeled perfluorocarbon-exposed sonicated dextrose albumin microbubbles. J Ultrasound Med. 1996;15(8):577–84. doi: 10.7863/jum.1996.15.8.577. [DOI] [PubMed] [Google Scholar]
- Rapoport N, Pitt WG, Sun H, Nelson JL. Drug delivery in polymeric micelles: from in vitro to in vivo. J Control Release. 2003;91(1–2):85–95. doi: 10.1016/s0168-3659(03)00218-9. [DOI] [PubMed] [Google Scholar]
- Semple SC, Klimuk SK, Harasym TO, Dos Santos N, Ansell SM, Wong KF. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim Biophys Acta. 2001;1510(1–2):152–66. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101(22):2554–6. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. A method for radiation-force localized drug delivery using gas-filled lipospheres. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(7):822–31. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- Steponkus PL, Dowgert MF. Gas Bubble Formation During Intracellular Ice Formation. Cryo-Letters. 1981;2:42–7. [Google Scholar]
- Tao LR, Hua TC. Microscopic study of crystal growth in cryopreservation agent solutions and water. Visualization and Imaging in Transport Phenomena 1985. 2002;972:151–7. doi: 10.1111/j.1749-6632.2002.tb04566.x. [DOI] [PubMed] [Google Scholar]
- Toner M, Cravalho EG, Karel M. Thermodynamics and Kinetics of Intracellular Ice Formation During Freezing of Biological Cells. Journal of Applied Physics. 1990;67:1582–93. [Google Scholar]
- Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33(12):886–92. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Ye Q, Asherman J, Stevenson M, Brownson E, Katre NV. DepoFoam technology: a vehicle for controlled delivery of protein and peptide drugs. J Control Release. 2000;64(1–3):155–66. doi: 10.1016/s0168-3659(99)00146-7. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Maeda N, Craig VS. Physical properties of nanobubbles on hydrophobic surfaces in water and aqueous solutions. Langmuir. 2006;22(11):5025–35. doi: 10.1021/la0601814. [DOI] [PubMed] [Google Scholar]


