Abstract
The incidence of human papillomavirus (HPV)-associated epithelial lesions is substantially higher in human immunodeficiency virus (HIV)-infected individuals than in HIV-uninfected individuals. The molecular mechanisms underlying the increased risk of HPV infection in HIV-infected individuals are poorly understood. We found that HIV proteins tat and gp120 were expressed within the oral and anal mucosal epithelial microenvironment of HIV-infected individuals. Expression of HIV proteins in the mucosal epithelium was correlated with the disruption of epithelial tight junctions (TJ). Treatment of polarized oral and anal epithelial cells and tissue explants with tat and gp120 led to disruption of epithelial TJ and increased HPV pseudovirion (PsV) paracellular penetration into the epithelium. PsV entry was observed in the basal/parabasal cells, the cells in which the HPV life cycle is initiated. Our data suggest that HIV-associated TJ disruption of mucosal epithelia may potentiate HPV infection and subsequent development of HPV-associated neoplasia.
Introduction
Human papillomavirus (HPV) is the causative agent of cervical and anal cancers, and a subset of oral and other epithelial cancers (Palefsky, 2006). The prevalence and incidence of anogenital HPV infection in HIV-infected individuals are substantially higher than in HIV-uninfected individuals (Palefsky et al., 1998). HIV-infected individuals also have an increased risk of HPV-associated neoplasia (Palefsky, 2009, 2012). Once HPV infection is established, attenuated immunity may reduce viral clearance and ultimately contribute to development of HPV-associated neoplasia. However, direct and indirect interactions between HIV and HPV within the epithelium may also play a role in the first step of the process, i.e., initial HPV infection of the epithelium.
In contrast to other HIV-associated malignancies, the incidence of HPV-associated cancers such as anal cancer has increased, not decreased since the introduction of antiretroviral therapy (ART) (Palefsky, 2009). Several studies have shown that ART for HIV infection has done little to reduce the increased risk of anal HPV infection in men and women (Palefsky et al., 2005) (Hessol et al., 2009). Others have shown a benefit for clearance of cervical HPV infection in HIV-infected women and anal HPV infection in men upon initiation of ART but overall the prevalence of both cervical and anal HPV infection remains high among HIV-infected individuals in the ART era (de Pokomandy et al., 2009). The mechanisms underlying the limited benefit of ART with respect to the continued high prevalence and incidence of HPV infection in HIV-infected individuals are poorly understood (Kang and Cu-Uvin, 2012).
Development of HPV-associated neoplasia is initiated upon HPV entry into basal and parabasal cells of the epithelium. Animal model work suggests that HPV penetration through multiple layers of the stratified squamous epithelium requires a mechanical breach or tear (Roberts et al., 2007). It is possible that co-infection with viruses such as HIV may also lead to epithelial disruption. If so, HIV-associated epithelial disruption may be one mechanism contributing to the increased risk of HPV infection among HIV-infected individuals. Several studies have shown that HIV infection can disrupt intestinal mucosal epithelium (Kapembwa et al., 1996; Kapembwa et al., 1991; Maingat et al., 2011; Obinna et al., 1995; Sankaran et al., 2008). However, HPV is not known to infect the intestinal epithelium, and the effect of HIV infection on the integrity of oral and anogenital mucosal epithelia, which are both targets for HPV infection, has not been well investigated.
Tat and gp120 are HIV proteins that are secreted from HIV-infected intraepithelial immune cells. These proteins may play an important role in disruption of epithelial tight junctions (TJ) and HPV entry into epithelium. Tat is a transactivator protein that activates integrin and mitogen-activated protein kinases (MAPK) signaling, which in turn may disrupt endothelial and epithelial cell junctions through aberrant internalization of TJ proteins (Andras et al., 2005; Bai et al., 2008; Pu et al., 2005; Song et al., 2007; Toschi et al., 2006; Zhong et al., 2008). HIV gp120 is an envelope protein that binds to galactosyl ceramide of epithelial cells, induces intracellular calcium elevation (Bozou et al., 1989; Dayanithi et al., 1995; Fantini et al., 2000) and activates protein kinase C (PKC). PKC activates p38 mitogen-activated protein kinases, phosphoinositide 3 kinases/protein kinase B (PI3K/AKT), and c-Jun N-terminal kinases (JNK) (Ali et al., 2009; Cai et al., 1997; Kawakami et al., 2004; Lopez-Bergami et al., 2005; Marshall, 1996; Preiss et al., 2007; Wang et al., 2004; Zhou et al., 2003). This leads to the disruption of epithelial TJ (Gonzalez-Mariscal et al., 2008; Kanmogne et al., 2005; Kanmogne et al., 2007; Kevil et al., 2000; Kevil et al., 2001; Wang et al., 2004; Yang et al., 2009) through modulation of TJ protein internalization and/or TJ gene expression (Alcorn et al., 2008; Shintani et al., 2006). HIV gp120 also disrupts TJs (Kanmogne et al., 2005; Kanmogne et al., 2007) through induction of proteasome-mediated degradation of ZO-1 and ZO-2 (Nakamuta et al., 2008) and internalization of occludin and claudins (Shen and Turner, 2005; Shin et al., 2006; Umeda et al., 2006).
If HIV infection is indeed a biologically-relevant contributor to mucosal epithelium disruption and entry of HPV into epithelium, then HIV proteins should be present in the mucosal environment, even among HIV-infected individuals with well-controlled HIV viral load on ART. Cell-free HIV-1 virions and viral DNA/RNA can be isolated from oral and genital mucosal epithelium, as well as the saliva and cervicovaginal secretions of HIV-infected individuals (Chou et al., 2000; Clemetson et al., 1993; Crowe and Sonza, 2000; Goto et al., 1991; Henning et al., 2010; Kakizawa et al., 1996; Liuzzi et al., 1996; Maticic et al., 2000; Nuovo et al., 1993; Qureshi et al., 1997; Qureshi et al., 1995; Rodriguez-Inigo et al., 2005; Sonza et al., 2001; Zuckerman et al., 2003). HIV-infected lymphocytes, and Langerhans cells can be detected in the mucosal and submucosal layers of oral and genital epithelium (Chou et al., 2000; Jayakumar et al., 2005; Qureshi et al., 1997; Qureshi et al., 1995; Rodriguez-Inigo et al., 2005), and HIV virions can be detected by electron microscopy, within the TJs of oral epithelium (Qureshi et al., 1997). Finally, replicating HIV and HIV-infected cells have been found within cervical epithelia of HIV-infected women, including women on ART (Crowe and Sonza, 2000; Henning et al., 2010; Sonza et al., 2001).
To model the role of tat and gp120 on mucosal epithelial TJ integrity and penetration of HPV into epithelium, we used HPV 16 pseudovirions (PsV) containing a plasmid expressing red fluorescent protein (RFP) (Buck et al., 2006; Roberts et al., 2007). We used polarized oral, anal and cervical epithelial cells to model passage of PsV through a single layer of squamous epithelial cells with intact TJ, as might be found in the stratum granulosum or spinosum of a multi-stratified epithelium. We also used oral mucosal epithelial tissue explants to model the effect of HIV proteins through intact, multi-stratified squamous epithelium. Our data show that HIV tat and gp120 disrupt TJ in mono-stratified and multi-stratified mucosal epithelium, facilitating PsV paracellular penetration between cells with TJs. Paracellular penetration of HPV-16 PsV in oral mucosal tissues through superficial cell layers with disrupted TJ led to entry of PsV into basal and parabasal cells, the site of initiation of the HPV lifecycle. Together our data indicate that HIV tat and gp120 proteins may disrupt mucosal epithelial TJ and potentiate development of HPV-associated neoplasia by facilitating HPV entry into mucosal epithelium.
Results
Disruption of tight junctions (TJ) in vivo in oral and anal mucosal epithelia of HIV-infected individuals
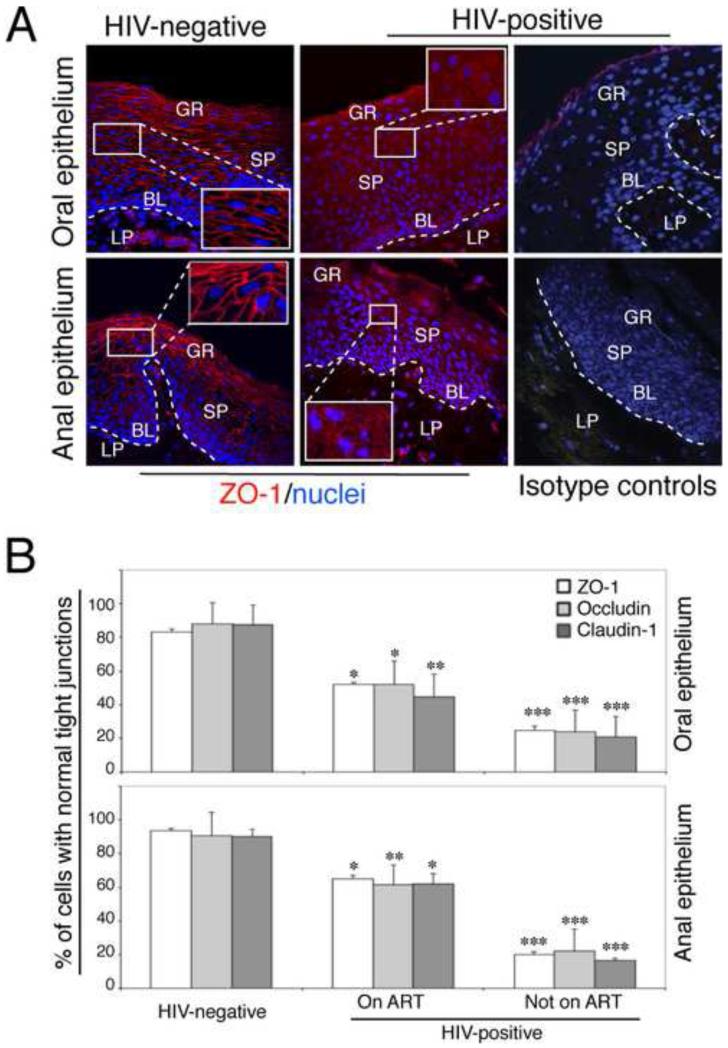
To determine whether mucosal epithelium TJ disruption occurs in vivo naturally in the setting of HIV infection, cryosections of clinically and histologically normal oral biopsies from 12 HIV-infected and 4 HIV-uninfected individuals were immunostained for TJ markers: zonula occludens (ZO-1), occludin and claudin-1. Six of the 12 HIV-infected donors (50%) were on antiretroviral therapy (ART). Expression and localization of ZO-1 (Fig. 1), occludin and claudin-1 (data not shown) in tissues of all four HIV-uninfected donors were strong and in a ring shape (Fig. 1A, upper left panel), indicating the presence of intact TJs. However, in oral tissues from 8 of 12 (67%) HIV-positive donors including 3 donors on ART, these proteins were found in a diffuse, dot-like pattern in the cytoplasm, indicating TJ disruption (Fig. 1A, upper middle panel). Quantitation of cells with a normal pattern of ZO-1, occludin and claudin-1 within the oral epithelium from ART-treated HIV-infected individuals showed that the number of cells with a normal pattern was reduced by approximately 25-30% compared with HIV-uninfected donors (ZO-1 p=0.03, occludin p=0.02 and claudin p=0.01). Analysis of TJ disruption in HIV-infected donors not on ART showed that all had substantial disruption of TJs, i.e., the number of epithelial cells with a normal pattern of ZO-1, occludin and claudin-1 was reduced by approximately 65-70% compared with the epithelia of HIV-uninfected individuals (ZO-1, occludin and claudin-1 p<0.001).
Fig. 1.
Disruption of TJs of HIV-infected oral and anal mucosal epithelia. (A) Oral and anal biopsies from HIV-infected and HIV-uninfected individuals were immunostained for ZO-1 (red). As a negative control, sections were stained with rabbit IgG. GR, granulosum; SP, spinosum; BL, basal; LP, lamina propria. Nuclei are stained in blue. Original magnification was ×400. Representative immunofluorescence images are shown. (B) For quantitative evaluation of TJ protein expression in oral and anal tissues of HIV-infected and HIV-uninfected individuals, epithelial cells expressing ZO-1, occludin, and claudin-1 in ring-shaped patterns were counted from 10 randomly-selected regions of mucosal epithelia. Cells were counted in 10 fields of each tissue section. Results were obtained from oral biopsies (4 HIV-uninfected, 6 HIV-infected on ART and 6 HIV-infected not on ART) and anal biopsies (5 HIV –negative, 6 HIV-infected on ART and 3 HIV-infected not on ART). Results are represented as a percentage of epithelial cells with a normal pattern (ring shape) of ZO-1, occludin and claudin-1 expression. Error bars represent standard errors of the means. * P<0.05, **P<0.01, ***P<0.001, all compared with the HIV-uninfected control group.
Similar observations of TJ disruption were found in the anal epithelium of HIV-infected individuals. We examined the status of TJs in normal anal biopsies from 5 HIV-uninfected donors, and normal anal biopsies from 9 HIV-infected individuals, 6 of whom were on ART. Each of the histologically-normal anal biopsies from the 5 HIV-uninfected individuals showed intact TJs (Fig 1A, lower panel), whereas all of the histologically-normal anal biopsies from HIV-infected individuals had disrupted TJs. The anal epithelium of HIV-infected patients on ART contained 20-30% fewer cells with a normal pattern of ZO-1, occludin and claudin-1 (ZO-1 p=0.03, occludin p=0.01, claudin-1, p=0.03) compared with biopsies from HIV-uninfected donors. Biopsies from each of the 6 HIV-infected individuals not on ART showed even more pronounced TJ disruption. Compared with HIV-uninfected donors, approximately 70% of anal epithelial cells in HIV-infected individuals lost a normal ring pattern of ZO-1, occludin and claudin-1, (ZO-1, occludin, claudin-1, p<0.001). These data indicate that HIV infection was accompanied by disruption of TJs within normal oral and anal epithelia, even among those on ART. However, junctional disruption at both mucosal sites was most pronounced among HIV-infected individuals not on ART.
HIV tat- and gp120-mediated TJ disruption potentiates PsV penetration into oral multi-stratified epithelium
HIV proteins tat and gp120 have been shown to disrupt endothelial, retinal, intestinal and endometrial epithelial TJs (Andras et al., 2005; Bai et al., 2008; Nakamuta et al., 2008; Nazli et al.; Pu et al., 2005; Song et al., 2007; Zhong et al., 2008). To determine if these proteins are playing a role in disrupting epithelial TJ as seen in oral and anal biopsies, we used mono-stratified-polarized oral (Fig. 2), anal and cervical (data not shown) epithelial cells with intact TJ. Previous in vitro studies of the effect of HIV proteins on TJ function used concentrations of tat (1 μg/ml and higher) and gp120 (100 ng/ml) (Andras et al., 2005; Bai et al., 2008; Nazli et al.; Pu et al., 2005; Song et al., 2007; Toschi et al., 2006; Zhong et al., 2008), that were substantially higher than physiological concentrations. Serum HIV-tat and gp120 levels may reach up to 40 ng/ml and 15 ng/ml in HIV-infected individuals, respectively (Poggi and Zocchi, 2006; Rychert et al., 2010; Westendorp et al., 1995; Xiao et al., 2000). Therefore, in our experiments we used concentrations of tat and gp120 at 10 ng/ml of each, similar to physiological levels in HIV-infected individuals.
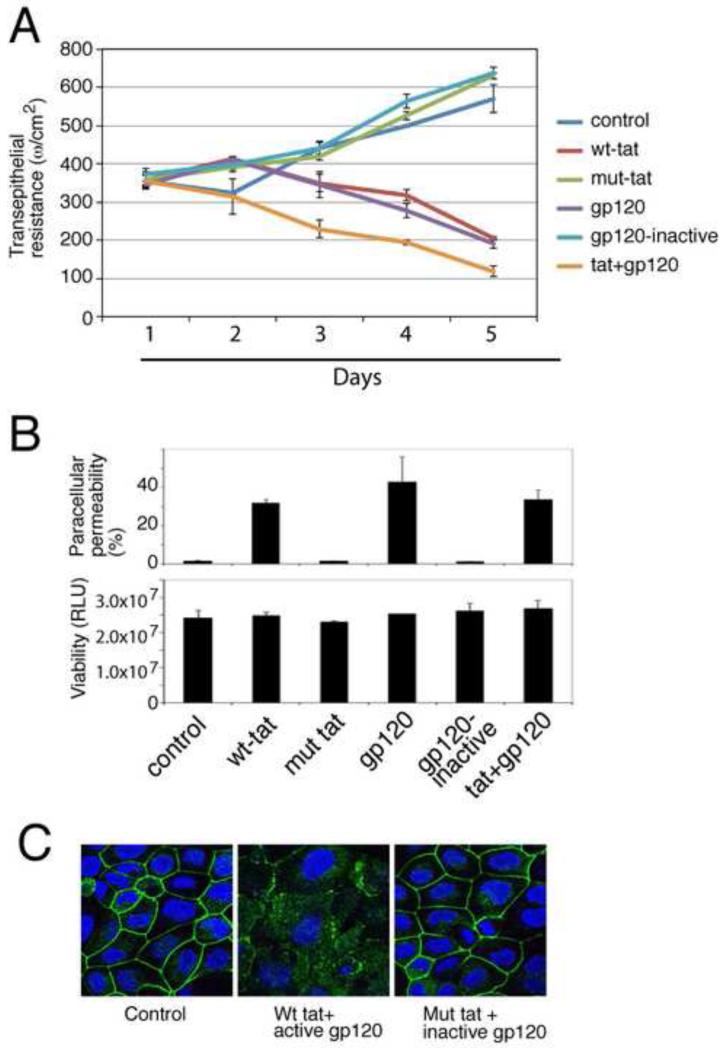
Fig. 2.
Role of HIV proteins tat and gp120 in the disruption of oral epithelial tight junctions. (A) Polarized oral epithelial cells were treated with an active and inactive recombinant HIV tat or gp120, separately or in combination. TER was measured daily. (B) (upper panel) The same cells were used to measure paracellular permeability after 5 days of treatment. IgG leakage into lower chambers is presented as % of apical value of tracer. (Lower panel) After measuring paracellular permeability, cells were examined for viability using an MTT assay. RLU, relative luminescence units. (C) At 5 days, untreated control cells and cells treated with inactive and active tat and gp120 were immunostained for occludin (green). Cell nuclei are stained in blue. (A and B) Error bars show ± s.e.m. (n=3).
Oral, cervical and anal cells were grown until they polarized sufficiently to form fully functional TJ as indicated by trans-epithelial resistance (TER). We added recombinant tat and gp120 separately and together to the polarized cells for 5 days and monitored TER (Fig. 2A). As a negative control we used mutant tat protein (HIV-1BAL strain) that lacks the basic and integrin-binding domains, and is not internalized into cells (Barillari et al., 1999a; Barillari et al., 1999b; Nagahara et al., 1998; Toschi et al., 2006). Heat-inactivated gp120 (HIV-1BAL strain) was used as a negative control for gp120 experiments. We added recombinant tat and gp120 to the apical surface of polarized cells. Culture media were changed daily with fresh proteins. Consistent with the disruption of TJs, TER gradually declined in polarized cells treated with tat and gp120 (Fig. 2A) independently and synergistically. In contrast, the TER of polarized cells increased over a 5-day period when untreated or treated with the inactive tat and heat-inactivated gp120 proteins. Tat- and gp120-induced TER reduction was detected only with prolonged exposure to active proteins (4-5 days); shorter treatment (1-3 days) did not lead to TER reduction.
As an additional measure of TJ functional integrity, we also examined paracellular permeability of polarized oral epithelial cells to horseradish peroxidase-conjugated goat anti-donkey IgG (Tugizov et al., 2011). At 5 days IgG was added to the apical surface and measured in the lower chamber media. Paracellular leakage of IgG was detected only in those cells treated with active tat and/or gp120 (Fig. 2B, upper panel). IgG leakage was not detected in untreated cells, or cells treated with the inactive tat and/or heat-inactivated gp120 proteins. Treatment with tat and/or gp120 proteins was not toxic to the polarized cells (Fig. 2B, lower panel). Polarized cells treated with active tat and gp120 showed complete TJ disruption on confocal microscopy after 5 days (Fig. 2C) in contrast to untreated cells and cells treated with inactive tat and gp120, which showed intact TJ. Four independent experiments with polarized oral epithelial cells showed similar data. HIV tat- and/or gp120- mediated disruption of TJs was also detected using paracellular IgG leakage in polarized anal and cervical epithelial cells (data not shown).
HIV tat- and gp120-associated TJ disruption of polarized monolayer epithelial cells facilitates paracellular passage of HPV PsV
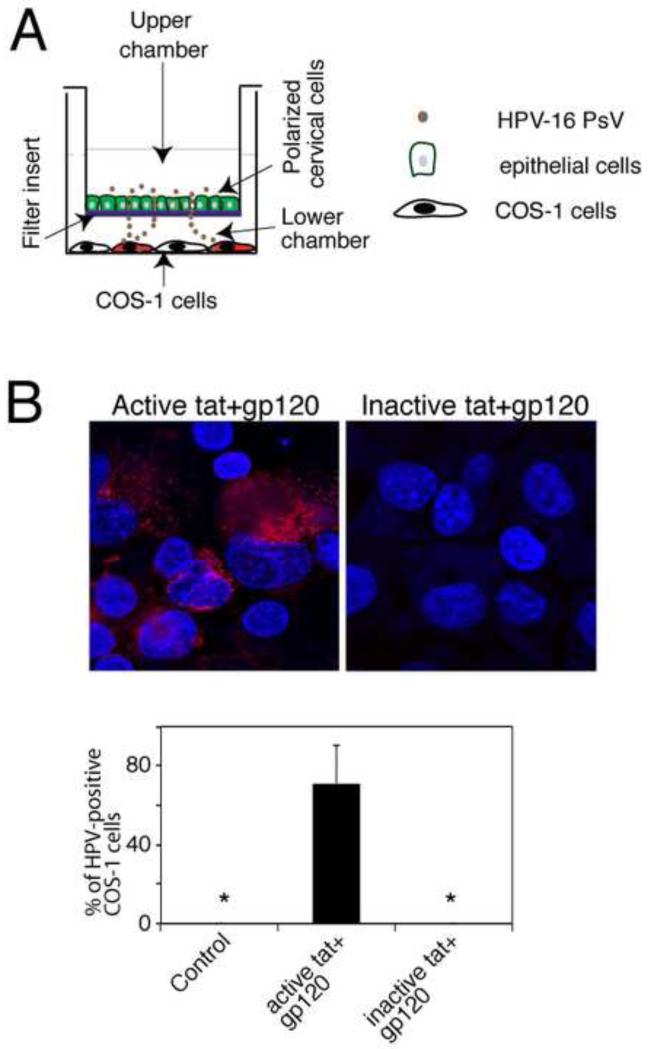
To determine whether tat/gp120-mediated TJ disruption promotes paracellular passage of HPV through polarized oral epithelial cells, the cells were treated with a combination of tat and gp120 for 5 days, and when TER values were reduced by 90% we exposed the apical surfaces to HPV-16 PsVs. To examine paracellular passage of PsV via disrupted polarized cells, COS-1 cells were grown in the lower chamber of 12 well plates where Transwell inserts were placed (Fig. 3A).
Fig. 3.
Role of tat- and gp120- disrupted epithelial TJ in paracellular spread HPV-16 PsVs. (A) Model of HPV-16 PsV paracellular passage between disrupted mucosal epithelia. Polarized cells were grown in the upper chambers of two-chamber Transwell filter inserts. COS-1 cells were grown in the lower chambers of the filter inserts, in the well where the filter inserts were then placed. To examine paracellular passage of HPV-16 PsVs, PsVs were added to the apical surfaces of polarized epithelial cells. At 2 h post-inoculation, filter inserts were removed, and the extent of paracellular passage of PsVs was determined in the COS-1 cells by detecting RFP fluorescence. (B, upper panel) Polarized oral epithelial cells were incubated for 5 days with HIV-tat and gp120 or their inactive forms. HPV-16 PsVs were then added to the apical surfaces and paracellular passage of HPV-16 PsVs across disrupted oral epithelium was examined by detection of RFP-positive COS-1 cells. (B, lower panel) Paracellular passage of HPV-16 PsVs across disrupted oral epithelium was quantitated by counting RFP-positive COS-1 cells. Results are presented as a % of RFP-positive cells. Error bars show ± s.e.m. (n=3). *, not detected.
Paracellular PsV passage was examined by detection of RFP fluorescence in COS-1 cells (Fig. 6A, and 6B upper panel). Although COS-1 cells are not normal host cell types for authentic HPV infection they were used for the evaluation of HPV PsV paracellular passage via TJ-disrupted epithelial cells because they are highly sensitive for measurement of HPV-16 PsV entry and detection of RFP expression. Quantitative analysis of RFP-positive COS-1 cells showed that polarized oral epithelial cells treated with tat/gp120 had PsV paracellular passage leading to infection of approximately 40% of the COS-1 cells. In contrast, no RFP-positive COS-1 cells were detected after PsV were added to control cells with intact TJs (Fig. 3B lower panel). Similar data were obtained with polarized anal and cervical epithelial cells (data not shown).
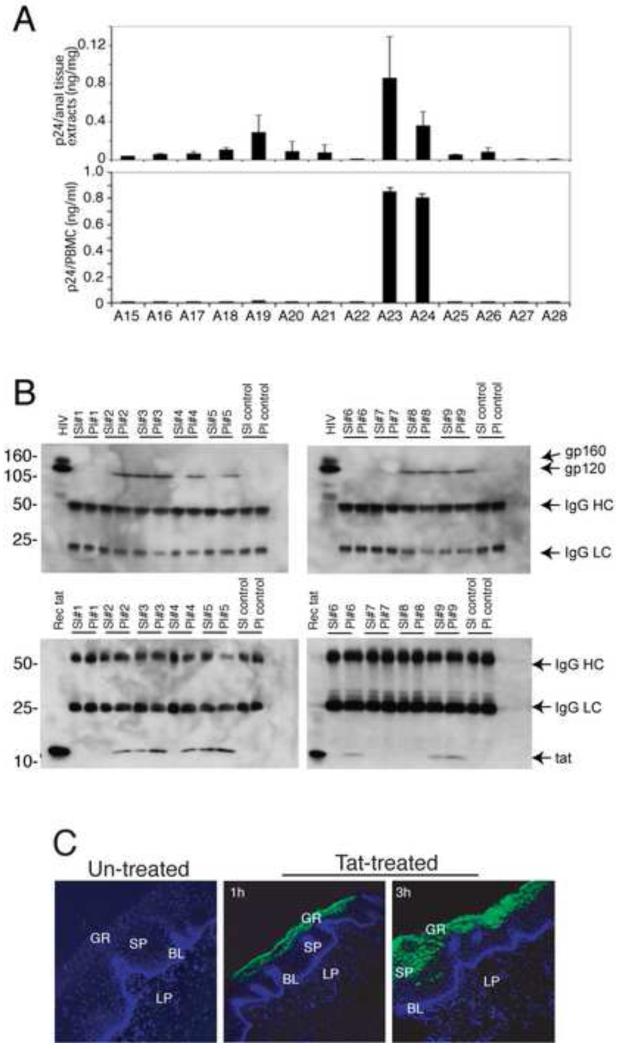
Fig. 6.
Mucosal epithelium of HIV-infected individuals contains infectious HIV, which express HIV tat and gp120 proteins with the mucosal environment. (A, upper panel) To detect HIV in the mucosal epithelium, anal biopsies were homogenized, and supernatants were examined for HIV p24 by ELISA. The p24 value was expressed in ng of p24 per mg of tissue. (A, lower panel) To examine the infectivity of intra-mucosal HIV, PBMCs were infected with homogenates, and p24 was quantified after 2 weeks. (B) HIV tat and gp120 in saliva and plasma samples of HIV-infected patients were detected by immunoprecipitation and Western blot assays. IgG HC, IgG heavy chain. IgG LC, IgG light chain. (C) Polarized, oriented buccal explants were treated with GFP-labeled recombinant tat at 30 ng/explant for 1 and 3 h and sectioned and examined for tat penetration by confocal microscopy. GR, granulosum; SP, spinosum; BL, basal; LP, lamina propria.
HIV tat- and gp120-mediated TJ disruption potentiates PsV penetration into oral multi-stratified epithelium
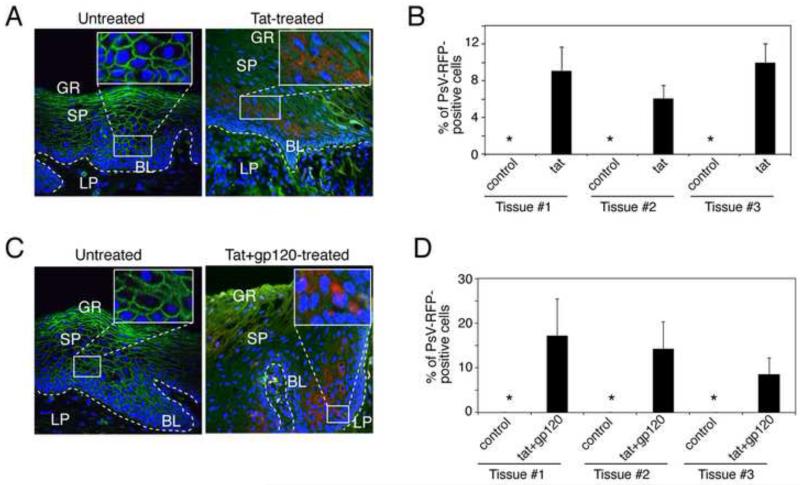
Having shown that HIV tat and gp120 disrupt TJs in mono-stratified polarized oral, anal, and cervical epithelial cells and facilitate paracellular passage of HPV-16 PsV between the cells, we next sought to determine whether these proteins were playing a role in PsV entry into multi-stratified mucosal epithelium. For these experiments we used buccal explants from HIV-uninfected donors. We exposed buccal explants from 12 different HIV-uninfected donors to HIV tat at 30 ng/ml for 2 days. Matching buccal explants were maintained without tat and served as controls. The tissue explants were then exposed to HPV-16 PsVs for 3 days. Culture media were replaced every day with media containing fresh tat. Tat was therefore present in the apical media during the entire 5-day experimental period. After 5 days, the tissues were fixed, sectioned and analyzed for ZO-1 and PsV-RFP expression. Confocal microscopy showed a diffuse cytoplasmic ZO-1 pattern in the tat-treated epithelia of 7 of 12 (58%) explants (Fig. 4A, right panel). All matching explants not treated with tat exhibited normal ring-shaped ZO-1 localization within the lateral membranes, indicating intact TJ. In 3 of 7 tat-disrupted explants (42%), PsV-RFP expression was detected within the basal and parabasal layers of epithelium (Fig. 4A). Quantitative analysis showed that approximately 5-8% of basal/parabasal cells were positive for PsV-RFP (Fig. 4B). PsV penetration was not detected in 4 of the tat-disrupted tissues. No PsV entry was detected in any the 5 tissues treated with tat but which retained normal ZO-1 localization, or in control explants not treated with tat.
Fig. 4.
HIV-associated disruption of mucosal epithelium facilitates PsV entry into basal and parabasal cells. (A and B) Buccal explants were treated with HIV tat alone (A) or HIV tat and gp120 in combination (B) for 2 days and then exposed to HPV-16 PsVs for the next 3 days. Tissues were immunostained for ZO-1 (red) and analyzed for PsV-RFP by confocal microscopy. GR, granulosum; SP, spinosum; BL, basal; LP, lamina propria. (C and D) PsV penetration of HIV tat- and gp120-treated explants was quantified by counting RFP-positive epithelial cells; data are presented as the percentage of cells positive for PsV-RFP. Error bars show [g940] s.e.m. (n=10). *, not detected.
We next exposed buccal explants from 5 different HIV-uninfected donors to a combination of tat and gp120 (30 ng/ml of each), and PsV penetration was examined as described above. After 5 days tissues were analyzed by confocal microscopy, which revealed a diffuse cytoplasmic ZO-1 pattern in the tat/gp120- treated epithelium of 3 of the 5 explants (Fig. 4C). In these disrupted tissues, PsV-RFP expression was detected within the basal and parabasal layers of epithelium. Approximately 15% of parabasal cells expressed PsV-RFP (Fig. 4D). No PsV entry was detected in the 3 explants treated with tat and gp120 but which retained normal ZO-1 localization, or in control tissues not treated with tat and gp120.
Expression of HIV tat and gp120 in oral and anal epithelial tissues of HIV-infected individuals
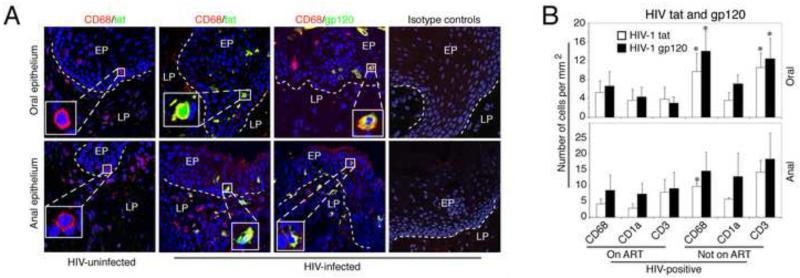
Above we showed that oral and anal epithelial TJs are disrupted in normal tissues in HIV-infected individuals in vivo, and that disruption of TJs by HIV tat and gp120 potentiates PsV entry when added exogenously to multi-stratified epithelium. We then sought to determine if these proteins can be detected in vivo in oral and anal biopsies, and whether their expression is associated with TJ disruption. In the Fig.1 we showed that the TJs were disrupted in the oral and anal tissues from HIV-infected individuals. Here, we examined the presence of HIV tat- and gp120-expressing macrophages, T lymphocytes and Langerhans cells (LCs) in similar oral and anal biopsy samples. Confocal immunofluorescence microscopy of 12 oral and 9 anal biopsies from HIV-infected donors showed HIV-tat-positive and gp120-positive intraepithelial and submucosal macrophages (Fig. 5A), T lymphocytes and LCs (data not shown) in all biopsies, including all of the donors on ART. Tat and gp120- expressing intraepithelial immune cells were detected in tissues from donors with a wide range of HIV plasma viral load, including 4 of 21 donors with an undetectable plasma viral load. Cells expressing tat and gp120 were not detected in any of the 4 oral and 5 anal biopsies from HIV-uninfected individuals.
Fig. 5.
Distribution of HIV-tat- and gp120- expressing immune cells in oral and anal epithelia of HIV- infected individuals. (A) Oral and anal tissue sections from HIV-uninfected and -infected individuals were co-immunostained for HIV-tat or gp120 (both in green) and CD68 (in red), a marker for macrophages. Yellow indicates co-localization of viral proteins with cellular markers. Nuclei are in blue (A-C). EP, epithelium; LP, lamina propria. The dotted white line indicates the boundary between the epithelium and the lamina propria. Original magnification was ×400. Representative immunofluorescence images are shown. (B) For quantitative evaluation, we counted CD3-, CD68-, and CD1a-positive cells expressing HIV-tat or gp120 in oral and anal tissue sections obtained from HIV-infected individuals on ART (6 oral and 6 anal biopsies) and not on ART (6 oral and 3 anal biopsies). Cells expressing HIV tat and gp120 were counted in 10 microscopic fields, and results are represented as the average number of cells per mm2. Error bars show the standard error of means. P<0.05, **P<0.01, ***P<0.001, all compared with control group.
Quantitative analysis of HIV tat- and gp120-expressing cells in anal and oral biopsies from HIV-infected individuals on ART and not on ART showed that tissues from individuals not on ART contained a higher number of macrophages and T lymphocytes expressing tat and gp120 than tissues from ART-treated individuals (p<0.05) (Fig. 5B). The numbers of LCs in oral and anal tissues from donors on or not on ART were not significantly different.
Mucosal epithelium of HIV-infected individuals contains infectious HIV
HIV virions could be a possible direct source of secreted tat and gp-120 within tissues. To determine if tat- and gp120-positive immune cells within the mucosal epithelium also contain intact, infectious HIV virions, we looked for the presence of infectious HIV in tissues shown to contain gp120 and tat. Protein was extracted from 14 anal biopsies from HIV-infected individuals on ART and 5 anal biopsies from HIV-uninfected donors, and examined for HIV p24 using an ELISA assay. Our data showed that 11 of 14 anal biopsies from HIV-infected donors contained HIV-1 p24 (Fig. 6A, upper panel), indicating that virions were present within the mucosal environment. HIV p24 was not detected in the tissues obtained form HIV-uninfected donors (data not shown). We then infected peripheral blood mononuclear cell (PBMC) with extracts of the HIV-infected anal biopsies. At 2 weeks post-infection p24 ELISA assays showed that virions from 2 of 11 (18%) HIV-infected biopsies were infectious in PBMCs (Fig. 6A lower panel). These data show that the anal mucosal epithelium and/or submucosal lamina propria of at least some HIV-infected individuals on ART contain infectious virus.
HIV tat and gp120 are detectable in saliva and HIV tat can penetrate into stratified mucosal epithelium
Above we showed that the oral and anal mucosal epithelium contain HIV that could shed tat and gp120 proteins within the epithelial environment. Since saliva may represent another potential source of tat and gp120 for the epithelium we analyzed 9 whole saliva and matching plasma samples from HIV-infected individuals not on ART to determine if these proteins are detectable in saliva. Using a combination of immunoprecipitation and Western blotting, we showed that 3 of 9 (33%) saliva samples were positive for gp120 or tat (Fig. 6B). Two saliva samples positive for gp120 were also positive for tat.
To determine if salivary tat at physiologic concentrations could penetrate into mucosal epithelium we exposed the normal mucosal surface of polarized buccal tissue explants from HIV-uninfected donors to 30 ng/ml GFP-labeled HIV-tat in saliva. After 1 h and 3 h the tissues were analyzed by confocal microscopy, and showed that tat-GFP from saliva penetrated the mucosal epithelium from the apical surface to granulosum and spinosum layers in a time-dependent manner (Fig. 6C).
Discussion
The granulosum and spinosum layers of oral and anal mucosal epithelium have well-developed TJs that form a barrier in the upper part of the stratified epithelium (Tugizov et al., 2012). The role of this barrier in preventing HPV from reaching the HPV-susceptible basal and parabasal cell layers during initial infection is poorly understood. Here we show that TJ disruption oral and anal epithelium in HIV-infected individuals due to expression of the HIV tat and gp120 serves as a mechanism for potentiating HPV penetration into epithelium. In turn this may contribute to the higher prevalence of anogenital and oral HPV infection, and ultimately, increased risk of HPV-associated neoplasia in these individuals.
HPV-16 PsVs readily penetrated into HIV tat- and gp120-disrupted oral epithelium as far down as the basal/parabasal layer, whereas no penetration was seen in intact tissues not treated with these proteins. We observed that all of tat/gp120-disrupted epithelia facilitate PsV penetration; however, approximately half of tat-disrupted tissues did not allow PsV penetration. Lack of PsV penetration in some of the disrupted epithelia could be due to clearance or damage of PsVs by intraepithelial macrophages and/or innate immune molecules, respectively (Buck et al., 2006; Ishii, 2013). Efficient paracellular passage of HPV-16 PsVs through HIV tat/gp120-disrupted TJs of mono-stratified epithelial cells is consistent with our demonstration that PsV migration into basal/parabasal cells of stratified mucosal epithelium occurred by TJ disruption in the granulosum and spinosum layers. The detection of RFP expression predominately in parabasal cells suggested that initial PsV entry may occur in basal cells, which may later become stratified into parabasal layers. Epithelial lesions arise when basal/parabasal cells infected with HPV rise through the epithelium, following a well-described sequence of coordinated viral transcription and host cell differentiation.
The presence of HIV and HIV gene products are well described in epithelium (Chou et al., 2000; Clemetson et al., 1993; Crowe and Sonza, 2000; Goto et al., 1991; Henning et al., 2010; Kakizawa et al., 1996; Liuzzi et al., 1996; Maticic et al., 2000; Nuovo et al., 1993; Qureshi et al., 1997; Qureshi et al., 1995; Rodriguez-Inigo et al., 2005; Sonza et al., 2001; Zuckerman et al., 2003), largely in circulating HIV-infected immune cells (Crowe and Sonza, 2000; Henning et al., 2010; Sonza et al., 2001). We detected immune cells expressing tat and gp120 in the oral and anal mucosa of all HIV-infected individuals, including those on ART. Similarly we showed that HIV in anal tissues was replicating and infectious, including tissues from ART-treated donors. Furthermore, tat and gp120 were detected in the saliva, and salivary tat penetrated into mucosal epithelium. HIV tat may penetrate into cells and tissues through its protein transduction domain (PTD), based on the basic amino acids arginine and lysine, which facilitates protein internalization into cells and tissues by multiple mechanisms, including endocytosis and macropinocytosis (Ferrari et al., 2003; Fittipaldi et al., 2003; Kaplan et al., 2005; Mann and Frankel, 1991; Wadia et al., 2004). Mucosal epithelium may therefore be exposed to tat and gp120 from multiple sources, including saliva and circulating immune cells, even among individuals with HIV viral load suppression on ART. Mucosal epithelium also serve as a viral reservoir (Henning et al., 2010), and a source of proteins that reduce epithelial integrity in the setting of HIV infection.
Detection of tat and gp120 in oral and anal tissues from HIV-infected donors on ART is consistent with previous studies, in which HIV RNA, DNA and virions in the oral and cervical tissues have been demonstrated (Chou et al., 2000; Clemetson et al., 1993; Crowe and Sonza, 2000; Goto et al., 1991; Henning et al., 2010; Kakizawa et al., 1996; Liuzzi et al., 1996; Maticic et al., 2000; Nuovo et al., 1993; Qureshi et al., 1997; Qureshi et al., 1995; Rodriguez-Inigo et al., 2005; Sonza et al., 2001; Zuckerman et al., 2003). Secretion of HIV tat into blood was first shown by Westendorp et al., (Westendorp et al., 1995) and the finding was confirmed by others (Poggi and Zocchi, 2006; Xiao et al., 2000). Detection of gp120 in the blood and lymphoid tissues was also shown (Montagnier et al., 1985; Oh et al., 1992; Rychert et al., 2010; Santosuosso et al., 2009). A recent report showed that ART did not inhibit secretion of tat (Mediouni et al., 2012). ART also did not eliminate gp120 expression in lymph nodes (Crowe and Sonza, 2000; Hatano et al., 2010; Henning et al., 2010; Schupbach et al., 2005; Zhang and Crumpacker, 2001). Low levels of replicating HIV and viral p24 were detected in peripheral blood mononuclear cells of ART-treated patients (Crowe and Sonza, 2000; Hatano et al., 2010; Henning et al., 2010; Schupbach et al., 2005; Zhang and Crumpacker, 2001), which may migrate into mucosal epithelium leading to spread of HIV (Crowe and Sonza, 2000; Henning et al., 2010; Sonza et al., 2001).
HIV tat- and/or gp120-mediated TJ disruption was detected only after prolonged incubation of cells (4-5 days) and this effect was not observed during shorter incubation periods (1-3 days). Earlier studies have shown that these proteins may disrupt epithelial TJs during a shorter time (24 h) incubation (Andras et al., 2005; Bai et al., 2008; Nazli et al.; Pu et al., 2005; Song et al., 2007; Toschi et al., 2006; Zhong et al., 2008); however, in those studies, the concentration of proteins was substantially higher than in our experiments, which were performed at physiological concentrations. Furthermore, in our studies, the development of TJs in polarized cells was much stronger (TER reached up to 800 Ω/cm2) than in the earlier studies (TER was about 200- 300 Ω/cm2). These factors may account for the differences of TJ disruption by HIV proteins in our work compared with previously published work.
In summary, we have observed HPV entry into disrupted mucosal epithelium facilitated by HIV tat and gp120 proteins, which may have a synergistic and/or additive effect on TJ disruption. HIV-associated TJ disruption is likely to play an important role in increasing the risk of HPV infection. Combined with other factors such as attenuated immune response to HPV antigen, this may increase the risk of subsequent development of HPV-associated neoplasia. Topical inhibitors of HIV tat- and gp120-activated signaling molecules including MAPK, PI3K/AKT and JNK (Ali et al., 2009; Andras et al., 2005; Bai et al., 2008; Cai et al., 1997; Kawakami et al., 2004; Lopez-Bergami et al., 2005; Marshall, 1996; Preiss et al., 2007; Pu et al., 2005; Song et al., 2007; Toschi et al., 2006; Wang et al., 2004; Zhong et al., 2008; Zhou et al., 2003) that contribute to TJ disruption in HIV-infected individuals may be useful for improving mucosal epithelial integrity and reducing the risk of exposure to HPV and potentially other infectious agents in the environment.
Materials and Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Committee on Human Research of the University of California San Francisco (IRB approval #: H8597-30664-03). All subjects provided written informed consent for the collection of samples and subsequent analysis.
Collection of oral and anal tissues and establishment of polarized oriented tissue explants
Buccal and anal biopsies containing stratified squamous mucosal epithelium and lamina propria from HIV-uninfected and HIV-infected individuals were collected from the UCSF Oral AIDS Center and UCSF Anal Neoplasia Clinic. To establish polarized organ cultures, biopsies were used approximately 1-2 h after biopsy procedures. Explants were placed with the mucosal side facing up in the top chamber of Millicell filter inserts of 12 mm diameter and 0.4-μm pore size (Millipore). The lateral edges of the explants were sealed with 3% agarose as described previously (Tugizov et al., 2012).
Establishment of polarized epithelial cells
Primary cervical keratinocytes from ectocervical tissues were purchased from Lonza. Primary anal and tonsil keratinocytes were established in our laboratory as described (Tugizov et al., 2011). Polarized cells were established in 0.4-μm Transwell two-chamber filter inserts (Tugizov et al., 2003; Tugizov et al., 2011).
Treatment of polarized cells and tissue explants with HIV proteins tat and gp120
Recombinant HIV-1 (Bal strain) wt tat and inactive mutant tat proteins were purchased from Immunodiagnostic Inc. Mutant tat was generated by substitution of the basic arginine-rich domain at 49-57 aa and the integrin-binding RGD motif in the C terminus with alanines. HIV-1 (Bal strain) gp120 was provided by the NIH AIDS Research reagent program. Gp120 was inactivated by incubation of protein at 85°C for 30 min (Bai et al., 2008).
To examine penetration of GFP-labeled HIV tat into oral epithelium, polarized oriented adult buccal explants were incubated at their mucosal surfaces with 30 ng/ml of HIV tat-GFP fusion protein (pEGFP/HXB-2 kindly provided by Dr. Ashok Chauhan, University of South Carolina) in the presence of whole saliva for 1 and 3 h at 37°C. Whole saliva was collected from 5 HIV-uninfected healthy donors and pooled and then used for tat reconstitution.
Recombinant tat and gp120 were added to polarized cells or tissue explants separately and in combination. Culture media were changed every day with fresh virus or proteins. In polarized cells disruption of tight junctions was monitored by measuring transepithelial electrical resistance (TER) and paracellular permeability (Tugizov et al., 2003; Tugizov et al., 2011). Briefly, TER was measured with an epithelial Millicell-ERS voltohmmeter (Millipore). Paracellular permeability was evaluated by adding horseradish peroxidase-conjugated goat anti-donkey IgG (Fab)2 (Jackson ImmunoResearch) to the upper filter compartment and photometrically assaying the medium from the lower compartment for horseradish peroxidase with o-phenylenediamine dihydrochloride as the substrate (Gulino et al., 1998). Detection of IgG in the lower chamber indicated leakage of IgG from the upper chamber. MTT assay was used to determine the viability of polarized cells treated with proteins (Biotium Inc.). Disruption of polarized cells in the tissue explants was examined by immunostaining of tissue sections for ZO-1.
Preparation of HPV-16 pseudovirions (PsVs)
HPV-16 pseudovirions (PsV) consist of the major L1 and minor L2 capsid proteins of the virion, with a plasmid expressing red fluorescent protein (RFP) packaged inside. Plasmids encoding the PsV components, as well as human sera positive and negative for HPV-16 antibodies, were the kind gift of Dr. John Schiller, National Cancer Institute. HPV-16 PsV were generated by co-transfection of HPV-16 genes encoding major L1 and minor L2 capsid proteins and RFP as described (Buck et al., 2004; Buck et al., 2005; Roberts et al., 2007). Cells were exposed to PsVs, and RFP expression – indicating PsV entry into epithelial cells – was detected after 3 days.
HPV paracellular passage assays
For HPV paracellular penetration assays, polarized epithelial cells were exposed to HPV-16 PsV-RFP at 1 ng per insert or explant at the apical surface of cells. To detect paracellular passage of PsVs, COS-1 cells were seeded on cover glass placed in the basolateral chambers of inserts containing polarized epithelial cells. After 2 h the inserts were removed. COS-1 cells were grown for 3 days with basolateral media from polarized culture. The cells were then fixed and PsV infection was determined by detection of RFP-positive cells using confocal microscopy. For quantitative analysis RFP-positive COS-1 cells were counted and results are presented as a % of RFP-positive cells. PsV penetration into tissue explants was evaluated by detecting RFP signals after 3 days of incubation of tissue explants. Tissues were fixed, sectioned and analyzed by confocal microscopy.
Confocal immunofluorescence
Immunostaining of tissue sections was performed as previously described (Tugizov et al., 2007; Tugizov et al., 2012). Mouse anti-HIV-1 tat (Immunodiagnostic Inc.) and goat anti-gp120 (Thermo Scientific) were used for detection of HIV-1 proteins. To detect tight junction proteins and immune cells, we used rabbit anti-ZO-1, anti-occludin, and anti-claudin-1 (all from Zymed) and mouse anti-CD3, anti-CD68 and anti-CD1a (all from BD Bioscience). Secondary antibodies labeled with fluorescein isothiocyanate (FITC), tetramethyl rhodamine isothiocyanate (TRITC), or cyanine 5 (Cy5), were purchased from Jackson Immunoresearch. The specificity of each antibody was confirmed by negative staining with the corresponding primary isotype control antibody. Cell nuclei were counterstained with TO-PRO-3 iodide (Molecular Probes) (blue). Cells and tissue sections were analyzed using a Leica SP5 laser confocal microscope.
For quantitative analysis of ZO-1-, occludin-, and claudin-1-expressing epithelial cells, tissue sections were immunostained for these proteins and we counted positive epithelial cells with a ring-shaped staining pattern. The ring-shaped pattern of localization of tight junction proteins indicates their association with intact tight junctions. Cells with normal TJs were counted within the basal and parabasal layers in 10 microscopic fields (200×) of each tissue section. Results are represented as a percentage of cells with ring shaped localization of TJ proteins.
For quantitative evaluation of HIV tat- and gp120-expressing immune cells, sections were co-immunostained for viral proteins or cytokines with immune cell markers (CD68 for macrophages, CD1a for Langerhans cells, CD3 for lymphocytes), and immune cells expressing HIV proteins were counted. Cells were counted in 10 randomly selected microscopic fields (200×) per section in at least 3 sections for each explant. Results were presented as the average number of positive cells per mm2.
For quantitative analysis of HPV-16 PsV penetration into oral or anal epithelia, sections of tissue explants exposed to PsVs were immunostained with anti-ZO-1 or anti-occludin antibodies, and the cells containing red signals were counted under various experimental conditions. All cell quantification was performed blindly by two investigators (ST, RH).
Western blot and immunoprecipitation assays
Matching saliva and plasma samples (2 ml of each) from HIV-infected (UCSF Options Study (Owotade et al., 2008)) and HIV-uninfected individuals were subjected to immunoprecipitation using sheep anti-tat (Abcam) and mouse monoclonal anti-gp120 antibodies (ID6) (NH AIDS Reagents). These samples (concentrated by approximately 20 times) were then analyzed by immunoblotting with mouse anti-tat monoclonal (Immunodiagnostics Inc) and mouse anti-gp120 antibodies (ID6) (NIH AIDS Research reagent program). As positive controls for gp120 and tat were used HIV-133 -infected PBMC extract and recombinant tat (HIV-1BAL strain) (Immunodiagnostics Inc), respectively. Bands were visualized using ECL-detection system (GE Healthcare).
Detection of infectious HIV from anal tissue biopsies
Anal biopsies from consenting HIV-uninfected and HIV-infected donors at the UCSF Anal Neoplasia Clinic and were weighed and homogenized using sterile glass powder in 1 ml phosphate-buffered saline (pH, 7.2). Tissue homogenates were centrifuged for 10 min at 2000 RPM, and supernatants were divided into 2 parts. One part was used for detection of HIV-1 p24. The other part was used for infection of PBMCs. HIV infection in PBMCs were examined after 2 weeks by p24 ELISA.
Statistical analysis
To determine the disruption of epithelial TJs and intraepithelial immune cells expressing HIV tat and gp120 proteins, data were obtained from HIV-uninfected donors, and HIV-infected individuals not on ART or treated with ART. The results were compared using the Student’s t-test; p values <0.05 were considered significant.
Acknowledgments
We thank Dr. Teresa Darragh (UCSF) for histological evaluation of tissues, Dr. Fred Fishman (UCSF) for providing the tissue biopsies, and Ashok Chauhan (University of South Carolina) for providing GFP-tat expressing plasmid pEGFP/HXB-2. This project was supported by National Institute of Dental and Craniofacial Research (R21 DE021011), California HIV/AIDS Research Program (ID10-SF-030), the UCSF Center for AIDS Research (P30-AI027763), the Oral HIV/AIDS Research Alliance (U01-AI-68636), the NCI AIDS Malignancy Consortium (U01 CA121947) and the Helen Diller Family Comprehensive Cancer Center (P30 CA 82103-13).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci. 2008;121:1036–1045. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AS, Ali S, El-Rayes BF, Philip PA, Sarkar FH. Exploitation of protein kinase C: a useful target for cancer therapy. Cancer Treat Rev. 2009;35:1–8. doi: 10.1016/j.ctrv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. doi: 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- Bai L, Zhang Z, Zhang H, Li X, Yu Q, Lin H, Yang W. HIV-1 Tat protein alter the tight junction integrity and function of retinal pigment epithelium: an in vitro study. BMC Infect Dis. 2008;8:77. doi: 10.1186/1471-2334-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillari G, Sgadari C, Fiorelli V, Samaniego F, Colombini S, Manzari V, Modesti A, Nair BC, Cafaro A, Sturzl M, Ensoli B. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the alpha5beta1 and alphavbeta3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood. 1999a;94:663–672. [PubMed] [Google Scholar]
- Barillari G, Sgadari C, Palladino C, Gendelman R, Caputo A, Morris CB, Nair BC, Markham P, Nel A, Sturzl M, Ensoli B. Inflammatory cytokines synergize with the HIV-1 Tat protein to promote angiogenesis and Kaposi’s sarcoma via induction of basic fibroblast growth factor and the alpha v beta 3 integrin. J Immunol. 1999b;163:1929–1935. [PubMed] [Google Scholar]
- Bozou JC, Rochet N, Magnaldo I, Vincent JP, Kitabgi P. Neurotensin stimulates inositol trisphosphate-mediated calcium mobilization but not protein kinase C activation in HT29 cells. Involvement of a G-protein. Biochem J. 1989;264:871–878. doi: 10.1042/bj2640871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci U S A. 2006;103:1516–1521. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005;79:2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco MT, Moscat J, Rapp U, Cooper GM. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou LL, Epstein J, Cassol SA, West DM, He W, Firth JD. Oral mucosal Langerhans’ cells as target, effector and vector in HIV infection. J Oral Pathol Med. 2000;29:394–402. doi: 10.1034/j.1600-0714.2000.290805.x. [DOI] [PubMed] [Google Scholar]
- Clemetson DB, Moss GB, Willerford DM, Hensel M, Emonyi W, Holmes KK, Plummer F, Ndinya-Achola J, Roberts PL, Hillier S, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA : the journal of the American Medical Association. 1993;269:2860–2864. [PubMed] [Google Scholar]
- Crowe SM, Sonza S. HIV-1 can be recovered from a variety of cells including peripheral blood monocytes of patients receiving highly active antiretroviral therapy: a further obstacle to eradication. Journal of leukocyte biology. 2000;68:345–350. [PubMed] [Google Scholar]
- Dayanithi G, Yahi N, Baghdiguian S, Fantini J. Intracellular calcium release induced by human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein in human intestinal epithelial cells: a putative mechanism for HIV-1 enteropathy. Cell Calcium. 1995;18:9–18. doi: 10.1016/0143-4160(95)90041-1. [DOI] [PubMed] [Google Scholar]
- de Pokomandy A, Rouleau D, Ghattas G, Vezina S, Cote P, Macleod J, Allaire G, Franco EL, Coutlee F. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. The Journal of infectious diseases. 2009;199:965–973. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- Fantini J, Maresca M, Hammache D, Yahi N, Delezay O. Glycosphingolipid (GSL) microdomains as attachment platforms for host pathogens and their toxins on intestinal epithelial cells: activation of signal transduction pathways and perturbations of intestinal absorption and secretion. Glycoconj J. 2000;17:173–179. doi: 10.1023/a:1026580905156. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Pellegrini V, Arcangeli C, Fittipaldi A, Giacca M, Beltram F. Caveolae-mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Molecular therapy : the journal of the American Society of Gene Therapy. 2003;8:284–294. doi: 10.1016/s1525-0016(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Fittipaldi A, Ferrari A, Zoppe M, Arcangeli C, Pellegrini V, Beltram F, Giacca M. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. The Journal of biological chemistry. 2003;278:34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yeh CK, Notkins AL, Prabhakar BS. Detection of proviral sequences in saliva of patients infected with human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1991;7:343–347. doi: 10.1089/aid.1991.7.343. [DOI] [PubMed] [Google Scholar]
- Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, Sulpice E, Scaife R, Alemany M, Vernet T. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem. 1998;273:29786–29793. doi: 10.1074/jbc.273.45.29786. [DOI] [PubMed] [Google Scholar]
- Hatano H, Delwart EL, Norris PJ, Lee TH, Neilands TB, Kelley CF, Hunt PW, Hoh R, Linnen JM, Martin JN, Busch MP, Deeks SG. Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals. AIDS. 2010;24:2535–2539. doi: 10.1097/QAD.0b013e32833dba03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. The Journal of infectious diseases. 2010;202:1543–1552. doi: 10.1086/656720. [DOI] [PubMed] [Google Scholar]
- Hessol NA, Holly EA, Efird JT, Minkoff H, Schowalter K, Darragh TM, Burk RD, Strickler HD, Greenblatt RM, Palefsky JM. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS. 2009;23:59–70. doi: 10.1097/QAD.0b013e32831cc101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y. Electron microscopic visualization of autophagosomes induced by infection of human papillomavirus pseudovirions. Biochemical and biophysical research communications. 2013;433:385–389. doi: 10.1016/j.bbrc.2013.02.130. [DOI] [PubMed] [Google Scholar]
- Jayakumar P, Berger I, Autschbach F, Weinstein M, Funke B, Verdin E, Goldsmith MA, Keppler OT. Tissue-resident macrophages are productively infected ex vivo by primary X4 isolates of human immunodeficiency virus type 1. J Virol. 2005;79:5220–5226. doi: 10.1128/JVI.79.8.5220-5226.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa J, Ushijima H, Oka S, Ikeda Y, Schroder HC, Muller WE. Detection of human immunodeficiency virus-1 DNA, RNA and antibody, and occult blood in inactivated saliva: availability of the filter paper disk method. Acta Paediatr Jpn. 1996;38:218–223. doi: 10.1111/j.1442-200x.1996.tb03473.x. [DOI] [PubMed] [Google Scholar]
- Kang M, Cu-Uvin S. Association of HIV viral load and CD4 cell count with human papillomavirus detection and clearance in HIV-infected women initiating highly active antiretroviral therapy. HIV Med. 2012;13:372–378. doi: 10.1111/j.1468-1293.2011.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapembwa MS, Fleming SC, Orr M, Wells C, Bland M, Back D, Griffin GE. Impaired absorption of zidovudine in patients with AIDS-related small intestinal disease. Aids. 1996;10:1509–1514. doi: 10.1097/00002030-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Kapembwa MS, Fleming SC, Sewankambo N, Serwadda D, Lucas S, Moody A, Griffin GE. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991;81:327–334. doi: 10.1042/cs0810327. [DOI] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. Journal of controlled release : official journal of the Controlled Release Society. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Nishimoto H, Kitaura J, Maeda-Yamamoto M, Kato RM, Littman DR, Leitges M, Rawlings DJ, Kawakami T. Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem. 2004;279:47720–47725. doi: 10.1074/jbc.M408797200. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)0(2)- mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol. 2000;279:C21–30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Oshima T, Alexander JS. The role of p38 MAP kinase in hydrogen peroxide mediated endothelial solute permeability. Endothelium. 2001;8:107–116. doi: 10.3109/10623320109165320. [DOI] [PubMed] [Google Scholar]
- Liuzzi G, Chirianni A, Clementi M, Bagnarelli P, Valenza A, Cataldo PT, Piazza M. Analysis of HIV-1 load in blood, semen and saliva: evidence for different viral compartments in a cross-sectional and longitudinal study. Aids. 1996;10:F51–56. doi: 10.1097/00002030-199612000-00001. [DOI] [PubMed] [Google Scholar]
- Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C [corrected] Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingat F, Halloran B, Acharjee S, van Marle G, Church D, Gill MJ, Uwiera RR, Cohen EA, Meddings J, Madsen K, Power C. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:2211–2220. doi: 10.1096/fj.10-175992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat protein. The EMBO journal. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Cell signalling. Raf gets it together. Nature. 1996;383:127–128. doi: 10.1038/383127a0. [DOI] [PubMed] [Google Scholar]
- Maticic M, Poljak M, Kramar B, Tomazic J, Vidmar L, Zakotnik B, Skaleric U. Proviral HIV-1 DNA in gingival crevicular fluid of HIV-1-infected patients in various stages of HIV disease. J Dent Res. 2000;79:1496–1501. doi: 10.1177/00220345000790071101. [DOI] [PubMed] [Google Scholar]
- Mediouni S, Darque A, Baillat G, Ravaux I, Dhiver C, Tissot-Dupont H, Mokhtari M, Moreau H, Tamalet C, Brunet C, Paul P, Dignat-George F, Stein A, Brouqui P, Spector SA, Campbell GR, Loret EP. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein. Infect Disord Drug Targets. 2012;12:81–86. doi: 10.2174/187152612798994939. [DOI] [PubMed] [Google Scholar]
- Montagnier L, Clavel F, Krust B, Chamaret S, Rey F, Barre-Sinoussi F, Chermann JC. Identification and antigenicity of the major envelope glycoprotein of lymphadenopathy-associated virus. Virology. 1985;144:283–289. doi: 10.1016/0042-6822(85)90326-5. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuovo GJ, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- Obinna FC, Cook G, Beale T, Dave S, Cunningham D, Fleming SC, Claydon E, Harris JW, Kapembwa MS. Comparative assessment of small intestinal and colonic permeability in HIV-infected homosexual men. Aids. 1995;9:1009–1016. doi: 10.1097/00002030-199509000-00005. [DOI] [PubMed] [Google Scholar]
- Oh SK, Cruikshank WW, Raina J, Blanchard GC, Adler WH, Walker J, Kornfeld H. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. Journal of acquired immune deficiency syndromes. 1992;5:251–256. [PubMed] [Google Scholar]
- Owotade FJ, Shiboski CH, Poole L, Ramstead CA, Malvin K, Hecht FM, Greenspan JS. Prevalence of oral disease among adults with primary HIV infection. Oral diseases. 2008;14:497–499. doi: 10.1111/j.1601-0825.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- Palefsky J. Biology of HPV in HIV infection. Adv Dent Res. 2006;19:99–105. doi: 10.1177/154407370601900120. [DOI] [PubMed] [Google Scholar]
- Palefsky JM. Anal cancer prevention in HIV-positive men and women. Current opinion in oncology. 2009;21:433–438. doi: 10.1097/CCO.0b013e32832f511a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palefsky JM. Antiretroviral therapy and anal cancer: the good, the bad, and the unknown. Sexually transmitted diseases. 2012;39:501–503. doi: 10.1097/OLQ.0b013e31825f7921. [DOI] [PubMed] [Google Scholar]
- Palefsky JM, Holly EA, Efirdc JT, Da Costa M, Jay N, Berry JM, Darragh TM. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus HIV)-positive and HIV-negative homosexual men. The Journal of infectious diseases. 1998;177:361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- Poggi A, Zocchi MR. HIV-1 Tattriggers TGF-beta production and NK cell apoptosis that is prevented by pertussis toxin B. Clin Dev Immunol. 2006;13:369–372. doi: 10.1080/17402520600645712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss S, Namgaladze D, Brune B. Critical role for classical PKC in activating Akt by phospholipase A2-modified LDL in monocytic cells. Cardiovasc Res. 2007;73:833–840. doi: 10.1016/j.cardiores.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B, Toborek M. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab. 2005;25:1325–1335. doi: 10.1038/sj.jcbfm.9600125. [DOI] [PubMed] [Google Scholar]
- Qureshi MN, Barr CE, Hewlitt I, Boorstein R, Kong F, Bagasra O, Bobroski LE, Joshi B. Detection of HIV in oral mucosal cells. Oral Dis. 1997;3(Suppl 1):S73–78. doi: 10.1111/j.1601-0825.1997.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Qureshi MN, Barr CE, Seshamma T, Reidy J, Pomerantz RJ, Bagasra O. Infection of oral mucosal cells by human immunodeficiency virus type 1 in seropositive persons. J Infect Dis. 1995;171:190–193. doi: 10.1093/infdis/171.1.190. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Inigo E, Jimenez E, Bartolome J, Ortiz-Movilla N, Bartolome Villar B, Jose Arrieta J, Manzarbeitia F, Carreno V. Detection of human immunodeficiency virus type 1 RNA by in situ hybridization in oral mucosa epithelial cells from anti-HIV-1 positive patients. J Med Virol. 2005;77:17–22. doi: 10.1002/jmv.20409. [DOI] [PubMed] [Google Scholar]
- Rychert J, Strick D, Bazner S, Robinson J, Rosenberg E. Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines. AIDS research and human retroviruses. 2010;26:1139–1145. doi: 10.1089/aid.2009.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, Dandekar S. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosuosso M, Righi E, Lindstrom V, Leblanc PR, Poznansky MC. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. The Journal of infectious diseases. 2009;200:1050–1053. doi: 10.1086/605695. [DOI] [PubMed] [Google Scholar]
- Schupbach J, Gunthard H, Joos B, Fischer M, Boni J, Tomasik Z, Yerly S, Perrin L, Battegay M, Furrer H, Vernazza P, Bernasconi E, Hirschel B. HIV-1 p24 may persist during long-term highly active antiretroviral therapy, increases little during short treatment breaks, and its rebound after treatment stop correlates with CD4(+) T cell loss. Journal of acquired immune deficiency syndromes. 2005;40:250–256. doi: 10.1097/01.qai.0000181281.75670.56. [DOI] [PubMed] [Google Scholar]
- Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Molecular biology of the cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annual review of cell and developmental biology. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745–11753. doi: 10.1158/0008-5472.CAN-06-2322. [DOI] [PubMed] [Google Scholar]
- Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. 2007;109:1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S, Mutimer HP, Oelrichs R, Jardine D, Harvey K, Dunne A, Purcell DF, Birch C, Crowe SM. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. Aids. 2001;15:17–22. doi: 10.1097/00002030-200101050-00005. [DOI] [PubMed] [Google Scholar]
- Toschi E, Bacigalupo I, Strippoli R, Chiozzini C, Cereseto A, Falchi M, Nappi F, Sgadari C, Barillari G, Mainiero F, Ensoli B. HIV-1 Tat regulates endothelial cell cycle progression via activation of the Ras/ERK MAPK signaling pathway. Mol Biol Cell. 2006;17:1985–1994. doi: 10.1091/mbc.E05-08-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov S, Herrera R, Veluppillai P, Greenspan J, Greenspan D, Palefsky JM. Epstein-Barr Virus (EBV)-Infected Monocytes Facilitate Dissemination of EBV within the Oral Mucosal Epithelium. J Virol. 2007;81:5484–5496. doi: 10.1128/JVI.00171-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9:307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology. 2011;409:211–222. doi: 10.1016/j.virol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. Journal of Virology. 2012;86:2556–2570. doi: 10.1128/JVI.06578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang J, Yi XJ, Yu FS. Activation of ERK1/2 MAP kinase pathway induces tightjunction disruption in human corneal epithelial cells. Exp Eye Res. 2004;78:125–136. doi: 10.1016/j.exer.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad SciU SA. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Akhter S, Chaudhuri A, Kanmogne GD. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: modulatory effects of STAT1 signaling. Microvasc Res. 2009;77:212–219. doi: 10.1016/j.mvr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Crumpacker CS. Human immunodeficiency virus type 1 RNA in peripheral blood mononuclear cells of patients receiving prolonged highly active antiretroviral therapy. The Journal of infectious diseases. 2001;184:1341–1344. doi: 10.1086/324002. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Smart EJ, Weksler B, Couraud PO, Hennig B, Toborek M. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci. 2008;28:7788–7796. doi: 10.1523/JNEUROSCI.0061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Das S, Murthy KS. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G472–480. doi: 10.1152/ajpgi.00345.2002. [DOI] [PubMed] [Google Scholar]
- Zuckerman RA, Whittington WL, Celum CL, Collis T, Lucchetti A, Sanchez JL, Hughes JP, Coombs RW. Factors associated with oropharyngeal human immunodeficiency virus shedding. J Infect Dis. 2003;188:142–145. doi: 10.1086/375741. [DOI] [PubMed] [Google Scholar]