Abstract
Background
Distinguishing cellular abnormalities in reactive and malignant lesions is challenging. We compared the incidence and severity of cytological abnormalities in malignant/premalignant and benign epidermal lesions.
Methods
One hundred fifty-two biopsies representing 69 malignant/premalignant squamous lesions and 83 benign conditions were studied. Cytological features, including nuclear hyperchromasia, nuclear overlap (crowding), irregular nuclei, high nuclear/cytoplasmic (N/C) ratio, conspicuous nucleoli, delicate inconspicuous nucleoli, clumped chromatin, pleomorphic parakeratosis, normal and abnormal mitotic figures and necrotic keratinocytes, were evaluated and graded. Statistical analysis was performed.
Results
Irregular nuclei, increased N/C ratio, conspicuous single prominent nucleoli, nuclear overlap (crowding), pleomorphic parakeratosis, nuclear hyperchromasia, necrotic keratinocytes, normal and abnormal mitotic figures and coarse chromatin were seen more frequently in malignant neoplasms (p < 0.05). Abnormal mitotic figures, although uncommon (20.3%), were only noted in the malignant/premalignant group. Certain cytological features were common among both malignant and benign lesions, suggesting that they are of little value.
Conclusion
In the setting of an atypical cutaneous squamous proliferation, nuclear irregularity, increased N/C ratio, conspicuous nucleoli, crowding and hyperchromasia are the most useful indicators of malignancy. In contrast, mitotic figures, necrotic cells and coarse chromatin are less useful. The presence of abnormal mitotic figures is very helpful when present; however, their overall rarity limits their utility.
Keywords: atypical features, cutaneous squamous cell carcinoma, non-melanoma skin cancer, squamous cell carcinomas
Cytopathologic features of malignancy are well described in the literature on cervical neoplasia,1 – 5 but there are only few articles that address this issue in skin lesions. It is well known that cytological abnormalities commonly encountered in malignant neoplasms can also be seen in certain inflamed benign neoplasms and inflammatory skin lesions, but differences in the frequency and expression of such features have not been statistically evaluated to the best of our knowledge.
Cytological ‘atypia’, more precisely defined as the specific cellular abnormalities discussed in this article, is often cited as diagnostic criterion for malignancy. In non-neoplastic epidermal proliferations and benign epidermal neoplasms with inflammation, reactive cytological changes may overlap with those found in malignant conditions.6,7 Often, the architectural pattern of a lesion is the best way to establish the correct diagnosis, but the architectural patterns of some conditions overlap,6,7 and in small specimens an evaluation of the architecture is sometimes not possible.
When the architecture is not analyzable, cytologic features such as nuclear hyperchromasia, nuclear density and overlapping of nuclei (crowding), irregular shape of nuclei, increased nuclear-cytoplasmic ratio (N/C ratio), coarse chromatin, prominent nucleoli and increased mitotic rate are often used as criteria to distinguish between malignant and benign conditions.4,8,9,10 – 18
This article focuses on two main questions. First, is it possible to distinguish between benign and malignant processes based on cellular features alone? Second, which cytopathologic features are most reliable in distinguishing benign and malignant entities? To answer these questions, we graded various abnormal cytological features in malignant and benign lesions, and compared those using contemporary statistical methods.
Materials and methods
We performed a search in the database of Ackerman Academy of Dermatopathology, NY, USA using the following terms: ‘actinic keratosis’, ‘Bowen’s disease’, ‘squamous cell carcinoma’, ‘spongiotic dermatitis’, ‘psoriasis’, ‘seborrheic keratosis, inflamed’, ‘lichen planus-like keratosis’ and ‘lichen simplex chroni-cus’. Lesions were randomly selected from those diagnosed at the Academy between the years 2008 and 2011 and divided into two groups – malignant/premalignant epidermal squamous neoplasms and benign epidermal lesions. These two broad categories were further subclassified into malignant invasive neoplasms, malignant/premalignant intraepidermal lesions, benign epidermal neoplasms with inflammation and inflammatory skin lesions with prominent epidermal changes (Table 1).
Table 1.
Groups evaluated in the study
| Groups | Subgroups | Diagnosis | Number of cases |
|---|---|---|---|
| Benign epidermal lesions (n = 83) | Inflammatory epidermal lesions (n = 44) | Psoriasis | 10 |
| Lichen planus-like keratosis | 10 | ||
| Lichen simplex chronicus | 10 | ||
| Chronic eczema | 14 | ||
| Benign proliferations with inflammation (n = 39) | Inflamed seborrheic keratosis | 39 | |
| Malignant/premalignant epidermal lesions (n = 69) | Malignant/premalignant intraepidermal lesions (n = 28) | Actinic keratosis | 14 |
| Bowen’s disease | 14 | ||
| Malignant invasive neoplasms (n = 41) | Squamous cell carcinoma | 41 |
Hematoxylin/eosin-stained slides were evaluated independently by the authors of this article with regards to 11 cytologic criteria. Precise definitions of each cytological feature were used (Table 2). Affected cells were compared with the adjacent uninvolved epidermis as an internal control for factors such as fixation, processing and staining.9
Table 2.
Definition of features used for the evaluation of benign and malignant processes
| Cytological feature | Definition | Grading scheme used |
|---|---|---|
| Pleomorphic parakeratosis10 | Pleomorphic, hyperchromatic nuclei of varying diameter in the stratum corneum | Present or absent |
| Mitotic figures11 – 13 | Condensed and separating chromosomes, typically arranged in a bipolar spindle | Quantity/five HPFs (mitotic index) |
| Abnormal mitotic figures12 – 15 | Mitotic figures were recognized as abnormal when they had tri/quadri/multipolar spindle instead of a bipolar spindle | Present or absent |
| Nuclear overlap (crowding)9,16,17 | Adjacent nuclei in contact or overlapping one another | Grading system 0–4 |
| Irregular nuclei9,17 | Deviations from round or oval shapes of nuclei (sharp angulations/clefts and curves in nuclear membrane), compared with cells in the uninvolved adjacent epidermis | Grading system 0–4 |
| Increased nuclear-cytoplasmic ratio5,9,18 | Ratio greater than 1 : 1 (i.e. when nucleus occupied more than half of the cell volume). (Normal nuclear-cytoplasmic ratio in squamous cells ranges 1 : 3–4 in different layers of the epidermis.) | Grading system 0–4 |
| Nuclear hyperchromasia2,9,13 | Increase in tinctorial properties of a nucleus or intensity of chromatin staining because of an increase in its chromatin content. To minimize the effect of staining, fixation and processing artifact, we evaluated this feature in lesional cells in comparison with uninvolved cells in the adjacent epidermis | Grading system 0–4 |
| Conspicuous nucleoli8,9 | Enlarged nucleoli easily visible at ×100 magnification or nucleoli that were approximately 2–3 μm or more in diameter were included in this category | Grading system 0–4 |
| Inconspicuous nucleoli8,9 | Small single delicate nucleoli visible only at higher magnifications (×200 or ×400) | Grading system 0–4 |
| Coarse chromatin2,9,12 | Multiple chromatin clumps, some of which may represent poorly formed nucleoli | Grading system 0–4 |
| Necrotic cells12 | Hypereosinophilic cells with pyknotic nuclei | Quantity/five HPFs (necrotic index) |
HPF, high-power field.
We graded expression of the cellular features in areas with the most prominent abnormality seen at low (×100) magnification (target areas). Nuclear features including crowding, irregularity of nuclei, increased N/C ratio, nuclear hyperchromasia, conspicuous and inconspicuous nucleoli and coarse chromatin were graded on a scale of 0–4. The grading was performed in five high-power fields (HPFs), irrespective of the size of specimen: 0 –absent, 1 – occasionally present (one or two cells showing the feature), 2 – more than two cells but less than 50% of the cells showing the feature, 3 – more than 50% but less than 75% of the cells showing the feature and 4 – more than 75% of the cells showing the feature (Table 2). We chose five HPFs based on the average size of the specimens. Mitotic figures and necrotic keratinocytes were graded based on their actual number in five HPFs (mitotic and necrotic indices; Table 2). Pleomorphic parakeratosis and abnormal mitoses were evaluated as ‘present’ or ‘absent’ in the lesion (Table 2).
A statistical analysis program STATISTICA 10.0 (StatSoft® Inc., Tulsa, OK, USA) was used for the data processing. The method of 2 × 2 tables, with chi-square test and Yates-corrected chi-square test for small samples, was used to compare frequencies of the features. The Spearman correlation method was used to study the strength of association of the features with benign or malignant lesions. Sensitivity, specificity, positive and negative likelihood ratios [LR (+) and LR (−)] and classification tree analysis were performed to study the significance of the features commonly seen in malignant neoplasms with squamous differentiation. A LR (+) of more than 10 indicated that the feature had a strong association with malignancy, while 5–10 indicated a moderate association with malignancy and less than 5 indicated a weak association with malignancy. A LR (−) of less than 0.1 was strongly associated with benign conditions, 0.1–0.5 had a moderate association and more than 0.5 a weak association with benign conditions.19 To rank the features according to their overall usefulness/predictive value for diagnosing malignancy, a classification tree analysis was performed.20
Results
The frequencies of cytologic features in inflamma-tory, benign proliferative, malignant/premalignant intraepidermal and invasive neoplasms are presented in Table 3.
Table 3.
Comparison of inflammatory epidermal lesions, benign epidermal proliferations with inflammation, intraepidermal malignant/premalignant squamous lesions and invasive malignant squamous lesions
| Features | Inflammatory epidermal lesions, n (%) | Benign epidermal proliferations with inflammation, n (%) | Malignant/premalignant intraepidermal squamous lesions, n (%) | Malignant invasive squamous lesions, n (%) |
|---|---|---|---|---|
| Pleomorphic parakeratosis | 2 (4.55) | 3 (7.69) | 25 (89.29) | 18 (43.9) |
| Mitotic figures | 30 (68.18) | 16 (41.03) | 23 (82.14) | 37 (90.24) |
| Abnormal mitotic figures | 0 | 0 | 5 (17.86) | 9 (21.95) |
| Nuclear overlap (crowding) | 2 (4.55) | 9 (23.08) | 27 (96.43) | 35 (85.37) |
| Irregular nuclei | 1 | 0 | 22 (78.57) | 39 (95.12) |
| Increased nuclear-cytoplasmic ratio | 7 (15.91) | 5 (12.82) | 28 (100) | 41 (100) |
| Nuclear hyperchromasia | 20 (45.45) | 24 (61.54) | 28 (100) | 37 (90.24) |
| Conspicuous nucleoli | 9 (20.45) | 5 (12.82) | 26 (92.86) | 41 (100) |
| Inconspicuous nucleoli | 44 (100) | 39 (100) | 27 (96.43) | 23 (56.10) |
| Coarse chromatin | 43 (97.73) | 22 (56.41) | 26 (92.86) | 41 (100) |
| Necrotic cells | 28 (63.64) | 25 (64.10) | 26 (92.86) | 40 (97.56) |
Statistical calculations, comparing the two main broad categories (malignant/premalignant and benign groups as a whole), showed that irregular nuclei, increased N/C ratio, conspicuous single prominent nucleoli, nuclear overlap (crowding), pleomorphic parakeratosis, nuclear hyperchromasia, necrotic keratinocytes, normal and abnormal mitotic figures and coarse chromatin were seen more frequently in malignant neoplasms (p < 0.05) (Table 4). In contrast, small delicate nucleoli were seen more frequently in benign lesions (p < 0.05) (Table 4, Fig. 1).
Table 4.
Comparison between malignant/premalignant and benign epidermal lesions
| Features | Benign epidermal lesions, n (%) | Malignant/premalignant epidermal lesions, n (%) | Differences in frequency of features*, p-value | Correlation† r; p-value | Sensitivity | Specificity | LR (+) | LR (−) |
|---|---|---|---|---|---|---|---|---|
| Pleomorphic parakeratosis | 5 (6.02) | 43 (62.32) | <0.0001 | — | 0.62 | 0.94 | 10.34 | 0.40 |
| Mitotic figures | 46 (55.42) | 60 (86.96) | <0.0001 | 0.40; <0.05 | 0.87 | 0.45 | 1.57 | 0.29 |
| Abnormal mitotic figures | 0 | 14 (20.29) | <0.0001 | — | 0.20 | 1.0 | Infinity** | 0.80 |
| Nuclear overlap (crowding) | 11 (13.25) | 62 (89.86) | <0.0001 | 0.82; <0.05 | 0.90 | 0.87 | 6.78 | 0.12 |
| Irregular nuclei | 1 (1.20) | 61(88.41) | <0.0001 | 0.85; <0.05 | 0.88 | 0.99 | 73.38 | 0.12 |
| Increased nuclear–cytoplasmic ratio | 12 (14.46) | 69 (100) | <0.0001 | 0.89; <0.05 | 1.0 | 0.86 | 6.92 | 0** |
| Nuclear hyperchromasia | 44 (53.01) | 65 (94.20) | <0.0001 | 0.70; <0.05 | 0.94 | 0.47 | 1.78 | 0.12 |
| Conspicuous nucleoli | 14 (16.87) | 67 (97.10) | <0.0001 | 0.86; <0.05 | 0.97 | 0.83 | 5.76 | 0.03** |
| Inconspicuous nucleoli | 83 (100) | 50 (72.46) | <0.0001 | −0.81; <0.05 | 0.72 | 0 | 0.72 | Infinity |
| Coarse chromatin | 65 (78.31) | 67 (97.10) | 0.0015 | 0.39; <0.05 | 0.97 | 0.22 | 1.24 | 0.13 |
| Necrotic cells | 53 (63.86) | 66 (95.65) | <0.0001 | 0.48; <0.05 | 0.97 | 0.36 | 1.52 | 0.08** |
LR, likelihood ratio.
Chi-square test and Yates-corrected chi-square test.
Spearman correlation.
Significant diagnostic evidence.
Fig. 1.
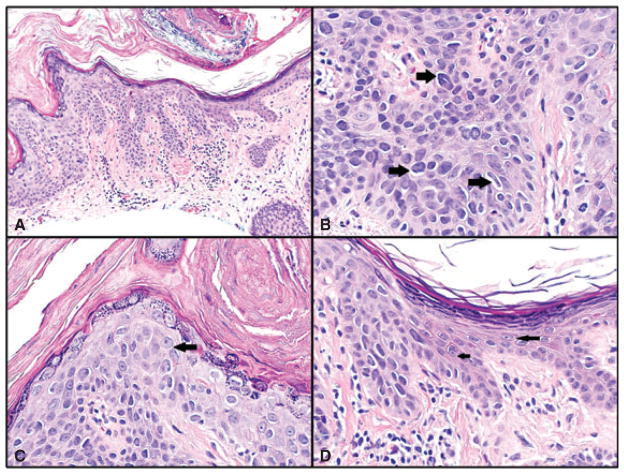
A–D) Bowen’s disease. A) Bowen’s disease on left and adjacent normal epidermis on right. Hematoxylin/eosin (H&E)-stained sections, magnification ×200. B) Multiple hyperchromatic cells in all layers of the epidermis, with crowding and irregular nuclei (sharply angulated pentagon shaped and curved cigar shaped – right-sided arrows). H&E sections, magnification ×600, cropped image. C) Another high-power field from same case showing cell with prominent nucleolus (arrow), which was visible at lower magnification. Irregular nuclei and crowding are also seen in this field. H&E sections, magnification ×600. D) Junction between Bowen’s disease and adjacent uninvolved epidermis for comparison. Cells in the uninvolved portion are round or oval in shape, without hyperchromasia and with delicate small nucleoli (arrows). In comparison, cells in the area of Bowen’s disease show irregular angulated shapes, hyperchromasia, increased nuclear/cytoplasmic ratio and crowding. H&E sections, magnification ×600.
All the examined features, except inconspicuous nucleoli, had a positive correlation with the malignant conditions (Table 4).
Sensitivity, specificity, LR (+) and LR (−) calculations are presented in Table 4. Abnormal mitotic figures, pleomorphic parakeratosis, crowding and irregular nuclei were among the most specific features for malignancy (specificity 94–100%). Abnormal mitotic figures were only rarely encountered in malignant neoplasms (14 of 69 cases), limiting their utility. We employed classification tree analysis (importance plot) to evaluate the significance, as well as practical usefulness (ranking predictive importance) of all the features.
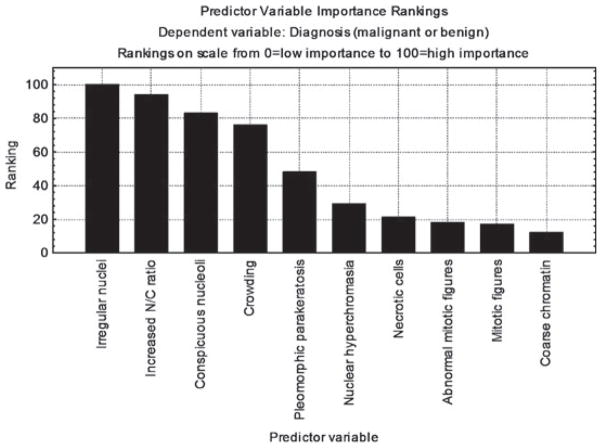
Classification tree analysis favored irregular nuclei, increased N/C ratio, conspicuous nucleoli and crowding as the most predictive cytological features of malignant/premalignant lesions (based on their relative high frequency in malignant conditions and low frequency in benign conditions), followed by pleomorphic parakeratosis and nuclear hyperchromasia. In contrast, mitotic figures (normal and abnormal), necrotic cells and coarse chromatin showed less overall predictive value for malignancy (Fig. 2). Results of the classification tree analysis were consistent with the chi-square p-values presented in Table 4, with the order of importance of features based on smallest to largest p-values. Comparison of the benign group with the malignant/premalignant intraepidermal lesions (excluding the invasive malignancies) yielded statistically significant p-values (p < 0.05) for 9 of the 11 features, with only coarse chromatin and inconspicuous nucleoli having a p-value >0.05.
Fig. 2.
Ranking of the most frequent features of malignancy. Irregular nuclei, increased nuclear/cytoplasmic ratio, conspicuous nucleoli, crowding, pleomorphic parakeratosis and nuclear hyperchromasia were the most important/useful features to predict malignancy. Necrotic cells, normal and abnormal mitotic figures and coarse chromatin are important clues to malignancy, but had more limited usefulness based on their relative frequencies in malignant and benign conditions.
Discussion
The histopathologic architecture or epidermal silhouette of a tumor is of great significance in establishing a correct diagnosis in dermatopathology. However, in certain settings (e.g. superficial/partially fragmented biopsies or curettage material, fractured specimens and tangentially embedded sections) distinction based on architecture alone becomes challenging. In addition, certain conditions, for example, clonal seborrheic keratosis and clonal Bowen’s disease, have overlapping architectural features.6 Cytological criteria can help establish an accurate diagnosis in such settings.
Descriptions of malignant epidermal cells often include large nuclear size, loss of keratinocyte polarity, altered N/C ratio, nuclear hyperchromasia, prominent nucleoli and a thick irregular nuclear envelope.12,13,21 The presence of large, pleomorphic and hyperchromatic nuclei in the stratum corneum (‘malignant’ parakeratosis) can also be helpful in establishing the diagnosis of malignancy, as these features are rare in inflammatory epidermal lesions and benign epidermal proliferations with inflammation.10 These features are often grouped together under the broad umbrella term ‘atypia’, a term that has invoked criticism by some prominent dermatopathologists in spite of its widespread use.22 In this study, we sought to distinguish the cytological features that are most useful to dermatopathologists, when faced with an atypical squamous proliferation.
Studies have confirmed the usefulness of cytopathological criteria in differential diagnosis of lesions of the esophageal mucosa,23,24 anal squamous mucosa,25 – 27 vaginal squamous mucosa,28 pancreas29 and thyroid.16 Some studies have evaluated cytopathological features of epidermal cells using instruments for quantitation of the parameters30 or have attempted to create an ‘index of atypia’ to distinguish malignant from benign conditions.31 The complexity of these methods limits their application to routine practice. In this study, we analyzed various cytological criteria, utilizing just the light microscope. We included only cases that had a definitive diagnosis, so that we could evaluate the importance of different features in lesions without any ambiguity, creating a body of data that can be subsequently applied either to equivocal lesions or to small/fragmented biopsies lacking analyzable architecture. We used definitions established in the cytology literature and compared affected areas with the uninvolved epidermis, as in cervical pathology, to provide an internal control for factors such as fixation, processing and staining.9 As external factors such as chemotherapeutic agents, radiation and trauma are well known to produce cytological abnormalities that mimic malignancy,9,32 – 34 studies evaluating cytological features in a broader range of conditions are needed.
There are different ways to rank the importance of criteria. In our study, we used a combination of statistical tests: calculation of frequencies and their correlation with the diagnoses, specificity and sensitivity, as well as LR (+) and LR (−). We also ranked features based on their relative frequencies by means of the classification tree method (importance plot).
Our study confirmed a statistically higher frequency of abnormal cytological features in malignant lesions. The degree of expression of these features correlated with malignancy. The same features, with the exception of coarse chromatin, were also statistically significant when benign lesions were compared with just the intraepidermal premalignant/malignant lesions. Abnormal mitotic figures, irregular nuclei and pleomorphic parakeratosis showed the highest specificity (1.0, 0.99 and 0.94, respectively) for malignancy. These features also showed high LR (+). LR (+) is a method that has been employed to show diagnostic significance of variables.6 It should be noted that some ‘abnormal’ cytological features were common among benign lesions. For example, 55% of benign lesions had mitotic figures, 65% had clumped chromatin and 53% had necrotic cells and focal nuclear hyperchromasia (Figs. 3 and 4).
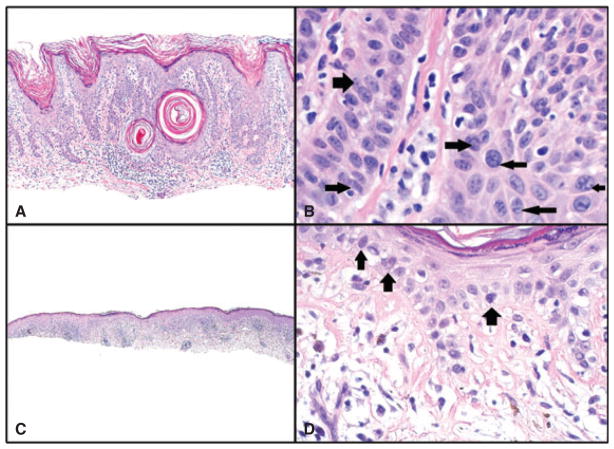
Fig. 3.
A and B) Seborrheic keratosis with inflammation. A) Hematoxylin/eosin (H&E) sections, magnification ×100. B) Nuclear crowding (right-sided arrows) and a clumped chromatin pattern (left-sided arrows) are present in areas of this benign lesion. Although some nuclei are compressed and elongated (left lower arrow), they did not meet our criteria for irregular nuclei, which require sharp angulations or curves in the nuclear membrane. Magnification ×600, cropped image. C and D) Benign lichenoid keratosis (lichen planus-like keratosis). C) H&E sections, magnification ×40. D) Hyperchromatic cells at the basal layer of the epidermis. Such cells were found focally in lesions with inflammation; however, their frequency and expression were much lower than in malignant lesions. Also, no foci show crowding, high nuclear/cytoplasmic ratio or irregular nuclei. H&E sections, magnification ×200.
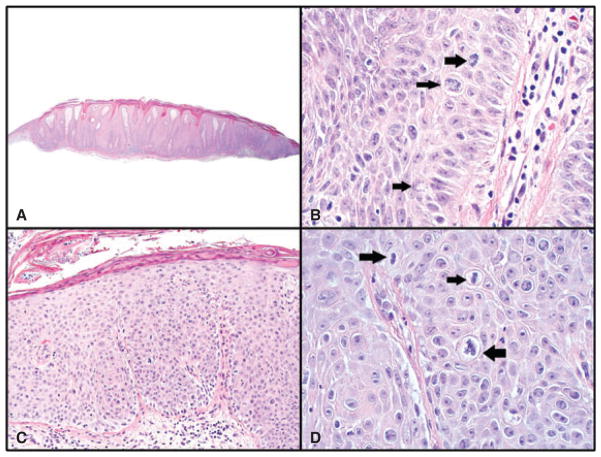
Fig. 4.
A and B) Prurigo nodule. A) Psoriasiform acanthosis, vertical collagen streaks in dermal papillae and compact parakeratosis. Hematoxylin/eosin (H&E) sections, magnification ×40. B) Three mitotic figures are present in one high-power field (HPF; all normal bipolar). Some crowding is seen in the basal layer. Nuclear-cytoplasmic ratio of cells is not increased (<1 : 1) and irregular nuclei (sharply angulated nuclei) are not present. H&E sections, magnification ×600. C and D) Bowen’s disease. C) H&E sections, magnification ×100. D) Three mitotic figures are present in one HPF: one is abnormal and tripolar (right-sided arrow) and two are normal bipolar (left-sided arrow). Crowding is present in all epidermal layers. Cells have high N/C ratio (>1 : 1). H&E sections, magnification ×600.
Using the classification tree analysis, irregularity of the nuclei was found to be a highly predictive feature of malignancy. The role of nuclear irregularities in the development of cancer has been shown in in vitro models of thyroid carcinoma, which showed that changes in chromatin distribution and nuclear envelope were significant factors in papillary thyroid carcinomas.17 Nuclear irregularities were also shown to be induced in normal thyroid cells by RET/PTC oncogene.17 In breast pathology, nuclear irregularities play a role in predicting outcome.35 Nuclear hyperchromasia and prominent nucleoli are closely related to the amount of DNA in neoplastic cells and may explain their more rapid growth, division and protein synthesis.36 Nuclear overlapping (crowding) has been shown to be an important sign of malignancy in cytological assessment of fine needle aspirations of bronchioloalveolar carcinoma and thyroid malignancies.8,16 This feature has also been found useful (100% sensitive and 64% specific) in the differential diagnosis of seborrheic keratosis and Bowen’s disease.6
While high mitotic index has been cited as an important criterion for malignancy,11 various benign lesions such as psoriasis show many mitotic figures37,38 and the lack of specificity was confirmed in our study. Abnormal mitotic figures are reported to be more specific for malignancy.14 Our study confirms that abnormal mitotic figures are highly specific for malignancy and had a high LR (+), but this feature has limited utility and a lower predictive variable rank in the importance plot (Fig. 4B).
In summary, nuclear irregularity, increased N/C ratio, conspicuous nucleoli, crowding and hyperchromasia proved to be the most useful indicators of malignancy. In contrast, mitotic figures, necrotic cells and coarse chromatin proved to be less useful. The presence of abnormal mitosis is very helpful when present; however, their overall rarity limits their utility.
Acknowledgments
We would like to thank Munir Hassen Idriss, MD for his valuable suggestions and help in this study.
References
- 1.Reagan JW, Seidemann IL, Saracusa Y. The cellular morphology of carcinoma in situ and dysplasia of atypical hyperplasia of the uterine cervix. Cancer. 1953;6:224. doi: 10.1002/1097-0142(195303)6:2<224::aid-cncr2820060203>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Reagan JW, Moore RD. Morphology of the malignant squamous cell. A study of six thousand cells derived from squamous cell carcinomas of the uterine cervix. Am J Pathol. 1952;28:105. [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyama C, Liu P, Ostrzega N, Holschneider CH. Histologic and immunohistochemical characteristics of neoplastic and nonneoplastic subgroups of atypical squamous lesions of the uterine cervix. Am J Clin Pathol. 2005;123:699. [PubMed] [Google Scholar]
- 4.Johnston EI, Logani S. Cytologic diagnosis of atypical squamous cells of undetermined significance in perimenopausal and postmenopausal women: lessons learned from human papillomavirus DNA testing. Cancer. 2007;111:160. doi: 10.1002/cncr.22687. [DOI] [PubMed] [Google Scholar]
- 5.Foraker AG, Reagan JW. Nuclear size and nuclear: cytoplasmic ratio in the delineation of atypical hyperplasia of the uterine cervix. Cancer. 1956;9:470. doi: 10.1002/1097-0142(195605/06)9:3<470::aid-cncr2820090307>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Böer-Auer A, Jones M, Lyasnichaya OV. Cytokeratin 10-negative nested pattern enables sure distinction of clonal seborrheic keratosis from pagetoid Bowen’s disease. J Cutan Pathol. 2012;39:225. doi: 10.1111/j.1600-0560.2011.01825.x. [DOI] [PubMed] [Google Scholar]
- 7.Prieto VG, Casal M, McNutt NS. Immuno-histochemistry detects differences between lichen planus-like keratosis, lichen planus, and lichenoid actinic keratosis. J Cutan Pathol. 1992;20:143. doi: 10.1111/j.1600-0560.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald LL, Yazdi HM. Fine-needle aspiration biopsy of bronchioloalveolar carcinoma. Cancer. 2001;93:29. [PubMed] [Google Scholar]
- 9.Demay RM, editor. The art & science of cytopathology. Vol. 1. Chicago: ASCP Press; 2012. The pap smear; p. 7. [Google Scholar]
- 10.Song J, Shea CR. Benign versus malignant parakeratosis: a nuclear morphometry study. Mod Pathol. 2010;23:799. doi: 10.1038/modpathol.2010.52. [DOI] [PubMed] [Google Scholar]
- 11.Batistatou A. Mitoses and cancer. Med Hypotheses. 2004;63:281. doi: 10.1016/j.mehy.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Abbas A, Fausto N, Aster J, editors. Robbins and Cotran pathologic basis of disease. Philadelphia: Elsevier; 2004. Neoplasia; p. 274. [Google Scholar]
- 13.Milikowski C, Berman I, editors. Color atlas of basic histopathology. New York: Appleton & Lange; 1997. Neoplasia; p. 38. [Google Scholar]
- 14.Steinbeck RG. Atypical mitoses interpretation in lesions of the oral mucosa: a new interpretation of their impact upon tumorigenesis. Science. 1997;33:110. doi: 10.1016/s0964-1955(96)00068-1. [DOI] [PubMed] [Google Scholar]
- 15.Barry M, Sinha S, Leader M, Kay E. Poor agreement in recognition of abnormal mitoses: requirement for standardized and robust definitions. Histopathology. 2001;38:68. doi: 10.1046/j.1365-2559.2001.01034.x. [DOI] [PubMed] [Google Scholar]
- 16.Mijovic T, Gologan O, Rochon L, et al. Fine-needle aspiration biopsy of the thyroid: review of cytopathologic features predictive of malignancy. J Otolaryngol Head Neck Surg. 2009;28:348. [PubMed] [Google Scholar]
- 17.Fischer AH, Taysavang P, Jhiang SM. Nuclear envelope irregularity is induced by RET/PTC during interphase. Am J Pathol. 2003;163:1091. doi: 10.1016/S0002-9440(10)63468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reagan JW. The cytological recognition of carcinoma in situ. Cancer. 1951;4:255. doi: 10.1002/1097-0142(195103)4:2<255::aid-cncr2820040208>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Sonis J. How to use and interpret interval likelihood ratios. Fam Med. 1999;31:432. [PubMed] [Google Scholar]
- 20.Camp NJ, Slattery ML. Classification tree analysis: a statistical tool to investigate risk factor interactions with an example for colon cancer (United States) Cancer Causes Control. 2002;13:813. doi: 10.1023/a:1020611416907. [DOI] [PubMed] [Google Scholar]
- 21.Jemec G, Miech D, Kemeny L. Non-surgical treatment of keratinocyte cancer. New York: Springer; 2010. [Google Scholar]
- 22.Milette F, Hurt MA, Ackerman AB. “Dysplasia” and “atypia”: impediments inordinate to understanding in pathology. New York: Ardor Shribendi; 2009. [Google Scholar]
- 23.Howell LP, Wright AL, Calafati SA, Rosen S, Koprowska I. Cytodiagnosis of in situ and early carcinoma of the upper gastrointestinal tract. Acta Cytol. 1985;29:269. [PubMed] [Google Scholar]
- 24.Bishop D, Lushpihan A, Louis C. The cytology of carcinoma in situ and early invasive carcinoma of the esophagus. Acta Cytol. 1977;21:298. [PubMed] [Google Scholar]
- 25.Zhao C, Domfeh AB, Austin RM. Histopatho-logic outcomes and clinical correlations for high-risk patients screened with anal cytology. Acta Cytol. 2012;56:62. doi: 10.1159/000331431. [DOI] [PubMed] [Google Scholar]
- 26.Bean SM, Chhieng DC, Roberson J, et al. Anal-rectal cytology: correlation with human papillomavirus status and biopsy diagnoses in a population of HIV-positive patients. J Low Genit Tract Dis. 2010;14:90. doi: 10.1097/LGT.0b013e3181ba9bcd. [DOI] [PubMed] [Google Scholar]
- 27.Longacre TA, Kong CS, Welton ML. Diagnostic problems in anal pathology. Adv Anat Pathol. 2008;15:263. doi: 10.1097/PAP.0b013e318183234b. [DOI] [PubMed] [Google Scholar]
- 28.Sherman ME, Paull G. Vaginal intraepider-mal neoplasia. Reproducibility of pathologic diagnosis and correlation of smears and biopsies. Acta Cytol. 1993;37:699. [PubMed] [Google Scholar]
- 29.Scarlett CJ, Salisbury EL, Biankin AV, Kench J. Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathology. 2011;43:183. doi: 10.1097/PAT.0b013e3283445e3a. [DOI] [PubMed] [Google Scholar]
- 30.Olemans C, Pierard-Franchimont C, Del-venne P, Pierard GE. Comparative karyome-try in Bowen’s disease and bowenoid papulosis. Anal Quant Cytol Histol. 1994;16:284. [PubMed] [Google Scholar]
- 31.Barton SP, Pearse AD, Marks R. Derivation of a dysplasia index for epidermal neoplasia. Dermatology. 1992;185:190. doi: 10.1159/000247445. [DOI] [PubMed] [Google Scholar]
- 32.Westra WH, Holmes GF, Eisele DW. Bizarre epithelial atypia of the sinonasal tract after chemotherapy. Am J Surg Pathol. 2001;25:652. doi: 10.1097/00000478-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Salomão DR, Mathers WD, Sutphin JE, Cuevas K, Folberg R. Cytologic changes in the conjunctiva mimicking malignancy after topical mitomycin C chemotherapy. Ophthalmology. 1999;106:1756. doi: 10.1016/S0161-6420(99)90355-X. [DOI] [PubMed] [Google Scholar]
- 34.Reddy VB, Ramsay D, Garcia JA, Kamino H. Atypical cutaneous changes after topical treatment with nitrogen mustard in patients with mycosis fungoides. Am J Dermatopathol. 1996;18:19. doi: 10.1097/00000372-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Giardina C, Renzulli G, Serio G, et al. Nuclear morphometry in node-negative breast carcinoma. Anal Quant Cytol Histol. 1996;18:374. [PubMed] [Google Scholar]
- 36.Gröntoft O, Hellquist H, Olofsson J, Nordström G. The DNA content and nuclear size in normal, dysplastic and carcinomatous laryngeal epithelium. A spectrophotometric study. Acta Otolaryngol. 1978;86:473. doi: 10.3109/00016487809107528. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg LH, Cox AJ, Abel EA. The mitotic index in psoriatic plaques and their response to PUVA therapy. Br J Dermatol. 1980;102:401. doi: 10.1111/j.1365-2133.1980.tb06552.x. [DOI] [PubMed] [Google Scholar]
- 38.Rico MJ, Halprin KM, Baker L, Cayer M, Taylor JR. Stimulated mitotic counts in the non-lesional skin of patients with psoriasis and controls. Br J Dermatol. 1985;113:185. doi: 10.1111/j.1365-2133.1985.tb02063.x. [DOI] [PubMed] [Google Scholar]