Abstract
Herpes Simplex virus, type 1 (HSV-1) causes cold sores, keratitis and rarely, fatal encephalitis. The infection is life long, with sensory ganglia serving as reservoirs of latent infection. Recently, exposure to HSV-1 has also been repeatedly associated with reduced cognitive function among healthy individuals without prior encephalitis. Though HSV-1 does not elevate risk for schizophrenia (SZ) per se, exposure is likewise associated with impaired cognitive functions among SZ patients. The range of cognitive changes observed in HSV-1 exposed persons has not been investigated systematically, nor is it known whether interaction between HSV-1 exposure and SZ related factors contributes to the impairment among SZ patients. Persons with or without schizophrenia/schizophreniform disorder (N = 298 total, DSM IV criteria) were assessed for HSV-1 exposure using serum HSV-1 antibody titers. The Penn Computerized Neurocognitive battery was used to assess eight cognitive domains with respect to accuracy and speed. There were no significant case-control differences in HSV-1 exposure. The SZ/schizophreniform disorder cases were significantly impaired in all cognitive domains compared with the controls. HSV-1 exposure was also associated with reduced cognitive function in the entire sample, but the magnitude of the effects and their patterns differed from the SZ related changes. Further, statistically significant interactions between HSV-1 exposure and SZ case status were not detected. HSV-1 exposure does not elevate risk for SZ, but it is associated with reduced function in specific cognitive domains regardless of SZ diagnostic status. An ‘epidiagnostic’ model for the association is proposed to explain the results.
Keywords: schizophrenia, herpes simplex, virus, cognition, HSV-1, memory
Introduction
Herpes Simplex Virus, type 1 (HSV-1), a human specific double stranded DNA virus infects mucosal membranes, corneal tissues and the central nervous system (CNS). Beginning in the intra-uterine period, HSV-1 exposure increases cumulatively with age, with exposure rates exceeding 70% in older adults (Smith & Robinson, 2002). Following primary infection through mucosal membranes, HSV-1 virions migrate to the trigeminal ganglion located within the blood brain barrier, culminating in lifelong cycles of latent infection and reactivation in the CNS (Cleator & Klapper, 2004). While latent infection is asymptomatic and has been considered to be harmless, viral particles replicate during the reactivation phase and migrate along sensory nerves to cause recurrent mucosal and skin lesions such as ‘cold sores’ and rarely, encephalitis (Steiner, Kennedy, & Pachner, 2007). The trigeminal ganglia (located inside the blood brain barrier) are favored sites for latent HSV-1 infection, but viral DNA has also been found in the fronto-temporal gray matter in 34% of individuals dying from causes other than HSV-1 encephalitis, suggesting that cortical brain regions may be targeted for persistent infection (Baringer & Pisani, 1994).
Because it can cause lifelong infection in the CNS, HSV-1 infection is a plausible risk factor for cognitive impairment (Nelson & Demmler, 1997). While severe cognitive impairment and other neurological deficits are prominent sequelae among survivors of HSV-1 induced encephalitis, Becker has suggested that such dysfunction can occur even in the absence of acute HSV-1 encephalitis, because repeated reactivation can lead to neuronal cell death (Becker, 1995). Consistent with Becker’s predictions, two studies of apparently healthy individuals and one community based study indicate that reduced cognitive function does occur in HSV-1 exposed individuals (Dickerson et al., 2008) (Strandberg, Pitkala, Linnavuori, & Tilvis, 2003) (Watson et al., 2012); one other community based study did not detect a significant association (Aiello, et al, 2006). Though HSV-1 has not convincingly been demonstrated as a risk factor for schizophrenia (SZ), five other studies have reported alterations in working memory as well as executive functions among SZ patients exposed to HSV-1; we are not aware of any studies that reported non-significant associations (Dickerson et al., 2003); (Shirts et al., 2008); (Yolken, Torrey, Lieberman, Yang, & Dickerson, 2011); (Gur et al., 2007b); (Watson et al., 2012); (Schretlen et al., 2010) (reviewed by Prasad, Watson, Dickerson, Yolken, & Nimgaonkar, 2012b)). Among HSV-1 exposed first-episode antipsychotic-naïve schizophrenia patients, a temporal decline in executive function has also been reported (Prasad et al., 2011). In a previous study of 413 individuals with schizophrenia, it was found that individuals who had levels of CRP>=5.0 µg/ml had significantly lower cognitive scores than individuals with schizophrenia who did not have elevated CRP levels (Dickerson et al., 2007).
Three MRI studies indicate reduced gray matter volume in fronto-temporal regions among persons with early course schizophrenia exposed to HSV-1 infection (SZ) (Prasad, Shirts, Yolken, Keshavan, & Nimgaonkar, 2007) (Schretlen et al., 2010) (Pandurangi, Pelonero, Nadel, & Calabrese, 1994). Recently, a double-blind placebo-controlled trial among HSV-1 exposed SZ patients indicated improvement in cognitive performance after antipsychotic medications were supplemented with valacylovir (a specific anti-herpes medication) or placebo (Prasad et al., 2012a; Prasad et al., 2012b). Another study also reported impaired cognitive function among patients with bipolar I disorder exposed to HSV-1 compared with unexposed patients (Dickerson et al., 2004). A substantial body of evidence thus supports the notion that persistent HSV-1 infection is associated with impaired cognitive function among persons with or without psychiatric illnesses (Prasad et al., 2012b). However, the range of cognitive changes has not been evaluated comprehensively because several studies have used composite measures of cognitive function (reviewed by Prasad et al., 2012b). Therefore, in the present study a range of cognitive domains were evaluated using a computerized neurocognitive battery (Gur et al., 2001b). It is also unclear whether individuals with SZ are particularly prone to the cognitive effects observed in relation to HSV-1 infection, reflecting interactions between HSV-1 exposure and other illness related factors that impair cognitive function. The present study was thus conducted in order to evaluate ‘epidiagnostic’ interaction between SZ/schizophreniform disorder and HSV-1 exposure, in other words, whether the cognitive impairment well known in SZ/schizophreniform disorder patients reflect an interaction between HSV-1 exposure and other illness related variables. We investigated HSV-1 exposure and cognitive function in a group of patients with SZ/schizophreniform disorder, as well as controls screened for the absence of SZ/schizophreniform disorder.
Methods
Design
The study included individuals with schizophrenia and non-psychotic control participants. A series of univariate analyses were initially used to evaluate associations between individual cognitive variables and diagnostic status (SZ/control), as well as HSV-1 exposure (present/absent), including demographic variables as covariates. For cognitive variables that were significantly associated with diagnostic status as well as HSV-1 exposure, interactions between diagnostic status and HSV-1 exposure were tested further.
Site
The study was conducted at the Department of Psychiatry, Post-graduate Institute of Medical Education and Research (PGIMER) - Dr Ram Manohar Lohia Hospital, Delhi (RMLH), a publicly funded facility that caters to all strata of Delhi.
Recruitment strategy
Individuals diagnosed with SZ/schizophreniform disorder were referred by treating clinicians for screening and inclusion in the study. For inclusion in the control group, healthy individuals without a history of SZ/schizophreniform disorder or substance abuse were sought from communities in Delhi and evaluated in the same manner as the cases (Thomas et al., 2007). The control sample was recruited from all socio economic groups by approaching community leaders, non-governmental community organizations and physicians working in the area. Individuals with a history of substance abuse, medical or neurological disorders (head injury, encephalitis, and epilepsy) and those unable to complete cognitive tests were excluded from the SZ /schizophreniform disorder case and the control groups. So the SZ cases referred henceforth include schizophreniform disorder cases also in our sample.
Clinical assessment
The primary diagnostic interview schedule was the Hindi version of the Diagnostic Interview for Genetic Studies (DIGS) (Deshpande et al., 1998), a semi-structured interview schedule. The DIGS was administered to all the cases and diagnosis was established on the basis of DSM IV criteria by a team of psychiatrists and psychologists after discussing the detailed information. Schizophreniform disorder was diagnosed when the symptom criteria for schizophrenia were met, but the duration was less than six months, and social and occupational decline was uncertain. The DIGS was administered to all controls in the same manner as the cases.
Cognitive assessment
The University of Pennsylvania Neurocognitive Computerized Battery (Penn CNB) has been validated to yield quantitative measures of cognitive domains in healthy subjects (Gur et al., 2001a, 2010, 2012). It has also been used to show deficits in persons with SZ (Gur et al., 2001a) and in probands and family members (Gur et al., 2007, Calkins et al., 2010, 2013).
Cognitive domains assessed
Abstraction and mental flexibility (ABF)
The Penn Conditional Exclusion Test measures concept formation and flexibility. Subjects decide which of four objects does not belong with the other three. The 3 sorting principles change, and feedback is used to develop new strategies.
Attention (ATT)
The Penn Continuous Performance Test (Penn CPT) uses a CPT paradigm where participants respond to 7-segment displays whenever one forms a digit or letter.
Working Memory (WM)
The Letter N Back Test consists of 0-back, 1-back and 2-back conditions.
Face Memory (FMEM)
The Penn Face Memory Test presents 20 digitized faces that are then mixed with 20 distractors equated for age, gender and ethnicity. The procedure is repeated at 20 min delay.
Spatial Memory (SMEM)
The Visual Object Learning Test uses Euclidean shapes as stimuli with the same paradigm as the word and face.
Spatial Processing (SPA)
Judgment of Line Orientation presents two lines at an angle, and participants indicate the corresponding lines on a simultaneously presented array.
Sensory-motor Dexterity (S-M)
The task requires moving the cursor to click as quickly as possible on a green square that disappears after the click. The square gets increasingly smaller.
Emotion Processing (EMO)
Facial displays of 4 emotions (Happy, Sad, Anger, Fear) and Neutral faces, 8 each, are presented and the subject identifies the emotion in a multiple-choice format. The facial stimuli are balanced for gender, age, and ethnicity.
We used a Hindi version of the Penn CNB that does not include verbal memory and language processing (Bhatia et al., 2012) as Hindi versions of these tests were not available. All instructions for the CNB were provided in Hindi for clarity. Each cognitive domain in the Penn CNB has three indices: accuracy, speed and efficiency. For the present study, we used the data generated from the indices of accuracy and speed, as the efficiency variables are composites derived from the speed and accuracy indices (Weiner et al., 2011). The CNB domain scores were normalized using data obtained from Indian control individuals (Bhatia et al., 2012).
Immunological assays
Each participant provided a venous blood sample to assess HSV-1 exposure based on antibodies to HSV-1 in the serum (Whitley, 2011). Serological analysis was performed in a certified commercial laboratory (SRL, India, http://www.srl.in/srl/srl.asp) using Euroimmun anti-HSV-1 Type specific glycoprotein C1 Elisa (IgG) kits. The sensitivity and specificity of IgG ELISA Euroimmun are 91% and 90% respectively, compared to an anti PT-IgG in house-ELISA (Riffelmann, Thiel, Schmetz, & Wirsing von Koenig, 2010). For quality control, 28 randomly selected samples were analyzed in duplicate. The duplicate samples matched perfectly with regard to the categorical HSV-1 status (positive, negative or equivocal). There were also significant correlations for antibody titers between the values of samples provided in duplicate (r=0.921, p< 0.001). All but one of the repeat samples matched for HSV-1 status (positive/negative). Based on these analyses, the reference ranges are as follows: less than 16.0 international units (negative), 16 to 100 (equivocal) and above 100 (positive).
Statistical analysis
Case-control differences were assessed using the Student’s t-test, chi-square statistics or non-parametric tests as appropriate. Relationships between HSV-1 exposure status and cognitive function were analyzed using univariate statistics. All analyses were conducted using the Statistical Package for Social Sciences version 20.0 (SPSS 20).
The Ethics Committee at RMLH, as well as the University of Pittsburgh Institutional Review Board approved the study. All participants provided written informed consent after the experimental procedures were explained.
Results
Case-control comparisons for demographic variables and HSV-1 exposure
The sample included 198 cases (SZ n=171; Schizophreniform n=27) and 100 controls (Table 1). There were significantly more men among the cases, and the cases were less likely to be married (p = 0.005 and p = 0.002, respectively). Age, duration of education or HSV-1 exposure status did not significantly differ between the cases and controls. The duration of illness was not significantly different between the HSV-1 exposed and unexposed cases (exposed: 282.76 ± 284.41 weeks, not exposed: 226.17 ± 280.56 weeks; mean ± standard deviation). Patients exposed to HSV-1 also did not significantly differ from those not unexposed with regard to the following items from the DIGS questionnaire: age at onset of psychotic features, global assessment of function (GAF) scores pattern of psychotic symptoms, pattern of severity, delusions (present/absent), auditory hallucination (present/absent), visual hallucinations (present/absent) (data not shown). All the patients with schizophrenia patients were receiving antipsychotic treatment at the time of the study, but the patients with schizophreniform disorder were either not on treatment or had just started treatment.
Table 1.
Clinical and demographic characteristics of the sample.
| Cases (N = 198) |
Controls (N = 100) |
t-value/ X2 value |
p-value | |
|---|---|---|---|---|
| Age | 30.79 (9.1) | 32.4 (9.6) | −1.375 | 0.170 |
| Education (years) | 10.01 (4.2) | 10.7 (5.7) | −1.126 | 0.215 |
| Gender (Male/ female) | 134/64 (67.7/32.3%) | 51 /49 (51/49%) | 7.850 | 0.005 |
| Marital status (Ever married/ never married) | 85/111 (43.4/56.6%) | 60/36 (62.5/37.5%) | 9.436 | 0.002 |
| HSV-1 Status (positive/negative) | 105/87 (55/45%) | 54/38 (59/41%) | 0.41 | 0.306 |
Standard deviations or proportions are provided in brackets. HSV-1 status was ambiguous for 13 cases; they were not used for case-control comparisons. Data regarding marital status of 2 cases and 4 controls were unavailable.
Variables associated with cognitive performance
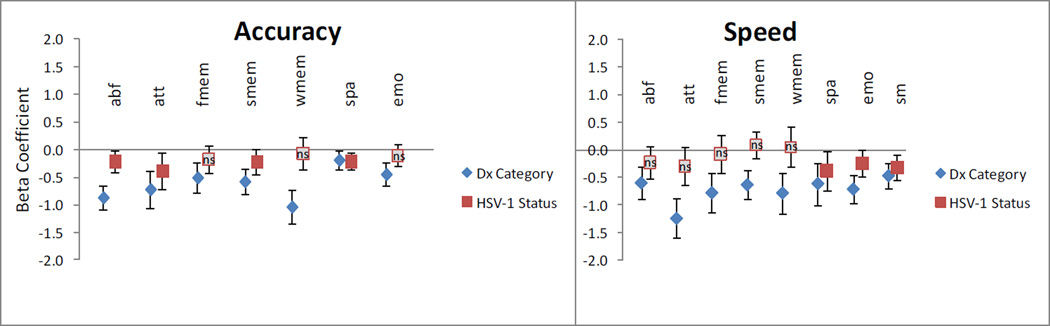
To evaluate the relative impact of case-control status and HSV-1 exposure on cognitive function, a series of linear regression analyses were conducted. The normalized value for each cognitive domain (accuracy or speed estimate) was used as the outcome variable in each analysis, with diagnostic status (SZ case or control), HSV-1 exposure (exposed or unexposed), age and gender as covariates. The sensorimotor dexterity test was used for training; therefore the accuracy component of this domain was not used for the analyses. Speed and accuracy for all the cognitive domains in the Penn CNB were significantly associated with diagnostic status, with cases performing less well than the controls (Figure 1 and Supplementary Table 1). Age and gender were not significantly associated with HSV-1 exposure.
Figure 1.
Associations between HSV-1 exposure and cognitive impairment.
Cognitive domains tested: Abstraction and mental flexibility (abf), Attention (att), Face memory (fmem), Spatial memory (smem), Working memory (wmem), Spatial ability (spa), Sensorimotor dexterity (sm), Emotion processing (emo).
A series of linear regression analyses were conducted, with each cognitive domain as the outcome, and the following variables as covariates: age, gender, diagnostic status (control/ schizophrenia) and HSV-1 exposure (present / absent). To illustrate the relative sizes of the key covariates associated with cognitive function, beta coefficients for diagnostic status (shown as diamonds) and HSV-1 exposure (shown as squares). All associations were statistically significant, except where indicated (ns: not significant). The impact of age and gender in the analyses is not shown (see Supplementary Table 1). Sensorimotor dexterity was analyzed for the speed component only.
Associations between cognitive variables and HSV-1 exposure varied when the entire case-control sample was analyzed. For accuracy estimates, HSV-1 exposed individuals performed less well than unexposed individuals with regard to abstraction and mental flexibility, attention, spatial memory and spatial processing (p ≤0.05). With regard to speed estimates, HSV-1 exposed persons performed less well with regard to spatial processing, emotional processing and sensorimotor dexterity. No significant associations were noted for the other speed related cognitive variables (Figure 1 and Supplementary Table 1).
For the variables significantly associated with diagnostic status as well as HSV-1 exposure, linear regression analyses were repeated using the outcome variable of interest and the covariates listed above, with the addition of a variable indicating interaction between diagnostic and exposure status. The interaction effect did not significantly predict any of the variables (Supplementary Table 2).
Discussion
The pattern of associations with HSV-1 exposure suggests modest and localized, rather than global deficits in cognitive function. Reduced function in six accuracy or speed for cognitive domains was associated with HSV-1 exposure, out of a total of fifteen variables assessed. The changes occurred for five accuracy variables and three variables related to speed of responses. The associations varied in magnitude across the cognitive domains; the largest effect sizes were observed in relation to attention (accuracy variable) and spatial processing (speed). Other domains, such as facial memory were not significantly associated with HSV-1 exposure. The results are consistent with earlier cross-sectional studies that indicate HSV-1 associated changes predominantly in attention and working memory (reviewed in Prasad et al., 2012b). Using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) battery, Dickerson and her colleagues reported reduced function in immediate memory, visuospatial / constructional tests, and attention among healthy individuals and patients with bipolar disorder (Dickerson et al., 2004). Our prior study of patients with US SZ patients indicated slower time to completion and larger number of errors with the Trail making test, part B; this test reflects attention and working memory (Shirts et al., 2008). Others have reported total scores on tests that also reflect attention and different indices of memory, such as the RBANS (Dickerson et al., 2003) and the Mini-Mental State Examination (MMSE) (Strandberg et al., 2003). One study reported reduction in verbal memory scores; this domain was not assessed in the present study (Schretlen et al., 2010). To our knowledge, only one study that investigated a community based sample of elderly Mexican-Americans did not detect significant associations between HSV-1 exposure and cognitive function, this study used the MMSE alone (Aiello et al., 2006).
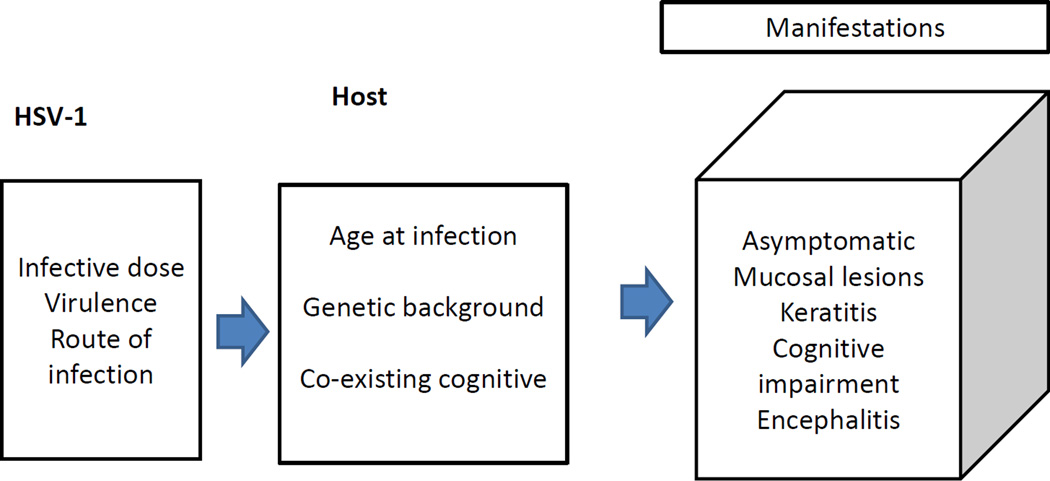
The profile of HSV-1 associated cognitive changes differs from the pattern with regard to SZ diagnostic status; the changes are also smaller in magnitude (Figure 1). No significant interaction was observed between HSV-1 exposure and SZ diagnostic status, though such an effect may be detectable in larger samples. While the published observations support several Bradford Hill criteria used to assess a causal relationship (Prasad et al., 2012b), they do not conclusively prove such a relationship, nor do they explain the range and pattern of abnormalities noted. A number of pathogenic mechanisms could explain the observed associations. Cognitive dysfunction could be due to cumulative damage to the brain from cycles of latency/reactivation during persistent adult infection, host immunological reactions during reactivation or developmental abnormalities following prenatal infection (Prasad et al., 2012b). It is unlikely to represent sequelae of prior acute encephalitis, which is rare (estimate 2–4/100,000/year). We have attempted to represent the range of abnormalities observed in relation to HSV-1 infections (Figure 2). In this model, the range, the type and severity of clinical effects following HSV-1 infection vary, based on individual innate susceptibility or other risk factors for cognitive impairment. Several studies can be conducted to test predictions based on this model. Prospective incident cohort studies are needed to definitively test causal links between HSV-1 exposure and cognitive dysfunction.
Figure 2.
Spectrum of effects observed in relation to infection with Herpes Simplex virus, type 1/.
There were no significant associations between HSV-1 exposure and a range of clinically relevant variables. Whether HSV-1 exposure is associated with changes in day to day function in addition to the reduced cognitive function is another important question. If a causal relationship between HSV-1 exposure and reduced cognitive function is present, it represents a clinical problem with broad clinical implications for the entire community.
It is unclear why the association with exposure has a greater effect size among the patients with schizophrenia. We favor the possibility that it occurs because the patients have reduced ‘cognitive reserve’ due to illness related variables. In support, patients with bipolar disorder, another group with illness related cognitive impairments also show the association (Dickerson et al, 2008). Another possibility is host susceptibility, such as genetic variants over-represented among the patients, that predisposes them to greater cognitive impairment. We did not conduct genetic analysis in this manuscript as the sample size was relatively small for genome wide analyses. Notably, Dickerson et al (2008) reported association of an exonic polymorphism of the Catechol-O-methyl transferase (COMT) gene with cognitive impairment as well as with HSV-1 exposure.
The prior published studies relating to HSV-1 exposure were all conducted in Europe or North America. Like other parts of the world, schizophrenia (SZ) can present as a chronic, disabling illness in India (Math, Chandrashekar, & Bhugra, 2007). The pattern of cognitive deficits among Indians with SZ is similar to those observed in other countries (Bhatia et al., 2012). Though prior studies suggest elevated prevalence of several viruses, including HSV-1 and CMV among Indian psychiatric inpatients (Srikanth et al., 1994), our analyses do not support a causal link between HSV-1 exposure and SZ risk per se in agreement with several other reports (Yolken & Torrey, 1995); (Brown & Derkits, 2010). The present analyses, the first in a developing country thus support the published associations in a very different environmental context. Dickerson et al. reported that the difference in cognitive functioning between HSV-1 positive and negative SZ patients could be attributed to immediate verbal memory, and that the difference in the controls by HSV-1 status was in delayed memory (Dickerson et al., 2003).We could not study the verbal memory domain in the CNB as it was unavailable in the Hindi version.
Several variables need to be considered when evaluating the observed associations. Since HSV-1 exposure rates vary by socio-economic status (SES), we sought control individuals from the same socio-economic strata as the patients. No significant case-control differences were noted with regard to educational status, used here as a proxy for SES. There was a relative excess of men among the patients, but gender did not account significantly for variation in cognitive function in this sample. A significant association between cognitive dysfunction and exposure to CMV, another herpes virus has also been reported in US samples (Shirts et al., 2008). CMV exposure was not assessed in the present sample and could contribute to the association observed here. Our preliminary analyses suggest that CMV exposure exceeds 90% among both cases and controls in India (Deshpande et al, unpublished). Thus, the impact of CMV exposure, if present would be expected to influence almost the entire sample, regardless of HSV-1 exposure. The patients analyzed here include those with multiple psychotic episodes. As some psychoactive drugs may impair cognitive function, treatment related variables are important. It is difficult to include drug treatment as a precise variable in the analyses, as the names and types of medications that the patients received were known, but the precise amounts they actually ingested or the plasma levels of the medications were not available. Therefore, treatment and other factors related to chronicity, such as medication effects may explain a portion of the cognitive impairments observed among the patients. Whether these factors interact with HSV-1 exposure is uncertain, though our statistical analyses do not provide any evidence in support. For example, the duration of illness was similar among HSV-1 exposed and unexposed persons.
In conclusion, we did not find a significant association between exposure to HSV-1 and risk for SZ. On the other hand, significant cognitive dysfunction was detected with respect to several variables among HSV-1 exposed patients and controls, compared with unexposed persons. The basis for the associations, as well as their impact on daily social functions needs to be explored further.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Baringer JR, Pisani P. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann Neurol. 1994;36:823–829. doi: 10.1002/ana.410360605. [DOI] [PubMed] [Google Scholar]
- Becker Y. HSV-1 brain infection by the olfactory nerve route and virus latency and reactivation may cause learning and behavioral deficiencies and violence in children and adults: a point of view. Virus Genes. 1995;10:217–226. doi: 10.1007/BF01701811. [DOI] [PubMed] [Google Scholar]
- Bhatia T, Agarwal A, Shah G, Wood J, Richard J, Gur RE, Gur RC, Nimgaonkar VL, Mazumdar S, Deshpande SN. Adjunctive cognitive remediation for schizophrenia using yoga: an open, non-randomized trial. Acta Neuropsychiatr. 2012;24:91–100. doi: 10.1111/j.1601-5215.2011.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Tepper P, Gur RC, Ragland JD, Klei L, Wiener HW, Richard J, Savage RM, Allen TB, O'Jile J, Devlin B, Kwentus J, Aliyu MH, Bradford LD, Edwards N, Lyons PD, Nimgaonkar VL/, Santos AB, Go RC, Gur RE. Project among African-Americans to explore risks for schizophrenia (PAARTNERS): evidence for impairment and heritability of neurocognitive functioning in families of schizophrenia patients. Am J Psychiatry. 2010;167(4):459–472. doi: 10.1176/appi.ajp.2009.08091351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Ray A, Gur RC, Freedman R, Green MF, Greenwood TA, Light GA, Nuechterlein KH, Olincy A/, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar C/, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL, Lazzeroni LC, Gur RE. Sex Differences in Familiality Effects on Neurocognitive Performance in Schizophrenia. Biol Psychiatry. 2013 Feb 5; doi: 10.1016/j.biopsych.2012.12.021. 2012.12.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleator GM, Klapper PE. Herpes simplex. In: Zuckerman AJ, Banatvala JE, Pattison J R, editors. Principles and Practice of Clinical Virology. New York: John Wiley & Sons, Ltd.; 2004. [Google Scholar]
- Deshpande SN, Mathur MN, Das SK, Bhatia T, Sharma S, Nimgaonkar VL. A Hindi version of the Diagnostic Interview for Genetic Studies. Schizophr Bull. 1998;24:489–493. doi: 10.1093/oxfordjournals.schbul.a033343. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1–3):261–265. doi: 10.1016/j.schres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Sullens A, Origoni A, Leister F, Krivogorsky B, Yolken R. Association between cognitive functioning, exposure to Herpes Simplex Virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain Behav Immun. 2008;22:1103–1107. doi: 10.1016/j.bbi.2008.04.156. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Cole S, Krivogorsky B, Yolken RH. Infection with herpes simplex virus type 1 is associated with cognitive deficits in bipolar disorder. Biol Psychiatry. 2004;55:588–593. doi: 10.1016/j.biopsych.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60:466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001a;25:777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001b;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007a;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007b;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;30;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Math SB, Chandrashekar CR, Bhugra D. Psychiatric epidemiology in India. Indian Journal of Medical Research. 2007;126:183–192. [PubMed] [Google Scholar]
- Nelson CT, Demmler GJ. Cytomegalovirus infection in the pregnant mother, fetus, and newborn infant. Clin Perinatol. 1997;24:151–160. [PubMed] [Google Scholar]
- Pandurangi AK, Pelonero AL, Nadel L, Calabrese VP. Brain structure changes in schizophrenics with high serum titers of antibodies to herpes virus. Schizophr Res. 1994;11:245–250. doi: 10.1016/0920-9964(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Eack SM, Goradia D, Pancholi KM, Keshavan MS, Yolken RH, Nimgaonkar VL. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: a longitudinal study. Am J Psychiatry. 2011;168:822–830. doi: 10.1176/appi.ajp.2011.10101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Eack SM, Keshavan MS, Yolken RH, Iyengar S, Nimgaonkar VL. Antiherpes Virus-Specific Treatment and Cognition in Schizophrenia: A Test-of-Concept Randomized Double-Blind Placebo-Controlled Trial. Schizophr Bull. 2012a doi: 10.1093/schbul/sbs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12:105–113. doi: 10.1038/sj.mp.4001915. 1. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Watson AM, Dickerson FB, Yolken RH, Nimgaonkar VL. Exposure to herpes simplex virus type 1 and cognitive impairments in individuals with schizophrenia. Schizophr Bull. 2012b;38:1137–1148. doi: 10.1093/schbul/sbs046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffelmann M, Thiel K, Schmetz J, Wirsing von Koenig CH. Performance of commercial enzyme-linked immunosorbent assays for detection of antibodies to Bordetella pertussis. J Clin Microbiol. 2010;48:4459–4463. doi: 10.1128/JCM.01371-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Vannorsdall TD, Winicki JM, Mushtaq Y, Hikida T, Sawa A, Yolken RH, Dickerson FB, Cascella NG. Neuroanatomic and cognitive abnormalities related to herpes simplex virus type 1 in schizophrenia. Schizophr Res. 2010;118:224–231. doi: 10.1016/j.schres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106:268–274. doi: 10.1016/j.schres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Ravi V, Poornima KS, Shetty KT, Gangadhar BN, Janakiramaiah N. Viral antibodies in recent onset, nonorganic psychoses: correspondence with symptomatic severity. Biol Psychiatry. 1994;36:517–521. doi: 10.1016/0006-3223(94)90615-7. [DOI] [PubMed] [Google Scholar]
- Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34:2126–2131. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- Thomas P, Mathur P, Gottesman II, Nagpal R, Nimgaonkar VL, Deshpande SN. Correlates of hallucinations in schizophrenia: A cross-cultural evaluation. Schizophr Res. 2007;92:41–49. doi: 10.1016/j.schres.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AM, Prasad KM, Klei L, Wood JA, Yolken RH, Gur RC, Bradford LD, Calkins ME, Richard J, Edwards N, Savage RM, Allen TB, Kwentus J, McEvoy JP, Santos AB, Wiener HW, Go RC, Perry RT, Nasrallah HA, Gur RE, Devlin B, Nimgaonkar VL. Persistent infection with neurotropic herpes viruses and cognitive impairment. Psychol Med. 2012:1–9. doi: 10.1017/S003329171200195X. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Sadowsky C, Saxton J, Hofbauer RK, Graham SM, Yu SY, Li S, Hsu HA, Suhy J, Fridman M, Perhach JL. Magnetic resonance imaging and neuropsychological results from a trial of memantine in Alzheimer's disease. Alzheimers Dement. 2011;7:425–435. doi: 10.1016/j.jalz.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Whitley R. Herpes simplex virus infections. In: Goldman L, Schafer AI, editors. Cecil Medicine. chap 382. Philadelphia: Saunders Elsevier; 2011. [Google Scholar]
- Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res. 2011;128:61–65. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.