Summary
Background
Pseudoxanthoma elasticum (PXE) is characterized by aberrant mineralization of connective tissues, causing considerable morbidity and mortality. The disease is typically of late onset, the skin manifestations first being noted in teens or later. Another aberrant mineralization disorder, generalized arterial calcification of infancy (GACI), is present at birth and can demonstrate a phenotypic overlap with PXE.
Objectives
A patient with PXE was noted to have skin findings as early as at 6-years of age, with cardiovascular involvement. The purpose of this study was to examine the genetic basis of this phenotypic presentation in the spectrum of PXE/GACI.
Methods
The patient’s genotype was studied by sequencing ABCC6 and ENPP1, genes known to be associated with PXE and/or GACI.
Results
Screening of the ABCC6 gene revealed two pathogenetic mutations, p.R1141X and g.del23-29. Analysis of the ENPP1 gene failed to demonstrate the presence of mutations.
Conclusions
This study demonstrates the presence of cutaneous findings of PXE in an 8-year old pediatric patient, with cardiovascular involvement, illustrating the phenotypic spectrum of PXE.
Introduction
Pseudoxanthoma elasticum (PXE) is a rare multisystem disorder characterized by aberrant mineralization manifesting primarily in the skin, the eyes, and the cardiovascular system1,2. The early skin findings consist of yellowish papules on the predilection sites, such as the sides of the neck, axillary areas, and antecubital fossae. Characteristic ocular findings include angioid streaks and retinal neovascularization, which can result in loss of visual acuity and lead to blindness in a significant number of patients if left untreated. The cardiovascular manifestations consist primarily of calcification of arterial blood vessels in the lower extremities. The clinical manifestations are of late onset and slowly progressive, and the definitive diagnosis can often be made on the basis of cutaneous, ocular, histopathologic and genetic considerations3.
PXE, an autosomal recessive disorder, is typically associated with mutations in the ABCC6 gene which encodes a putative efflux transporter expressed primarily in the liver and kidneys, and consequently, PXE is considered to be a metabolic disorder2. However, the precise pathomechanistic pathways leading from mutations in the ABCC6 gene to aberrant mineralization in the peripheral tissues are currently unknown, and no effective or specific treatment for the systemic manifestations of this disorder is currently available.
Another heritable mineralization disorder, generalized arterial calcification of infancy (GACI), is often diagnosed by prenatal ultrasound or shortly after birth4,5. The extensive vascular manifestations in GACI result in early demise of the affected individuals usually within the first year of life. The majority of cases with GACI were initially shown to harbor mutations in the ENPP1 gene which encodes a phosphatase capable of converting ATP to AMP and PPi, the latter being a powerful anti-mineralization factor. More recently, cases with GACI have also been shown to harbor mutations in the ABCC6 gene6. A number of patients with clinical features of both PXE and GACI have also been reported, suggesting a spectrum of aberrant mineralization due to mutations in the ABCC6 and ENPP1 genes7,8.
In this brief case study we report a patient with PXE with several interesting and unusual features, including overlap with the GACI phenotype.
Materials and methods
Blood samples were obtained from the patient and three family members, including an older unaffected brother and parents who are obligate heterozygote carriers. For mutation analysis, DNA was isolated by standard procedures, polymerase chain reaction (PCR) primers were utilized to amplify segments of the ABCC6 and ENPP1 genes, and the PCR products were sequenced by using automated sequencer 3730 (Applied Biosystems, Foster City, CA, USA). The primer sequences for amplification of these genes are available from the corresponding author upon request. Allelic nucleotide sequence variants were compared with the sequences in the exome variant server and dbSNP (NCBI) databases. The presence for g.del23-29 deletion mutation in the ABCC6 gene was examined, as described previously9.
Results
Clinical aspects. An 8-year old female was seen at the University of Miami Pediatric Dermatology Clinic with no significant past medical history, except that the parents reported low exertion tolerance and mild shortness of breath. The mother reported no episodes of cyanosis, diaphoresis, syncope, chest pain, or palpitations. There was no evidence for congenital heart disease, premature coronary artery disease, systemic hypertension, or hyperlipidemia. Gastrointestinal, hematologic and neurologic examinations were unremarkable. The mother stated that she had noted a rash on the patient’s skin for at least two years.
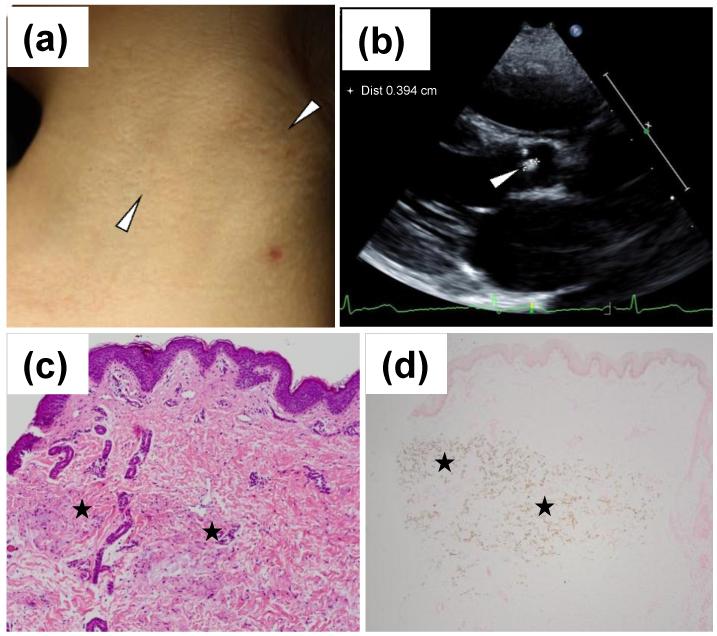
On physical examination, skin findings consistent with PXE were noted consisting of yellowish papules coalescing into thin cobblestone plaques bilaterally on the sides of the neck (Fig. 1a). A skin biopsy from the lesional area revealed the presence of pleiomorphic elastic structures with evidence of calcification by hematoxylin-eosin and von Kossa stains (Fig. 1c,d). The patient had normal fundoscopic eye examination with no evidence of angioid streaks or peau d’orange. Cardiovascular examination was significant for decreased radial and femoral pulses bilaterally. On cardiac examination, there was a single S1, and S2 was normally split with a normal P2 component. There were no cardiac clicks, however, there was a grade 2/6 vibratory systolic ejection murmur at the mid-left sternal border. A 12-lead electrocardiogram revealed normal sinus rhythm, no atrial enlargement and no ventricular hypertrophy. ST-wave changes were not present. An echocardiogram revealed an echo bright area on the aortic valve (Fig. 1b). On the basis of these results, the diagnosis of PXE with possible aortic valve and arterial calcification was made.
Figure 1.
Cutaneous presentation, histopathology and echocardiographic findings in the heart of a pediatric patient with PXE. Skin findings are characterized by yellowish papules and plaques on the side of the neck (a; arrowheads). Histopathology by hematoxylin-eosin stain (c) and by von Kossa stain (d) reveals aberrant mineralization in the mid dermis (asterisks). Echocardiography reveals an echo bright area on the aortic valve (b, arrowhead).
The patient had an older 11-year old brother with no apparent skin rash, and the parents were unrelated, clinically normal, with no evidence of skin or cardiovascular findings related to PXE (Fig. 2a).
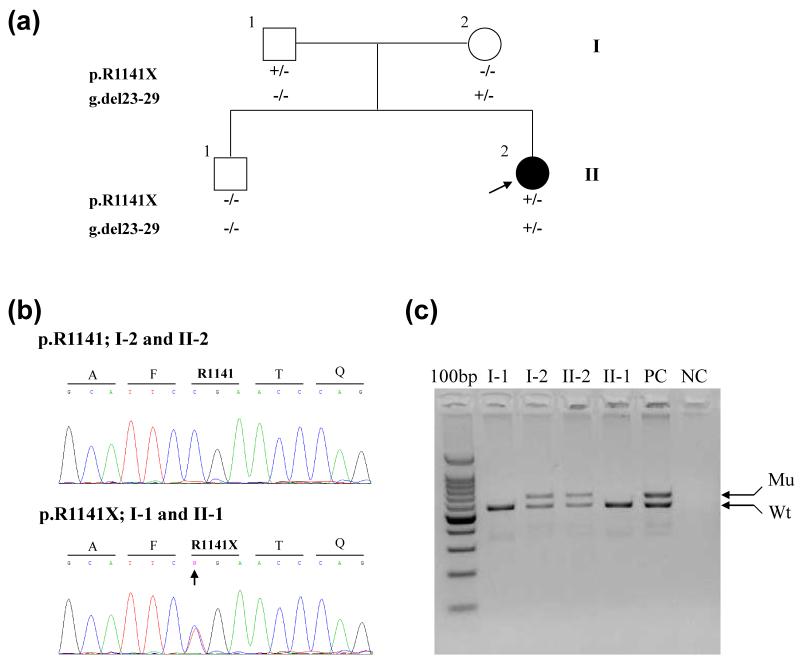
Figure 2.
Nuclear pedigree and mutation detection in the ABCC6 gene. The proband, an 8-year old female (II-2), is indicated by an arrow (a). Mutation analysis revealed that she is a compound heterozygote for the p.R1141X nonsense mutation (b, arrow) and the g.del23-29 deletion mutation (c, Mu). The parents, I-1 and II-2, are heterozygous carriers of the corresponding mutations, and the older brother (II-1) has the wild-type (Wt) alleles only. “+” refers to the presence of the mutation, and “−” to its absence.
Mutation Analysis. Based on putative clinical diagnosis of PXE, mutation analysis in the ABCC6 gene was initiated. The screening of the ABCC6 gene consisted of sequencing the “hotspots” (exons 18, 21, 24, 27 and 28, as well as g.del23-29) and revealed pathogenetic compound heterozygous mutations p.R1141X and g.del23-29 (Fig. 2b,c); the rest of the gene was not sequenced. Both mutations have been previously reported in patients with PXE10. The combination of characteristic skin findings and histopathology, together with ABCC6 mutations confirmed the diagnosis of PXE in the proband. Analysis of the ENPP1 gene by sequencing all 25 exons and the flanking intronic sequences failed to identify mutations.
Discussion
The patient presented in this case study has cutaneous findings and skin histopathology consistent with PXE, and the diagnosis was confirmed by identification of two mutations, g.del23-29 and p.R1141X, in the ABCC6 gene; these findings allow the diagnosis of PXE3.
There are several interesting features in this case. First, PXE is characteristically of late onset, and the diagnosis is usually made in mid-teens but occasionally not until the patient is in his/her 20s or 30s1. This patient’s first clinical findings were noted around 6 years of age. While cases of pediatric PXE have been reported, there appears to be a phenotypic overlap with GACI, a disorder which in its typical form is characterized by extensive vascular calcification. The majority of patients with GACI die within the first year of life from cardiovascular and renal complications5-7. Some patients with GACI have also been noted to have cutaneous findings consistent with PXE, and in many of these cases mutations in the ABCC6 gene have been disclosed4.
An unusual feature in this case is the presence of cardiovascular mineralization at the early stages of PXE, particularly mineralization in the aortic valve and possibly in radial and femoral arteries, although ultrasound examination of the extremities was not performed. The presence of cardiovascular mineralization in this patient with PXE again emphasizes the phenotypic spectrum of this disease.
Another interesting observation in this patient relates to the types of mutations disclosed in her ABCC6 gene. Specifically, the two mutations, g.del23-29 and p.R1141X, are the most common mutations accounting for ~14 and 30% of all ABCC6 mutations found in PXE10. These same mutations have been found in patients in the whole spectrum of the age at onset, with varied phenotypes, emphasizing the fact that no genotype/phenotype correlations have been established in larger cohorts of patients with PXE10. These observations suggest the influence of modifier genes, epigenetic factors, and environmental variables, including diet2,11. In this context, it should be noted that PXE-like cutaneous findings have been encountered in patients with mutations in the GGCX gene, associated with vitamin K-dependent coagulation factor deficiency, as well as in patients with β-thalassemia without detectable mutations in the ABCC6 gene12,13. Finally, the mineral composition of diet, particularly magnesium, has been shown to modulate the mineralization phenotype in Abcc6−/− mice, a model system recapitulating features of PXE14,15.
In conclusion, the present case illustrates the phenotypic spectrum of PXE, including the early onset of aberrant vascular mineralization in the pediatric population, and attests to the complexity of this, currently intractable disorder, at the genome/environment interface.
What is already known about this topic?
Pseudoxanthoma elasticum (PXE), an aberrant mineralization disease, characteristically has a late-onset of manifestations primarily with skin findings
Generalized arterial calcification of infancy (GACI) is characterized by extensive arterial calcification noted by prenatal ultrasound or at birth
While PXE and GACI in their classic forms are associated with mutations in the ABCC6 and ENPP1 genes, recently both genotypic and phenotypic overlap has been noted
What does this study add?
A patient was noted to have skin findings characteristic of PXE as early as at 6 years of age, associated with cardiovascular mineralization
Sequence analysis revealed compound heterozygosity for two mutations, g.del23-29 and p.R1141X, in the ABCC6 gene
This case illustrates the phenotypic spectrum of PXE, with early manifestations in the pediatric population with cardiovascular mineralization, with overlapping clinical features with GACI
Acknowledgements
We thank the family for their participation, and Carol Kelly for manuscript preparation.
Funding Sources: This study was supported by NIH/NIAMS Grant R01 AR28450 (JU). Dr. Li is the recipient of a Research Career Development Award from Dermatology Foundation.
Footnotes
Conflict of Interest: None declared
References
- 1.Neldner KH. Pseudoxanthoma elasticum. Clin. Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J. Invest. Dermatol. 2010;130:661–70. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plomp AS, Toonstra J, Bergen AA, et al. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am. J. Med. Genet. A. 2010;152A:1049–58. doi: 10.1002/ajmg.a.33329. [DOI] [PubMed] [Google Scholar]
- 4.Nitschke Y, Rutsch F. Genetics in arterial calcification: lessons learned from rare diseases. Trends Cardiovasc. Med. 2012;22:145–9. doi: 10.1016/j.tcm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Ruf N, Uhlenberg B, Terkeltaub R, et al. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI) Hum. Mutat. 2005;25:98. doi: 10.1002/humu.9297. [DOI] [PubMed] [Google Scholar]
- 6.Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitschke Y, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front. Genet. 2012;3:302. doi: 10.3389/fgene.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Schumacher W, Siegel D, et al. Cutaneous features of pseudoxanthoma elasticum in a patient with generalized arterial calcification of infancy due to a homozygous missense mutation in the ENPP1 gene. Br. J. Dermatol. 2012;166:1107–11. doi: 10.1111/j.1365-2133.2012.10811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Saux O, Beck K, Sachsinger C, et al. A spectrum of ABCC6 mutations is responsible for pseudoxanthoma elasticum. Am. J. Hum. Genet. 2001;69:749–64. doi: 10.1086/323704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfendner EG, Vanakker OM, Terry SF, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J. Med. Genet. 2007;44:621–8. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uitto J, Varadi A, Bercovitch L, et al. Pseudoxanthoma elasticum: progress in research toward treatment: summary of the 2012 PXE International Research Meeting. J. Invest. Dermatol. 2013 doi: 10.1038/jid.2013.20. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Vanakker OM, Martin L, Gheduzzi D, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J. Invest. Dermatol. 2007;127:581–7. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 13.Hamlin N, Beck K, Bacchelli B, et al. Acquired Pseudoxanthoma elasticum-like syndrome in beta-thalassaemia patients. Br. J. Haematol. 2003;122:852–4. doi: 10.1046/j.1365-2141.2003.04484.x. [DOI] [PubMed] [Google Scholar]
- 14.LaRusso J, Li Q, Jiang Q, et al. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) J. Invest. Dermatol. 2009;129:1388–94. doi: 10.1038/jid.2008.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Q, Uitto J. Restricting dietary magnesium accelerates ectopic connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) Exp. Dermatol. 2012;21:694–9. doi: 10.1111/j.1600-0625.2012.01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]