Abstract
Background
A comprehensive assessment of the association of patients’ renal replacement therapy (RRT) modality on their participation in life activities (physical function, travel, recreation, freedom, work) is needed.
Study Design
Systematic review of peer-reviewed published studies.
Setting & Population
Adults undergoing RRT (hemodialysis, peritoneal dialysis, or transplantation).
Selection Criteria for Studies
We searched PubMed, Cochrane Library, and EMBASE from January 1980 through April 2012 for English-language articles that compared participation in life activities among patients receiving 1) hemodialysis compared with peritoneal dialysis, 2) hemodialysis compared with kidney transplantation, or 3) peritoneal dialysis compared with kidney transplantation.
Predictor
RRT modality.
Outcomes
Reported rates of physical function, travel, recreation, freedom, and work-related activities by RRT modality.
Results
A total of 46 studies (6 prospective cohort, 38 cross-sectional, and 2 pre-post transplantation) provided relevant comparisons of life participation activities among patients treated with hemodialysis, peritoneal dialysis, and kidney transplantation. Studies were conducted from 1985 to 2011 among diverse patient populations in 16 distinct locations. A majority of studies reported greater life participation rates among patients with kidney transplants compared to patients receiving either hemodialysis or peritoneal dialysis. In contrast, a majority of studies reported no differences in outcomes between patients receiving hemodialysis and patients receiving peritoneal dialysis. These results were consistent throughout the study period, across diverse populations, and among the subset of studies that performed appropriate adjustments for potential confounding factors.
Limitations
Many studies included in the review had significant design weaknesses.
Conclusions
Evidence suggests patients with kidney transplants may experience better rates of life participation compared to patients receiving dialysis, while patients receiving hemodialysis and patients receiving peritoneal dialysis may experience similar rates of life participation. Rigorously performed studies are needed to better inform patients about the association of RRT on these important patient reported outcomes.
Keywords: dialysis, ESRD treatment, kidney transplantation, physical functioning, quality of life, social participation
Patients initiating renal replacement therapy (RRT) for end-stage renal disease (ESRD) experience significant morbidity and limitations in quality of life1,2. Limitations include often-substantial decrements in patients’ involvement in social and recreational activities, freedom, and abilities to work and travel, which have been associated with poorer overall health status and survival1–7. While their declining involvement in life activities may be attributed, in part, to patients’ significant ESRD-associated morbidity8, the extent to which patients’ mode of RRT might independently influence their life participation has not been well-quantified.
The various RRT modalities (hemodialysis, peritoneal dialysis and kidney transplantation) have distinct characteristics, including different delivery methods (e.g., treatment in a center versus at home), requirements for self-care (e.g., clinician directed versus self-directed), levels of physical invasiveness (e.g., need for catheters or surgery), and associated symptoms (e.g., fatigue with dialysis or transplantation medication side effects). Each of these RRT characteristics could substantially influence patients’ abilities to engage in social and recreational activities9–12, and they are frequently presented to patients as important factors they should consider while approaching decisions regarding initiating or switching RRT modalities13–17.
Prior studies suggested patients who undergo transplantation generally experience better quality of life than dialysis patients18–20, while there may be no significant differences for patients on hemodialysis compared with peritoneal dialysis21,22. However, these studies broadly examined quality of life without a specific focus on systematically examining the independent association of RRT modality with patients’ physical activity, freedom, and their abilities to participate in key activities of daily living, such as their abilities to work, travel, and participate in social and recreational activities, all important but distinct aspects which contribute to patients’ global quality of life. Patients with ESRD and their families view information about the influence of RRT selection on these life activities as important to include in educational material informing patients’ RRT selection decisions23. Systematic reviews summarizing evidence of associations between RRT modality choice and patients’ abilities to participate in these important life activities could therefore greatly enhance informed decisions about RRT selection.
We performed a systematic literature review to provide an evidence-based summary of the association of patients’ RRT modality with their rates of life participation activities across a variety of outcomes measures, settings, and patient populations.
Methods
Study Design
We performed a systematic review of published, peer-reviewed studies describing differences in rates of five types of activities reflecting various aspects of life participation (i.e., physical function, travel, recreation, freedom, and work outcomes) reported by adults with ESRD receiving different RRTs. We assessed factors that could influence the validity of study findings, and we quantified the direction and magnitude of differences in life participation outcomes among patients receiving different RRTs.
Populations Studied
Eligible articles reported on adults receiving RRT (hemodialysis, peritoneal dialysis, and kidney transplantation). Hemodialysis modalities considered eligible in our study included both in-center hemodialysis and ‘non-specific’ hemodialysis (i.e., patients on in-center hemodialysis plus one or more alternative modes of hemodialysis, such as satellite hemodialysis, home hemodialysis, nocturnal dialysis, etc.). We included both deceased donor and living donor kidney transplantation.
Data Sources and Literature Search Strategy
We identified studies potentially eligible for inclusion in our review through a search of all studies in PubMed, EMBASE, and the Cochrane Library (trials only) from January 1980 through April 2012. An expert methodologist and content experts within our team developed comprehensive search strategies to identify relevant studies. Our search terms consisted of key words for each treatment modality and terms for each of the five life participation outcomes. We hand-searched bibliographies of all potentially-relevant studies to identify additional articles that our electronic search might have missed. Our initial hand search of bibliographies revealed that there were missed studies reporting primarily on ‘quality of life’ outcomes but also reporting relevant life participation outcomes as secondary outcomes. Thus, we repeated our electronic search with additional terms consisting of key words to identify studies primarily reporting on ‘quality of life’ outcomes. We conducted this expanded search in all three databases and screened all studies for their potential inclusion in our review. The detailed search strategies are included within Table S1 (provided as online supplementary material).
We identified studies as reporting on physical function outcomes if they reported data on patients’ limitations in performing activities of daily living, patients’ self-reported physical functioning assessed via quality of life sub-scales (e.g., in SF-36), or other measures of physical activity. We identified studies as reporting on travel outcomes if they reported on patients’ travel abilities or restrictions. We identified studies as reporting on recreation outcomes if they reported on patients’ abilities to engage in recreational or social activities (e.g., in SF-36). We identified studies as reporting on freedom outcomes if they reported on patients’ perceived independence, ability to perform usual tasks, or intrusiveness. We identified studies as reporting on work outcomes if they reported on employment status or working capacity.
Study Inclusion and Exclusion Criteria, Data Extraction
We reviewed titles and abstracts of identified citations for potential inclusion. We then reviewed the full text of any citation deemed potentially relevant. We included studies if they reported on relevant outcomes (physical function, travel, recreation, freedom, and work) as a primary or secondary outcome, and if they compared relevant outcomes for participants on at least two different ESRD treatment modalities (i.e., hemodialysis, peritoneal dialysis, or kidney transplantation). We excluded articles if they 1) were not written in English, 2) did not include relevant outcomes, 3) included only participants younger than 18 years old, 4) contained no original data (i.e. review, commentary, editorial, meeting abstract, or letter) 5) were case reports, or 6) did not compare differences in relevant outcomes among patients receiving different RRT modalities. We also excluded studies of special populations (e.g., studies including only home hemodialysis patients but not in-center hemodialysis patients) to prevent expected small study size bias. For each article that met our inclusion criteria, two reviewers independently extracted data, including information on study design, follow-up, RRT modalities compared, locations, sample sizes, participant characteristics, and outcomes. Reviewers resolved disagreements by discussion and adjudication with a third party.
Classification of Study Designs
We classified eligible studies into one of four main design types: randomized controlled trial (RCT), longitudinal cohort (prospective/retrospective), cross sectional, and pre-post transplantation24. We classified a study as RCT if it contained two or more groups receiving different RRT modalities, and patients were randomly allocated to RRT modality as indicated by investigators. We classified a study as cohort if there were at least two groups receiving different RRT modalities (without random allocation), and investigators reported repeated assessments of relevant outcomes. Such studies could be prospective or retrospective in nature. We classified a study as cross-sectional if there were at least two groups receiving different RRT modalities (without random allocation), and investigators assessed relevant outcomes at only one point in time. Finally, we classified a study as pre-post transplantation if there was only a single group of kidney transplant recipients, and investigators reported relevant outcomes for patients both prior to and after receiving kidney transplants (i.e., at least two assessments of relevant outcomes with participants serving as their own controls). For this design, we only included studies where investigators explicitly described which RRT modality patients received prior to transplantation.
Assessment of Studies’ External and Internal Validity for Relevant Outcomes
Two reviewers used a modified version (Item S1) of a previously published instrument25 to independently assess studies’ reporting on factors which could influence the validity of findings, including studies’ external validity (i.e., inclusion and exclusion criteria, recruitment response rate) and factors influencing studies’ internal validity (i.e., potential for selection bias, validity and appropriateness of outcome assessment, and rigor of statistical analyses to account for potential confounding). We considered studies to have described the inclusion and exclusion criteria well if they clearly reported their criteria or if they specified that all consecutive subjects were enrolled. We also categorized studies’ response rates (<45%, 45%–59%, 60%–79%, or ≥80%), and we considered adequate response rates to be present if they reported 60% or greater response. We considered studies to have minimal potential for selection bias if investigators reported no significant or only minor differences in participant characteristics that could influence relevant outcomes. We considered assessments of relevant outcomes to be valid if studies clearly defined ascertainment of relevant outcomes using standard and previously validated instruments. We considered studies’ statistical analyses to have been appropriately conducted if analyses attempted to account for factors potentially confounding the association between participants’ RRT modality and relevant outcomes (e.g., using multivariable adjustment within regression models), or if important confounding was unlikely within studies. Two reviewers independently assessed study quality, and reviewers resolved disagreements with the aid of a third party.
Data Synthesis and Analysis
We decided a priori not to statistically combine results in a meta-analysis because we expected studies to be methodologically and clinically diverse. For instance, some studies reported outcomes as means on scales (e.g. SF-36 physical function score) while others reported the percentage of participants achieving a particular physical activity threshold or percentage of patients who were employed. Therefore, we qualitatively synthesized results for individual studies within summary evidence tables to help clarify the similarities and differences among studies that appear to address similar research questions across a variety of measures and patient populations.
In an effort to assess the magnitude and direction of reported associations in a standard manner across studies reporting these heterogeneous outcomes, we calculated Cohen’s d effect size indices and 95% confidence intervals (CIs) for each treatment comparison using Microsoft Excel spreadsheets containing published formulas for calculations26,27. Cohen’s d is an index commonly used in research synthesis that represents the sample estimate of the standardized mean difference in outcomes between groups reported within studies26. We classified statistically significant Cohen’s d effect sizes as small (0.2–0.49), moderate (0.5–0.79), or large (≥ 0.8) using standard criteria28. We considered a two-sided p-value of <0.05 to be statistically significant for studies that reported p-values for results of analyses testing differences in relevant outcomes. We used the calculated 95% CI of the Cohen’s d effect size to determine statistical significance for studies that did not report p-values for results29. We considered 95% CIs that did not contain zero to be statistically significant. We considered non-statistically significant results to indicate that RRT modalities were no different with respect to life participation outcomes.
Results
Search Results
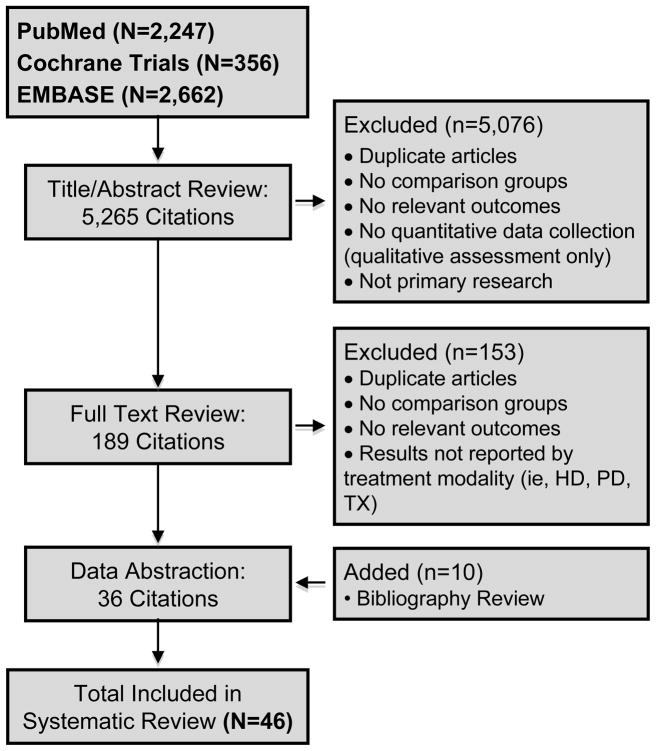
Our electronic search of potentially relevant citations identified 2,247 in PubMed, 2,662 in EMBASE, and 356 (trials) in the Cochrane Library. After reviewing a total of 5,265 titles and abstracts identified through our electronic searches, 189 articles were eligible for full text review. We retained 36 articles that met our inclusion criteria. Our hand-search of bibliographies yielded an additional 10 articles. We included a total of 46 studies in the final review2,7,9,19,20,30–70. (Figure 1)
Figure 1.
Summary of Literature Search and Article Review Process
Studies’ Characteristics
Eligible studies were conducted over a period of some 3 decades (1985–2011), with greater than half published since 2000. The studies were heterogeneous in design (6 cohort, 38 cross-sectional, and 2 pre-post transplantation), their sample sizes (ranging from 46 to 18,015 total participants), and their participants’ demographic characteristics. Approximately one third of studies were performed in the United States, while the remaining studies were from the United Kingdom, Malaysia, Thailand, Iran, Greece, Japan, The Netherlands, Turkey, Denmark, Taiwan, Poland, Italy, Spain, Australia, and Germany. (Table 1) We also collected data on additional patient characteristics, such as mean treatment time, primary ESRD cause, employment status, and education. Unfortunately, these data were not systematically reported within the articles, and thus we included these data within Table S2.
Table 1.
Study Characteristics and Outcome Domains Addressed
| Study | Earliest Year of Study Data | Location | Study Design (Follow Up) | Treatment Modality | Sample Size | Patient Demographics | Outcome Domains | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | Female Sex | US Racial/Ethnic Minority | Married* | Physical | Travel | Rec | Freedom | Work | ||||||
| 2011 Ibrahim | 2011 | Malaysia; Multi-Site | Cross-Sectional | HD | 183 | 37.6% aged 51–60 y | 48.5% | N/A | 75.9% | X | X | |||
| PD | 91 | |||||||||||||
| 2010 Johansen | 2005 | US; Multi-Site | Cross-Sectional | HD | 161 | M:60.7 ±14.6; F:59.8 ±13.8 | 45% | M: 28%; F: 36% | N/R | X | ||||
| PD | 1,386 | |||||||||||||
| 2009 Aiyasanon | 2005 | Thailand; Single-Site | Cross-Sectional | HD | 87 | 54.05 | 49.4% | N/A | 66.7% | X | X | X | X | X |
| PD | 23 | 61.39 | 47.8% | N/A | 69.6% | |||||||||
| 2009 Alavi | 2007 | Iran; Single-Site | Cross-Sectional | HD | 63 | 55.3 ± 14.5 | 55.6% | N/A | 81% | X | ||||
| TX | 100 | 40.6 ± 14 | 41% | N/A | 77% | |||||||||
| 2009 Borowiak | 2009 | Poland; Multi-site | Cross-Sectional | HD | 50 | 59.6 ± 13.4 | 56% | N/A | N/R | X | X | |||
| PD | 50 | 58.9 ± 13.2 | 48% | |||||||||||
| 2009 Kontodimoloulos | N/R | Greece; Multi-Site | Cross-Sectional | HD | 642 | 58.1 ± 14.9 | 38.7% | N/A | 65.7% | X | X | |||
| PD | 65 | 58.7 ± 12.9 | 49.2% | N/A | 75.4% | |||||||||
| TX | 167 | 43.7 ± 12.5 | 39.5% | N/A | 63.6% | |||||||||
| 2009 Masuda | 2009 | Japan; Single-Site | Cross-Sectional | HD | 35 | 58.3 ± 14.7 | 31.4% | N/A | N/R | X | ||||
| PD | 26 | 47.5 ± 14.2 | 46.2% | |||||||||||
| 2009 Panagopoulou | 2009 | Greece; Single-Site | Pre-Post Tx | HD | 40 | 57 ± 25 | 50% | N/A | 67.5% | X | ||||
| PD | 36 | 59 ± 25 | 41.6% | N/A | 72.2% | |||||||||
| TX | 48 | 39 ± 20 | 33% | N/A | 47.9% | |||||||||
| 2009 Thong | 1997 | Netherlands; Multi-Site | Cross-Sectional | HD | 1010 | 63.2 ± 13.8 | 43.3% | N/A | 65% | X | ||||
| PD | 543 | 53.3 ± 14.6 | 36.1% | N/A | 73.5% | |||||||||
| 2009 Basok | N/R | Turkey; Single-Site | Cross-Sectional | HD | 24 | 43.08 ± 12.44 | 100% | N/A | N/R | X | X | |||
| PD | 21 | 45.19 ± 8.92 | ||||||||||||
| TX | 20 | 36.45 ± 8.55 | ||||||||||||
| 2007 Apostolou | 2007 | Greece; Single-Site | Cross-Sectional | PD | 26 | 53.6 ± 12.7 | 42.3% | N/A | 65% | X | X | |||
| TX | 20 | 49.2 ± 11.4 | 50% | N/A | 89% | |||||||||
| 2007 Molsted | N/R | Denmark; Single-Site | Cross-Sectional | HD | 71 | 59 ± 16 | 24% | N/A | N/R | X | ||||
| PD | 59 | 59 ± 13 | 44% | |||||||||||
| 2007 Sayin | 2007 | Turkey; Single-Site | Cross-Sectional | HD | 75 | 46.91 ± 15.77 | 28% | N/A | 74.7% | X | X | |||
| PD | 41 | 46.15 ± 15.29 | 61% | N/A | 70.7% | |||||||||
| TX | 20 | 33.15 ± 10.61 | 65% | N/A | 45.0% | |||||||||
| 2006 Juergensen | N/R | US; Multi-Site | Cross-Sectional | HD | 84 | 69.6 ± 13.3 | N/R | 20% | 60% | X | ||||
| PD | 62 | 55 ± 14 | N/R | 17% | 57% | |||||||||
| 2006 Ogutmen | 2003 | Turkey; Multi-Site | Cross-Sectional | HD | 64 | 48.14 ± 15.5 | 42.2% | N/A | 70% | X | X | |||
| PD | 207 | 46.00 ± 13.88 | ||||||||||||
| TX | 302 | 38.22 ± 11.52 | ||||||||||||
| 2005 Barendse | N/R | UK; Single-Site | Cross-Sectional | HD | 35 | 52.8 ± 14.3 | 39% | N/A | 76% | X | ||||
| PD | 57 | |||||||||||||
| TX | 46 | |||||||||||||
| 2005 Kutner | 1996 | US; Multi-Site | Prospective Cohort (1 y) | HD | 455 | 61.2 ± 15.6 | 43.3% | 29.9% | 53.4% | X | ||||
| PD | 413 | 56.1 ± 14.7 | 47.2 % | 19.9% | 65.6% | |||||||||
| 2005 Lee | 2002 | UK; Single-Site | Cross-Sectional | HD | 99 | M: 62.4 ± 14.5; F: 64.0 ± 13.8 | 39% | N/A | N/R | X | X | X | X | |
| PD | 74 | M: 63.5 ± 13.6; F: 53.7 ± 15.5 | 49% | |||||||||||
| TX | 209 | M: 53.6 ± 13.8; F: 51.6 ± 14.1 | 40% | |||||||||||
| 2005 Niu | 2002 | Taiwan; Multi-Site | Cross-Sectional | HD | 80 | 54.7 ± 13.5 | 57.9% | N/A | 72.5% | X | ||||
| PD | 80 | 50.8 ± 12.2 | ||||||||||||
| TX | 80 | 43.3 ± 8.8 | ||||||||||||
| 2005 Van de Ham | N/R | Netherlands; Multi-Site | Cross-Sectional | HD | 16 | 49.0 ± 11.9 | 48.57% | N/A | N/R | X | ||||
| TX | 35 | 52.3 ± 10.4 | 37.5% | |||||||||||
| 2004 Wu | 1995 | US; Multi-Site | Prospective Cohort (1 y) | HD | 698 | 54 | 48% | 37% | 51% | X | X | X | X | X |
| PD | 230 | 59 | 46% | 20% | 67% | |||||||||
| 2003 Manns | 1999 | UK; Single-Site | Prospective Cohort (1 y) | HD | 151 | 62.2 | 13% | N/A | 64% | X | X | |||
| PD | 41 | 56.1 | 80% | N/A | 70% | |||||||||
| 2003 Tomasz | N/R | Poland; Single-Site | Cross-Sectional | HD | 61 | 57.84 ± 11.85 | 39.34% | N/A | 14.75% | X | X | X | ||
| TX | 83 | 43.3 ± 11.73 | 48.19% | N/A | 71.08% | |||||||||
| 2002 Baiardi | 1997 | Italy; Single-Site | Prospective Cohort (16+ mo) | HD | 171 | 61.9 ± 13.4 | 37.4% | N/A | N/R | X | ||||
| PD | 30 | 64.0 ± 15.7 | 36.7% | |||||||||||
| TX | 34 | 44.0 ± 12.0 | 35.3% | |||||||||||
| 2002 Harris | 2002 | UK; Multi-Site | Prospective Cohort (1 y) | HD | 96 | 77.0 ± 4.4 | 38% | N/A | N/R | X | ||||
| PD | 78 | 76.8 ± 4.0 | 30% | |||||||||||
| 2000 Carmichael | N/R | UK; Single-Site | Cross-Sectional | HD | 49 | 57.8 ± 13 | 34.7% | N/A | 71.4% | X | X | |||
| PD | 97 | 57 ± 15 | 40.2% | N/A | 70.1% | |||||||||
| 2000 Diaz-Buxo | 1996 | US; Multi-Site | Cross-Sectional | HD | 16,755 | 59.44 ± 15.28 | 48.22% | 47.07% | N/R | X | X | |||
| PD | 1,260 | 53.45 ± 15.31 | 49.52% | 31.03% | ||||||||||
| 2000 Fujisawa | N/R | Japan; Multi-Site | Cross-Sectional | HD (awaiting TX) | 114 | 45.8 ± 11.9 | 24% | N/A | N/R | X | ||||
| HD (not awaiting TX) | 45.7 ± 6.8 | 31% | ||||||||||||
| TX | 117 | 43.9 ± 9.1 | 57% | |||||||||||
| 1999 Merkus | 1993 | Netherlands; Multi-Site | Cross-Sectional | HD | 120 | 59 ± 16 | 43% | N/A | N/R | X | X | |||
| PD | 106 | 52 ± 14 | 35% | |||||||||||
| 1998 Jofre | 1993 | Spain; Multi-Site | Pre-Post Tx | Pre-TX (HD) | 93 | 45 ± 13.2 | 45% | N/A | N/R | X | ||||
| Post-TX | ||||||||||||||
| 1997 Merkus | 1997 | Netherlands; Multi-Site | Cross-Sectional | HD | 120 | 59.3 ± 15.5 | 43% | N/A | 68% | X | X | |||
| PD | 106 | 52.3 ± 14.0 | 35% | N/A | 79% | |||||||||
| 1996 Curtin | 1996 | US; Multi-Site | Cross-Sectional | HD | 238 | 43 ± 10.6 | 50.1% | 49.6% | N/R | X | ||||
| PD | 30 | |||||||||||||
| 1996 Lok | N/R | Australia; Single-Site | Cross-Sectional | HD | 56 | 42.5 | 37.5% | N/A | N/R | X | ||||
| PD | 8 | |||||||||||||
| 1995 Khan | N/R | UK; Single-Site | Cross-Sectional | HD | 43 | 48.8 | 41.6% | N/A | N/R | X | ||||
| PD | 27 | |||||||||||||
| TX | 102 | |||||||||||||
| 1995 Tell | N/R | US; Single-Site | Cross-Sectional | HD (in-center) | 186 | 54.9 ± 15.3 | 52% | 51% | M: 70%; F: 49% |
X | ||||
| HD (home) | 12 | |||||||||||||
| PD | 58 | |||||||||||||
| 1994 Holley | 1993 | US; Single-Site | Cross-Sectional | HD | 46 | N/R | 53% | 48% | N/R | X | ||||
| PD | 31 | |||||||||||||
| 1992 Pietrabissa | 1982 | Italy; Single-Site | Cross-Sectional | HD | 172 | N/R | N/R | N/A | N/R | X | ||||
| TX | 71 | |||||||||||||
| 1991 Tucker | N/R | US; Single-Site | Cross-Sectional | HD | 22 | 50.4 ± 13.8 | 62% | 72% | 62% | X | ||||
| PD | 29 | 47.1 ± 13.9 | 45% | 27% | 73% | |||||||||
| 1990 Devins | N/R | Canada; Single-Site | Prospective Cohort** (6 wk) | HD (in-center) | 39 | 41 | 42% | N/A | 63% | X | X | |||
| HD (home) | 15 | |||||||||||||
| PD | 11 | |||||||||||||
| TX | 34 | |||||||||||||
| 1990 Koch | N/R | Germany; Multi-Site | Cross-Sectional | HD | 290 | 50.1 | 43% | N/A | 68% | X | ||||
| PD | 68 | 51.8 | 32% | N/A | 79% | |||||||||
| TX | 761 | 43.5 | 41% | N/A | 70% | |||||||||
| 1990 Simmons | 1970 | US; Multi-Site | Cross-Sectional | HD | 83 | Range, 19-–56 | N/R | N/R | N/R | X | X | X | X | |
| PD | 510 | |||||||||||||
| TX (current) | 91 | |||||||||||||
| TX (historical) | 82 | |||||||||||||
| 1989 Bremer | N/R | US; Multi-Site | Cross-Sectional | HD (in-center) | 105 | 57.1 ± 13.4 | 49% | 38% | N/R | X | ||||
| HD (self-care) | 41 | 54.5 ± 11.4 | 46% | 37% | ||||||||||
| HD (home) | 47 | 53.2 ± 12.9 | 49% | 19% | ||||||||||
| PD | 79 | 54.9 ± 13.7 | 35% | 13% | ||||||||||
| TX (first) | 166 | 37.6 ± 11.4 | 42% | 15% | ||||||||||
| TX (failed) | 30 | 36.3 ± 10.3 | 60% | 20% | ||||||||||
| TX (>1) | 21 | 31.2 ± 8.3 | 38% | 10% | ||||||||||
| 1989 Julius | 1984 | US; Multi-Site | Cross-Sectional | HD | 95 | 60% >44 y | 41.1% | 49.5% | 61.1% | X | ||||
| PD | 119 | 60.5% >44 y | 48.7% | 23.3% | 63.0% | |||||||||
| 1988 Wolcott | N/R | US; Multi-Site | Cross-Sectional | HD | 33 | 47.4 ± 15.1 | 30% | 39% | 49% | X | ||||
| PD | 33 | 46.2 ± 14.4 | 30% | 33% | 64% | |||||||||
| 1987 Hart | N/R | US; Multi-Site | Cross-Sectional | HD (in-center) | 347 | 51.9 | 50% | 46.5% | N/R | X | X | |||
| HD (home) | 287 | 47.0 | 35.9% | 13.6% | ||||||||||
| PD | 81 | 49.8 | 54.3% | 16.1% | ||||||||||
| TX | 144 | 37.2 | 43.8% | 16.6% | ||||||||||
| 1985 Evans | N/R | US; Multi-Site | Cross-Sectional | HD (in-center) | 347 | 47.6 | 44.5% | 27.2% | NR | X | ||||
| HD (home) | 287 | 75% | ||||||||||||
| PD | 81 | 75% | ||||||||||||
| TX | 44 | 66.7% | ||||||||||||
Note: Unless otherwise indicated, age is given as mean or mean ± standard deviation; other patient demographics given as percentage.
HD = hemodialysis; PD = peritoneal dialysis; Tx = transplant; M=Male; F=Female; NR = not reported; NA = not applicable; US, United States; UK, United Kingdom; Physical, physical functioning; Rec, recreation;
Or Living with a Partner.
Factors Influencing Studies’ Internal and External Validity
Most studies described their inclusion and exclusion criteria well (Table S3). A majority of studies reported response rates of 60% or greater and conducted valid outcome assessments. However, many studies were influenced by potential selection bias. Also, the comparative groups of participants within many studies were deemed to be different enough in aspects other than selection of RRT modality such that observed associations between RRT modality and life participation activities could be confounded by group differences. Few studies were judged to have performed appropriate statistical analyses to account for these differences, which could potentially confound observed associations between study participants’ RRT modalities and life participation outcomes. (Table 2)
Table 2.
Summary of Study Quality Assessment for Relevant Outcomes
| Outcome Domain | Total Articles | Well-Described Criteria* | Response Rate >60% | Minimal Risk of Bias** | Valid Outcome Assessment | Appropriate Adjustment^ |
|---|---|---|---|---|---|---|
| Physical Function | 35 | 25 (71%) | 18 (51%) | 6 (17%) | 34 (97%) | 13 (37%) |
| Travel | 2 | 2 (100%) | 1 (50%) | 2 (100%) | 2 (100%) | 1 (50%) |
| Recreation | 20 | 15 (75%) | 13 (65%) | 5 (25%) | 19 (95%) | 5 (25%) |
| Freedom | 7 | 6 (86%) | 3 (43%) | 3 (43%) | 7 (100%) | 1 (14%) |
| Work | 13 | 8 (61%) | 8 (61%) | 2 (15%) | 13 (100%) | 4 (31%) |
Note: Quality Assessment: number of articles that met criteria (% of total articles)
Inclusion and exclusion criteria
Selection bias.
For potential confounders. Total articles.
Measures Used to Assess Associations between RRT Modality and Life Participation Outcomes
Studies used a variety of measures to capture life participation outcomes. Physical function was measured using several tools, including the SF-36 physical functioning and role physical measures, author-developed difficulties in activities of daily living and physical well-being scales, and Karnofsky self-reported scores2,7,9,20,30–32,35–38,40,43–49,51–58,60–69. Travel was measured using the CHOICE (Choices for Healthy Outcomes in Caring for ESRD) Health Experience Questionnaire (CHEQ), and the Thai version of the CHEQ2,30. Recreation was measured using several tools, including the SF-36 social functioning measure, Thai CHEQ, Intrusiveness Ratings Scale, Sickness Impact Profile, and author-developed patient questionnaires 2,9,20,30,33,35,36,38,41,42,45,48,49,52,55,58,59,64–67. Freedom was measured using several tools, including the CHEQ, Thai CHEQ, Renal Treatment Satisfaction Questionnaire, Index of Well-Being and author-developed social well-being scale2,20,30,32,34,41,55,62. Work was also measured using several tools, including the CHEQ, Thai CHEQ, Sickness Impact Profile, Intrusiveness Ratings Scale, and patient-reported work status2,9,19,20,30,38,39,42,53,55,58,70. We provide a detailed description of the included outcome measures (i.e., whether the measure is validated, outcome type, range of scores, and whether a higher score indicates a better outcome) within Table S4.
Comparison of Life Participation Activities
Patients Receiving Hemodialysis Versus Peritoneal Dialysis
A total of 39 studies evaluated life participation activities between patients receiving hemodialysis compared to patients receiving peritoneal dialysis2,9,16,19,20,30,32–36,38,41,42,44–53,55–57,59,61–63,65–70. Most studies reported on multiple outcomes, thus providing 41 physical function, 2 travel, 18 recreation, 8 freedom, and 13 work-related comparisons. The majority of comparisons demonstrated no significant differences in physical function outcomes (76%), recreation outcomes (78%), freedom outcomes (75%), and work outcomes (69%). (Table 3) These findings of no differences in outcomes were consistent across study designs, location, and quality ratings with 100% of comparisons from cohort studies, 81% of comparisons from US-based studies, 83% of comparisons from studies that properly adjusted for potential confounders, and 70% of comparisons from studies published after 2000 favoring neither RRT modality. (Table 4)
Table 3.
Physical Function and Life Participation Outcome Measures and Study Results: HD versus PD
| Study; Design | Outcome Measure | HD | PD | Statistical Results | Treatment Favored | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Estimate@ | N | Estimate@ | Reported P** | Effect Size^ | Calculated CI**** | |||
| Physical Function | |||||||||
| Aiyasanon; 2009; CS | Thai SF-36 Physical Functioning | 87 | 52 | 23 | 29 | 0.001 | 0.79 | ---- | HD |
| Aiyasanon; 2009 (CS) | Thai SF-36 Role Physical | 87 | 37 | 23 | 17 | <0.05 | 0.46 | ---- | HD |
| Aiyasanon; 2009 (CS) | CHEQ (Thai) Role Physical | 87 | 77 | 23 | 59 | 0.001 | 0.79 | ---- | HD |
| Tell; 1995 (CS) | Karnofsky self-report | 186 | 72.9 | 58 | 78 | 0.02 | −0.34 | ---- | PD |
| Masuda; 2009 (CS) | Average no. steps/d | 35 | 3,391 | 26 | 6,336 | <0.05 | −0.83 | (−4784.1, −1105.9) | PD |
| Merkus; 1997 (CS) | SF-36 Physical Functioning | 120 | 50.7 | 120 | 60.9 | <0.05 | −0.37 | (−17.3, −3.1) | PD |
| Merkus; 1999 (CS) | SF-36 Physical Functioning | 120 | 50.7 | 106 | 60.9 | <0.05 | −0.36 | ---- | PD |
| Ogutmen; 2006 (CS) | SF-36 Role Physical | 64 | 35.71 | 207 | 55.10 | <0.05 | −0.43 | (−32.1, −6.7) | PD |
| Thong; 2009 (CS) | SF-36 Physical Functioning | 1,010 | 48.3 | 543 | 61.2 | <0.05 | −0.47 | (−15.8, −10.0) | PD |
| Thong; 2009 (CS) | SF-36 Role Physical | 1,010 | 25.9 | 543 | 35.7 | <0.05 | −0.26 | (−13.7, −5.9) | PD |
| Harris; 2002 (Cohort) | UK-version SF-36 PCS | 96 | 31.6 | 78 | 32.0 | 0.2 | −0.03 | ---- | ---- |
| Kutner; 2005 (Cohort) | SF-36 Physical Functioning | 32 | N/R | 141 | N/R | 0.3 | −3.48 | --- | --- |
| Kutner; 2005 (Cohort) | SF-36 Role Physical | 32 | N/R | 141 | N/R | 0.3 | −2.90 | --- | --- |
| Wu; 2004 (Cohort) | SF-36 Physical Functioning | 452 | 1.0# | 133 | 0.72# | 0.1 | --- | ---- | --- |
| Wu; 2004 (Cohort) | SF-36 Role Physical | 452 | 1.0# | 133 | 0.84# | 0.7 | --- | ---- | --- |
| Baiardi; 2002 (CS)*** | SF-36 Physical Functioning | 171 | 59.3 | 30 | 59.7 | N/R | −0.01 | (−11.2, 10.4) | ---- |
| Basok; 2009 (CS) | SF-36 Physical Functioning | 24 | 62.27 | 21 | 56.61 | N/R | 0.25 | (−7.9, 19.2) | ---- |
| Basok; 2009 (CS) | SF-36 Role Physical | 24 | 58.83 | 21 | 39.29 | N/R | 0.46 | (−6.1, 45.2) | ---- |
| Borowiak; 2009 (CS) | EQ 5D (mobility) | 50 | 38## | 50 | 44## | NS | −0.14 | (−0.6, 0.3) | ---- |
| Carmichael;2000 (CS) | SF-36 Physical Functioning | 49 | 44.4 | 97 | 40.3 | NS | 0.13 | (−6.4, 14.6) | --- |
| Carmichael;2000 (CS) | SF-36 Role Physical | 49 | 21.4 | 97 | 19.7 | NS | 0.05 | (−9.8, 13.2) | --- |
| Devins; 1990 (CS)*** | Author-developed difficulties in ADL scale | 39 | 2 | 11 | 2.6 | NS | −0.40 | (−1.6, 0.4) | --- |
| Diaz-Buxo; 2000 (CS) | SF-36 Physical Functioning | 16,755 | 41.4 | 1,260 | 42.1 | N/R | −0.11 | (−2.4, 1.0) | --- |
| Diaz-Buxo; 2000 (CS) | SF-36 Role Physical | 16,755 | 33.1 | 1,260 | 33 | N/R | −0.01 | (−2.4, 2.1) | --- |
| Ibrahim; 2011 (CS) | SF-36 Physical Functioning | 183 | 72.568 | 91 | 75.275 | N/R | −0.15 | (−7.2, 1.8) | ---- |
| Ibrahim; 2011 (CS) | SF-36 Role Physical | 183 | 68.443 | 91 | 76.786 | N/R | −0.41 | (13.5, −3.2) | ---- |
| Koch; 1990 (CS) | Satisfaction with physical function | 290 | 0.51 | 68 | 0.47 | N/R | 0.09 | (−0.2, 0.4) | --- |
| Kontodimopoulos; 2008 (CS) | Greek SF-36 Physical Functioning | 642 | 49.2 | 65 | 49.2 | 0.9 | 0.00 | (−7.8, 7.8) | --- |
| Kontodimopoulos; 2008 (CS) | Greek SF-36 Role Physical | 642 | 40.3 | 65 | 30.9 | 0.08 | 0.21 | (−1.8, 20.6) | --- |
| Lee; 2005 (CS) | SF-36 Physical Functioning | 99 | 26.5 | 74 | 30.9 | NS | −0.16 | (−12.9, 4.1) | --- |
| Lee; 2005 (CS) | SF-36 Role Physical | 99 | 60.4 | 74 | 60.6 | NS | 0.00 | (−13.4, 12.9) | --- |
| Lok; 1996 (CS) | HD Stressor Scale | 56 | 3.76 | 8 | 3.43 | NS | 0.32 | (−0.4, 1.1) | --- |
| Manns; 2003 (CS) | SF-36 Physical Functioning | 151 | 46.2 | 41 | 40 | 0.2 | 0.22 | ---- | --- |
| Manns; 2003 (CS) | SF-36 Role Physical | 151 | 27 | 41 | 29.3 | 0.7 | −0.06 | ---- | --- |
| Merkus; 1997 (CS) | SF-36 Role Physical | 106 | 28.6 | 106 | 31.7 | NS | −0.08 | (−13.3, 7.1) | ---- |
| Ogutmen; 2006 (CS) | SF-36 Physical Functioning | 64 | 56.99 | 207 | 57.06 | N/R | −0.83 | (−46.9, −23.8) | --- |
| Niu; 2005 (CS) | WHOQOL-BREF (Taiwan) | 80 | 11.96 | 80 | 11.61 | N/R | −0.99 | (−0.4, 1.1) | --- |
| Sayin; 2007 (CS) | SF-36 Physical Functioning | 75 | 55.90 | 41 | 55.76 | 0.1 | 0.00 | (−10.8, 11.1) | ---- |
| Sayin; 2007 (CS) | SF-36 Role Physical | 75 | 40.69 | 41 | 39.10 | 0.9 | 0.04 | (−13.7, 16.9) | ---- |
| Simmons; 1990 (CS) | Author developed physical well-being scale (summary score) | 83 | 14.04 | 510 | 14.64 | N/R | −1.00 | (−1.5, 0.3) | --- |
| Wolcott; 1988 (CS) | Karnofsky (clinical performance) | 33 | 72.5 | 33 | 72.9 | NS | --- | --- | --- |
| Travel | |||||||||
| Aiyasanon; 2009 (CS) | CHEQ (Thai) (travel restrictions) | 87 | 68 | 23 | 51 | <0.05 | 0.46 | ---- | HD |
| Wu; 2004 (Cohort) | CHEQ-Travel | 452 | 1.0 | 133 | 1.07 | 0.8 | −0.03 | ---- | --- |
| Recreation | |||||||||
| Carmichael;2000(CS) | SF-36 Social Functioning | 49 | 44.9 | 97 | 53.2 | <0.05 | −0.29 | (−18.2, 1.6) | PD |
| Ibrahim; 2011 (CS) | SF-36 Social Functioning | 183 | 77.322 | 91 | 83.516 | N/R | −0.31 | (−11.3, −1.1) | PD |
| Lee; 2005 (CS) | SF-36 Social Functioning | 99 | 36.7 | 74 | 46.5 | <0.05 | −0.33 | (−18.8, 1.6) | PD |
| Tucker; 1991 (CS) | Quality of Life Assessment Battery (mean recreation/wk) | 29 | 5.1 | 22 | 7.3 | <0.05 | −0.34 | (−5.9, 1.5) | PD |
| Wu; 2004 (Cohort) | CHEQ (limitations to recreation) | 452 | 1.0 | 133 | 0.85 | 0.5 | 0.06 | ---- | --- |
| Aiyasanon; 2009(CS) | CHEQ (Thai) (recreation) | 87 | 65 | 23 | 62 | NS | 0.46 | ---- | --- |
| Basok; 2009 (CS) | SF-36 Social Functioning | 24 | 68.75 | 21 | 63.69 | 0.1 | 0.19 | (−10.8, 20.9) | --- |
| Devins; 1990 (CS)*** | Intrusiveness Ratings Scale | 39 | 2.5 | 11 | 3.2 | NS | −0.51 | (−1.6, 0.2) | --- |
| Diaz-Buxo; 2000 (CS) | SF-36 Social Functioning | 16,755 | 64 | 1,260 | 66.1 | NS | −0.04 | (−2.8, 0.6) | --- |
| Hart; 1987 (CS) | Sickness Impact Profile | 343 | 23.7 | 77 | 24 | NS | −0.25 | ---- | --- |
| Juergensen; 2006 (CS) | Author developed questions (recreation) | 84 | 4.95 | 52 | 5.12 | NS | −0.09 | (−0.8, 0.5) | --- |
| Kontodimopoulos 2008 (CS) | Greek SF-36 Social Functioning | 642 | 58.1 | 65 | 54.9 | 0.4 | 0.11 | (−4.5, 10.9) | --- |
| Manns; 2003 (CS) | SF-36 Social Functioning | 151 | 60.7 | 41 | 62.2 | 0.8 | −0.06 | ---- | --- |
| Merkus; 1997 (CS) | SF-36 Social Functioning | 120 | 63.1 | 120 | 68.9 | NS | −0.21 | (−12.9, 1.3) | ---- |
| Merkus; 1999 (CS) | SF-36 Social Functioning | 120 | 63.1 | 106 | 68.9 | NS | −0.26 | ---- | --- |
| Ogutmen; 2006 (CS) | SF-36 Social Functioning | 64 | 66 | 207 | 71.9 | NS | −0.27 | (−11.9, 0.3) | --- |
| Sayin; 2007 (CS) | SF-36 Social Functioning | 75 | 62.62 | 41 | 56.32 | 0.5 | 0.22 | (−4.6, 17.2) | ---- |
| Simmons; 1990 (CS) | Author-developed scale | 83 | 2.24 | 510 | 2.21 | NS | 0.03 | (−0.2, 0.3) | --- |
| Freedom | |||||||||
| Aiyasanon;2009 (CS) | CHEQ (Thai) (freedom) | 87 | 57 | 23 | 42 | <0.05 | 0.46 | ---- | HD |
| Juergensen; 2006 (CS) | Author-developed scale (independence) | 84 | 5.14 | 52 | 6.18 | 0.02 | −0.41 | ---- | PD |
| Wu; 2004 (Cohort) | CHEQ (freedom) | 452 | 1.0 | 133 | 1.03 | 0.9 | −0.01 | ---- | --- |
| Borowiak; 2009 (CS) | EQ-5D (usual activity) | 50 | 40 | 50 | 46 | NS | −0.13 | (−0.6, 0.3) | ---- |
| Barendse; 2005 (CS) | Renal Treatment Satisfaction Questions | 35 | 4.3 | 57 | 4.3 | NS | 0.00 | (−0.7, 0.7) | --- |
| Bremer; 1989 (CS) | Index of Well-Being (tied-down, free) | 105 | 4.9 | 79 | 4.5 | NS | −0.55 | (−0.2, 1.0) | --- |
| Lee; 2005 (CS) | EQ-5D (usual activities) | 99 | 18.09 | 74 | 20.55 | NS | −0.20 | (−0.5, 0.1) | --- |
| Simmons; 1990 (CS) | Author-developed social well-being scale | 83 | 5.17 | 510 | 5.29 | NS | −0.14 | (−0.3, 0.1) | --- |
| Work | |||||||||
| Evans; 1985 (CS) | Reported ability to work | 347 | 44.8## | 81 | 27.8## | N/R | 0.41 | (0.1, 0.7) | HD |
| Hart; 1987 (CS) | Sickness Impact Profile (work) | 338 | 45 | 81 | 51 | <0.01 | −0.32 | ---- | HD |
| Julius; 1989 (CS) | Reported working or looking | 95 | 0.096## | 119 | 0.227## | <0.05 | −0.56 | (−1.0, −0.1) | PD |
| Molsted; 2007 (CS) | KDQOL (work status) | 71 | 17.9 | 59 | 33.6 | <0.05 | −0.42 | (−28.8, −2.6) | PD |
| Wu; 2004 (Cohort) | CHEQ (work) | 452 | 1.0 | 133 | 1.17 | 0.5 | −0.07 | ---- | --- |
| Aiyasanon; 2009 (CS) | CHEQ (Thai) (limitations to work) | 87 | 67 | 23 | 57 | NS | 0.46 | ---- | --- |
| Curtin; 1996 (CS) | Percent of employed pts | 311 | 23.4 | 42 | 28.6 | 0.2 | −0.14 | (−0.5, 0.2) | --- |
| Devins; 1990 (CS)*** | Intrusiveness Ratings Scale | 39 | 4 | 11 | 3.6 | NS | 0.19 | (−1.0, 1.8) | --- |
| Hart; 1987 (CS) | Pt-reported work | 46 | 0.37## | 31 | 0.52## | NS | −0.34 | (−0.8, 0.2) | --- |
| Lee; 2005 (CS) | KDQOL (work status) | 99 | 20 | 74 | 28.2 | NS | −0.23 | (−18.8, 2.4) | --- |
| Panagopoulou2009 (CS)*** | Pt-reported full time employment | 40 | 0.05## | 36 | 0.138## | N/R | −0.61 | (−1.6, 0.3) | --- |
| Panagopoulou2009 (CS)*** | Pt-reported part-time employment | 40 | 0.15## | 36 | 0.17## | N/R | −0.08 | (−0.8, 0.6) | --- |
| Simmons; 1990 (CS) | Author developed scale (job) | 83 | 2.18 | 510 | 2.38 | NS | −0.17 | (−0.5, 0.1) | --- |
Estimates are means unless otherwise noted.
Author-reported p-values.
Calculated Cohen’s d.
Odds ratio.
Percentage.
denotes studies were originally designed as longitudinal cohort but authors only reported cross-sectional assessments of relevant outcomes
We calculated the CI for the Cohen’s d effect size difference for studies that reported all of the needed estimates (e.g., means plus standard deviations) We reported ‘---‘ for studies that we could not calculate the CIs for due to missing estimates.
Abbreviations and definitions: ADL, activities of daily living; CS, cross sectional study design; NS, not statistically significantly different at P>0.05 level; NR, not reported within the article; Pt, patient; Pts, patients; CI, confidence interval; HD, hemodialysis; PD, peritoneal dialysis; CHEQ, CHOICE (Choices for Healthy Outcomes in Caring for ESRD) Health Experience Questionnaire; SF-36, 36-Item Short-Form Health Survey; PCS, physical component summary; UK, United Kingdom; KDQOL, Kidney Disease Quality of Life scale; WHOQOL-BREF, World Health Organization Quality of Life instrument, short version,
Table 4.
Summary of Published Evidence Comparing Life Participation Outcomes by RRT Modality and Study Characteristics
| HD vs. PD | HD vs. Tx | PD vs. Tx | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No. of outcomes that | No. of outcomes that | No. of outcomes that | |||||||
|
| |||||||||
| Favors HD | Favors Neither | Favors PD | Favors HD | Favors Neither | Favors Tx | Favors PD | Favors Neither | Favors Tx | |
|
| |||||||||
|
Physical Function Domain
| |||||||||
| Study Design | |||||||||
| Randomized Trial | |||||||||
| Prospective Cohort | 5 | ||||||||
| Cross-Sectional | 3 | 26 | 6 | 8 | 17 | 7 | 14 | ||
| Pre-Post Transplant | 1 | ||||||||
|
| |||||||||
| Location | |||||||||
| US | 8 | 1 | 1 | ||||||
| Non-US | 3 | 23 | 7 | 8 | 17 | 7 | 13 | ||
|
| |||||||||
| Study Quality | |||||||||
| Appropriate Adjustment* | 12 | 2 | 2 | 3 | 3 | 2 | |||
| No/Minimal Adjustment* | 3 | 19 | 5 | 6 | 15 | 4 | 12 | ||
|
| |||||||||
| Publication Year | |||||||||
| 1980–1990 | 4 | 1 | 2 | 1 | 2 | ||||
| 1991–2000 | 6 | 3 | 1 | 4 | 2 | ||||
| 2001–2012 | 3 | 21 | 4 | 6 | 12 | 6 | 10 | ||
|
| |||||||||
| Outcome Measures | |||||||||
| SF-36 (Thai/UK/Greek) | 2 | 24 | 5 | 5 | 10 | 5 | 10 | ||
| CHEQ (Thai) | 1 | ||||||||
| Karnofsky | 1 | 1 | |||||||
| EQ-5D | 1 | ||||||||
| WHOQOL | 1 | 2 | 1 | ||||||
| Sickness Impact Profile | 1 | ||||||||
| Nottingham | 1 | ||||||||
| HD Stressor Scale | 1 | ||||||||
| Author-developed scales | 2 | 2 | 1 | 1 | 1 | ||||
| Other patient reports | 1 | 1 | 2 | 1 | 2 | ||||
| Physical assessments | 1 | 1 | |||||||
|
| |||||||||
|
Travel Domain
| |||||||||
| Study Design | |||||||||
| Randomized Trial | |||||||||
| Prospective Cohort | 1 | ||||||||
| Cross-Sectional | 1 | ||||||||
| Pre-Post Transplant | |||||||||
|
| |||||||||
| Location | |||||||||
| US | 1 | ||||||||
| Non-US | 1 | ||||||||
|
| |||||||||
| Study Quality | |||||||||
| Appropriate Adjustment* | 1 | ||||||||
| No/Minimal Adjustment* | 1 | ||||||||
|
| |||||||||
| Publication Year | |||||||||
| 1980–1990 | |||||||||
| 1991–2000 | |||||||||
| 2001–2012 | 1 | 1 | |||||||
|
| |||||||||
| Outcome Measures | |||||||||
| CHEQ (Thai) | 1 | 1 | |||||||
|
| |||||||||
|
Recreation Domain
| |||||||||
| Study Design | |||||||||
| Randomized Trial | |||||||||
| Prospective Cohort | 1 | ||||||||
| Cross-Sectional | 13 | 4 | 4 | 3 | 2 | 5 | |||
| Pre-Post Transplant | |||||||||
|
| |||||||||
| Location | |||||||||
| US | 5 | 1 | 2 | 2 | |||||
| Non-US | 9 | 3 | 4 | 1 | 2 | 3 | |||
|
| |||||||||
| Study Quality | |||||||||
| Appropriate Adjustment* | 4 | 1 | 1 | 1 | |||||
| No/Minimal Adjustment* | 10 | 4 | 3 | 3 | 1 | 4 | |||
|
| |||||||||
| Publication Year | |||||||||
| 1980–1990 | 1 | 2 | 3 | ||||||
| 1991–2000 | 2 | ||||||||
| 2001–2012 | 2 | 3 | 1 | 2 | 2 | ||||
|
| |||||||||
| Outcome Measures | |||||||||
| SF-36 (Greek) | 3 | 2 | 1 | 2 | 2 | ||||
| Sickness Impact Profile | 1 | 1 | |||||||
| Intrusiveness Ratings Scale | 1 | 1 | |||||||
| WHOQOL-100 | 1 | ||||||||
| Quality of Life Assessment Battery | 1 | ||||||||
| Author-developed scales | 1 | 1 | |||||||
|
| |||||||||
|
Freedom Domain
| |||||||||
| Study Design | |||||||||
| Randomized Trial | |||||||||
| Prospective Cohort | 1 | ||||||||
| Cross-Sectional | 1 | 5 | 1 | 4 | 4 | ||||
| Pre-Post Transplant | |||||||||
|
| |||||||||
| Location | |||||||||
| US | 3 | 1 | 2 | 2 | |||||
| Non-US | 1 | 3 | 2 | 2 | |||||
|
| |||||||||
| Study Quality | |||||||||
| Appropriate Adjustment* | 1 | ||||||||
| No/Minimal Adjustment* | 1 | 5 | 1 | 4 | 4 | ||||
|
| |||||||||
| Publication Year | |||||||||
| 1980–1990 | 2 | 2 | 2 | ||||||
| 1991–2000 | |||||||||
| 2001–2012 | 1 | 4 | 1 | 2 | 2 | ||||
|
| |||||||||
| Outcome Measures | |||||||||
| CHEQ (Thai) | 1 | 1 | |||||||
| EQ 5D | 2 | 1 | 1 | ||||||
| Renal Treatment Satisfaction | 1 | 1 | 1 | ||||||
| Index of Well-Being | 1 | 1 | 1 | ||||||
| Author-developed scales | 1 | 1 | 1 | 1 | |||||
|
| |||||||||
|
Work Domain
| |||||||||
| Study Design | |||||||||
| Randomized Trial | |||||||||
| Prospective Cohort | 1 | ||||||||
| Cross-Sectional | 2 | 8 | 2 | 6 | 5 | ||||
| Pre-Post Transplant | |||||||||
|
| |||||||||
| Location | |||||||||
| US | 2 | 4 | 1 | 3 | 3 | ||||
| Non-US | 5 | 1 | 3 | 2 | |||||
|
| |||||||||
| Study Quality: | |||||||||
| Appropriate Adjustment* | 1 | 2 | 1 | 1 | 1 | ||||
| No/Minimal Adjustment* | 1 | 7 | 1 | 5 | 4 | ||||
|
| |||||||||
| Publication Year | |||||||||
| 1980–1990 | 2 | 3 | 1 | 4 | 4 | ||||
| 1991–2000 | 1 | ||||||||
| 2001–2012 | 5 | 1 | 2 | 1 | |||||
|
| |||||||||
| Outcome Measures | |||||||||
| CHEQ (Thai) | 2 | ||||||||
| Sickness Impact Profile | 1 | 1 | 1 | ||||||
| KDQOL | 1 | 1 | 1 | 1 | |||||
| Intrusiveness Ratings Scale | 1 | 1 | 1 | ||||||
| WHOQOL-100 | 1 | ||||||||
| Author-developed scales | 1 | 1 | 1 | ||||||
| Other patient reports | 1 | 4 | 1 | 1 | 1 | ||||
RRT, renal replacement therapy; HD, hemodialysis; PD, peritoneal dialysis; Tx, transplantation; US, United States; SF-36, 36-Item Short-Form Health Survey; CHEQ, CHOICE (Choices for Healthy Outcomes in Caring for ESRD) Health Experience Questionnaire; WHOQOL, World Health Organization Quality of Life instrument; UK, United Kingdom; KDQOL, Kidney Disease Quality of Life scale;
For confounders.
Patients Receiving Hemodialysis Versus Transplant Recipients
A total of 22 studies evaluated life participation activities between patients receiving hemodialysis compared to patients with kidney transplants9,19,20,31–34,37,38,40,43–45,51–55,58,60,63,66. Most studies reported on multiple outcomes, thus providing 26 physical function, 7 recreation, 4 freedom, and 6 work-related comparisons. The majority of comparisons demonstrated small to large differences in activities among patients with kidney transplants compared to patients receiving hemodialysis, with transplant patients having better physical function (90%), freedom (100%), and work outcomes (100%). (Table 5) These findings of better outcomes in transplant patients were observed among 71% of comparisons from cross-sectional studies, 100% of comparisons from US-based studies, 57% of comparisons from studies that properly adjusted for potential confounders, and 65% of comparisons from studies published after 2000 favoring kidney transplantation. (Table 4)
Table 5.
Physical Function and Life Participation Outcome Measures and Study Results: HD versus Tx
| Study; Design | Outcome Measure | HD | Tx | Statistical Results | Treatment Favored | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Estimate* | N | Estimate* | Reported P** | Effect Size^ | Calculated CI**** | |||
| Physical Function | |||||||||
| Alavi; 2009; CS | Nottingham Extended ADL | 63 | 31.7 | 100 | 51.4 | 0.00 | −1.62 | (−23.6, −15.8) | TX |
| Basok; 2009 (CS) | SF-36 Physical Functioning | 24 | 62.27 | 20 | 77.78 | N/R | −0.73 | (−28.4, −2.6) | TX |
| Fujisawa; 2000 (CS) | SF-36 Physical Functioning | 114 | 81.6 | 117 | 86.2 | <0.05 | −0.29 | (−8.7, −0.5) | TX |
| Khan; 1995 (CS) | SF-36 Physical Functioning | 43 | 46.35 | 102 | 67.51 | <0.05 | −0.36 | ---- | TX |
| Khan; 1995 (CS) | SF-36 Role Physical | 43 | 51.09 | 102 | 63.22 | <0.05 | −0.36 | ---- | TX |
| Koch; 1990 (CS) | Satisfaction with life questions | 290 | 0.51## | 761 | 0.18## | N/R | 0.86 | (0.7, 1.0) | TX |
| Kontodimopoulos; 2008 (CS) | SF-36 (Greek) Physical Functioning | 642 | 49.2 | 167 | 72.9 | <0.001 | −0.83 | (−28.6, −18.8) | TX |
| Kontodimopoulos 2008 (CS) | SF-36 (Greek) Role Physical | 642 | 40.3 | 167 | 62.6 | <0.001 | −0.51 | (−29.8, −14.8) | TX |
| Lee; 2005 (CS) | SF-36 Physical Functioning | 99 | 26.5 | 209 | 56.4 | <0.05 | −0.99 | (−37.1, −22.7) | TX |
| Lee; 2005 (CS) | SF-36 Role Physical | 99 | 60.4 | 209 | 86.7 | <0.05 | −0.76 | (−34.6, −18.0) | TX |
| Niu; 2005 (CS) | WHOQOL-BREF (Taiwan; physical health) | 80 | 11.96 | 80 | 14.34 | <0.05 | −0.99 | (−3.1, −1.6) | TX |
| Ogutmen; 2006 (CS) | SF-36 Role Physical | 64 | 35.71 | 302 | 71.09 | <0.05 | −0.83 | (−47.0, −23.8) | TX |
| Panagopoulou 2009 (CS)*** | Pt-reported full time employment | 40 | 0.05# | 48 | 0.375# | N/R | −1.34 | (−2.2, −0.5) | TX |
| Sayin; 2007 (CS) | SF-36 Physical Functioning | 75 | 55.90 | 20 | 68.75 | N/R | −0.51 | (−25.5, −0.2) | TX |
| Simmons; 1990 (CS) | Author-scale physical well-being score | 83 | 14.04 | 91 | 17.55 | <0.01 | −0.99 | (−4.6, −2.4) | TX |
| Tomasz; 2003 (CS) | WHOQOL-100 | 61 | 12.28 | 83 | 13.3 | <0.05 | −0.37 | (−1.9, −0.1) | TX |
| Van den Ham; 2005 (CS) | Symptom-limited graded cycle-ergometry test (ie, exercise test) | 16 | 6.2 | 35 | 7.2 | <0.05 | −0.67 | (−1.9, −0.1) | TX |
| Jofre; 1998; (Pre-Post) | Sickness Impact Profile | 93 | 5.5 | 93 | 3.6 | <0.01 | 0.29 | (0.0, 3.8) | TX |
| Baiardi; 2002(CS)*** | SF-36 Physical Functioning | 171 | 59.3 | 34 | 64.6 | N/R | −0.19 | (−15.7, 5.1) | ---- |
| Basok; 2009 (CS) | SF-36 Role Physical | 24 | 58.83 | 20 | 60 | N/R | −0.04 | (−26.6, 23.3) | --- |
| Devins; 1990 (CS)** | Author-scale (difficulties daily living) | 39 | 2 | 34 | 1.7 | NS | 0.17 | (−0.5, 1.1) | --- |
| Fujisawa; 2000 (CS) | SF-36 Role Physical | 114 | 68 | 117 | 77.6 | NS | −0.26 | (−19.2, 0.0) | --- |
| Ogutmen; 2006 (CS) | SF-36 Physical Functioning | 64 | 56.99 | 302 | 59.92 | NS | −0.11 | (−10.2, 4.4) | --- |
| Panagopoulou; 2009 (CS)*** | Pt-reported part time employment | 40 | 0.15# | 48 | 0.19# | N/R | −0.16 | (−0.8, 0.5) | --- |
| Pietrabissa; 1992 (CS) | Physical well-being scale developed by Simmons et al. (summary score) | 172 | 12.38 | 71 | 11.81 | NS | 0.28 | ---- | --- |
| Sayin; 2007 (CS) | SF-36 Role Physical | 75 | 40.69 | 20 | 42.50 | N/R | −0.05 | (−20.7, 17.1) | ---- |
| Recreation | |||||||||
| Hart; 1987 (CS) | Sickness Impact Profile (recreation/pastimes) | 343 | 23.7 | 146 | 9 | N/R | ---- | ---- | TX |
| Kontodimopoulos 2008 (CS) | SF-36 (Greek) Social Functioning | 642 | 58.1 | 167 | 72.4 | <0.001 | −0.49 | (−19.3, −9.3) | TX |
| Simmons; 1990 (CS) | Author-developed scale (recreation) | 83 | 2.24 | 91 | 2.86 | <0.05 | −0.67 | (−0.9, −0.3) | TX |
| Basok; 2009 (CS) | SF-36 Social Functioning | 24 | 68.75 | 20 | 64.38 | 0.1 | 0.14 | (−14.6, 23.3) | --- |
| Devins; 1990 (CS)*** | Intrusiveness Ratings Scale (recreation/social) | 39 | 2.5 | 34 | 2 | NS | 0.32 | (−0.2, 1.0) | --- |
| Sayin; 2007 (CS) | SF-36 Social Functioning | 75 | 62.62 | 20 | 57.93 | 0.5 | 0.18 | (−8.5, 17.9) | ---- |
| Tomasz; 2003 (CS) | WHOQOL-100 (environment) | 61 | 11.93 | 83 | 12.43 | NS | −0.26 | (−1.1, 0.1) | --- |
| Freedom | |||||||||
| Barendse; 2005(CS) | Renal Treatment Satisfaction Questionnaire | 35 | 4.3 | 46 | 5.7 | <0.05 | −1.12 | (−2.0, −0.8) | TX |
| Bremer; 1989 (CS) | Index of Well-Being (tied-down, free) | 105 | 4.2 | 166 | 5.3 | <0.05 | −0.55 | (−1.6, −0.6) | TX |
| Lee; 2005 (CS) | EQ-5D (usual activities) | 99 | 18.09 | 209 | 54.95 | <0.05 | −0.94 | (−1.3, −0.6) | TX |
| Simmons; 1990 (CS) | Author-developed scale (doing most things) | 83 | 5.17 | 91 | 5.78 | <0.05 | −0.83 | (−0.8, −0.4) | TX |
| Work | |||||||||
| Devins; 1990 (CS)*** | Intrusiveness Ratings Scale (work/finances) | 39 | 4 | 34 | 2.1 | <0.05 | 1.03 | (1.0, 2.8) | TX |
| Evans; 1985 (CS) | Patient reported ability to work | 347 | 44.8# | 144 | 62.3# | N/R | −0.39 | (−0.6, −0.2) | TX |
| Hart; 1987 (CS) | Sickness Impact Profile (work) | 338 | 45 | 144 | 28.4 | N/R | ---- | ---- | TX |
| Tomasz; 2003 (CS) | WHOQOL-100 (working capacity) | 61 | 10.4 | 83 | 12.18 | <0.05 | −0.46 | (−3.1, −0.5) | TX |
| Lee; 2005 (CS) | KDQOL (work status) | 99 | 20 | 209 | 46.8 | <0.05 | −0.65 | (−36.7, −16.9) | TX |
| Simmons; 1990 (CS) | Author-developed scale (job satisfaction) | 83 | 2.18 | 91 | 2.66 | <0.01 | −0.44 | (−0.8, −0.1) | TX |
Calculated Cohen’s d.
Estimates are means unless otherwise noted.
Author-reported p-values.
Percentage.
Percentage with low satisfaction with physical performance.
denotes studies were originally designed as longitudinal cohort but authors only reported cross-sectional assessments of relevant outcomes
We calculated the confidence interval for the Cohen’s d effect size difference for studies that reported all of the needed estimates (e.g., means plus standard deviations) We reported ‘---‘ for studies that we could not calculate the confidence intervals for due to missing estimates.
Abbreviations and definitions: ADL, activities of daily living; CS, cross sectional study design; Pre-Post, pre-post transplantation; NS, not statistically significantly different at P>0.05 level; NR, not reported within article; Pt, patient; Pts, patients; CI, confidence interval; HD, hemodialysis; KDQOL, Kidney Disease Quality of Life scale; SF-36, 36-Item Short-Form Health Survey; WHOQOL, World Health Organization Quality of Life instrument; BREF, short version, Tx, transplantation
Patients Receiving Peritoneal Dialysis Versus Transplant Recipients
A total of 17 studies evaluated life participation activities between patients receiving peritoneal dialysis compared to patients with kidney transplants9,19,20,32–34,38,43–45,51–53,55,63,64,66. Most studies reported on multiple outcomes, thus providing 21 physical function, 7 recreation, 4 freedom, and 5 work-related comparisons. The majority of comparisons demonstrated small to large differences in activities among patients with kidney transplants compared to patients receiving peritoneal dialysis, with transplant patients having better physical function (90%), freedom (100%), and work outcomes (100%). (Table 6) These findings of better outcomes in transplant patients were observed among 76% of comparisons from cross-sectional studies, 100% of comparisons from US-based studies, 50% of comparisons from studies that properly adjusted for potential confounders, and 65% of comparisons from studies published after 2000 favoring kidney transplantation. (Table 4)
Table 6.
Physical Function and Life Participation Outcome Measures and Study Results: PD versus Tx
| Study; Design | Outcome Measure | PD | Tx | Statistical Results | Treatment Favored | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Estimate* | N | Estimate* | Reported P** | Effect Size^ | Calculated CI**** | |||
| Physical Function | |||||||||
| Apostolou; 2007; CS | SF-36 Physical Functioning | 26 | 36 | 20 | 51.5 | 0.01 | −2.77 | (−18.9, −12.1) | TX |
| Apostolou; 2007 (CS) | SF-36 Role Physical | 26 | 12 | 20 | 47.5 | 0.001 | −4.95 | (−39.8, −31.2) | TX |
| Basok; 2009 (CS) | SF-36 Physical Functioning | 21 | 56.61 | 20 | 77.78 | N/R | −1.09 | (−33.4, −8.9) | TX |
| Khan; 1995 (CS) | SF-36 Physical Functioning | 27 | 38.99 | 102 | 67.51 | <0.05 | −0.43 | ---- | TX |
| Khan; 1995 (CS) | SF-36 Role Physical | 27 | 29.7 | 102 | 63.22 | <0.05 | −0.43 | ---- | TX |
| Koch; 1990 (CS) | Pt-reported low satisfaction with physical performance | 68 | 0.47# | 761 | 0.18# | N/R | 0.80 | (0.49, 1.0) | TX |
| Kontodimopoulos; 2008 (CS) | SF-36 (Greek) Physical Functioning | 65 | 49.2 | 167 | 72.9 | <0.001 | −1.04 | (−30.3, −17.1) | TX |
| Kontodimopoulos; 2008 (CS) | SF-36 (Greek) Role Physical | 65 | 30.9 | 167 | 62.6 | <0.001 | −0.76 | (−43.7, −19.7) | TX |
| Lee; 2005 (CS) | SF-36 Physical Functioning | 74 | 30.9 | 209 | 56.4 | <0.05 | −0.83 | (−33.6, −17.1) | TX |
| Lee; 2005 (CS) | SF-36 Role Physical | 74 | 60.6 | 209 | 86.7 | <0.05 | −0.79 | (−34.8, −17.3) | TX |
| Niu; 2005 (CS) | WHOQOL-BREF (Taiwan; Scale physical health) | 80 | 11.61 | 80 | 14.34 | <0.05 | −1.17 | (−3.5, −2.0) | TX |
| Ogutmen; 2006 (CS) | SF-36 Role Physical | 207 | 55.1 | 302 | 71.09 | <0.05 | −0.36 | (−23.8, −8.1) | TX |
| Panagopoulou; 2009 (CS)*** | Pt-reported full time employment | 36 | 0.14 | 48 | 0.375 | N/R | −0.71 | (−1.3, −0.1) | TX |
| Simmons; 1990 (CS) | Author-scale physical well-being score | 510 | 14.64 | 91 | 17.55 | <0.01 | −0.72 | (−3.8, −2.0) | TX |
| Baiardi; 2002 (CS)*** | SF-36 Physical Functioning | 30 | 59.7 | 34 | 64.6 | N/R | −0.17 | (−19.4, 9.6) | ---- |
| Basok; 2009 (CS) | SF-36 Role Physical | 21 | 45.81 | 20 | 60 | N/R | −0.47 | (−48.6, 7.2) | --- |
| Devins; 1990 (CS)*** | Author-developed difficulties in ADL scale | 11 | 2.6 | 34 | 1.7 | NS | 0.49 | (−0.4, 2.2) | --- |
| Ogutmen; 2006 (CS) | SF-36 Physical Functioning | 207 | 57.06 | 302 | 59.92 | N/R | −0.12 | (−7.1, 1.4) | --- |
| Panagopoulou; 2009 (CS)*** | Pt-reported employment | 36 | 0.17# | 48 | 0.19# | N/R | −0.07 | (−0.7, 0.5) | --- |
| Sayin; 2007 (CS) | SF-36 Physical Functioning | 41 | 55.76 | 20 | 68.75 | 0.1 | −0.47 | (−27.9, 2.0) | ---- |
| Sayin; 2007 (CS) | SF-36 Role Physical | 41 | 39.10 | 20 | 42.50 | 0.9 | −0.08 | (−25.3, 18.5) | ---- |
| Recreation | |||||||||
| Apostolou; 2007 (CS) | SF-36 Social Functioning | 26 | 49.3 | 20 | 76.7 | 0.001 | −5.67 | (−30.3, −24.5) | TX |
| Devins; 1990 (CS)*** | Intrusiveness Ratings Scale (recreation/social activities) | 11 | 3.2 | 34 | 2 | <0.05 | 0.82 | (0.2, 2.0) | TX |
| Hart; 1987 (CS) | Sickness Impact Profile (recreation) | 77 | 24 | 146 | 9 | <0.05 | ---- | ---- | TX |
| Kontodimopoulos; 2008 (CS) | SF-36 (Greek) Social Functioning | 65 | 54.9 | 167 | 72.4 | <0.001 | −0.67 | (−25.0, −10.0) | TX |
| Simmons; 1990 (CS) | Author-scale (recreation satisfaction) | 510 | 2.21 | 91 | 2.86 | <0.05 | −0.66 | (−0.9, −0.4) | TX |
| Basok; 2009 (CS) | SF-36 Social Functioning | 21 | 63.39 | 20 | 64.38 | NS | −0.03 | (−16.3, 14.9) | --- |
| Sayin; 2007 (CS) | SF-36 Social Functioning | 41 | 56.32 | 20 | 57.93 | 0.5 | −0.05 | (−18.5, 15.3) | ---- |
| Freedom | |||||||||
| Barendse; 2005 (CS) | Renal Treatment Satisfaction | 57 | 4.3 | 46 | 5.7 | <0.05 | −1.05 | (−1.9, −0.9) | TX |
| Bremer; 1989 (CS) | Index of Well-Being (tied-down, free) | 79 | 4.5 | 166 | 5.3 | <0.05 | −0.44 | (−1.3, −0.3) | TX |
| Lee; 2005 (CS) | EQ-5D (usual activities) | 74 | 20.55 | 209 | 54.95 | <0.05 | −0.85 | (−1.2, −0.5) | TX |
| Simmons; 1990 (CS) | Author-scale (doing most things) | 510 | 5.29 | 91 | 5.78 | <0.05 | −0.59 | (−0.7, −0.3) | TX |
| Work | |||||||||
| Devins; 1990 (CS)*** | Intrusiveness Ratings Scale (work) | 11 | 3.6 | 34 | 2.1 | <0.05 | 1.01 | (0.5, 2.5) | TX |
| Evans; 1985 (CS) | Pt-reported ability to work | 81 | 27.8# | 144 | 62.3# | N/R | −0.80 | (−1.1, −0.5) | TX |
| Hart; 1987 (CS) | Sickness Impact Profile (work) | 81 | 51 | 144 | 28.4 | <0.01 | ---- | ---- | TX |
| Lee; 2005 (CS) | KDQOL (work status) | 74 | 28.2 | 209 | 46.8 | <0.05 | −0.43 | (−30.2, −7.0) | TX |
| Simmons; 1990 (CS) | Author-scale (job satisfaction) | 510 | 2.38 | 91 | 2.66 | N/R | −0.23 | (−0.5, −0.01) | TX |
Calculated Cohen’s d.
Estimates are means unless otherwise noted.
Percentage.
Author-reported p-values.
denotes studies were originally designed as longitudinal cohort but authors only reported cross-sectional assessments of relevant outcomes
We calculated the CI for the Cohen’s d effect size difference for studies that reported all of the needed estimates (e.g., means plus standard deviations) We reported ‘---‘ for studies that we could not calculate the CIs for due to missing estimates.
Abbreviations and definitions: ADL, activities of daily living; CS, cross sectional study design; Pre-Post, pre-post transplantation; NS, not statistically significantly different at P>0.05 level; NR, not reported within article; Pt, patient; Pts, patients; CI, confidence interval; PD, peritoneal dialysis; KDQOL, Kidney Disease Quality of Life scale; SF-36, 36-Item Short-Form Health Survey; WHOQOL, World Health Organization Quality of Life instrument; BREF, short version, Tx, transplantation
Discussion
In this systematic review, a majority of studies consistently reported better physical functioning, greater engagement in social and recreational activities, greater independence, and better ability to work among patients with kidney transplants compared to patients receiving dialysis. Included studies did not report significant differences in outcomes between patients receiving hemodialysis and patients receiving peritoneal dialysis. Studies used a variety of measures to assess outcomes and were conducted among patients from diverse demographic backgrounds and clinical settings.
To our knowledge, this is the most comprehensive and recent systematic review to explore differences in rates of life participation activities among patients receiving various RRT modalities. With a carefully designed literature search and rigorous methods, we synthesized the results of 46 studies published over nearly three decades. Our findings, which summarize evidence on a broad range of life participation outcomes, should help patients and physicians better understand the quality and quantity of evidence available to inform their RRT selection choices. Outcomes were assessed both objectively (e.g. symptom-limited graded cycle ergometry tests) and subjectively reported by patients themselves (e.g. patient-reported questionnaires). We found that the magnitude and direction of associations we observed were similar for subjective patient-reported outcomes and objective clinical outcome measures. This may provide evidence of the importance and intrinsic similarity of both subjective and objective measurements, which attempt to comprehensively capture the extent to which patients are able to assimilate normal activities after initiating therapies.
As with every systematic review, the strength of our conclusions depends on the quality of available studies. Substantial limitations in the studies we identified indicate the evidence should be interpreted with caution. First, a majority of the studies reported outcomes at a single time point among patients often being observed for other (i.e. non-life participation) primary outcomes. Thus, we were only able to assess potential associations (versus true causal links) between RRT modality and outcomes of interest. It is possible that other important clinical characteristics influencing patients’ initial selection of RRT modalities (e.g., comorbid disease burden and medical eligibility for certain RRT modalities) could have also influenced their rates of life participation. For instance, patients enrolled in these studies who had received kidney transplants could have been healthier than those who may not have received kidney transplants. Studies variably accounted for these and other related factors, such as the presence or absence of diabetes or peripheral vascular disease, which could influence observed associations between RRT modality and rates of life participation. Ideally, randomized controlled trials would be performed to quantify differences in life participation among patients randomly selected to receive different RRTs. However, the feasibility of performing such trials is low, particularly since choice of RRT is influenced by a variety of factors, including patient and provider preferences, patients’ families’ capacities to support certain RRTs (e.g., managing peritoneal dialysis supplies and equipment at home), and patients’ medical suitability for transplants. One randomized trial conducted to compare mean quality-adjusted life-year (QALY) and survival among patients receiving hemodialysis compared to peritoneal dialysis reported statistically-equivalent QALY scores between the two groups of patients; however, the trial was prematurely stopped due to low inclusion rate71.
Additional potential limitations deserve consideration. First, we excluded non-English articles, which could introduce potential language bias. However, only 15% of potential articles were not published in English, and our included studies were conducted among a heterogeneous patient population representing 16 distinct locations worldwide. Therefore, we anticipate that the exclusion of these non-English articles will not significantly change our observed findings. Second, our review did not fully assess some potential treatment characteristics that might influence life participation outcomes, such as treatment intensity. Third, several of our included studies were identified from our hand-search of bibliographies but were not retrieved through our initial search of electronic databases. Although our electronic search yielded 5,265 citations that were potentially content-relevant, many of these studies were excluded due to a lack of comparative reporting of outcomes. We encountered difficulties devising electronic search terms to explicitly distinguish studies that are both content-relevant and inherently comparative in nature (i.e., reported relevant outcomes by RRT modality). Further, we speculate that some missed articles may have also been indexed prior to the inclusion of key search terms (e.g., MeSH headings and subheadings in PubMed) for our outcomes of interest72. Finally, we did not seek unpublished data from investigators who may have studied life participation among patients on RRT. It is possible studies reporting better outcomes among patients with transplants were more likely to be published. Notwithstanding these limitations, we believe our review provides a comprehensive summary of the most recent evidence regarding rates of life participation among patients receiving various RRTs and could serve as a valuable resource to patients and clinicians seeking to understand the current state of evidence informing this area.
In summary, a majority of studies reported better rates of life participation among patients with kidney transplants compared to patients receiving dialysis. Studies reported no significant differences in activities among patients receiving hemodialysis and patients receiving peritoneal dialysis. Many studies featured significant weaknesses in their design, limiting inferences. Rigorously performed studies incorporating randomized or longitudinal designs allowing for causal inferences and appropriately accounting for factors which could confound observed differences in outcomes among patients on different RRTs could better guide patients’ and nephrologists’ selection decisions.
Supplementary Material
Table S1: Search strategy and terms.
Table S2: Additional study participant characteristics.
Table S3: Assessment of individual study quality for relevant outcomes of interest.
Table S4: Description of outcome measures.
Item S1: Structured quality assessment questions.
Acknowledgments
The authors sincerely thank Blair Anton, MLIS, MS (Associate Director, Clinical Informationist Services, Johns Hopkins Welch Medical Library) and Victoria Goode, MLIS (Clinical Informationist, Johns Hopkins Welch Medical Library) for their methodological expertise in helping to develop the search terms.
Support: Dr Purnell was supported by grant F31DK084840 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). Dr Crews was supported by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation, Princeton, NJ. Dr Greer was supported by the National Center for Research Resources of the NIH and grant 5KL2RR025006 from the NIH Roadmap for Medical Research. Dr Powe was supported, in part, by grants R01DK079682 and K24DK02643 from the NIH-NIDDK. Dr Rabb was supported by grant R01DK079682 from the NIH-NIDDK. Dr Boulware was supported by grants R01DK079682 and K23DK070757 from the NIH-NIDDK.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007 Jan;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Wu AW, Fink NE, Marsh-Manzi JVR, et al. Changes in Quality of Life during Hemodialysis and Peritoneal Dialysis Treatment: Generic and Disease Specific Measures. Journal of the American Society of Nephrology. 2004;15(3):743–753. doi: 10.1097/01.asn.0000113315.81448.ca. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007 Aug;72(3):247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 4.Blake C, Codd MB, Cassidy A, O’Meara YM. Physical function, employment and quality of life in end-stage renal disease. J Nephrol. 2000 Mar-Apr;13(2):142–149. [PubMed] [Google Scholar]
- 5.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997 Aug;30(2):204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 6.Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003 Jun;41(6):1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 7.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010 Dec;78(11):1164–1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korevaar JC, Jansen MA, Merkus MP, Dekker FW, Boeschoten EW, Krediet RT. Quality of life in predialysis end-stage renal disease patients at the initiation of dialysis therapy. The NECOSAD Study Group. Perit Dial Int. 2000 Jan-Feb;20(1):69–75. [PubMed] [Google Scholar]
- 9.Devins GM, Mandin H, Hons RB, et al. Illness intrusiveness and quality of life in end-stage renal disease: comparison and stability across treatment modalities. Health Psychol. 1990;9(2):117–142. doi: 10.1037//0278-6133.9.2.117. [DOI] [PubMed] [Google Scholar]
- 10.Kurella M, Suri RS, Chertow GM. Frequent hemodialysis and psychosocial function. Semin Dial. 2005 Mar-Apr;18(2):132–136. doi: 10.1111/j.1525-139X.2005.18216.x. [DOI] [PubMed] [Google Scholar]
- 11.Ting GO, Kjellstrand C, Freitas T, Carrie BJ, Zarghamee S. Long-term study of high-comorbidity ESRD patients converted from conventional to short daily hemodialysis. Am J Kidney Dis. 2003 Nov;42(5):1020–1035. doi: 10.1016/j.ajkd.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Kutner NG. Quality of life and daily hemodialysis. Semin Dial. 2004 Mar-Apr;17(2):92–98. doi: 10.1111/j.0894-0959.2004.17203.x. [DOI] [PubMed] [Google Scholar]
- 13.Morton RL, Tong A, Howard K, Snelling P, Webster AC. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ. 2010;340:c112. doi: 10.1136/bmj.c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanouzas D, Ng KP, Fallouh B, Baharani J. What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant. 2012 Apr;27(4):1542–1547. doi: 10.1093/ndt/gfr452. [DOI] [PubMed] [Google Scholar]
- 15.Halpern SD, Berns JS, Israni AK. Willingness of patients to switch from conventional to daily hemodialysis: looking before we leap. Am J Med. 2004 May 1;116(9):606–612. doi: 10.1016/j.amjmed.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Lee A, Gudex C, Povlsen JV, Bonnevie B, Nielsen CP. Patients’ views regarding choice of dialysis modality. Nephrology Dialysis Transplantation. 2008;23(12):3953–3959. doi: 10.1093/ndt/gfn365. [DOI] [PubMed] [Google Scholar]
- 17.Bass EB, Jenckes MW, Fink NE, et al. Use of focus groups to identify concerns about dialysis. Choice Study. Med Decis Making. 1999 Jul-Sep;19(3):287–295. doi: 10.1177/0272989X9901900307. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011 Oct;11(10):2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans RW, Manninen DL, Garrison LP, Jr, et al. The quality of life of patients with end-stage renal disease. N Engl J Med. 1985 Feb 28;312(9):553–559. doi: 10.1056/NEJM198502283120905. [DOI] [PubMed] [Google Scholar]
- 20.Lee AJ, Morgan CL, Conway P, Currie CJ. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin. 2005 Nov;21(11):1777–1783. doi: 10.1185/030079905X65277. [DOI] [PubMed] [Google Scholar]
- 21.Boateng EA, East L. The impact of dialysis modality on quality of life: a systematic review. J Ren Care. 2011 Dec;37(4):190–200. doi: 10.1111/j.1755-6686.2011.00244.x. [DOI] [PubMed] [Google Scholar]
- 22.Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2008 Jul-Aug;11(4):733–741. doi: 10.1111/j.1524-4733.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 23.Depasquale N, Ephraim PL, Ameling J, et al. Selecting renal replacement therapies: what do African American and non-African American patients and their families think others should know? A mixed methods study. BMC Nephrol. 2013;14:9. doi: 10.1186/1471-2369-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ. In: Modern epidemiology. 3. Greenland S, Lash TL, editors. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. thoroughly rev. and updated. ed. [Google Scholar]
- 25.Chalmers TC, Smith H, Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981 May;2(1):31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 26.Cooper HM, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. 2. New York: Russell Sage Foundation; 2009. [Google Scholar]
- 27.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiologic reviews. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 28.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological reviews of the Cambridge Philosophical Society Nov. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 30.Aiyasanon N, Premasathian N, Nimmannit A, Jetanavanich P, Sritippayawan S. Validity and reliability of CHOICE Health Experience Questionnaire: Thai version. J Med Assoc Thai. 2009 Sep;92(9):1159–1166. [PubMed] [Google Scholar]
- 31.Alavi NM, Aliakbarzadeh Z, Sharifi K. Depression, anxiety, activities of daily living, and quality of life scores in patients undergoing renal replacement therapies. Transplant Proc. 2009 Nov;41(9):3693–3696. doi: 10.1016/j.transproceed.2009.06.217. [DOI] [PubMed] [Google Scholar]
- 32.Barendse SM, Speight J, Bradley C. The Renal Treatment Satisfaction Questionnaire (RTSQ): a measure of satisfaction with treatment for chronic kidney failure. Am J Kidney Dis. 2005 Mar;45(3):572–579. doi: 10.1053/j.ajkd.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Basok EK, Atsu N, Rifaioglu MM, Kantarci G, Yildirim A, Tokuc R. Assessment of female sexual function and quality of life in predialysis, peritoneal dialysis, hemodialysis, and renal transplant patients. Int Urol Nephrol. 2009;41(3):473–481. doi: 10.1007/s11255-008-9475-z. [DOI] [PubMed] [Google Scholar]
- 34.Bremer BA, McCauley CR, Wrona RM, Johnson JP. Quality of life in end-stage renal disease: a reexamination. Am J Kidney Dis. 1989 Mar;13(3):200–209. doi: 10.1016/s0272-6386(89)80053-8. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael P, Popoola J, John I, Stevens PE, Carmichael AR. Assessment of quality of life in a single centre dialysis population using the KDQOL-SF questionnaire. Qual Life Res. 2000 Mar;9(2):195–205. doi: 10.1023/a:1008933621829. [DOI] [PubMed] [Google Scholar]
- 36.Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang H, Lazarus JM. Quality-of-life evaluation using Short Form 36: comparison in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2000 Feb;35(2):293–300. doi: 10.1016/s0272-6386(00)70339-8. [DOI] [PubMed] [Google Scholar]
- 37.Fujisawa M, Ichikawa Y, Yoshiya K, et al. Assessment of health-related quality of life in renal transplant and hemodialysis patients using the SF-36 health survey. Urology. 2000 Aug 1;56(2):201–206. doi: 10.1016/s0090-4295(00)00623-3. [DOI] [PubMed] [Google Scholar]
- 38.Hart LG, Evans RW. The functional status of ESRD patients as measured by the Sickness Impact Profile. J Chronic Dis. 1987;40 (Suppl 1):117S–136S. doi: 10.1016/s0021-9681(87)80041-3. [DOI] [PubMed] [Google Scholar]
- 39.Holley JL, Nespor S. An analysis of factors affecting employment of chronic dialysis patients. Am J Kidney Dis. 1994 May;23(5):681–685. doi: 10.1016/s0272-6386(12)70278-0. [DOI] [PubMed] [Google Scholar]
- 40.Jofre R, Lopez-Gomez JM, Moreno F, Sanz-Guajardo D, Valderrabano F. Changes in quality of life after renal transplantation. Am J Kidney Dis. 1998 Jul;32(1):93–100. doi: 10.1053/ajkd.1998.v32.pm9669429. [DOI] [PubMed] [Google Scholar]
- 41.Juergensen E, Wuerth D, Finkelstein SH, Juergensen PH, Bekui A, Finkelstein FO. Hemodialysis and peritoneal dialysis: patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin J Am Soc Nephrol. 2006 Nov;1(6):1191–1196. doi: 10.2215/CJN.01220406. [DOI] [PubMed] [Google Scholar]
- 42.Julius M, Kneisley JD, Carpentier-Alting P, Hawthorne VM, Wolfe RA, Port FK. A comparison of employment rates of patients treated with continuous ambulatory peritoneal dialysis vs in-center hemodialysis (Michigan End-Stage Renal Disease Study) Arch Intern Med. 1989 Apr;149(4):839–842. [PubMed] [Google Scholar]
- 43.Khan IH, Garratt AM, Kumar A, et al. Patients’ perception of health on renal replacement therapy: Evaluation using a new instrument. Nephrology Dialysis Transplantation. 1995;10(5):684–689. [PubMed] [Google Scholar]
- 44.Koch U, Muthny FA. Quality of life in patients with end-stage renal disease in relation to the method of treatment. Psychother Psychosom. 1990;54(2–3):161–171. doi: 10.1159/000288390. [DOI] [PubMed] [Google Scholar]
- 45.Kontodimopoulos N, Pappa E, Niakas D. Gender- and age-related benefit of renal replacement therapy on health-related quality of life. Scand J Caring Sci. 2009 Dec;23(4):721–729. doi: 10.1111/j.1471-6712.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 46.Kutner NG, Zhang R, Barnhart H, Collins AJ. Health status and quality of life reported by incident patients after 1 year on haemodialysis or peritoneal dialysis. Nephrol Dial Transplant. 2005 Oct;20(10):2159–2167. doi: 10.1093/ndt/gfh973. [DOI] [PubMed] [Google Scholar]
- 47.Lok P. Stressors, coping mechanisms and quality of life among dialysis patients in Australia. J Adv Nurs. 1996 May;23(5):873–881. doi: 10.1046/j.1365-2648.1996.00893.x. [DOI] [PubMed] [Google Scholar]
- 48.Manns B, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C. Quality of life in patients treated with hemodialysis or peritoneal dialysis: what are the important determinants? Clin Nephrol. 2003 Nov;60(5):341–351. doi: 10.5414/cnp60341. [DOI] [PubMed] [Google Scholar]
- 49.Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT. Physical symptoms and quality of life in patients on chronic dialysis: results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD) Nephrol Dial Transplant. 1999 May;14(5):1163–1170. doi: 10.1093/ndt/14.5.1163. [DOI] [PubMed] [Google Scholar]
- 50.Molsted S, Prescott L, Heaf J, Eidemak I. Assessment and clinical aspects of health-related quality of life in dialysis patients and patients with chronic kidney disease. Nephron Clin Pract. 2007;106(1):c24–33. doi: 10.1159/000101481. [DOI] [PubMed] [Google Scholar]
- 51.Niu SF, Li IC. Quality of life of patients having renal replacement therapy. J Adv Nurs. 2005 Jul;51(1):15–21. doi: 10.1111/j.1365-2648.2005.03455.x. [DOI] [PubMed] [Google Scholar]
- 52.Ogutmen B, Yildirim A, Sever MS, et al. Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc. 2006 Mar;38(2):419–421. doi: 10.1016/j.transproceed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Panagopoulou A, Hardalias A, Berati S, Fourtounas C. Psychosocial issues and quality of life in patients on renal replacement therapy. Saudi J Kidney Dis Transpl. 2009 Mar;20(2):212–218. [PubMed] [Google Scholar]
- 54.Pietrabissa A, Ciaramella A, Carmellini M, et al. Effect of kidney transplantation on quality of life measures. Transpl Int. 1992;5 (Suppl 1):S708–710. doi: 10.1007/978-3-642-77423-2_207. [DOI] [PubMed] [Google Scholar]
- 55.Simmons RG, Anderson CR, Abress LK. Quality of life and rehabilitation differences among four end-stage renal disease therapy groups. Scand J Urol Nephrol Suppl. 1990;131:7–22. [PubMed] [Google Scholar]
- 56.Tell GS, Mittelmark MB, Hylander B, Shumaker SA, Russell G, Burkart JM. Social support and health-related quality of life in black and white dialysis patients. ANNA J. 1995 Jun;22(3):301–308. discussion 309–310. [PubMed] [Google Scholar]
- 57.Thong MS, van Dijk S, Noordzij M, et al. Symptom clusters in incident dialysis patients: associations with clinical variables and quality of life. Nephrol Dial Transplant. 2009 Jan;24(1):225–230. doi: 10.1093/ndt/gfn449. [DOI] [PubMed] [Google Scholar]
- 58.Tomasz W, Piotr S. A trial of objective comparison of quality of life between chronic renal failure patients treated with hemodialysis and renal transplantation. Ann Transplant. 2003;8(2):47–53. [PubMed] [Google Scholar]
- 59.Tucker CM, Ziller RC, Smith WR, Mars DR, Coons MP. Quality of life of patients on incenter hemodialysis versus continuous ambulatory peritoneal dialysis. Perit Dial Int. 1991;11(4):341–346. [PubMed] [Google Scholar]
- 60.Van Den Ham ECH, Kooman JP, Schols AMWJ, et al. Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. American Journal of Transplantation. 2005;5(8):1957–1965. doi: 10.1111/j.1600-6143.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 61.Wolcott DL, Nissenson AR. Quality of life in chronic dialysis patients: a critical comparison of continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis. Am J Kidney Dis. 1988 May;11(5):402–412. doi: 10.1016/s0272-6386(88)80053-2. [DOI] [PubMed] [Google Scholar]
- 62.Borowiak E, Braksator E, Nowicki M, Kostka T. Quality of life of chronic hemodialysis and peritoneal dialysis patients. Clinical and Experimental Medical Letters. 2009;50(1):37–42. [Google Scholar]
- 63.Baiardi F, Esposti ED, Cocchi R, et al. Effects of clinical and individual variables on quality of life in chronic renal failure patients. Journal of Nephrology. 2002;15(1):61–67. [PubMed] [Google Scholar]
- 64.Apostolou T, Hutchison AJ, Boulton AJM, et al. Quality of life in CAPD, transplant, and chronic renal failure patients with diabetes. Renal Failure. 2007;29(2):189–197. doi: 10.1080/08860220601098862. [DOI] [PubMed] [Google Scholar]
- 65.Ibrahim N, Chiew-Tong NK, Desa A. Symptoms and health-related quality of life in patients with heamodialysis and continuous ambulatory peritoneal dialysis. Research Journal of Medical Sciences. 2011;5(5):252–256. [Google Scholar]
- 66.Sayin A, Mutluay R, Sindel S. Quality of Life in Hemodialysis, Peritoneal Dialysis, and Transplantation Patients. Transplantation Proceedings. 2007;39(10):3047–3053. doi: 10.1016/j.transproceed.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 67.Merkus MP, Jager KJ, Dekker FW, et al. Quality of life in patients on chronic dialysis: Self-assessment 3 months after the start of treatment. American Journal of Kidney Diseases. 1997;29(4):584–592. doi: 10.1016/s0272-6386(97)90342-5. [DOI] [PubMed] [Google Scholar]
- 68.Masuda R, Imamura H, Mizuuchi K, Miyahara K, Kumagai H, Hirakata H. Physical activity, high-density lipoprotein cholesterol subfractions and lecithin:cholesterol acyltransferase in dialysis patients. Nephron. Clinical practice. 2009;(4):c253–259. doi: 10.1159/000209152. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/876/CN-00704876/frame.html. [DOI] [PubMed]
- 69.Harris SAC, Lamping DL, Brown EA, et al. Clinical outcomes and quality of life in elderly patients on peritoneal dialysis versus hemodialysis. Peritoneal Dialysis International. 2002;22(4):463–470. [PubMed] [Google Scholar]
- 70.Curtin RB, Oberley ET, Sacksteder P, Friedman A. Differences between employed and nonemployed dialysis patients. American Journal of Kidney Diseases. 1996;27(4):533–540. doi: 10.1016/s0272-6386(96)90164-x. [DOI] [PubMed] [Google Scholar]
- 71.Korevaar JC, Feith GW, Dekker FW, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney International. 2003;(6):2222–2228. doi: 10.1046/j.1523-1755.2003.00321.x. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/154/CN-00446154/frame.html. [DOI] [PubMed]
- 72.Vincent B, Vincent M, Ferreira CG. Making PubMed searching simple: learning to retrieve medical literature through interactive problem solving. The oncologist. 2006 Mar;11(3):243–251. doi: 10.1634/theoncologist.11-3-243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Search strategy and terms.
Table S2: Additional study participant characteristics.
Table S3: Assessment of individual study quality for relevant outcomes of interest.
Table S4: Description of outcome measures.
Item S1: Structured quality assessment questions.