Abstract
Appropriate animal models are critical to conduct translational studies of human disorders without variables that can confound clinical studies. Such analytic methods as patch-clamp electrophysiological and voltammetric recordings of neurons in brain slices require living brain tissue. In order to obtain viable tissue from nonhuman primate brains, tissue collection methods must be designed to preserve cardiovascular and respiratory functions for as long as possible. This paper describes a method of necropsy in three species of monkeys that satisfies this requirement. At necropsy, animals were maintained under a deep surgical plane of anesthesia while a craniotomy was conducted to expose the brain. Following the craniotomy, animals were perfused with ice-cold, oxygenated artificial cerebrospinal fluid to displace blood and to reduce the temperature of the entire brain. The brain was removed within minutes of death and specific brain regions were immediately dissected for subsequent in vitro electrophysiology or voltammetry experiments. This necropsy method also provided for the collection of tissue blocks containing all brain regions that were immediately frozen and stored for subsequent genomic, proteomic, autoradiographic and histological studies. An added benefit from the design of this necropsy method is that all major peripheral tissues were also collected and are now being utilized in a wide range of genomic, biochemical and histological assays. This necropsy method has resulted in the establishment and growth of a nonhuman primate alcohol tissue bank designed to distribute central nervous system and peripheral tissues to the larger scientific community.
Keywords: Non-human primate, brain function, electrophysiology, voltammetry, hybridization array, autoradiography, living tissue, tissue banking
Introduction
Nonhuman primates (NHPs) provide unique research models to study complex behavioral issues. NHPs are phylogenetically close to humans and also have extensive capacities for complex social and cognitive behavior. Their relatively long lifespan, extended infancy, and social cognition parallel many aspects of human development and the physiological, behavioral, and neuroanatomical similarities to humans facilitate translation of findings in these animal models to a variety of human conditions (Grant and Bennett 2003; Shively and Clarkson 2009). The cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) macaque have been widely used as research models to examine multiple pathological conditions in humans. The African green vervet monkey (Chlorocebus aethiops) is an intermediate size NHP that physiologically and genetically resembles other old world monkeys (Disotell et al. 2000) and is currently gaining more focus as a translational research resource. Animal models provide an alternative to address or overcome limitations inherent with clinical research. In addition, behavioral, pharmacological, and neurobiological variables that mediate drug intake can be identified in an animal model with a greater degree of confidence that the consequences are attributable to the treatment in question rather than to comorbid conditions that can often confound clinical studies. These variables can be tightly managed in animal studies in ways that are not possible clinically. We have developed a standardized necropsy protocol for central nervous system (CNS) and peripheral tissue collection that has optimized the use of these valuable resources by the broader scientific community.
Histological, genomic and proteomic assays of brain regions typically utilize tissue that is freshly obtained and then flash frozen or prepared with a fixative. In vitro electrophysiology and voltammetry slice technologies applied to brain tissue, however, require living tissue in order to record physiological responses. Many of these studies have been conducted in rodent brains, which can be quickly removed from the skull and are small enough to chill fairly readily when bathed in cold buffer solutions. Similarly, clinical studies utilize tissues obtained during biopsy that are typically small enough to rapidly reduce temperature of the tissue block. The typical method of preparing tissues for recording studies in non-human primates involves a similar approach of applying cold buffer to brain regions prior to removal from the skull and then submerging the tissue block into the same cold buffer (González-Burgos et al. 2000; Altemus et al. 2005; Povysheva et al. 2006). The initial impetus for developing the current necropsy method was to harvest viable CNS tissue for electrophysiology and voltammetry studies in the brains of nonhuman primates that were subjects in long-term ethanol self-administration studies in order to determine the impact of chronic ethanol consumption on brain function. The present report describes a method for perfusing the entire NHP brain with cold artificial cerebral spinal fluid (ACSF), which results in the displacement of blood and other potential ischemic components while at the same time reducing the internal temperature of the entire brain from “inside-out”. This method allows for the global harvesting of brain regions in addition to the collection and banking of multiple peripheral tissues for subsequent histological, genomic, proteomic and biochemical assays. This approach provides for better use of multiple CNS and peripheral tissues to address specific questions from multiple avenues of research, thus providing a clearer picture of the physiological changes that occur following chronic ethanol exposure.
Methods
Subjects
The animals used to develop this method were cynomolgus macaques (Macaca fascicularis) that were subjects in long-term ethanol self-administration protocols using a well characterized drinking paradigm designed to elicit patterns and intake of ethanol that parallel those observed in human alcoholics. The details of the ethanol self-administration procedures are described in previous reports (Vivian et al. 2001; Grant et al. 2008). This experimental design elicits ethanol consumption that is normally distributed such that some animals consume low amounts of ethanol (~0.5–1.0 g/kg or the equivalent of 2–4 drinks/day) while other animals are on the opposite end of the spectrum, drinking upwards of 3.0–4.0 g/kg (12–16 drinks/day) as described previously (Vivian et al. 2001; Grant et al. 2008). All procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University Health Sciences. All experiments conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals in research (NIH Publications No. 80-23).
Craniotomy
Pre-mortem structural MRIs were used to guide the brain dissection in order to localize the precise location of discrete brain regions as previously described (Daunais et al. 2010). Prior to necropsy, coordinates for specific brain regions were identified for placement of the brain knives so that the brain would be blocked at targeted locations. At necropsy, each animal was sedated with ketamine (15 mg/kg i.m.) and transported to the necropsy suite. The monkey was placed on its abdomen and brought to a deep surgical plane of anesthesia with intravenous sodium pentobarbital administered to effect (30–50 mg/kg, i.v.) via an angiocatheter that was placed in the saphenous vein. Indicators that the plane of anesthesia was adequate to proceed with the procedure included deep steady respirations, a lack of corneal and palpebral reflexes, and the absence of deep pain withdrawal reflexes. At that point, the scalp was incised longitudinally in a rostral to caudal direction along the sagittal suture beginning at the bregma cranial suture and extending caudally beyond the foramen magnum to approximately the 3rd cervical vertebrae. A second incision was made in the lateral to medial direction along the coronal suture, perpendicular to the initial incision. The temporal, frontal and occipital muscles were reflected bilaterally and the skull was exposed by blunt dissection. The occipital muscle at the back of the skull was carefully reflected beyond the occipital ridge. Once the muscle and fascia were reflected, a 1½″ 25g needle was inserted through the foramen magnum into the cisterna magna and approximately 1½–2 cc of CSF was collected, placed in a microfuge tube and immediately placed on dry ice. The top of the skull was cleaned and dried with 70% ethanol and gauze pads. Using a cordless drill and skull bit, a small circle (0.5 cm2) of bone was excised from the right parietal bone with great care taken not to disturb the dura mater. A squeeze bottle filled with saline was used to reduce aerosolization of bone dust during the craniotomy. The piece of bone was removed using small tipped forceps. Small tipped rongeurs were then introduced into the newly made craniotomy and the process of bone removal was started. Using the rongeurs, the skull was chipped away in small pieces so as not to fracture large sections of skull or pierce the dura mater. The bone was removed in a ventro-lateral direction to approximately the squamosal suture, and caudally to the occipital ridge. A periosteal elevator was utilized frequently to separate dural adhesions from the calvaria. The process of bone removal continued in a rostral direction along the greater wings of the sphenoid bone, across the midline taking care to not disturb the sagittal sinus. The process of bone removal was repeated on the contralateral side in a rostral to caudal direction to the occipital ridge. The area of bone between the occipital ridge and foramen magnum was left intact until immediately following the perfusion. The calvaria were carefully removed exposing the dura, and the brain was gently covered with saline-soaked gauze.
Transcardial perfusion
The monkey was turned over and placed in a supine position with the head and exposed brain resting on saline-soaked gauze. An incision was made between the clavicles extending to the lower pelvic region. A thoracotomy was conducted and multiple blood samples were immediately collected from the inferior vena cava. It should be noted that additional blood samples were collected at an earlier time during the week preceding the scheduled necropsy in order to reduce the volume that was collected the day of the procedure to avoid hypovolemia. The diaphragm was then opened to expose the heart. The descending aorta was clamped in the lower thorax to ensure adequate perfusion of the brain. The pericardium was opened and the right atrium was cut to decrease the blood pressure. The apex of the left ventricle was excised and a large bore cannula was introduced into the left ventricle, placed into the ascending aorta and clamped in place. Each animal was perfused for approximately 1.5 minutes with ice-cold ACSF buffer containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 23 NaHCO3, 3 NaH2PO4, 10 glucose (pH 7.4, osmolarity 290–300 mOsm) oxygenated with 95%O2:5% CO2. The ACSF was perfused via gravity as well as by cardiovascular function since the heart was still actively beating at the beginning of the procedure. The use of a large bore cannula for the perfusion allowed the brain to be perfused with approximately 1½–2L of cold ACSF. While the perfusion was ongoing, additional tissues, including liver and lung were collected. Immediately following the perfusion, the heart was quickly removed and together with the excised tip of the left ventricle was provided to a collaborator to investigate alcohol-induce cardiomyopathy (Cheng et al. 2004, 2010).
Brain extraction and micro-dissection
The animal was immediately turned over onto its abdomen and the remaining occipital bone was removed. Using small tipped forceps and curved scissors, the dura mater was cut at the posterior-most extent of the occipital lobes and along the midsagittal line and the falx cerebri was removed. The tentorium cerebelli was also removed. The cervical spinal cord was excised at the level of approximately C2–C3 and the flat end of the scalpel handle was used to blunt dissect the cranial nerves. The brain was then removed from the skull in a caudal to rostral direction.
The brain was immediately placed in an acrylic brain mold on ice and quickly rinsed with cold ACSF. Two brain blocking procedures were implemented, depending on the needs of the investigators conducting electrophysiology and voltammetry assays. Initial recording assays required blocks of tissue containing hippocampus and amygdala as well as caudate/putamen and lateral geniculate nucleus. For these studies, the brain was blocked in the coronal plane at three levels. The first cut was made through the rostral-most pole of the temporal lobe. The second cut was performed approximately 6–8 mm caudal to the first cut at the level of the rostral hippocampus. The third cut was at the caudal pole of the thalamus. The brain was removed from the mold and was hemisected to separate the hemispheres. At this point the hippocampus, amygdala and lateral geniculate nuclei were dissected and immediately placed into the same cold, oxygenated ACSF for in vitro electrophysiological recording in brain slices. The striatum (caudate, putamen and nucleus accumbens) was micro-dissected for in vitro voltammetry studies. The remainder of the hemisphere was dissected into an array of cortical fields and subcortical nuclei comprised of 30–35 brain structures that were immediately frozen and banked for future use (Table 1). Concurrent with the micro-dissection procedures, the brain blocks from the contralateral hemisphere were flash frozen in isopentane cooled to −35°C on dry ice and stored at −80°C. Removal and blocking of the brain occurred in two to three minutes. Total elapsed time from the beginning of the perfusion of the brain to removal, blocking and micro-dissection approximately 5 min, resulting in a very short post-mortem interval.
Table 1.
Regions of interest that have been micro-dissected from nonhuman primate brain
| Subcortical regions | Cortical regions |
|---|---|
|
| |
| Caudate | Area 4 (Primary motor cortex) |
| Cerebellum (anterior lobe) | Area 6 (Premotor cortex) |
| Cerebellum (posterior lobe) | Area 9 |
| Cerebellum (vermis) | Area 10 |
| Cerebellar hemispheres | Area 12 |
| Corpus callosum | Area 13a, b (medial orbital) |
| Dorsal Amygdala | Area 14 (Gyrus rectus) |
| Edinger-Westphal | Area 24 (anterior cingulate) |
| Frontal white matter | Area 25 |
| Globus pallidus | Area 32 |
| Hippocampus | Area 45 |
| Hypothalamus (lateral) | Area 46 (Principle sulcus) |
| Hypothalamus (medial) | Entorhinal cortex |
| Inferior Olive | Occipital cortex |
| Lateral Amydgala | Primary visual cortex |
| Lateral Geniculate | |
| Locus coeruleus | |
| Medial Amydgala | |
| Nucleus accumbens core | |
| Nucleus accumbens shell | |
| Periaqueductal gray | |
| Putamen | |
| Raphe nucleus | |
| Substantia nigra pars compacta | |
| Substantia nigra pars reticulata | |
| Thalamus (medial dorsal) | |
| Thalamus (midline) | |
| Ventral tegmental area | |
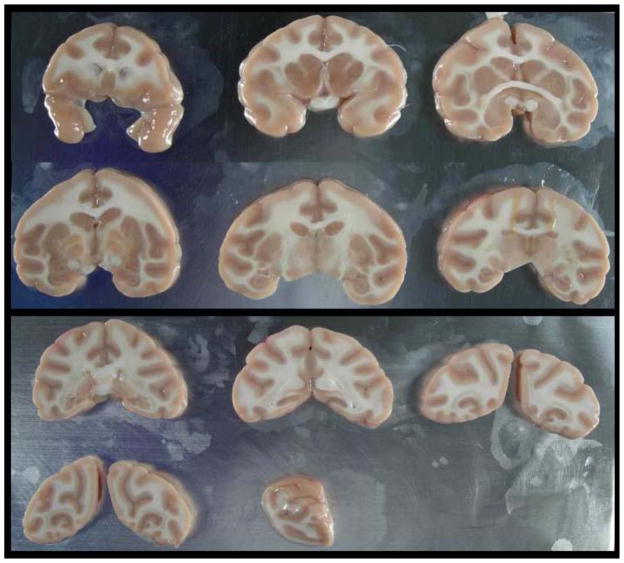
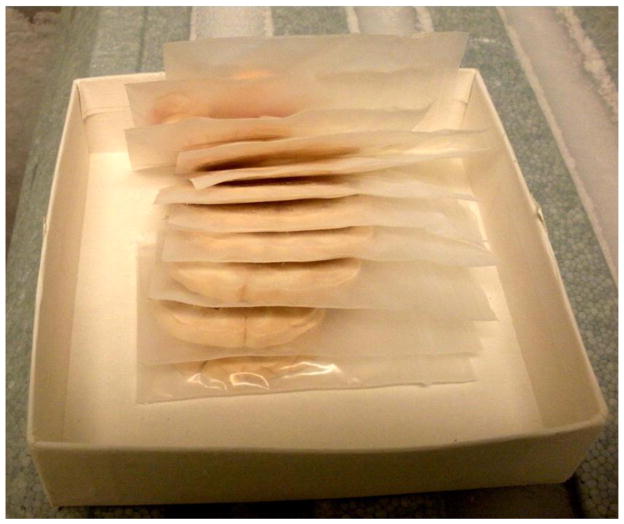
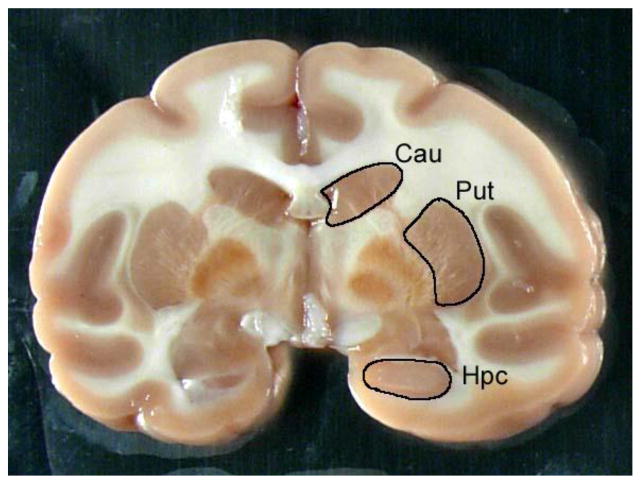
An alternate strategy for brain sectioning was also developed in response to needs of investigators conducting electrophysiology or voltammetry. For these requests, the frontal block was removed by placing the first cut at the anterior tips of the temporal poles. The frontal block was then microdissected into 10–12 frontal cortical fields (Table 1) that were individually stored in microfuge tubes at −80°C immediately after microdissection. The remainder of the brain was then cut into 4 mm slabs and placed onto aluminum plates (Figure 1). The brain slabs were then digitally archived and the aluminum plates were placed onto dry ice in order to flash freeze the tissues. Each brain slab was placed into individual small zippered bags, boxed together and stored at −80°C (Figure 2). This strategy allows us to readily identify banked tissues and easily retrieve specific blocks of tissue for future requests. Figure 3 illustrates the strategy for dissecting discrete brain regions. This image demonstrates that specific brain regions such as the caudate nucleus (Cau), putamen (Put) and hippocampus (Hpc) can be dissected either at the time of the necropsy procedure or after the brain slab is banked at −80°C. Figure 4 illustrates the caudate nucleus and putamen (outlined in black) at the level of the crossing of the anterior commissure in a frozen brain slab (Fig 4A). Figure 4B demonstrates that the putamen has been dissected from this frozen block. Using this strategy as opposed to the initial strategy of micro-dissecting brain regions at the time of the necropsy allows the capability of obtaining virtually any brain region that is requested following the necropsy.
Fig. 1.
Frontal block is removed and microdissected into multiple regions of interest. The remainder of the brain is cut into 4 mm blocks, placed on aluminum plates, photographed and then the plate is placed onto dry ice to freeze the brain slabs. Note: the occipital cortex from one hemisphere was provided to an investigator at the time of the necropsy and is therefore absent.
Fig. 2.
After freezing, each block of tissue is placed into individual zippered bags for storage at −80°C for subsequent microdissection at a later time.
Fig. 3.
This is a 4 mm coronal brain slab at the level of the globus pallidus. This image demonstrates that individual brain regions such as the caudate nucleus, putamen or hippocampus can be dissected either at the time of necropsy or after the slab has been frozen in order to retrieve specific regions of interest.
Fig. 4.
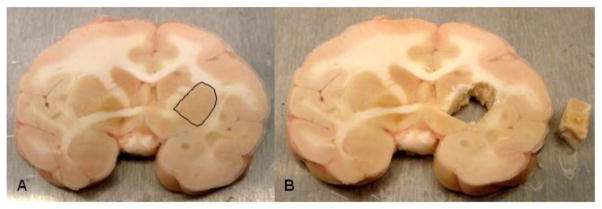
Figure 4A illustrates a frozen coronal brain slab at the level of the caudate nucleus and putamen where the anterior commissure begins to cross the midline. The putamen on the right side of the image (left hemisphere) is outlined in black. Figure 4B illustrates that the putamen on this hemisphere has been dissected from the brain block (next to brain slab).
Peripheral tissues were also collected during this procedure. During the perfusion, samples of liver and upper lung were harvested from the thorax, and quadriceps muscle was also excised in this time period. At the start of the transcardial perfusion stage, the apex of the left ventricle was excised to allow placement of the perfusion cannula. The excised apex was immediately provided to an investigator conducting cardiomyocyte function studies (Cheng et al. 2004). The heart was collected immediately following the perfusion and just before the animal was turned over onto its abdomen for harvesting of the brain. Following the removal of the brain, the animal was moved to a separate table and peripheral tissues were collected at the same time that brain micro-dissection and blocking was ongoing (Table 2).
Table 2.
Alphabetical list of peripheral tissues collected during or immediately following perfusion of the brain.
| Abdominal fat |
| Adrenal glands |
| Aorta |
| Axillary lymph node |
| Bile |
| Bladder |
| Blood |
| Bone marrow |
| Carotid arteries |
| Cerebral spinal fluid |
| Coronary arteries |
| Corpora |
| Esophagus |
| Eye |
| Femur |
| Gall Bladder |
| Gastrocnemius muscle |
| Hair |
| Heart |
| Iliac artery |
| Inguinal lymph node |
| Kidney |
| Large intestine |
| Liver |
| Lumbar vertebrae |
| Lung |
| Mesenteric lymph node |
| Ovaries |
| Ovary |
| Pancreas |
| Pituitary gland |
| Prostate gland |
| Quadriceps muscle |
| Retina |
| Retroperitoneal fat |
| Ribs |
| Sciatic nerve |
| Skin |
| Small intestine |
| Small intestine |
| Spinal cord (cervical) |
| Spinal cord (lumbar) |
| Spinal cord (thoracic) |
| Spleen |
| Stomach |
| Subcutaneous fat |
| Testes |
| Thymus |
| Tibia |
| Tongue |
| Urine |
| Uterus |
Results
Electrophysiology and Voltammetry
This euthanasia procedure was initially designed to provide viable brain tissue appropriate for conducting electrophysiological and voltammetric recording experiments. For these techniques, the hippocampus, striatum (caudate and putamen), amygdala and lateral geniculate nucleus of the thalamus were micro-dissected immediately following perfusion of the brain. These micro-dissected brain regions were transported by the requesting investigators to their laboratories where the tissues were then processed for their respective assays within thirty minutes of the necropsy (Ariwodola et al. 2003; Budgyin et al. 2003; Floyd et al. 2004; Alexander et al. 2006; Carden et al. 2006; Anderson et al. 2007).
Slices containing amygdala were dissected from the anterior half of the temporal lobe of one hemisphere for the conduction of whole-cell GABA-gated currents measured on individual acutely isolated basolateral amygdala neurons using patch-clamp electrophysiology and local application of agonists and modulators (McCool et al. 2003; Floyd et al. 2004). The results of these studies suggest that macaque amygdala GABAA receptors collected in this manner can be effectively measured in individual isolated neurons and that these receptors retain at least some of their native pharmacological properties.
Brain slices from the posterior half of the temporal lobe containing the hippocampus were prepared for whole-cell patch clamp methods to determine whether activation of serotonin 5HT1A receptors would inhibit glutamatergic synaptic transmission in nonhuman primate brain in a similar fashion to what had been previously reported in rodents (Schmitz et al. 1995, 1998; Bouryi and Lewis 2003). Application of the 5-HT1A receptor agonist 8-OH DPAT significantly inhibited AMPA EPSCs. The decrease in AMPA EPSCs was also associated with an increase in paired-pulse ratio (50 msec inter-pulse interval) at these synapses consistent with a presynaptic mechanism of action (Ariwodola et al. 2003).
Electrically stimulated release and uptake of dopamine were evaluated in blocks of tissue containing the caudate and putamen using Fast Scan Cyclic Voltammetry (Budygin et al., 2003). The rate and amount of release and the rate of uptake of dopamine were found to be similar to that observed in recordings from rat tissue. The caudate had greater release (peak height) and faster uptake (downward slope) of DA compared to the nucleus accumbens as expected (Letchworth et al., 2001). Voltammetry recordings were stable for 10 to 14 hours, which is similar to results in rats. This indicates that the tissues collected were viable and appropriate for these analytic methodologies..
Intracellular recordings were obtained from tissue blocks containing the lateral geniculate nucleus. Burst and tonic firing patterns of relay neurons within the lateral geniculate nucleus exhibited active membrane properties consistent with viable cells in monkey thalamic tissue slices. (Alexander et al. 2006; Carden et al. 2006) and are characteristic of T-type calcium currents recorded from thalamus of several mammalian species (Perez-Reyes 2003). These results are consistent with previous recordings from various animal models and demonstrate that the necropsy method used provided viable tissue for these methodologies. In addition, tissues collected by this method have been used to evaluate electrophysiological responses to chronic ethanol self-administration and periods of sustained abstinence in the inferior olivary nucleus. The brain is known to adapt to chronic ethanol exposure by altering synaptic and ion-channel function to increase excitability, in part to offset alcohol-induced inhibition. The results of this study demonstrated bi-directional plasticity of pace-making currents in the inferior olive induced by chronic heavy drinking and periods of abstinence (Welsh et al. 2011).
Molecular Methodologies Using Archived Tissue
In addition to the brain regions that were utilized for recording assays, our initial tissue collection strategy entailed harvesting 30–35 cortical and subcortical structures that were then snap frozen and stored at −80 for later use (Table 1). At the time of necropsy, micro-dissected tissue was either flash frozen in liquid nitrogen or submerged in RNAlater (Ambion) and stored at −80°C or −20°C, respectively, until processed for RNA isolation. For example, the effects of chronic ethanol consumption on GABAA receptor subunit mRNA expression were examined in orbitofrontal anterior cingulate and dorsolateral prefrontal cortices as described in Hemby et al. 2006. It was determined that RNA prepared from these tissues was routinely of sufficient quality for microarray analysis. Using a similar strategy, the effects of chronic ethanol self-administration on glutamate receptor ionotropic NMDA (GRIN), as well as GRIN1 splice variant mRNA expression was also studied in the same cortical regions (Acosta et al. 2010).
In vitro Receptor Autoradiography
At necropsy, the contralateral hemisphere was cut into three blocks (prefrontal, cortical and occipital) that were immediately flash frozen and stored at −80 for future histological and autoradiographic procedures. Select tissue blocks were subsequently processed for in vitro receptor autoradiographic and tissue homogenate assays to determine the impact of chronic EtOH self-administration on multiple neurotransmitter systems. Binding to cortical GABAA a1 and a4 receptors was determined in membrane preparations containing parietal and temporal lobes and a1 and a6 in preparations of cerebellar cortex using [3H] Ro15-4513. GABAA and subunit densities were then assessed in cryostat cut 20 micron serial sections from the hippocampus of these same animals, which showed layer and field specific changes in total GABAA and a4 subunit binding (Sullivan et al., 2005). The impact of chronic EtOH exposure on hippocampal serotonin transporter (SERT) binding was assessed in a different population of self-administering animals in a layer and field specific manner using in vitro receptor autoradiograpy. Serial sections collected at the same time were also successfully used for immunohistochemistry and for laser capture microdissection of specific hippocampal cell populations for receptor trafficking protein and mRNA analysis (Burnett et al., 2012). The tissue sections used for these studies were slide mounted cryostat cut sections and in one study were stored at −80°C for 4 years prior to processing for in vitro receptor autoradiography. This demonstrates the stability of the tissue for long periods when stored at −80°C under appropriate conditions.
Morphological studies
In addition to the neurobiological, functional and molecular studies described above, tissues from these animals were also used for morphological studies of dendritic branching and dendritic spine morphology using DiOLISTIC staining methods (Seabold et al. 2010), which uses a gene gun to introduce fluorescent dyes into neurons in brain slices. In the putamen, Cuzon-Carlson and colleagues (2011) reported a selective increase in dendritic spine density and enhanced glutamatergic transmission following chronic ethanol self-administration and periods of abstinence. These changes in morphology and physiology indicated a shift in excitatory/inhibitory balance that shifts the circuit towards an enduring increase in synaptic activation of output of the putamen as a result of chronic heavy drinking and relapse.
Peripheral Tissues
In addition to CNS tissues, whole blood and other peripheral tissues are routinely harvested and archived at the time of necropsy (Table 2) for later use. For example, liver samples collected during perfusion were assayed for the presence of alcoholic liver disease (Ivester et al. 2007). Cardiac tissue was successfully harvested for the study of cardiac myocytes (Cheng et al. 2004, 2010). Bone marrow samples were used to evaluate the effect of chronic ethanol exposure on production and mobilization of endothelial progenitor cells (Williams et al. 2008). Multiple other tissues were successfully harvested, many of which are currently being analyzed using a variety of genomic and biochemical assays. Taken together, these results demonstrate the global utility of this necropsy method.
Discussion
The primary goal of this study was to design a necropsy method that would optimize the viability of brain tissue that was collected for in vitro slice electrophysiology and voltammetry techniques. In order to preserve the integrity of the brain tissue, it was necessary to maintain cardiac and respiratory functions for as long as possible before extracting the brain. To accomplish this, we opted to conduct the craniotomy while the animal was maintained under a deep surgical plane of anesthesia followed by transcardial perfusion with cold ACSF in order to chill the brain and remove blood and other ischemic products. Previous studies have utilized a similar brain perfusion technique, but in those studies, the craniotomy is generally conducted following the perfusion, adding to the time frame in which the tissue is collected (St. John et al. 1997; but see González-Burgos et al. 2000). Using our strategy, the brain was removed and the regions of interest were collected with a post mortem interval (PMI) of less than 5 minutes after the beginning of the perfusion. Other studies report an average extraction time of 13–14 minutes (St. John et al. 1997) as opposed to the typical PMI of up to 72 hours in human postmortem studies (Sheedy et al. 2008). Critically, the use of pre-mortem structural MRIs allowed us to cut the brain in precise locations to harvest specific brain regions with 100% accuracy, further reducing the amount of time that elapsed between brain removal and providing specific brain regions for recording assays.
The method that we developed differs from previous reports (Buckmaster et al. 2004; Altemus et al. 2005) in which hippocampal slices were prepared by exposing the brain through a large craniotomy and applying cold ACSF to the left hemisphere prior to resecting the temporal lobe. In these studies a 1 cm tissue block containing the rostral half of the hippocampus was subsequently submerged in cold ACSF. As such, that strategy utilized an “outside-in” approach to chilling the tissue. That is, once the tissue was excised, it was quickly submerged in a cold buffer or ACSF solution and hence, cooled from the outside in. In contrast, our technique was designed to perfuse cold, oxygenated ACSF through the entire brain, chilling the brain in a more uniform manner from the “inside-out” while the animal was still alive. Once the brain was extracted, it was further chilled by briefly rinsing it in cold, oxygenated buffer. Using this method, the blood is displaced from the brain, removing potential ischemic byproducts and replaced with a cold buffer that contains the same constituents as ACSF. Depending on the assay utilized, the tissue obtained with this technique was found to remain viable up to 15 hours after the necropsy (Carden et al. 2006). Voltammetry recordings were stable for 10 to 14 hours which is similar to results in rats. Tissue samples from the 4 distinct brain regions (caudate nucleus, hippocampus, amygdala and lateral geniculate nucleus) that were provided to 4 different investigators yielded results that were characteristic for the experimental protocols that were utilized. This confirms that the monkey brain slices were viable and were not compromised by the necropsy and tissue dissection. This dissection protocol proved to be a valuable method for obtaining reproducible brain slices for neurochemical and neurophysiological analyses.
In addition to the brain regions that were collected and processed for recording purposes, multiple brain regions were microdissected and banked for subsequent analyses using genomic and proteomic techniques. A list of discrete brain regions that were microdissected using our initial strategy are listed in Table 1. Importantly, some tissues were processed for RNA at the time of necropsy using RNAlater (Ambion) and stored at −20°C, while others were flash-frozen and stored at −80°C for up to 3–4 years before being processed for RNA. Regardless of when the RNA was isolated, all tissues were found to yield high quality RNA suitable for microarray analysis or other downstream applications. Indeed, recent RNA quality control assays determined that tissues that have been stored for 10 years continue to provide high quality RNA.
As would be expected, using our necropsy protocol, the postmortem interval for collection of these samples was markedly shorter than typical PMI in clinical studies, avoiding such potential confounds as altered pH which can affect RNA integrity. Autoradiographic analyses were also successfully conducted on both tissue sections that derived from the hemisphere opposite from the one that was microdissected as well as cortical tissue homogenates obtained at the end of the microdissection procedure (Sullivan et al. 2005; Burnett et al. 2012). Taken together, these data suggest that the method of necropsy and the PMI were satisfactory for obtaining viable tissues for the conduct of multiple genomic and proteomic assays. The current strategy of cutting the brain into 4 mm slabs and storing those at −80°C now allows us to collect virtually any brain region since all regions are preserved within a 4 mm slab. This allows us to retrieve a particular slab of tissue containing specific brain regions once a discrete region is requested.
Our method also produced tissue samples from numerous other organ systems that were collected either immediately prior to the perfusion (CSF and blood), while the brain was being perfused (lung, liver) or immediately following the perfusion (see Table 2), while the brain was micro-dissected. These tissues were distributed to other investigators for use with their individual assays, and/or banked at −80°C for future use. Liver samples that were collected during the perfusion procedure for example, were processed for biochemical assays to assess liver function following chronic ethanol consumption (Ivester et al. 2007). Cardiac tissue that was harvested during the perfusion procedure was subsequently processed for the study of cardiac myocytes to determine the Beta3-adrenergic functional response to chronic ethanol self-administration (Cheng et al. 2004; 2010). Using this necropsy strategy, the effects of ethanol consumption on vasculogenesis in bone marrow has also been determined as described (Williams et al. 2008). Additional tissues collected using this necropsy strategy are currently being analyzed to determine the functional consequences of chronic ethanol exposure on a variety of systems, including among others, blood, lung, pancreas and quadriceps muscle. This demonstrates the far-reaching capabilities of the tissue collection method that we implemented.
Multiple considerations must be observed in order to optimize the viability of tissues collected. The first consideration is the plane of anesthesia. If the level of anesthesia is too deep the animal can overdose, or at a minimum, respirations will become slow and labored, reducing oxygenation and placing undue stress on brain function and tissue viability. The ability to intubate the animals and provide oxygen during the procedure can provide an additional level of protection from possible ischemic events. A second consideration is the amount of blood that is collected prior to the perfusion. If too large a volume is collected immediately prior to the procedure, the subject can easily become hypovolemic thus reducing cerebral perfusion, which can impact the integrity of the harvested tissues. Additionally, the removal of large amounts of blood reduces the patency of the vessels, making it more difficult to insert an angiocatheter for anesthesia purposes. Therefore, we typically collected at least part of the required blood during the days before the necropsy procedure, with the remainder collected during the procedure. A third factor to consider is the temperature of the perfusion buffer. We utilized ice cold, oxygenated ACSF in order to maximize the viability of the tissue. Perfusion of ice cold ACSF through the system removes blood while chilling the brain. Once the brain is harvested from the skull, quickly rinsing it with ACSF and placing it in cold ACSF while micro dissecting regions also helps maintain viability of the tissue. The final consideration to obtain viable tissue that is optimal for recording purposes is to limit the amount of time and handling of the tissue between the initiation of the perfusion and microdissection of the desired regions of interest. Tissue that is not being actively microdissected is placed in cold ACSF to prevent exposure to room temperature conditions.
In conclusion, we have developed a method for extracting living brain tissue from nonhuman primates that is viable for conducting in vitro electrophysiological and voltammetric studies in brain slices as evidenced by our recent reports (Ariwoldola et al. 2003; Budygin et al. 2003; Carden et al. 2006; Alexander et al. 2006). This method also produces fresh frozen tissue from which high quality mRNA can be extracted (Floyd et al. 2004; Walker et al. 2006). One distinct advantage of our necropsy method is that the postmortem interval between death and tissue acquisition can be measured in minutes as compared to the sometimes hours to days that can elapse in human studies. The standardization and implementation of this necropsy method in rhesus, cynomolgus and vervet monkeys across multiple institutions formed the basis for the development of an R24 resource grant that now provides standardized tissues to alcohol researchers across the world. This resource was recently funded through NIAAA as the Monkey Alcohol Tissue Research Resource (MATRR) and is available at www.matrr.com. The MATRR resource has been an invaluable source of tissues and accompanying bioinformatics that has resulted in multiple manuscripts, data presentations, and most importantly, multiple collaborations that have resulted in numerous funding opportunities. Collaborative efforts between our groups and over 50 investigators across the United States has allowed us to leverage multiple approaches to address the impact of chronic ethanol exposure on both CNS and peripheral organ function, thus providing a much larger overall picture of the neurobiological and physiological consequences of alcohol abuse than can be achieved by individual efforts.
Acknowledgments
This research was supported by National Institute of Alcohol Abuse and Alcoholism funding AA016748 (JBD), AA14106, AA011997 (DPF), AA11997 (KAG), AA019431 (KAG, JBD).
Contributor Information
April T Davenport, Department of Physiology & Pharmacology, Wake Forest University Health Sciences, Winston-Salem, NC, USA.
Kathleen A Grant, Department of Behavioral Neuroscience, Oregon Health & Science University, Portland, OR, USA.
Kendall T Szeliga, Department of Physiology & Pharmacology, Wake Forest University Health Sciences, Winston-Salem, NC, USA.
David P Friedman, Department of Physiology & Pharmacology, Wake Forest University Health Sciences, Winston-Salem, NC, USA.
James B Daunais, Email: jdaunais@wakehealth.edu, Department of Physiology & Pharmacology, Wake Forest University Health Sciences, Winston-Salem, NC, USA, Tele: 336-713-7185, Fax: 336-713-7186.
References
- Acosta G, Hasenkamp W, Daunais JB, Freidman DP, Grant KA, Hemby SE. Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res. 2010;1318:144–54. doi: 10.1016/j.brainres.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Carden WB, Mu J, Kurukulasuriya NC, McCool BA, Norskog BK, Friedman DP, Daunais JB, Grant KA, Godwin DW. The native T-type calcium current in relay neurons of the primate thalamus. Neuroscience. 2006;141:453–461. doi: 10.1016/j.neuroscience.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Altemus KL, Lavenex P, Ishizuka N, Amaral DG. Morphological characteristics and electrophysiological properties of CA1 pyramidal neurons in macaque monkeys. Neuroscience. 2005;136:741–56. doi: 10.1016/j.neuroscience.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Crowder TL, Grant KA, Daunais JB, Friedman DP, Weiner JL. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol Clin Exp Res. 2003;27:1632–40. doi: 10.1097/01.ALC.0000089956.43262.17. [DOI] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motorneurones, in vitro. J Physiol. 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Alonso A, Canfield DR, Amaral DG. Dendritic morphology, local circuitry, and intrinsic electrophysiology of principal neurons in the entorhinal cortex of macaque monkeys. J Comp Neurol. 2004;470:317–329. doi: 10.1002/cne.20014. [DOI] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse. 2003;50:266–8. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Burnett EJ, Davenport AT, Grant KA, Friedman DP. The effects of chronic ethanol self –administration on hippocampal serotonin transporter density in monkeys. Frontiers Psychiatry. 2012;3:38. doi: 10.3389/fpsyt.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden WB, Alexander GM, Friedman DP, Daunais JB, Grant KA, Mu J, Godwin DW. Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Res. 2006;1089:92–100. doi: 10.1016/j.brainres.2006.02.135. [DOI] [PubMed] [Google Scholar]
- Cheng H-J, Han Q-H, Daunais JB, Grant KA, Friedman DP, Little WC, Cheng C-P. Enhanced cardiac beta3-adrenergic functional response in alcoholic monkeys. J American Coll Cardiol. 2004;43(2):3: A456. [Google Scholar]
- Cheng HJ, Grant KA, Han QH, Daunais JB, Friedman DP, Masutani S, Little WC, Cheng CP. Up-regulation and functional effect of cardiac β3-adrenoreceptors in alcoholic monkeys. Alcohol Clin Exp Res. 2010;34:1171–1181. doi: 10.1111/j.1530-0277.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and Morphological Neuroadaptations in the Putamen Associated with Long-Term, Relapsing Alcohol Drinking in Primates. Neuropsychopharmacol. 2011;36(12):2513–28. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Kraft RA, Davenport AT, Burnett EJ, Maxey VM, Szeliga KT, Flory GS, Hemby SE, Kroenke CD, Grant KA, Friedman DP. MRI-guided dissection of the nonhuman primate brain: a case study. Methods. 2010;50:199–204. doi: 10.1016/j.ymeth.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disotell TR. Molecular systematics of the Cercopithecidae. In: FWCJJ, editor. Old World Monkeys. Cambridge University Press; Cambridge: 2000. pp. 29–56. [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–79. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex. 2000;10:82–92. doi: 10.1093/cercor/10.1.82. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Therap. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LSM, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, O’Conner JA, Acosta G, Floyd D, Anderson N, McCool BA, Friedman DP, Grant KA. Ethanol-induced regulation of GABAA subunit mRNAs in prefrontal fields of cynomolgus monkeys. Alcohol Clin Exp Res. 2006;30:1978–1985. doi: 10.1111/j.1530-0277.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Ivester P, Roberts LJ, Young T, Stafforini D, Vivian J, Lees C, Young J, Daunais J, Friedman D, Rippe RA, Parsons CJ, Grant K, Cunningham C. Ethanol self-administration and alterations in the livers of the cynomolgus monkey, Macaca fascicularis. Alcohol Clin Exp Res. 2007;31:144–155. doi: 10.1111/j.1530-0277.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–77. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Povysheva NV, González-Burgos G, Zaitsev AV, Kroner S, Barrionuevo G, Lewis DA, Krimer LS. Properties of excitatory synaptic responses in fast-spiking interneurons and pyramidal cells from monkey and rat prefrontal cortex. Cereb Cortex. 2006;16:541–552. doi: 10.1093/cercor/bhj002. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin and 8-OH-DPAT reduce excitatory transmission in rat hippocampal area CA1 via reduction in presumed presynaptic Ca2+ entry. Brain Res. 1995;701:249–254. doi: 10.1016/0006-8993(95)01005-5. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Gloveli T, Empson RM, Draguhn A, Heinemann U. Serotonin reduces synaptic excitation in the superficial medial entorhinal cortex of the rat via a presynaptic mechanism. J Physiol. 1998;508 (1):119–129. doi: 10.1111/j.1469-7793.1998.119br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold GK, Daunais JB, Rau A, Grant KA, Alvarez VA. DiOLISTIC labeling of neurons from rodent and non-human primate brain slices. J Vis Exp. 2010 Jul;6(41) doi: 10.3791/2081. doi:2081.10.3791/2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C. An Australian brain bank: a critical investment with a high return! Cell Tissue Bank. 2008;9:205–216. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Am J Primatol. 2009;71:715–721. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- St John JL, Rosene DL, Luebke JI. Morphology and electrophysiology of the dentate granule cells in the rhesus monkey: comparison with the rat. J Comp Neurol. 1997;387:136–147. doi: 10.1002/(sici)1096-9861(19971013)387:1<136::aid-cne11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Sable HJ, Strother WN, Friedman DP, Davenport AT, Tillman-Smith H, Kraft RA, Wyatt C, Szeliga KT, Buchheimer NC, Daunais JB, Adalsteinsson E, Pfefferbaum A, Grant KA. Neuroimaging of Rodent and Primate Models of Alcoholism: Initial Reports from the Integrative Neuroscience Initiative on Alcoholism. Alcohol Clin Exp Res. 2005;29:287–294. doi: 10.1097/01.alc.0000153546.39946.ec. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majersky LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Walker SJ, Wang Y, Grant KA, Chan F, Hellman GM. Long versus short oligonucleotide microarrays for the study of gene expression in nonhuman primates. J Neurosci Meth. 2006;152:179–189. doi: 10.1016/j.jneumeth.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Han VZ, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci USA. 2011;108:10314–10319. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Baptista PM, Daunais JB, Szeliga KT, Friedman DP, Soker S. The effects of ethanol consumption on vasculogenesis potential in nonhuman primates. Alcohol Clin Exp Res. 2008;32:155–161. doi: 10.1111/j.1530-0277.2007.00558.x. [DOI] [PubMed] [Google Scholar]