Abstract
Objectives: Acinetobacter spp. has emerged as an important opportunistic pathogen responsible for nosocomial infections in many health-care settings worldwide. The study describes the clinico-epidemiology and antimicrobial susceptibility of Acinetobacter spp. in a tertiary health-care institution.
Methodology: Acinetobacter spp. were isolated from 141 specimens of the patients who reported to Universiti Kebangsaan Medical Centre (UKMMC). The sources of specimens were wound, skin and soft tissue, respiratory and urinary tract from patients in various wards. Clinio-epidemiological features of patients infected with Acinetobacter spp. were recorded. Standard bacteriological techniques with API 20NE kits and disk diffusion method were followed for identification and antibiotic sensitivity of the organisms.
Results: One hundred and forty one patients with positive culture for Acinetobacter spp. were identified. Soft tissue/wound and respiratory tract were among the commonest sites of Acinetobacter spp. isolation. The isolates were most frequently obtained from ICU. All isolates were multi-drug resistant and had a resistance rate of more than 70% to most antibiotics, except polymyxin B.
Conclusion: High prevalence of multi-drug resistance Acinetobacter spp. provides essential information on judicious antibiotic selection for empirical therapy in our health-care institution.
Key Words: Acinetobacter spp., Antimicrobial susceptibility, Intensive care unit, Polymyxin B
INTRODUCTION
Infections caused by Acinetobacter spp. have become a serious concern in many health-care institutions worldwide. Acinetobacter calcoaceticus–baumannii complex has been recognized as one the most common species responsible for nosocomial bacteremia, meningitis, respiratory tract and urinary tract infections.1
The prevalence and antimicrobial susceptibility profiling of Acinetobacter spp. has been reported in Malaysian hospitals.2,3 However, the result of the studies might not represent our institution.
The aim of this study was to determine, the demographic and clinical profile of Acinetobacter spp. The antimicrobial resistance patterns of Acinetobacter strains will be assessed.
METHODOLOGY
Setting: This is a cross-sectional retrospective observational study conducted in a tertiary healthcare facility with 830 beds.
Bacterial isolates: From October 2010 to April 2011, non-duplicate isolates of Acinetobacter spp. grown from all clinical specimens of hospitalized patients were analyzed. The sources of isolates included blood, sputum, tracheal aspirate, bronchoalveolar lavage, pus, sterile body fluid and urine. In this study, nosocomial isolate was defined as isolate grown from specimen that was sampled after 48 hours of hospitalization. Non-nosocomial isolate was defined as isolate grown from specimen sampled within 48 hours of hospitalization. Colonizer was defined as isolate that had microscopy smear showing 0 to 1 pus cell/high power field. Isolate showing more than 1 pus cell/high power field was regarded as significant isolate.
Patient data: Medical and demographic data of hospitalized patients with culture-positive Acinetobacter spp. were retrieved from patients’ medical records. Data that were recorded include age, gender, ward location, date of hospitalization, transfer and discharge, date of specimen sampling, specimen site, ICU stay and antibiotic usage.
Laboratory identification: Microbiological data were obtained from laboratory records. Bacterial colonies grown on MacConkey plates were identified by its colonial morphology, Gram-staining and oxidase test. Genus identification was performed using conventional biochemical tests. For blood and sterile body fluid specimens, speciation was performed using API 20NE system, based on manufacturer’s instruction (bioMérieux, France).
Antimicrobial susceptibility test: The antimicrobial susceptibility testing was assessed by disk diffusion method, according to the guidelines of Clinical and Laboratory Standards Institute (CLSI).4 Antibiotic disks were obtained from Oxoid Ltd. (Basingstoke UK). Tigecycline susceptibility testing was assessed by disk diffusion method, according to the guidelines of The Asia Pacific Clinical Microbiology Working Group Laboratory Manual 2009. Antibiotic disks were obtained from Becton Dickinson (USA). Minimum inhibitory concentrations (MIC) for polymyxin B were determined by E-tests, based on manufacturer’s instructions (AB Biodisk, Solna Sweden). Quality control was performed with the following strains as recommended by the CLSI: Escherichia coli ATCC 25922, Escherichia coli ATCC 35218 and Pseudomonas aeruginosa ATCC 27853. Isolates were tested for susceptibility to sixteen relevant drugs. Multiple-drug resistant (MDR) Acinetobacter spp. was defined as resistance to 3 or more classes of antibiotic used to treat Acinetobacter infections.
Statistical analysis: Statistical analysis was performed by using Statistical Package for the Social Science for Windows (version 18.0; SPSS Inc, Chicago, IL, USA). Parametric variables were assessed using chi-squared test, as appropriate. A difference was considered statistically significant if the p-value < 0.05.
Ethical considerations: The institution’s medical research and ethical committee had approved this study.
RESULTS
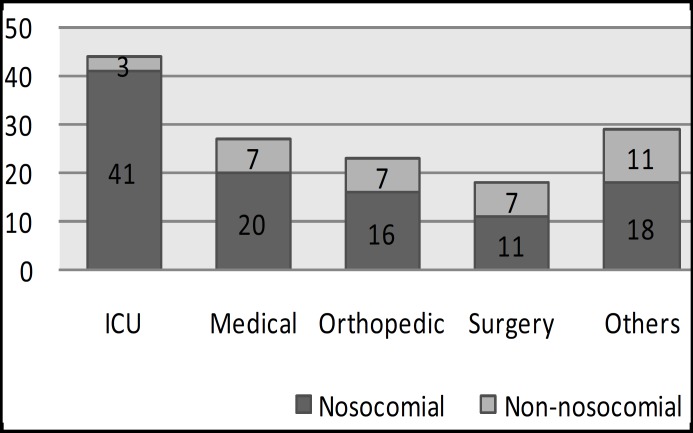
A total of 141 non-duplicate isolates of Acinetobacter spp. grown from all clinical specimens were included in this study. Distribution for the 141 isolates was as shown in Fig.1. The isolates were most frequently derived from ICU, followed by medical, orthopedics and surgery wards. Soft tissue/wound (43.3%) and respiratory tract (31.2%) were among the commonest sites of isolation (Table-I). The isolates were predominantly colonizers (53.2%). Only 66/141 (46.8%) isolates were regarded as significant based on the presence of pus cells in Gram stain smear.
Fig.1.
Distribution of Acinetobacter spp. Isolates
Table-I.
Distribution of the isolates in relation to specimen site
| Specimen Site | No. of Acinetobacter spp. (%) |
|---|---|
| Total | 141 (100) |
| Blood Respiratory tract Soft tissue/wound |
8 (5.7) 44 (31.2) 61 (43.3) |
| Sterile body fluid Urinary tract |
4 (2.8) 12 (8.5) |
| Others | 12 (8.5) |
The demographic characteristics and clinical epidemiology profile of hospitalized patients with culture-positive Acinetobacter spp. are shown in Table-II. Bacterial isolates were mostly from males 85 (60.3%). The median age of patients was 54 (IQR 31-66). Overall, 75.2% (106/141) of the isolates were of nosocomial origin. There were 24.8% (35/141) non-nosocomial isolates and 45.6% (17/35) of these strains were from patients whom had previous hospitalization. The duration taken for patients to acquire nosocomial Acinetobacter spp. infection/colonization varied from 3 days to 78 days, median duration was 13 days (IQR 7-23).
Table-II.
Characteristics of the study population (n=141
| Age | 54 (31-66) |
| Male | 85 (60.3) |
| Female | 56 (39.7) |
| Nosocomial isolates | 106/141(75.2) |
|
41 (38.6) |
|
20 (18.9) |
|
11 (10.4) |
|
16 (15.1) |
|
18 (17.0) |
| Days taken to acquire nosocomial Acinetobacter | |
|
13 days (7-23) |
|
3 – 78 |
| Non-nosocomial isolates | 35/141 (24.8) |
| Non-nosocomial isolates from patients whom were hospitalized during previous 1 year | 17/35 (48.6) |
| Colonizer | 75/141 (53.2) |
| Significant isolate | 66/141(46.8) |
| Prior antibiotics use | 93/141 |
|
37 (39.8) |
|
30 (32.3) |
|
26 (28.0) |
| Quantitative variables, i.e. age, duration of stay in hospital, duration of stay in ICU, number of surgery are presented as median (interquartile range) because they do not have a normal distribution; categorical variables are presented as number (%). | |
In this study, 93/141 patients (66.0%) received antibiotics before the isolation of Acinetobacter spp. Thirty-seven of them (39.8%) had one course of antibiotic therapy. Thirty out of ninety-three (32.3%) patients had 2 courses of antibiotic therapy and 26/93 (28.0%) patients had three or more courses of antibiotic therapy prior to Acinetobacter spp. isolation. The most common antibiotic used was meropenem (22.5%), followed by ceftriaxone (17.6%), piperacillin-tazobactam (13.4%) and amoxicillin-clavulanate (12.0%).
The susceptibility profile of 141 Acinetobacter spp. isolates is shown in Table-III. In general, Acinetobacter isolates had more than 70% resistance to most antibiotics tested. The rates of more than 70% resistance for antibiotics were ampicillin (95.0%), cefuroxime-parenteral (80.8%), cefotaxime (78.0%), amoxicillin-clavulanate (75.9%), ciprofloxacin (73.8%), ceftazidime (73.0%), meropenem (73.0%), imipenem (72.3%), piperacillin-tazobactam (72.7%), cefepime (73.1%), ampicillin-sulbactam (70.9%) and gentamicin (70.2%). Out of 141 isolates, 79.4% were MDR Acinetobacter spp. Twenty-eight isolates were tested for polymyxin B and were 100% sensitive. The MIC90 was 2 mcg/mL. Comparison of the rate of antibiotic resistance between ICU and non-ICU isolates did not show any significant difference (p>0.05).
Table-III.
Antimicrobial susceptibility of Acinetobacter spp. isolated from 141 patients
| Antibiotics |
Acinetobacter spp. Isolates
|
||
|---|---|---|---|
| No. Resistance (%) | No. Intermediate (%) | No. Sensitive (%) | |
| Ampicillin | 134 (95) | 3 (2.1) | 4 (2.9) |
| Cefuroxime-paranteral | 114 (80.8) | 17 (12.1) | 10 (7.1) |
| Cefotaxime | 110 (78) | 27 (19.2) | 4 (2.8) |
| Amoxicillin-clavulanate | 107 (75.9) | 14 (9.9) | 20 (14.2) |
| Ciprofloxacin | 104 (73.8) | 2 (1.4) | 35 (24.8) |
| Meropenem | 103 (73) | 1 (0.7) | 37 (26.3) |
| Ceftazidime | 103 (73) | 1 (0.7) | 37 (26.3) |
| Imipenem | 102 (72.3) | - | 39 (27.7) |
| Piperacillin tazobactam | 101 (72.7) | 6 (4.3) | 32 (23) |
| Ampicillin sulbactam | 100 (70.9) | - | 41 (29.1) |
| Gentamicin | 99 (70.2) | 4 (2.8) | 38 (27) |
| Cefepime | 95 (73.1) | 1 (0.7) | 34 (26.2) |
| Cefoperazone sulbactam | 92 (65.2) | 8 (5.7) | 41 (29.1) |
| Netilmicin | 88 (62.4) | 3 (2.1) | 50 (35.5) |
| Amikacin | 82 (62.7) | 3 (2.1) | 46 (35.2) |
| Polymyxin B | - | - | 28 (100) |
| Tigecycline (for 121 samples) | 9 (7.4) | 27 (22.3) | 85 (70.3) |
DISCUSSION
In the past two decades, Acinetobacter spp. have been considered as important opportunistic pathogens responsible for nosocomial infections, especially among patients in intensive care units (ICUs).5 The data in this study showed that most of these isolates were obtained from soft tissue and wound followed by respiratory tract. Most of them were isolated from the intensive care unit (ICU), which suggests that seriously ill patients in ICUs have a greater chance of becoming colonized or infected by Acinetobacter spp. especially through the soft tissue/wound and respiratory tract. Similarly Falagas et al6 reported that infections caused by Acinetobacter spp. are more common in the ICUs in Asian and European hospitals and are lower in the United States hospitals, also Carlet et al7 and Peleg et al8 mentioned the prevalence of hospital-acquired infections could be as high as 25% in an ICU and there is a problem with nosocomial infection which is only one third of hospital-acquired infections that are avoidable.
Our data show that the median age of patients is 54 (31-66) which indicates the infection by Acinetobacter spp. occurs in elderly patients. One study documented the distribution of MDR-Acinetobacter was greatest in the >65 age group and long term care facilities.9 Also, duration of stay in hospital effected to acquire Acinetobacter infections, as in our data point out that this duration is from 3 days to 78 days. In several studies which examined nosocomial, blood stream, and burn infections explained antibiotic-resistance Acinetobacter infections are associated with longer hospital stays.10,11
The present study showed that most patients (75.2%) exposed to Acinetobacter infection after 48 hours during hospitalization are considered as nosocomial patients. Reports of Chang et al12 and Enoch et al13 mentioned that Acinetobacter spp. emerged as a crucial pathogen in health care - associated and nosocomial infections with high mortality, and was difficult to treat efficiently. Munoz-Price5 also reported that hospital acquired Acinetobacter is often multidrug resistance and widespread.
In the present study, the isolates were predominantly colonizers (53.2%). Thus, Acinetobacter spp. colonization of the hospital environmental may lead to infection because they survive on both moist and dry surfaces for long periods in the hospital environment.14 This feature of Acinetobacter is helpful to survive in hospital environments and cause infection and eliminating Acinetobacter spp. from clinical materials is difficult.15
In this study, the frequency of patients who received antibiotics before the isolation of Acinetobacter spp. is 66.0%. The most common antibiotics that patients received before the diagnosis of Acinetobacter spp. were meropenem (22.5%), followed by ceftriaxone (17.6%), piperacillin-tazobactam (13.4%). Other studies16,17 have reported that prior exposure to antibiotics as one of the risk factors for acquisition of Acinetobacter infections. Besides prior exposure to antibiotics18, other factors include long stay in hospital19, ICU admission, using tubes and catheter18 and furthermore, transmission between colonized or infected patients directly from hospital equipment or through the hands of health care workers, were also reported as risk factors for acquisition of Acinetobacter spp.20
In general, treatment options for Acinetobacter infections are limited and there are not any controlled trials to show therapeutic choices. Also, carbapenems and colistin are the options of choice for the most drug-resistant infections.1 In view of increasing resistance of Acinetobacter to carbapenem, polymyxins have been considered an option for the treatment of multidrug resistant Acinetobacter spp. infections.6
In our study, except for polymyxin B, tigecycline had high sensitivity rates to Acinetobacter isolates. Therefore, this new glycylcycline agent has bacteriostatic activity against multidrug Acinetobacter spp. to is the appropriate agent for skin and soft tissue infection caused by MDR Acinetobacter spp.23 However; there are some reports of high level resistance to tigecycline among MDR Acinetobacter spp.23
In our study, except for polymyxin B, tigecycline had high sensitivity rates to Acinetobacter isolates. Therefore, this new glycylcycline agent has bacteriostatic activity against multidrug Acinetobacter spp.23 However; there are some reports of high level resistance to tigecycline among MDR Acinetobacter spp.24
In conclusion, the present study provided some information about the patients that are prone to Acinetobacter infections based on their clinic-epidemiological features. It also showed that there were high resistant rates of Acinetobacter isolates to common antibiotics except for polymyxin B which becomes emerging problem in combating nosocomial infections in Malaysia. The current findings might be helpful to strategize infection control measures and guidance for prudent use of antibiotic against Acinetobacter infections.
ACKNOWLEDGMENTS
Authors are grateful to the Faculty of Medicine, University Kebangsaan Malaysia (UKM) for financial support as fellowship and research grant (FF-004-2012) of Shirin Biglari, Ph.D. student, Department of Medical Microbiology & Immunology, UKM.
Conflict of Interests: The authors declare that they have no competing interests.
References
- 1.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 2.Kong BH, Hanifah YA, Mohd Yusof MY, Thong KL. Antimicrobial Susceptibility Profiling and Genomic Diversity of Multidrug-Resistant Acinetobacter baumannii Isolates from a Teaching Hospital in Malaysia. Jpn J Infect Dis. 2011;64(4):337–340. [PubMed] [Google Scholar]
- 3.Deris ZZ, Harun A, Oman M, Johari MR. The prevalence and risk factors of nosocomial Acinetobacter blood stream infections in tertiary teaching hospital in north-eastern Malaysia. J Tropical Biomedicine. 2009;26(2):123–129. [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility testing. 17th informational supplement. CLSI document M100-S17; Wayna, Pa. Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- 5.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358(12):1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Mourtzoukou EG, Polemis M, Vatopoulos AC. Trends in antimicrobial resistance of Acinetobacter baumannii clinical isolates from hospitalized patients in Greece and treatment implications. Clin Microbiol Infect. 2007;13(8):816–819. doi: 10.1111/j.1469-0691.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 7.Carlet J, Fabry J, Amalberti R, Degos L. The “zero risk” concept for hospital-acquired infections: a risky business! Clin Infect Dis. 2009;49:747–749. doi: 10.1086/604720. [DOI] [PubMed] [Google Scholar]
- 8.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Queenan AM, Pillar CM, Deane J, Sahm DF, Lynch AS, Flamm RK, et al. Multidrug resistance among Acinetobacter spp. in the USA and activity profile of key agents: results from CAPITAL Surveillance 2010. Diagn Microbiol Infect Dis. 2010;73(3):267–270. doi: 10.1016/j.diagmicrobio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Giske CG, Monnet DL, Cars O, Carmeli Y. On behalf of ReAct-Action on Antibiotic Resistance. Clinical and economic impact of common multidrug-resistant Gram negative bacilli. J. Antimicrob Agents Chemother. 2008;52:813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012;39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Chang HL, Tang CH, Hsu YM, Wan L, Chang YF. Nosocomial outbreak of infection with multidrug-resistant Acinetobacter baumannii in a medical center in Taiwan. Infect. Control Hosp Epidemiol. 2009;30(1):34–38. doi: 10.1086/592704. [DOI] [PubMed] [Google Scholar]
- 13.Enoch DA, Summers C, Brown NM, Moore L, Gillham MI. Investigation and management of an outbreak of multidrug carbapenem-resistant Acinetobacter baumannii in Cambridge, UK. J Hosp Infect. 2008;70(2):109–118. doi: 10.1016/j.jhin.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Wendt C, Dietze B, Dietz E, Ruden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35(6):1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen K, Cannegieter SC, van der Reijden TJ, van Strijen B, You DM, Babel BS, et al. Diversity and clinical impact of Acinetobacter baumannii colonization and infection at a military medical center. J Clin Microbiol. 2011;49(1):159–166. doi: 10.1128/JCM.00766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smolyakov R, Borer A, Riesenberg K, Schlaeffer F, Alkan M, Porath A, et al. Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J Hosp Infect. 2003;54(1):32–38. doi: 10.1016/s0195-6701(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee SO, Kim NJ, Choi SH, Hyong Kim T, Chung JW. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: A case-control study. Antimicrobial Agents and Chemotherapy. 2004;48(1):224–228. doi: 10.1128/AAC.48.1.224-228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cisneros JM, Rodriguez-Bano J, Fernandez-Cuenca F, Ribera A, Vila J, Pascual A, et al. Risk-factors for the acquisition of imipenem-resistant Acinetobacter baumannii in Spain: a nationwide study. Clin Microbiol Infect. 2005;11(11):874–879. doi: 10.1111/j.1469-0691.2005.01256.x. [DOI] [PubMed] [Google Scholar]
- 19.Baran G, Erbay A, Bodur H, Onguru P. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. Int J Infect Dis. 2008;12(1):16–21. doi: 10.1016/j.ijid.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol. 2003;24(4):284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 21.Ko KS, Suh JY, Kwon KT, Jung SI, Park KH. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007;60(5):1163–1167. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 22.Tognim MC, Andrade SS, Silbert S, Gales AC, Jones RN, Sader HS. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int J Infect Dis. 2004;8(5):284–291. doi: 10.1016/j.ijid.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 23.NavonVenezia S, Leavitt A, Carmeli Y. High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2007;59(4):772–774. doi: 10.1093/jac/dkm018. [DOI] [PubMed] [Google Scholar]