Abstract
Objectives: Genetic factors and environmental factors play a role in pathogenesis of esophageal squamous cell carcinoma (ESCC). Previous studies regarding the association of folate intake and Methylenetetrahydrofolate reductase C677T polymorphism with ESCC was conflicting. We conducted a meta-analysis to investigate the association of MTHFR C677T and folate intake with esophageal cancer risk.
Methodology: MEDLINE, EMBASE and the Chinese Biomedical Database were searched in our study. The quality of studies were evaluated by predefined scale, and The association of polymorphisms of MTHFR C677T and folate intake and ESCC risk was estimated by Odds ratio (ORs) with 95% confidence intervals (CIs).
Results: Nineteen studies (4239 cases and 5575 controls) were included for meta-analysis. A significant association was seen between individuals with MTHFR 677 CT [OR(95%)=1.47(1.32-1.63)] and TT [OR(95%)=1.69(1.49-1.91)] genotypes and ESCC risk (p<0.05). Low intake of folate had significantly higher risk of esophageal cancer among individuals with CT/TT genotype [OR(95%)=1.65(1.1-2.49)], while high intake of folate did not find significant high risk of esophageal cancer among individuals with CT/TT genotype [OR(95%)=1.64 (0.82-3.26)].
Conclusions: Our meta-analysis indicated the folate intake and MTHFR 677CT/TT are associated with the risk of ESCC, and folate showed a significant interaction with polymorphism of MTHFR C677T.
Key Words: Methylenetetrahydrofolate reductase C677T, Polymorphism, Esophageal cancer, Folate intake, Meta-analysis
INTRODUCTION
Esophageal squamous cell and adenocarcinoma are common malignancies worldwide1, which is the sixth most commonly occurring cancer and sixth most common cause of cancer-related death in the world.1 The five-year survival rate for all stages combined was 15.6% from 1996 to 2003, which was much lower than most of other cancer types (ACS, 2008). Esophageal squamous cell carcinoma (ESCC) is one of the most prevalent cancer in China, and it is estimated 250,000 cases are diagnosed annually. Possible risk factors for ESCC include cigarette smoking, alcohol drinking, hot-temperature food, low intake of vegetable, salted food, pickled vegetables, chronic mucosal irritation and a family history of cancer.1-6 Deficiency of nutrients, such as vitamins and microelements, was suggested to be associated with an increased risk for ESCC.6
Folate is a water-soluble vitamin and naturally found in green leafy vegetables, cereals, legumes and fruits.7 Deficiency of folate could induce defective DNA repair and chromosomal fragile site expression, leading to chromosomal breaks and micronucleus formation.8 Methylenetetrahydrofolate reductase (MTHFR) C677T in the gene encoding the MTHFR enzyme, which converts dietary folate to its active cofactor in Hcy catabolism, has been studies as candidate genetic risk factor for esophageal cancer.9 As T allele dose increases, this functional polymorphism causes a graded elevation in individuals with low dietary folate consumption.10
Therefore, several previous studies have investigated the association of MTHFR C677T and folate intake with esophageal cancer risk, but the results are conflicting.9,11,12 The variation of these results might be induced by difference in ethnicities, sample size, study design and background of patients as well as random error. Therefore, we conducted a systematic review to investigate the association of MTHFR C677T and folate intake with esophageal cancer risk by reducing random error and obtaining precise estimates for some potential genetic associations.13
METHODOLOGY
We searched MEDLINE (from Jan. 1966 to Jan. 2011), EMBASE (from January 1988 to Jan. 2011), and the Chinese Biomedical Database (CBM; from January 1980 to Jan. 2011) by using the following search strategy for published papers: ‘esophageal squamous cell carcinoma’, ‘esophagus’, ‘oesophagus’, ‘carcinoma or cancer or neoplasm or tumour or tumor’, ‘Methylenetetrahydrofolate reductase’, or ‘MTHFR’. There was no restriction on the language of published paper. All references cited in studies and previously published review articles were retrieved for additional eligible studies. The eligible criteria for including studies were (1) a case-control study reporting an association between MTHFR C677T polymorphisms and ESCC; (2) original study and an available genotype or allele frequency of MTHFR C677T genotypes for estimating an odds ratio (OR) with a 95% confidence interval (CI). If the results of a study reported two or more times on the same patient populations, only the most recent and complete study was included in our study.
Data extraction: Two reviewers independently evaluated the retrieved articles, and the disagreements were resolved by discussion. Data retrieved from selected articles was included. In case the data were insufficient or missing, we attempted to contact the authors of the articles in order to request the relevant data. From those studies which werefinally selected, we extracted the following data: first author’s name, year of publication, country of origin, numbers of cases and controls, genotype frequencies of MTHFR C677T.
Quality score assessment: The quality of studies was evaluated by predefined scale in previous studies14 (Table-I). The quality score assessment criteria were evaluated by traditional epidemiological considerations and cancer genetic issues. The quality scores ranged from 0 to 15. Score<10 was defined as low quality, and score≥10 was defined as high quality.
Table-I.
Scale for Quality assessment
| Criterion Score | Score |
|---|---|
| Source of cases | |
| Selected from population or cancer registry | 3 |
| Selected from hospital | 2 |
| Selected from pathology archives, but without description | 1 |
| Not described | 0 |
| Source of control | |
| Population-based | 3 |
| Blood donors or volunteers | 2 |
| Hospital-based (cancer-free patients) | 1 |
| Not described | 0 |
| Specimens used for determining genotypes | |
| White blood cells or normal tissues | 3 |
| Tumor tissues or exfoliated cells of tissue | 0 |
| Hardy-Weinberg equilibrium in controls | |
| Hardy–Weinberg equilibrium | 3 |
| Hardy–Weinberg disequilibrium | 0 |
| Total sample size | |
| >1,000 | 3 |
| >500 and <1,000 | 2 |
| >200 and <500 | 1 |
| <200 | 0 |
Statistical analysis: Statistical analysis was conducted by using STATA Statistical Package (version 9, STATA, College Station, TX). The distributions of genotypes in controls were tested by Hardy-Weinberg equilibrium (HWE) using the Chi-square test. The association of polymorphisms of MTHFR C677T and folate intake and ESCC risk was estimated by Odds ratio (ORs) with 95% confidence intervals (CIs). The heterogeneity was tested by the Q-statistics with p-values < 0.1, and its possible sources of heterogeneity were assessed by subgroup analysis. If there was heterogeneity, the random effect model was used. Otherwise, a fixed-effect model was applied to obtain the summary OR and their 95% CI. One-way sensitivity analysis was performed to explore robustness of the results. All P values were two-sided and a P value of less than 0.05 was deemed statistically significant.
RESULTS
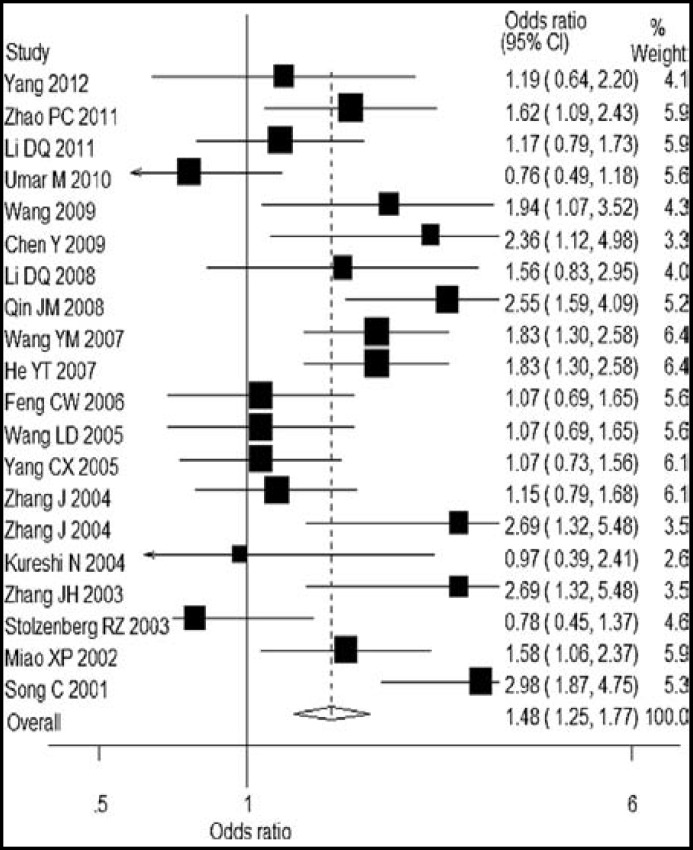
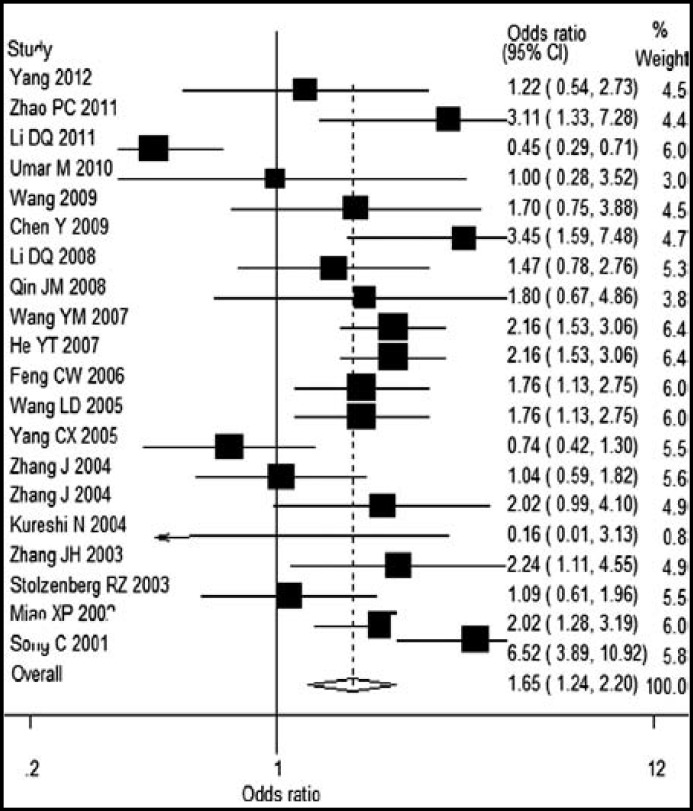
Characteristics of studies: Forty seven studies were initially identified after search, and 28 studies were excluded due to overlapping data and being without meeting the criteria. Finally, 19 studies (4239 cases and 5575 controls) were included for meta-analysis. The detailed characteristics of these studies are summarized in Table-II. Only two studies had high quality score, and the scores of other studies ranged from 7 to 10. Of the 19 case-control studies, 14 studies were conducted in China.
Table-II.
Characteristics of studies of MTHFR C677T polymorphism and ESCC
| Study ID | County | Control source | Case | Control |
Cases
|
Controls
|
P HWE | Quality score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |||||||
| Chen Y 2009 (16) | China | Hospital | 103 | 181 | 11 | 49 | 43 | 45 | 85 | 51 | 0.42 | 10 |
| Feng CW 2006 (17) | China | Population | 275 | 315 | 51 | 105 | 119 | 74 | 143 | 98 | 0.12 | 8 |
| Zhao PC 2011 (18) | China | Hospital | 155 | 310 | 68 | 74 | 13 | 179 | 120 | 11 | 0.09 | 9 |
| Li DQ 2011 (19) | China | Hospital | 226 | 246 | 112 | 113 | 45 | 95 | 82 | 85 | <0.1 | 9 |
| Li DQ 2008 (20) | China | Population | 126 | 169 | 22 | 52 | 52 | 41 | 62 | 66 | <0.1 | 10 |
| Wang YM 2007(21) | China | Population | 584 | 540 | 73 | 263 | 248 | 119 | 234 | 187 | <0.1 | 11 |
| Qin JM 2008 (22) | China | Population | 120 | 204 | 60 | 53 | 7 | 170 | 59 | 11 | 0.06 | 11 |
| He YT 2007 (23) | China | Population | 584 | 540 | 73 | 263 | 248 | 119 | 234 | 187 | <0.1 | 10 |
| Song C 2001 (9) | China | Population | 240 | 360 | 29 | 118 | 93 | 126 | 172 | 62 | 0.8 | 11 |
| Wang LD 2005 (24) | China | Population | 275 | 315 | 51 | 105 | 119 | 74 | 143 | 98 | 0.12 | 10 |
| Yang CX 2005 (12) | Japan | Hospital | 165 | 493 | 63 | 82 | 20 | 186 | 227 | 80 | 0.45 | 9 |
| Zhang J 2004 (25) | German | Population | 241 | 256 | 94 | 116 | 31 | 107 | 115 | 34 | 0.72 | 10 |
| Zhang J 2004 (25) | China | Population | 189 | 141 | 16 | 93 | 80 | 25 | 54 | 62 | <0.1 | 10 |
| Kureshi N 2004 (26) | Pakistan | Population | 34 | 54 | 22 | 12 | 0 | 32 | 18 | 4 | 0.52 | 8 |
| Zhang JH 2003(27) | China | Population | 198 | 141 | 16 | 93 | 89 | 25 | 54 | 62 | <0.1 | 7 |
| Stolzenberg RZ 2003 (11) | China | Population | 129 | 398 | 23 | 58 | 48 | 65 | 209 | 124 | 0.14 | 8 |
| Miao XP 2002 (28) | China | Population | 217 | 468 | 47 | 107 | 63 | 151 | 217 | 100 | 0.18 | 12 |
| Umar M 2010 (29) | India | Hospital | 208 | 223 | 155 | 48 | 5 | 155 | 63 | 5 | 0.63 | 13 |
| Total | 4239 | 5576 | 1008 | 1856 | 1375 | 1829 | 2353 | 1393 | ||||
| Results of meta-analysis (Random effect model), OR(95% CI) | CT vs CC | 1.47(1.32-1.63), P for heterogeneity: <0.05 | ||||||||||
| TT vs CC | 1.69(1.49-1.91), P for heterogeneity: <0.05 | |||||||||||
A significant association was seen between individuals with MTHFR 677 CT [OR(95%)=1.47(1.32-1.63)] and TT [OR(95%)=1.69(1.49-1.91)] genotypes and ESCC risk (p<0.05). There was significant heterogeneity between studies regarding MTHFR 677 CT and TT (P<0.05).
Subgroup analysis was taken according to folate intake, which indicated low intake of folate had significantly higher risk of esophageal cancer among individuals with CT/TT genotype [OR(95%)=1.65(1.1-2.49)] (Table-III). However, high intake of folate did not find significant high risk of esophageal cancer among individuals with CT/TT genotype [OR(95%)=1.64 (0.82-3.26)]. No significant heterogeneity was found between studies (P>0.05). These results indicated folate had a significant interaction with MTHFR C677T.
Table-III.
Subgroup analysis of MTHFR C677T polymorphism and ESCC
|
Cases
|
Control
|
|||
|---|---|---|---|---|
| Folate intake | CC | CT/TT | CC | CT/TT |
| Low folate intake | ||||
| Zhao 2011 | 21 | 21 | 37 | 26 |
| Yang 2005 | 12 | 28 | 35 | 70 |
| Qin 2008 | 41 | 81 | 37 | 33 |
| Results of meta-analysis (Random effect model), OR(95% CI): CT/TT vs CC | 1.65(1.1-2.49), P for heterogeneity: 0.41 | |||
| Moderate folate intake | ||||
| Zhao 2011 | 28 | 33 | 63 | 64 |
| Results of meta-analysis (Random effect model), OR(95% CI) | - | |||
| High folate intake | 2.98(1.76-7.73) | 0.25 | 3.35(1.84-6.12) | 0.20 |
| Zhao 2011 | 19 | 33 | 63 | 59 |
| Yang 2005 | 50 | 151 | 74 | 237 |
| Qin 2008 | 19 | 89 | 23 | 37 |
| Results of meta-analysis (Random effect model), OR(95% CI) | 1.64 (0.82-3.26), P for heterogeneity:0.13 | |||
A single study in this meta-analysis was deleted each time to reflect the impact of the individual data on the pooled ORs, and most of the results did not alter (Data not shown). Funnel plot an Egger’s test were used to assess the publication bias, and it provided evidence that there was no publication bias among studies regarding MTHFR 677 CT, but a significant publication bias was found in studies regarding MTHFR 677 TT genotype(P<0.05). The shape A of funnel plots was asymmetrical (Fig. 1 and 2).
Fig.1.
Publication bias on studies of MTHFR 677CT vs CC
Fig.2.
Publication bias on studies of MTHFR 677TT vs CC
DISCUSSION
Many epidemiologic studies which investigated the role of folate intake and MTHFR C677T for EC risk provided inconsistent results. Most of those studies involved few cases, and these few sample size limited the genetic effect reliabilty. Our meta-analysis recognized as an important tool to more precisely define the effect of selected genetic polymorphisms on risk of disease and to identify the potentially important sources of between-study heterogeneity. A previous meta-analysis in Asian population included 13 case-control studies which indicated MTHFR 677 CT and TT genotypes were significantly association with increased risk of esophageal cancer, especially in drinkers and smokers.30 However, this study did not explore the interaction between folate intake and MTHFR genotype. Therefore, we conducted an updated meta-analysis by critically reviewing 19 individual case-control studies on MTHFR C677T and folate intake with esophageal cancer risk. Compared with the last meta-analysis conducted in China by Fang et al, this updated meta-analysis included another 6 new case-control studies, and we explored the interaction between folate intake and MTHFR C677T. Our study showed that high intake of folate had a protective factor for esophageal cancer, and folate showed a significant interaction with polymorphism of MTHFR C677T.
Heterogeneity is a potential problems in the meta-analysis, and eliminating heterogeneity is an important factor during meta-analysis.31 In our study, we found there was significant heterogeneity between studies by using Q-statistics. However, after stratifying by the quantity of folate intake suggested folate was an important source of heterogeneity.
Previous studies have indicated folate mediates the transfer of one-carbon moieties both in the synthesis of nucleotides necessary for DNA synthesis, replication, repair and in DNA methylation reactions.32 These functions may play a critical role in carcinogenesis. Previous epidemiological studies have indicated an abundant intake of food stuffs full of folate could protect the development of various cancers.33 Ours study indicated that the folate intake was associated with a decreased risk of esophageal cancer, which proved previous hypothesis. Moreover, the activity of folate metabolic enzyme, such as MTHFR, are involved in the folate metabolic and DNA methylation process. As a key enzyme in folate metabolism, the product of MTHFR serves as the carbon donor for the methylation of homocysteine tomethionine, which is catalyzed by the enzyme MTR.34 The MTHFR gene is high polymorphic in the general population, the mutation of most common functional variant of 677C to T. This polymorphism results in an alanine to valine substitution, leading to a reduction in enzyme activity.35 The role of MTHFR in the folate metabolism decides the interaction between folate and polymorphisms of MTHFR, which was proved by our meta-analysis. Our study showed the MTHFR had strong risk of esophageal cancer in individuals with low intake of folate intake.
Possible limitations of this meta-analysis have to be considered in explaining our results. Firstly, most of the studies are conducted in China, and this could limit the power to find the difference in genotypes by different ethnicities. Secondly, publication bias may have occurred due to only published papers which were included in the meta-analysis. Thirdly, there might be misclassification during our study. Some controls in our study were selected from non-cancer inpatients, and some were selected from residents. Finally, there might be gene-environment interaction for esophageal cancer. However, we did not perform subgroup analysis due to lack of data on environmental factors. Further studies are warranted to interpreted this interaction.
In conclusion, our meta-analysis has indicated the folate intake and MTHFR 677CT/TT are associated with the risk of ESCC, and folate showed a significant interaction with polymorphism of MTHFR C677T.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Wang JM, Xu B, Rao JY, Shen HB, Xue HC, Jiang QW. Diet habits, alcohol drinking, tobacco smoking, green tea drinking, and the risk of esophageal squamous cell carcinoma in the Chinese population. Eur J Gastroenterol Hepatol. 2007;19(2):171–176. doi: 10.1097/MEG.0b013e32800ff77a. [DOI] [PubMed] [Google Scholar]
- 3.Morita M, Kumashiro R, Kubo N, Nakashima Y, Yoshida R, Yoshinaga K, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15(2):126–134. doi: 10.1007/s10147-010-0056-7. [DOI] [PubMed] [Google Scholar]
- 4.Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am. 2009;18(3):469–485. doi: 10.1016/j.soc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 5.De Stefani E, Aune D, Boffetta P, Deneo-Pellegrini H, Ronco AL, Acosta G, et al. Salted meat consumption and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10(5):853–857. [PubMed] [Google Scholar]
- 6.Yu X, Zhang T, Zhang H, Hu A, Hu Y, Guo W, et al. Comparison of lifestyle and living environment among high risk immigrant and low risk host residents: implications for esophageal cancer etiology. Asian Pac J Cancer Prev. 2010;11(6):1827–1831. [PubMed] [Google Scholar]
- 7.Aune D, Deneo-Pellegrini H, Ronco AL. Dietary folate intake and the risk of 11 types of cancer: a case-control study in Uruguay. Ann Oncol. 2011;22:444–451. doi: 10.1093/annonc/mdq356. [DOI] [PubMed] [Google Scholar]
- 8.Aune D, Deneo-Pellegrini H, Ronco AL, Boffetta P, Acosta G, Mendilaharsu M, et al. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Ann Oncol. 2011;22(2):444–451. doi: 10.1093/annonc/mdq356. [DOI] [PubMed] [Google Scholar]
- 9.Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001;61(8):3272–3275. [PubMed] [Google Scholar]
- 10.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease, a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 11.Stolzenberg-Solomon RZ, Qiao YL, Abnet CC, Ratnasinghe DL, Dawsey SM, Dong ZW, et al. Esophageal and gastric cardia cancer risk and folate- and vitamin B(12)-related polymorphisms in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1222–1226. [PubMed] [Google Scholar]
- 12.Yang CX, Matsuo K, Ito H, Shinoda M, Hatooka S, Hirose K, et al. Gene-environment interactions between alcohol drinking and the MTHFR C677T polymorphism impact on esophageal cancer risk: results of a case-control study in Japan. Carcinogenesis. 2005;26(7):1285–1290. doi: 10.1093/carcin/bgi076. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Smith GD, Phillips AN. Meta-analysis, principles and procedures. BMJ. 1997;315(7121):1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang DK, Ren WH, Yao L, Wang WZ, Peng B, Yu L. Meta-analysis of association between TP53 Arg72Pro polymorphism and bladder cancer risk. Urology. 2010;76(3):765.e1–7. doi: 10.1016/j.urology.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Cancer facts and figures 2008. American Cancer Society: American Cancer Society; 2008. [Google Scholar]
- 16.Chen Y, Yin D, Deng YC, A HL, Wang HJ, Ma YQ, et al. Relationship between MTHFR gene polymorphisms and susceptibility of esophageal cancer of Han nationality in Xinjiang. J Toxicol. 2009;23(6):429–432. [Google Scholar]
- 17.Feng CW, Fan ZM, Gao SS, He X, Huaqin G, Yin LM, et al. Analysis of methylenetetrahydrofolate reductase and thymidylate synthase gene polymorphisms of esophageal and cardia cancerpatients. J Zhengzhou Uni Med Sci. 2006;41(1):10–14. [Google Scholar]
- 18.Zhao P, Lin F, Li Z, Lin B, Lin J, Luo R. Folate intake, methylenetetrahydrofolate reductase polymorphisms, and risk of esophageal cancer. Asian Pac J Cancer Prev. 2011;12(8):2019–2023. [PubMed] [Google Scholar]
- 19.Li QD, Li H, Wang MS, Diao TY, Zhou ZY, Fang QX, et al. Multi-susceptibility genes associated with the risk of the development stages of esophageal squamous cell cancer in Feicheng County. BMC Gastroenterol. 2011;11 doi: 10.1186/1471-230X-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li DQ, Diao YT, Li H, Fang XQ, Li HQ. Association of the Polymorphisms of MTHFR C677T, VDR C352T, and MPO G463A with Risk for Esophageal Squamous Cell Dysplasia and Carcinoma. Arch Med Res. 2008(39):594–600. doi: 10.1016/j.arcmed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Guo W, He Y, Chen Z, Wen D, Zhang X, et al. Association of MTHFR C677T and SHMT(1) C1420T with susceptibility to ESCC and GCA in a high incident region of Northern China. Cancer Causes Control. 2007;18(2):143–152. doi: 10.1007/s10552-006-0097-4. [DOI] [PubMed] [Google Scholar]
- 22.Qin JM, Yang L, Chen B, Wang XM, Li F, Liao PH, et al. Interaction of methylenetetrahydrofolate reductase C677T, cytochrome P4502E1 polymorphism and environment factors in esophageal cancer in Kazakh population. World J Gastroenterol. 2008;14(45):6986–6992. doi: 10.3748/wjg.14.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He YT, Wang YM, Zhang JH, Li Y, Guo W, Wang N. Correlation between a Polymorphism in the Methylene Tetrahydrofolate Reductase Gene and Susceptibility to Carcinoma of the Esophagus and Gastric Cardia. Chinese J Clin Oncol. 2007;34(4):194–197. [Google Scholar]
- 24.Wang LD, Guo RF, Fan ZM, He X, Gao SS, Guo HQ, et al. Association of methylenetetrahydrofolate reductase and thymidylate synthase promoter polymorphisms with genetic susceptibility to esophageal and cardia cancer in a Chinese high-risk population. Dis Esophagus. 2005;18(3):177–184. doi: 10.1111/j.1442-2050.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Zotz RB, Li Y, Wang R, Kiel S, Schulz WA, et al. Methylenetetrahydrofolate reductase C677T polymorphism and predisposition towards esophageal squamous cell carcinoma in a German Caucasian and a northern Chinese population. J Cancer Res Clin Oncol. 2004;130(10):574–580. doi: 10.1007/s00432-004-0585-4. [DOI] [PubMed] [Google Scholar]
- 26.Kureshi N, Ghaffar S, Siddiqui S, Salahuddin I, Frossard PM. Head and neck cancer susceptibility: a genetic marker in the methylenetetrahydrofolate reductase gene. Orl J Otorhinolaryngol Relat Spec. 2004;66(5):241–245. doi: 10.1159/000081120. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JH, Li Y, Guo W, Wang R, Sarabia K, Wen DG, et al. The Association of Methylenetetrahydrofolate Reductase C677T Polymorphism and Esophageal Squamous Cell Carcinoma Analyzed by LightCycler. Progress In Biochemistry and Biophysics. 2003;30(4):555–559. [Google Scholar]
- 28.Miao X, Xing D, Tan W, Qi J, Lu W, Lin D. Susceptibility to Gastric Cardia Adenocarcinoma and Genetic Polymorphisms in Methylenetetrahydrofolate Reductase in an At-Risk Chinese Population. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1454–1458. [PubMed] [Google Scholar]
- 29.Umar M, Upadhyay R, Khurana R, Kumar S, Ghoshal UC, Mittal B. Evaluation of MTHFR677C>T polymorphism in prediction and prognosis of esophageal squamous cell carcinoma: a case-control study in a northern Indian population. Nutr Cancer. 2010;62(6):743–749. doi: 10.1080/01635581003605961. [DOI] [PubMed] [Google Scholar]
- 30.Fang Y, Xiao F, An Z, Hao L. Systematic review on the relationship between genetic polymorphisms of methylenetetrahydrofolate reductase and esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2011;12(7):1861–1866. [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Sasco AJ, Fu C, Xue H, Guo G, Hua Z, et al. Aberrant DNA methylation of P16, MGMT, and hMLH1 genes in combination with MTHFR C677T genetic polymorphism in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17(1):118–125. doi: 10.1158/1055-9965.EPI-07-0733. [DOI] [PubMed] [Google Scholar]
- 33.Mason JB. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr Rev. 2009;67(4):206–212. doi: 10.1111/j.1753-4887.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarbia M, Stahl M, von Weyhern C, Weirich G, Puhringer-Oppermann F. The prognostic significance of genetic polymorphisms (Methylenetetrahydrofolate Reductase C677T, Methionine Synthase A2756G, Thymidilate Synthase tandem repeat polymorphism) in multimodally treated oesophageal squamous cell carcinoma. Br J Cancer. 2006;94(2):203–207. doi: 10.1038/sj.bjc.6602900. [DOI] [PMC free article] [PubMed] [Google Scholar]