Abstract
Background
Excess adiposity and dietary factors may be important determinants of urinary albumin excretion (UAE).
Study Design
Observational analysis of PREMIER, a randomized trial designed to lower blood pressure using behavioral interventions (counseling on weight loss, healthy diet, and exercise).
Setting & Participants
481 participants with normal kidney function who provided adequate 24-hour urine collections at baseline and 6 months.
Predictors
Change in waist circumference, 24-hour urine sodium, potassium, phosphorus, and protein intake estimated from urea nitrogen.
Outcomes & Measurements
The primary outcome was change in log-transformed 24-hour UAE over 6 months.
Results
After 6 months, the proportion of individuals with UAE ≥10 mg/d decreased from 18.7% to 12.7% (p<0.001). Changes in mean waist circumference (−4.2±6.6 [SD] cm), 24-hour excretion of sodium (−28.2±71.7 mmol/d), potassium (+8.4±27.8 mmol/d), phosphorus (−27.7±314.1 mg/d), and protein intake(−1.7±19.4 g/d) were observed. After adjustment for relevant covariates, the following variables were significantly associated with reduction in ln(UAE) in separate models: decrease in waist circumference (p=0.001), decrease in 24-hour urine phosphorus (p<0.001), and decrease in protein intake (p=0.01). In a multivariable model including these three predictors, decreases in waist circumference (p=0.002) and 24-hour urine phosphorus (p=0.03), but not change in protein intake (p=0.5), remained significantly associated with reduction in ln(UAE). These associations remained significant even after adjustment for changes in blood pressure and insulin resistance. Baseline UAE and metabolic syndrome modified the relationship of waist circumference with ln(UAE); specifically, individuals with higher UAE and baseline metabolic syndrome experienced greater reductions in ln(UAE) from decreases in waist circumference.
Limitations
Observational study with potential for confounding.
Conclusions
In adults with normal kidney function, decreases in waist circumference and 24-hour urine phosphorus are associated with reductions in UAE. These findings support the rationale for clinical trials to determine whether reducing dietary phosphorus or waist circumference could prevent CKD or slow its progression.
Keywords: weight loss, waist circumference, protein, phosphorus, urinary albumin excretion
Elevated levels of urinary albumin excretion (UAE) are associated with increased risk of cardiovascular events, end-stage renal disease (ESRD), and mortality even at levels as low as 10 mg/d 1–5. Cohort studies of individuals with diabetes or vascular disease have shown that reductions in UAE are associated with decreased risk for cardiovascular outcomes 6, kidney disease outcomes and mortality 7. Potentially modifiable lifestyle factors that may impact UAE include excess adiposity 8–12 and diet 12–14. In a few small trials, weight loss interventions reduced proteinuria and albuminuria; however, most of these studies did not assess changes in diet, including protein and phosphorus intake 11,15–19. Hence, it is uncertain whether the benefit of these interventions resulted from weight loss or from changes in nutrient intake. Only one study of 30 individuals with overt proteinuria reported changes in 24-hour urine urea nitrogen (no significant change during the intervention) and found that a mean weight loss of 4.1% resulted in a mean decrease in proteinuria of 31.2% 20. Furthermore, little is known about the impact of weight loss on UAE in individuals without overt kidney disease.
Diet may play an important role in UAE because dietary patterns characterized by high intake of red meat, saturated fats, and sweets have been associated with incident microalbuminuria 12,13. While studies of protein restriction in individuals with chronic kidney disease (CKD) have suggested potential benefits on slowing progression of CKD 21, potential risks of high protein intake in persons with normal kidney function remain uncertain 22–24. One dietary factor intrinsically linked to protein is phosphorus, which at high levels of consumption can cause kidney injury in animal CKD models (independent of protein) 25. While serum phosphorus has been found to be associated with low-grade albuminuria 26, cardiovascular events, and mortality 27,28, little is known about risk associated with 24-hour urine phosphorus, which may more adequately reflect dietary intake 29.
The goal of this study was to examine whether changes in central obesity and dietary factors, estimated from 24-hour urine collections, were associated with changes in UAE, using data from a randomized controlled trial of dietary intervention in patients with pre-hypertension or stage I hypertension.
Methods
Study Population
The PREMIER study is a completed, 18-month, multicenter randomized trial that was designed to test the effect of two behavioral interventions on blood pressure (BP) in adults with pre-hypertension or stage I hypertension (systolic, 120–159 mmHg; diastolic, 80–95 mm Hg). Participants were eligible if they were not taking antihypertensive agents and had a systolic BP of 120–159mmHg or diastolic BP of 80–95mmHg. Exclusion criteria included use of BP medications, weight-loss or steroid medications, diabetes, decreased kidney function (estimated glomerular filtration rate <60ml/min using the Cockcroft-Gault equation), history of a cardiovascular event, congestive heart failure, angina, cancer diagnosis or treatment in the past 2 years, consumption of more than 21 alcoholic drinks per week, and pregnancy. More detailed information about the study methods and main results have been published 30.
Eligible participants were randomized to one of three groups: (1) an “established” group who received behavioral counseling on achieving weight loss of at least 15 lb at 6 months (for those with a body mass index [BMI] ≥ 25), engaging in ≥ 180 min/wk of moderate-intensity physical activity, and consuming ≤ 100 mEq/d of dietary sodium; (2) an “established plus Dietary Approaches to Stop Hypertension (DASH)” group who received the same recommendations as the established group as well as counseling on the DASH dietary pattern; and (3) an “advice only” comparison group who received a single 30-minute individual advice session at the time of randomization with verbal and written instructions on weight loss, increasing physical activity, sodium reduction, and the DASH dietary pattern. Both the established and the established-plus-DASH groups received weekly group counseling for the first 8 weeks, then biweekly through 6 months, that emphasized reduced total caloric intake and increased physical activity. The established group did not have goals for fruit, vegetable, and dairy intake; goals for saturated fat and total intake were set at ≤10% and ≤30% of energy intake, respectively. The established-plus-DASH group received additional instruction on following the DASH dietary pattern, which emphasized increased consumption of fruits and vegetables (9–12 servings/d), low-fat dairy products (2–3 servings/d), and reduced intake of saturated fat (≤7% of energy) and total fat (≤ 25% of energy).
Measurements
Baseline and six-month measurements were obtained by staff who were masked to randomization assignment. BP measurements were obtained by trained, certified individuals using a random-zero sphygmomanometer following a standardized protocol (9). All baseline BP measurements were obtained before randomization. BP at baseline and 6 months was defined as an average of six to eight readings.
Twenty-four hour urine collections were obtained at baseline and 6 month visits. Collections with urine volume <500mL or collection period <22 or >26 hours were repeated. Urinary sodium, potassium, phosphorus, urea nitrogen and creatinine were measured in a central laboratory on the Hitachi 917 analyzer using Roche reagents. Albumin was measured on urine samples that were stored at −70°C for three to five years before analysis using a Tina-Quant (Roche) albumin assay. All urine laboratory values were standardized to 24-hour measurements. Dietary protein intake was estimated using the Maroni equation 31–34: Estimated protein intake = [urinary urea nitrogen + (weight in kg * 0.031g nitrogen/kg/day)]*6.25
Blood samples for measurement of glucose, insulin and lipids were obtained by venipuncture in the morning after an overnight fast. Weight was measured to the nearest 0.1kg twice at each study visit and averaged, using a calibrated scale with individuals in light indoor clothing and no shoes. Height was measured using a wall-mounted stadiometer. Waist circumference was measured using a tape, according to a standardized protocol at baseline and six months.
Metabolic syndrome was defined by National Cholesterol Education Program (NCEP) criteria, which required 3 or more of the following: waist circumference >102 cm (men) or >88 cm (women); triglyceride level ≥150 mg/dL; high-density lipoprotein cholesterol level <40 mg/dL (men) or <50 mg/dL (women); BP≥130/≥85mmHg; and fasting glucose≥110 mg/dL 35. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the formula: (glucose × insulin)/405 36. Supplement use was measured by two unannounced 24-hour dietary recalls conducted by telephone interviews (one on a weekday; the other, on a weekend day) only during the baseline visit.
Analysis
Unpaired t-tests or Pearson chi-square tests were used to compare continuous and categorical baseline characteristics between individuals with elevated UAE (≥10mg/d) and those without elevated UAE. Median UAE and elevated UAE status at six months were compared to the baseline examination using paired t-test and McNemar’s test. UAE was natural log transformed due to its skewed distribution and expressed as ln(UAE) in longitudinal analyses examining associations between changes in waist circumference and dietary biomarkers with change in ln(UAE). To minimize the influence of over- and under-collection of urine on this analysis, we only included individuals with 24-hour urine creatinine coefficients of variation (CVs) <25%, which is the upper limit of the intraindividual CV found in previous studies 37. This substantially improved correlation between the baseline and 6 month 24-hour urine creatinine measurements from 0.65 to 0.85.
Covariates including age, sex, race, cohort (participants were recruited in 4 waves), site, current smoking, treatment group assignment, baseline systolic BP, ln(UAE), and metabolic syndrome were considered in our base model, which was created using backwards stepwise regression, retaining variables with p values <0.1. We then created separate models adding change in waist circumference and each dietary measure (estimated protein intake, 24-hour urinary sodium, potassium, sodium/potassium ratio, phosphorus) individually to the base model. We used waist circumference rather than weight because waist circumference better reflects central adiposity 38. Covariates were standardized in these models to facilitate relative comparisons among regression coefficients. Beta coefficients were then back-transformed and presented as percentage change in UAE associated with a 1 standard deviation (SD) change in the corresponding predictor variable.
We then created multivariable models adding significant predictors (p value<0.05) to the base model, which included age, race, site, current smoking, baseline systolic BP and ln(UAE): multivariable model 1: base model + change in waist circumference, change in protein intake, and change in 24-hour urine phosphorus; multivariable model 2: base model + change in waist circumference and change in protein intake; multivariable model 3: base model + change in waist circumference and change in 24-hour urine phosphorus. These latter 2 models were constructed because changes in protein intake and 24-hour urine phosphorus were strongly correlated (r=0.58; p<0.001). To test for mediators, we included change in systolic BP and change in HOMA-IR to multivariable model 1. We also tested for effect modification by baseline systolic BP, baseline UAE, and baseline metabolic syndrome by adding relevant interaction terms to multivariable model 1.
Sensitivity analyses were performed using different inclusion criteria: 1) Including those with 24-hour urine creatinine CV <40% (instead of 25%); 2) Including individuals with 24-hour urine collections within 30% of expected creatinine values (for men, 22.1mg/kg; women, 17.2mg/kg) 34,39. We also repeated analyses adjusting for self-reported baseline use of calcium and vitamin D supplements. For all analyses, we used complete case analysis, only including participants with complete data for each model using Stata version 11.1 (StataCorp LP, College Station, TX).
Results
Population Characteristics
A total of 810 participants were enrolled in the PREMIER trial, and 598 individuals provided complete 24-hour urine samples at baseline and at the 6 month visit. After exclusion of individuals who had 24-hour urine creatinine CV ≥25% (n=95), and individuals missing data on 24-hour urine dietary markers (n=16), or waist circumference (n=6), 481 participants were included in our analyses. A higher proportion of African-Americans (42.1% vs. 30.6%; p=0.03) were excluded due to urine collections with CV≥25%; otherwise, there were no other significant differences between those with adequate and inadequate urine collections. Table 1 shows baseline characteristics by elevated UAE status (≥ 10mg/d). Individuals with elevated UAE were more likely to be African-American, obese, current smokers, and have hypertension. They also had higher mean waist circumference, systolic and diastolic BPs, insulin resistance, higher 24-hour urine sodium excretion, higher sodium/potassium ratios, and higher 24-hour urine phosphorus excretion. (Table 1)
Table 1.
Baseline Characteristics by Presence or Absence of Elevated UAE)
| Elevated UAE (n=90) | Normal UAE (n=391) | P value | |
|---|---|---|---|
| Age (y) | 50.9 (8.6) | 51.0 (8.5) | 0.9 |
| Female sex | 57% | 62% | 0.4 |
| African-American | 42.2% | 29.4% | 0.02 |
| BMI (kg/m2) | 34.7 (6.2) | 32.5 (5.6) | 0.002 |
| Waist circumference (cm) | 114.0 (17.6) | 106.9 (14.5) | <0.001 |
| SBP (mmHg) | 138.6 (9.3) | 133.9 (9.6) | <0.001 |
| DBP (mmHg) | 85.5 (4.3) | 84.5 (4.1) | 0.04 |
| Hypertension | 51% | 34% | 0.002 |
| Glucose (mg/dL) | 102.4 (16.5) | 99.0 (13.9) | 0.05 |
| Insulin (μU/mL) | 17.8 (11.3) | 13.9 (9.7) | 0.001 |
| LDL cholesterol (mg/dL) | 136.2 (30.9) | 135.9 (34.8) | 0.9 |
| HOMA-IR | 4.7 (4.0) | 3.6 (3.6) | 0.01 |
| Current smoking | 9% | 3% | 0.01 |
| Metabolic syndrome | 61% | 51% | 0.07 |
| 24-h UAE (mg/d) | 22.0 [13.7–45.2] | 3.2 [2.1–5.0] | <0.001 |
| Estimated protein intake* (g/d) | 95.7 (27.3) | 90.2 (24.4) | 0.06 |
| Urine Na/K ratio | 3.2 (1.3) | 2.7 (1.2) | 0.003 |
| 24-h urine Na (mmol/d) | 198.0 (78.3) | 169.4 (69.1) | <0.001 |
| 24-h urine K (mmol/d) | 65.9 (22.4) | 67.5 (24.5) | 0.6 |
| 24-h urine P (mg/d) | 1007.8 (372.4) | 914.1 (325.6) | 0.02 |
Note: Elevated UAE is ≥10 mg/d. Values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Elevated UAE is ≥10 mg/d. Conversion factors for units: glucose in mg/dL to mmol/L, x0.05551; LDL cholesterol in mg/dL to mmol/L, x0.02586.
Estimated protein intake =(urinary urea nitrogen +0.031g nitrogen/kg body weight)* 6.25.
Abbreviations: body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), low-density lipoprotein (LDL), homeostasis model assessment of insulin resistance (HOMA-IR), urinary albumin excretion (UAE); phosphorus (P), sodium (Na), potassium (K)
Changes in UAE and Measures of Obesity and Diet After 6 Months
After 6 months, median UAE decreased from 4.0 (interquartile range, 2.3–7.4) to 3.0 (interquartile range, 1.9–5.5) (p<0.001) with no significant differences between treatment groups. Overall, the proportion of individuals with elevated UAE ≥ 10 mg/d decreased from 18.7% to 12.7% (p<0.001), and findings were similar using a UAE cutoff of ≥30 mg/d (6.4% to 4.4%; p=0.03). When defined in categories of UAE <10 mg/d, 10–29.9 mg/d, and ≥30 mg/d, 52 individuals had improvements in UAE category while 16 individuals changed to worse UAE categories after 6 months (Table 2). Over 6 months, waist circumference changed by a mean of −4.2±6.6 (SD) cm (Table 3). Changes in mean 24-hour urinary excretion of sodium (−28.2± 71.7 mmol/d), potassium (8.4± 27.8 mmol/d), phosphorus (−27.7±314.1 mg/d), and protein intake (−1.7±19.4 g/d) were also observed.
Table 2.
Cross-tabulation of Baseline and 6-Month UAE Categories
| Baseline UAE | 6-Month UAE | ||
|---|---|---|---|
| <10 mg/d | 10–29.9 mg/d | ≥30 mg/d | |
| <10 mg/d | 379 (78.8%)¶ | 11 (2.3%)§ | 1 (0.2%)§ |
| 10–29.9 mg/d | 37 (7.7%)* | 18 (3.7%)¶ | 4 (0.8%)§ |
| ≥30 mg/d | 4 (0.8%)* | 11 (2.3%)* | 16 (3.3%)¶ |
Note: N=481. Values are given as number (percentage).
Improvement in UAE category.
No change in UAE category.
Worsening in UAE category.
Abbreviation: urinary albumin excretion (UAE)
Table 3.
Changes in Obesity and Dietary Measures Over 6 Months
| Measure | Change |
|---|---|
| Waist circumference (cm) | −4.2 (6.6) |
| Weight (kg) | −4.3 (5.4) |
| 24-h urine Na (mmol/d) | −28.2 (71.7) |
| 24-h urine K (mmol/d) | +8.4 (27.8) |
| 24-h urine P (mg/d) | −27.7 (314.1) |
| Urine Na/K ratio | −0.58 (1.31) |
| Estimated protein intake (g/d)* | −1.7 (19.4) |
Note: Values are given as mean ± standard deviation.
Estimated protein intake =(urinary urea nitrogen +0.031g nitrogen/kg body weight)* 6.25
phosphorus (P), sodium (Na), potassium (K)
Associations Between Changes in Obesity and Dietary Measures With Change in ln(UAE)
Table 4 shows percentage changes in UAE for every 1 SD decrease in predictors, in an analysis adjusted for age, site, African-American race, current smoking, baseline systolic BP and baseline ln(UAE). In individual models, every 1-SD reduction (6.6cm) in waist circumference was associated with a −10.9% change (95% confidence interval [CI], −16.6% to −5.2%; p=0.001) in UAE; every 1-SD reduction in 24-hour urine phosphorus (314mg/d) and protein intake (19 g/d) were associated with -10.9% (95% CI, −16.4% to −4.9%; p<0.001) and −8.1% (95% CI, −13.9% to −2.0%; p=0.01) changes in UAE, respectively, in these individual models. Changes in 24-hour urine sodium, potassium, and sodium/potassium ratio were not significantly associated with changes in UAE (Table 4).
Table 4.
Changes in UAE Associated with Decreases in Waist Circumference and Dietary Measures
| Individual Models | Multivariable Model 1 | Multivariable Model 2 | Multivariable Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Decrease | ΔUAE (95% CI) | p-value | ΔUAE (95% CI) | p-value | ΔUAE (95% CI) | p-value | ΔUAE (95% CI) | p-value |
| Waist circumference (per 6.6 cm) | −10.9 (−16.6 to −5.2) | 0.001 | −9.9 (−15.6 to −3.8) | 0.002 | −9.9 (−15.4 to −3.8) | 0.002 | −10.6 (−16.3 to −4.6) | 0.001 |
| 24-h urine P (per 314.1 mg/d) | −10.9 (−16.4 to −4.9) | <0.001 | −8.2 (−15.2 to −0.6) | 0.03 | −9.8 (−15.4 to −3.8) | 0.002 | ||
| Protein intake (per 19.4 g/d) | −8.1 (−13.9 to −2.0) | 0.01 | −2.9 (−10.3 to 5.0) | 0.5 | −7.7 (−13.4 to −1.5) | 0.02 | ||
| 24-h urine Na (per 71.7 mmol/d) | −4.7 (−10.7 to 1.7) | 0.1 | ||||||
| 24-h urine K (per 27.8 mmol/d) | −2.3 (−8.5 to 4.4) | 0.5 | ||||||
| Na/K ratio (per 1.3 mmol/mmol) | −1.5 (−7.8 to 5.1) | 0.6 | ||||||
Note: N=481. Each model was adjusted for age, site, African-American race, current smoking, baseline systolic blood pressure and ln(UAE). Covariates were standardized in these models to facilitate relative comparisons between regression coefficients. Beta coefficients were then back-transformed and are presented as percent change in UAE associated with a one standard deviation decrease in the corresponding predictor variable.
Abbreviations and definitions: urinary albumin excretion (UAE), phosphorus (P), sodium (Na), potassium (K); CI, confidence interval, ΔUAE, change in UAE.
In multivariable model 1, decreases in waist circumference (−9.9% change in UAE per 1-SD decrease; 95% CI, −15.6% to −3.8%; p=0.002) and 24-hour urine phosphorus (−8.2% change in UAE per 1-SD decrease; 95% CI, −15.2% to -0.6%; p=0.03) remained significantly associated with change in UAE while decrease in estimated protein intake was not (Table 4). The association between decrease in 24-hour urine phosphorus and change in UAE was slightly stronger (−9.8% change in UAE per 1-SD decrease; 95% CI, −15.4% to −3.8%; p=0.002) in multivariable model 2, which does not include change in protein intake (r=0.58; p<0.001 for correlation with 24-hour urine phosphorus). Decrease in protein intake was significantly associated with a −7.7% change in UAE per 1-SD decrease (95% CI, −13.4% to −1.5%; p=0.02) in multivariable model 3, which does not include change in 24-hour urine phosphorus (Table 4). Thus, the association between protein intake and UAE was largely explained by 24-hour urine phosphorus whereas the association between 24-hour urine phosphorus and UAE was not explained solely by protein intake. When changes in systolic BP or insulin resistance were added to multivariable model 1, decreases in waist circumference and 24-hour urine phosphorus remained significantly associated with reductions in UAE (Table 5).
Table 5.
Multivariable Model 1 Adjusted for Potential Mediators
| Decrease | ΔUAE (95% CI) | p-value |
|---|---|---|
| Waist circumference (per 6.6 cm) | −10.5 (−16.6 to −4.0) | 0.002 |
| 24-h urine P (per 314.1 mg/d) | −8.6 (−15.6 to −1.0) | 0.03 |
| Protein intake (per 19.4 g/d) | −2.9% (−10.4 to 5.1) | 0.5 |
| SBP (per 9.2 mmHg) | +1.7 (−5.3 to 9.3) | 0.6 |
| HOMA-IR (per 3.2 U) | +3.3 (−3.2 to 10.3) | 0.3 |
Note: n=473. Potential mediators are changes in SBP and HOMA-IR. Adjusted for age, site, African-American race, baseline SBP, current smoking and baseline ln (UAE). Covariates were standardized in these models to facilitate relative comparisons between regression coefficients. Beta coefficients were then back-transformed and are presented as percent change in UAE associated with a one standard deviation decrease in the corresponding predictor variable.
Abbreviations and definitions: systolic blood pressure (SBP), homeostasis model assessment of insulin resistance (HOMA-IR), urinary albumin excretion (UAE); CI, confidence interval; P, phosphorus; ΔUAE, change in UAE.
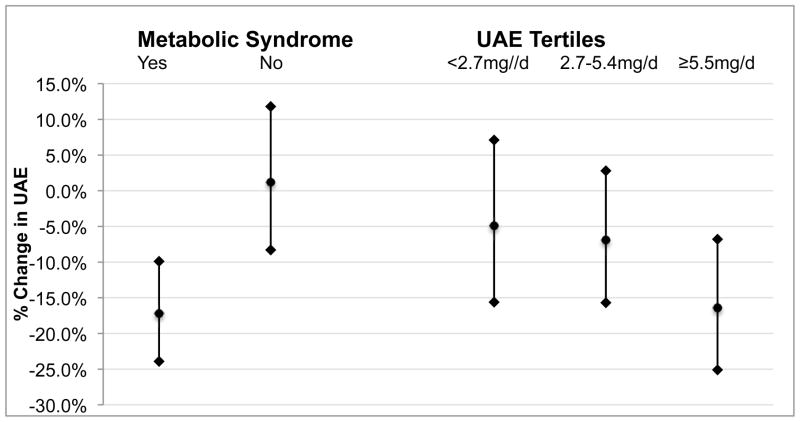
In tests for interaction, we found that the effect of decreased waist circumference on change in UAE was modified by baseline metabolic syndrome (p=0.01) and baseline UAE (p=0.02) but not baseline systolic BP (p=0.5) (Figure 1). In subgroup analyses adjusted for the same covariates as multivariable model 1, individuals in the highest tertile of baseline UAE (≥5.5mg/d) had a −16.4% (95% CI, −25.1% to −6.8%; p=0.001) change in UAE for every 1-SD decrease in waist circumference compared to −6.9% (95% CI, −15.7% to 2.8%; p=0.2) for the middle tertile (2.7–5.4mg/d) and −4.9% (95% CI, −15.6% to 7.1%; p=0.4) for the lowest tertile (<2.7mg/d) of baseline UAE. Similarly, individuals who had metabolic syndrome at baseline had a −17.2% (95% CI, −23.9% to −9.9%; p<0.001) change in UAE for every 1-SD decrease in waist circumference whereas no association (p=0.8) was observed for individuals without metabolic syndrome. Baseline metabolic syndrome, baseline UAE, and baseline systolic BP did not modify the effect of changes in 24-hour urine phosphorus or protein intake on UAE.
Figure 1.
Effect of Decreased Waist Circumference on UAE was Modified by Baseline Metabolic Syndrome and Baseline UAE. Changes in UAE (95% CI) associated with a 1-SD decrease (6.6 cm) in waist circumference stratified by baseline metabolic syndrome and UAE tertile are shown. All models adjusted for age, site, African-American race, baseline systolic BP, current smoking, baseline ln(UAE), changes in protein intake and 24-hour urine phosphorus. P value<0.05 for both interactions (baseline metabolic syndrome and baseline UAE as a continuous variable) on change in waist circumference.
Sensitivity analyses using different inclusion criteria (24-hour urine creatinine CV<40%; 24-hour urine creatinine values within 30% of expected sex-specific values 34,39) showed similar effect sizes and associations as our primary analysis as did analyses in which we adjusted for baseline calcium and vitamin D supplement use (data not shown).
Discussion
In this cohort of mostly overweight and obese individuals with pre-hypertension or stage I hypertension and normal kidney function, we found that reductions in waist circumference and 24-hour urine phosphorus were significantly associated with decreases in UAE. Our findings suggest that reducing central adiposity and phosphorus intake could be an important strategy in reducing UAE. In our study the association between change in waist circumference and UAE was greatest among those with baseline metabolic syndrome and higher baseline UAE. This latter finding is consistent with a meta-analysis of 5 controlled and 8 uncontrolled trials (n=528 participants) evaluating the effect of weight loss on UAE and proteinuria 11. Unlike most of these previous studies, our study was strengthened by having repeat 24-hour urine sodium, urea nitrogen, and phosphorus measurements. Thus, we were able to examine the impact of changes in diet as well as changes in central obesity on UAE.
The significant association we observed between changes in 24-hour urine phosphorus and UAE is novel and needs to be confirmed in other studies. These findings could reflect changes in total intake of phosphorus, the sources of phosphorus being consumed, or less likely, changes in gut absorption or bone metabolism. In a normal physiologic state, 24-hour urine phosphorus should approximate gut absorption of phosphorus 29,40. However, the bioavailability of phosphorus can be affected by vitamin D and calcium supplements 41,42 as well as the source of phosphorus. For example, inorganic phosphorus salts are believed to have much higher bioavailability (90%–100%) than naturally occurring forms of phosphorus (40%–60%) 43–45, and food additives have been estimated to contribute up to 30% of the phosphorus consumed in the U.S. diet 43. Phosphorus derived from animal protein is typically more bioavailable than vegetable protein because approximately 75% of plant protein exists as phytate, which is not readily digestible by humans 46. Indeed, feeding studies have shown that there is lower 24-hour urinary phosphorus excretion while eating a diet with equivalent amounts of phosphorus from vegetable protein compared to animal protein, although no collection of stool were done in these studies to confirm that this was due to differences in gut absorption 45,47. Thus, changes in 24-hour urine phosphorus could be due to changes in proportions of phosphorus derived from food processing, animal, and vegetable sources.
Cross-sectional studies have shown that UAE is highest among omnivores and lowest among vegans 48, and cross-over studies of individuals with normal kidney function 49 and individuals with diabetic nephropathy 50 have found lower rates of UAE while on vegetable protein diets compared to animal protein diets. Whether high protein intake of any type can cause long-term harm in individuals with normal kidney function remains unclear 23,24,33. A secondary analysis of OmniHeart (Optimal Macro-Nutrient Intake to Prevent Heart Disease), a randomized crossover feeding trial testing the effects of different macronutrient profiles on BP and lipids, found that a high protein diet (25% total calories) increased estimated glomerular filtration rate independent of changes in BP compared to the other two diets with lower protein (15% total calories) 24. In a randomized trial comparing a prescribed low-carbohydrate high-protein weight loss diet to a prescribed low-fat weight loss diet, creatinine clearance was significantly increased at 3 months and 1 year (when adherence was likely greatest) in the low-carbohydrate, high-protein diet group compared to the low-fat diet group 22. However, the long-term effects of glomerular hyperfiltration related to high protein intake are unknown; studying the effects of diet on hard kidney disease or cardiovascular outcomes in a trial would require many years of follow-up or a high-risk population. Secondary analyses of the Modification of Diet in Renal Disease (MDRD) study, and a meta-analysis of dietary protein restriction studies, suggest that restricting dietary protein intake may slow glomerular filtration rate decline in individuals with CKD 51,52.
While a direct causal link between excess dietary phosphorus intake and UAE remains speculative, in vitro and in vivo studies in animal and humans provide some supportive evidence that excess dietary phosphorus could have a detrimental effect on the endothelium. Bovine aortic endothelial cells exposed to higher phosphorus concentrations have been shown to have increased reactive oxygen species production and decreased nitric oxide production 53. These authors also reported that healthy volunteers who consumed a meal with 1200 mg of phosphorus (800mg as sodium phosphate supplement) developed post-prandial impaired flow-mediated dilatation, which correlated inversely with serum phosphorus levels 53. Observational studies have found that fasting serum phosphorus levels are associated with increased mortality even in individuals without CKD 27, and the phosphaturic hormone fibroblast growth factor 23 (FGF-23) is associated with albuminuria, CKD progression 54, incident heart failure, and all-cause mortality 55. However, self-reported dietary phosphorus intake is weakly associated with fasting serum phosphorus levels, making it difficult to extrapolate adverse associations with serum phosphorus to dietary phosphorus intake 56.
High phosphorus diets have been shown in animal models of CKD to cause renal injury (independent of protein intake) 25. Studies of reducing phosphorus intake in humans have been largely confounded by concomitant reductions in protein intake 21. However, one non-randomized study of protein restriction in proteinuric CKD patients found an interaction between 24-hour urine phosphorus and the anti-proteinuric effect of a very-low protein diet. Individuals in this study who attained lower 24-hour urine phosphorus while on the very low protein diet (0.3g/kg supplemented with keto-analogues) experienced greater reductions in proteinuria (p for interaction <0.001) 57, providing some support for our findings.
Our study has a few limitations. First, this is an observational analysis of PREMIER, which was not originally designed to study the effects of behavioral interventions on UAE. As multiple lifestyle factors changed simultaneously during the study, we cannot be certain that the observed associations were directly causal. We found no effect of the behavioral interventions on UAE, possibly because the “advice only” group also lost weight and had the most negative change in protein intake of the three groups. Second, we only included 59% of individuals originally randomized in PREMIER, which mainly reflects the difficulty in collecting repeated 24-hour urine collections. Third, participants had normal kidney function and most had baseline UAE of less than 10 mg/d, above which seems to confer risk for cardiovascular disease and mortality 1–5. Though reduction of albuminuria is associated with decreased risk of kidney and cardiovascular disease, 6,7 the effect of changes in UAE in this low range are unknown. Lastly, we adjusted for baseline supplement use in sensitivity analyses, but did not have information on changes in levels of parathyroid hormone, vitamin D, or FGF-23. Though we cannot definitively exclude the possibility that changes in 24-hour urine phosphorus were in part due to changes in phosphorus metabolism rather than dietary intake, we believe this possibility is unlikely in individuals with normal kidney function. Strengths of our study include repeat dietary measurements based on 24-hour urine dietary biomarkers in a well-characterized cohort and the wide generalizability of our study, as individuals with pre-hypertension or stage I hypertension comprise roughly two thirdss of the US population.
In conclusion, our findings suggest that reducing excess adiposity and phosphorus intake may be important in lowering UAE. More research is needed to understand determinants of phosphorus absorption and excretion and whether excessive dietary phosphorus intake could be harmful to individuals with normal kidney function.
Acknowledgments
We thank the PREMIER participants for their dedication; Gayle Meltesen, Alan Bauck, and Dave Gibson for their assistance in obtaining the laboratory data; and Gayane Yenokyan for reviewing an early draft.
Support: Dr Chang was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant T32DK007732.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 3.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 4.Kramer H, Jacobs DR, Jr, Bild D, et al. Urine albumin excretion and subclinical cardiovascular disease. the multi-ethnic study of atherosclerosis. Hypertension. 2005;46(1):38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 5.Warnock DG, Muntner P, McCullough PA, et al. Kidney function, albuminuria, and all-cause mortality in the REGARDS (reasons for geographic and racial differences in stroke) study. Am J Kidney Dis. 2010;56(5):861–871. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110(8):921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 7.Schmieder RE, Mann JF, Schumacher H, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22(7):1353–1364. doi: 10.1681/ASN.2010091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12(6):1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 9.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294(4):F685–96. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 10.Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 2007;71(2):159–166. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- 11.Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: Systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25(4):1173–1183. doi: 10.1093/ndt/gfp640. [DOI] [PubMed] [Google Scholar]
- 12.Chang A, Van Horn L, Jacobs D, Liu K, Bibbins-Domingo K. Lifestyle behaviors and incident chronic kidney disease (CKD): The CARDIA study [NKF abstract 56] Am J Kidney Dis. 57(4):B30. [Google Scholar]
- 13.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the nurses’ health study. Am J Kidney Dis. 2011;57(2):245–254. doi: 10.1053/j.ajkd.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs DR, Jr, Gross MD, Steffen L, et al. The effects of dietary patterns on urinary albumin excretion: Results of the dietary approaches to stop hypertension (DASH) trial. Am J Kidney Dis. 2009;53(4):638–646. doi: 10.1053/j.ajkd.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saiki A, Nagayama D, Ohhira M, et al. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes (Lond) 2005;29(9):1115–1120. doi: 10.1038/sj.ijo.0803009. [DOI] [PubMed] [Google Scholar]
- 16.Solerte SB, Fioravanti M, Schifino N, Ferrari E. Effects of diet-therapy on urinary protein excretion albuminuria and renal haemodynamic function in obese diabetic patients with overt nephropathy. Int J Obes. 1989;13(2):203–211. [PubMed] [Google Scholar]
- 17.Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: Long-term follow-up. J Am Soc Nephrol. 2006;17(12 Suppl 3):S213–7. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 18.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal V, Khan I, Rai B, et al. The effect of weight loss after bariatric surgery on albuminuria. Clin Nephrol. 2008;70(3):194–202. doi: 10.5414/cnp70194. [DOI] [PubMed] [Google Scholar]
- 20.Morales E, Valero MA, Leon M, Hernandez E, Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41(2):319–327. doi: 10.1053/ajkd.2003.50039. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the modification of diet in renal disease study. Am J Kidney Dis. 1996;27(5):652–663. doi: 10.1016/s0272-6386(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 22.Friedman AN, Ogden LG, Foster GD, et al. Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin J Am Soc Nephrol. 2012;7(7):1103–1111. doi: 10.2215/CJN.11741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138(6):460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 24.Juraschek SP, Appel LJ, Anderson CA, Miller ER., 3rd Effect of a high-protein diet on kidney function in healthy adults: Results from the OmniHeart trial. Am J Kidney Dis. 2013;61(4):547–554. doi: 10.1053/j.ajkd.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haut LL, Alfrey AC, Guggenheim S, Buddington B, Schrier N. Renal toxicity of phosphate in rats. Kidney Int. 1980;17(6):722–731. doi: 10.1038/ki.1980.85. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Oh SW, Heo NJ, et al. Serum phosphorus as a predictor of low-grade albuminuria in a general population without evidence of chronic kidney disease. Nephrol Dial Transplant. 2012;27(7):2799–2806. doi: 10.1093/ndt/gfr762. [DOI] [PubMed] [Google Scholar]
- 27.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 28.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167(9):879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 29.Houston J, Isakova T, Wolf M. Phosphate metabolism and fibroblast growth factor 23 in chronic kidney disease. In: Kopple J, Massry S, Kalantar-Zadeh K, editors. Nutritional management of renal disease. 3. Academic Press; 2012. pp. 285pp. 286–307. [Google Scholar]
- 30.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 31.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27(1):58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 32.Masud T, Manatunga A, Cotsonis G, Mitch WE. The precision of estimating protein intake of patients with chronic renal failure. Kidney Int. 2002;62(5):1750–1756. doi: 10.1046/j.1523-1755.2002.00606.x. [DOI] [PubMed] [Google Scholar]
- 33.Halbesma N, Bakker SJ, Jansen DF, et al. High protein intake associates with cardiovascular events but not with loss of renal function. J Am Soc Nephrol. 2009;20(8):1797–1804. doi: 10.1681/ASN.2008060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scialla JJ, Appel LJ, Astor BC, et al. Net endogenous acid production is associated with a faster decline in GFR in african americans. Kidney Int. 2012;82(1):106–112. doi: 10.1038/ki.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Stamler J. Assessment of sodium intake in epidemiological studies on blood pressure. Ann Clin Res. 1984;16 (Suppl 43):49–54. [PubMed] [Google Scholar]
- 38.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73(7):460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 39.Pak CY, Odvina CV, Pearle MS, et al. Effect of dietary modification on urinary stone risk factors. Kidney Int. 2005;68(5):2264–2273. doi: 10.1111/j.1523-1755.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 40.Spencer H, Menczel J, Lewin I, Samachson J. Effect of high phosphorus intake on calcium and phosphorus metabolism in man. J Nutr. 1965;86:125–132. doi: 10.1093/jn/86.2.125. [DOI] [PubMed] [Google Scholar]
- 41.Schiller LR, Santa Ana CA, Sheikh MS, Emmett M, Fordtran JS. Effect of the time of administration of calcium acetate on phosphorus binding. N Engl J Med. 1989;320(17):1110–1113. doi: 10.1056/NEJM198904273201703. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez JA, Emmett M, White MG, et al. The absorption of dietary phosphorus and calcium in hemodialysis patients. Kidney Int. 1986;30(5):753–759. doi: 10.1038/ki.1986.252. [DOI] [PubMed] [Google Scholar]
- 43.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical american diet: Does it matter in nephrology? Semin Dial. 2003;16(3):186–188. doi: 10.1046/j.1525-139x.2003.16037.x. [DOI] [PubMed] [Google Scholar]
- 44.Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107(1):42–50. doi: 10.1093/jn/107.1.42. [DOI] [PubMed] [Google Scholar]
- 45.Karp HJ, Vaihia KP, Karkkainen MU, Niemisto MJ, Lamberg-Allardt CJ. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: A whole-foods approach. Calcif Tissue Int. 2007;80(4):251–258. doi: 10.1007/s00223-007-9011-7. [DOI] [PubMed] [Google Scholar]
- 46.Iqbal TH, Lewis KO, Cooper BT. Phytase activity in the human and rat small intestine. Gut. 1994;35(9):1233–1236. doi: 10.1136/gut.35.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(2):257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiseman MJ, Hunt R, Goodwin A, Gross JL, Keen H, Viberti GC. Dietary composition and renal function in healthy subjects. Nephron. 1987;46(1):37–42. doi: 10.1159/000184293. [DOI] [PubMed] [Google Scholar]
- 49.Kontessis PA, Bossinakou I, Sarika L, et al. Renal, metabolic, and hormonal responses to proteins of different origin in normotensive, nonproteinuric type I diabetic patients. Diabetes Care. 1995;18(9):1233. doi: 10.2337/diacare.18.9.1233. [DOI] [PubMed] [Google Scholar]
- 50.Azadbakht L, Esmaillzadeh A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: A crossover, randomized clinical trial. J Ren Nutr. 2009;19(6):479–486. doi: 10.1053/j.jrn.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Greene T, Beck GJ, et al. Dietary protein restriction and the progression of chronic renal disease: What have all of the results of the MDRD study shown? modification of diet in renal disease study group. J Am Soc Nephrol. 1999;10(11):2426–2439. doi: 10.1681/ASN.V10112426. [DOI] [PubMed] [Google Scholar]
- 52.Kasiske BL, Lakatua JD, Ma JZ, Louis TA. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am J Kidney Dis. 1998;31(6):954–961. doi: 10.1053/ajkd.1998.v31.pm9631839. [DOI] [PubMed] [Google Scholar]
- 53.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20(7):1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lundberg S, Qureshi AR, Olivecrona S, Gunnarsson I, Jacobson SH, Larsson TE. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol. 2012;7(5):727–734. doi: 10.2215/CJN.10331011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix gla protein with mortality in coronary artery disease: The heart and soul study. Ann Intern Med. 2010;152(10):640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third national health and nutrition examination survey (NHANES III) Am J Kidney Dis. 2009;53(3):399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Iorio BR, Bellizzi V, Bellasi A, et al. Phosphate attenuates the anti-proteinuric effect of very low-protein diet in CKD patients. Nephrol Dial Transplant. 2013;28(3):632–640. doi: 10.1093/ndt/gfs477. [DOI] [PubMed] [Google Scholar]