Abstract
Background
Impaired exercise capacity is common in adults with congenital heart disease (ACHD). This impairment is progressive and is associated with increased morbidity and mortality. We studied the influence of the frequency of at least moderately strenuous physical activity (PhysAct) on changes in exercise capacity of ACHD patients over time.
Methods
We studied ACHD patients ≥21 years old who had repeated maximal (RER≥1.09) cardiopulmonary exercise tests within 6 to 24 months. On the basis of data extracted from each patient’s clinical records, PhysAct frequency was classified as (1) Low: minimal PhysAct, (2) Occasional: moderate PhysAct <2 times/week, or (3) Frequent: moderate PhysAct ≥2 times/week.
Results
PhysAct frequency could be classified for 146 patients. Those who participated in frequent exercise tended to have improved pVO2 (ΔpVO2=+1.63±2.67 ml/kg/min) compared to those who had low or occasional activity frequency (ΔpVO2=+0.06±2.13 ml/kg/min, p=0.003) over a median follow-up of 13.2 months. This difference was independent of baseline clinical characteristics, time between tests, medication changes, or weight change. Those who engaged in frequent PhysAct were more likely to have an increase of pVO2 of ≥1SD between tests as compared with sedentary patients (multivariable OR=7.4, 95%CI 1.5-35.7). Aerobic exercise capacity also increased for patients who increased activity frequency from baseline to follow-up; 27.3% of those who increased their frequency of moderately strenuous physical activity had a clinically significant (at least +1SD) increase in pVO2 compared to only 11% of those who maintained or decreased activity frequency.
Conclusions
ACHD patients who engage in frequent physical activity tend to have improved exercise capacity over time.
Keywords: congenital heart disease, adults, exercise capacity and physical activity
INTRODUCTION
As a consequence of improvements in medical and surgical care, adults with congenital heart disease (ACHD) now outnumber children with congenital heart disease in developed nations.[1] Survival is not, however, synonymous with optimal functional capacity and quality of life.[2] The importance of these less easily quantifiable outcomes is becoming increasingly appreciated.[3, 4]
ACHD patients have lower peak oxygen consumption (pVO2), a measure of exercise capacity, than seen in the general population. Low pVO2 in ACHD has strong independent prognostic value; it is associated with increased risk of morbidity and mortality and with worse quality of life.[5, 6] An accelerated age-related decline has also been described. Several factors may account for these observations. Residual hemodynamic and electrophysiological defects are often present following surgical “repair.” Congenital heart disease (and its treatments) may also cause, or be associated with, impairment of other organs including the pulmonary and systemic vascular beds, lung and airways, central nervous system, and neuroendocrine system,[7, 8, 9] that can affect exercise capacity.
The adverse physiological consequences of residual hemodynamic inefficiency and other medical problems are compounded by deconditioning related to a sedentary lifestyle.[10] The majority of ACHD patients do not engage in physical activity (PhysAct) as frequently as recommended by published guidelines for the general population.[11, 12]
While PhysAct frequency does seem to be associated with higher aerobic capacity in ACHD at a single point in time,[13] it is unclear whether increasing frequency of PhysAct results in maintenance of aerobic capacity over time.
To better characterize the impact of frequent PhysAct upon exercise capacity in ACHD patients, we undertook a retrospective study of the relationship between the frequency of at least moderately strenuous PhysAct and changes in objective measures of exercise capacity.
METHODS
Subjects
We identified ACHD patients≥21 years old that underwent cycle ergometry exercise testing at Boston Children’s Hospital between January 2006 and July 2011. Patients with 2 maximal (RER≥1.09) tests during the period of study, separated by 6-24 months (median=13.2mos, IQR 10.8-16.8), were included. Exclusion criteria included pregnancy at or between tests, intervening cardiac percutaneous or surgical interventions with the potential to impact exercise capacity, or acute illness at the time of exercise testing. The first 2 exercise tests fulfilling these requirements were used for this analysis.
Cardiopulmonary Testing
Cardiopulmonary exercise testing (CPX) involved symptom-limited cycle-ergometry using a standard ramp protocol with electrocardiographic monitoring and breath-by-breath expiratory gas analysis (CardiO2 exercise testing system, Medical Graphics, Minneapolis, Minnesota). Calculations and predicted values were obtained as described by Wasserman et al.[14]
Physical Activity Frequency Assessment
PhysAct frequency was assessed from patients’ clinical notes based upon the following guidelines (with representative clinical descriptions):
Low: minimal PhysAct, e.g., “He has a recumbent bike at home but has not been using this.”
Occasional: light activity, or moderately strenuous activity (activity that makes the patient sweat or breathe hard) <2 times weekly and/or <40 minutes per session, e.g., “He has been exercising regularly, spending 10-20 minutes on the treadmill about 3 times a week.”
Frequent: at least moderately strenuous activity ≥ 2 times weekly for ≥40 minutes, i.e. “He is now going to the gym two hours a day Monday, Wednesday, and Friday.”
Undetermined: inadequate documentation to assign a level.
Changes in PhysAct frequency as defined above (low, occasional, frequent), between the clinic note at the time of the first CPX and at the time of the second CPX, were assessed. Changes were classified as: stable, increased or decreased.
Two investigators (AUT, JR) independently classified PhysAct frequency and change in PhysAct frequency for 50 subjects to assess inter-observer variability. The weighted kappa for PhysAct frequency was 0.79; the value for change in PhysAct between visits was lower (0.66), but still acceptable.
The baseline median percent predicted pVO2 (pVO2-%pred) for univentricular (56.5%) and biventricular (66.7%) physiology was determined, and patients with pVO2-%pred above this value were classified as having “above average” exercise capacity for their underlying physiology; those with pVO2-%pred below this value were classified as having “below average” exercise capacity. The proportion of patients with values above/below the median pVO2-%pred baseline value at the second CPX was assessed.
Statistical Analysis
Change in pVO2 over time, including the change in absolute pVO2 (pVO2-abs expressed in l/min), weight normalized pVO2 (pVO2-perkg, expressed in ml/min/kg) and percent predicted pVO2 (pVO2-%pred), was the primary outcome of interest. Secondary outcomes of interest were changes in the peak heart rate (pHR), peak O2pulse, and VE/VCO2 slope.
Continuous variables are presented as mean±SD and categorical variables as counts and percentages. Because characteristics of the low and occasional PhysAct frequency groups were similar, these were combined into a single comparison group for most analyses. The relationships between PhysAct frequency categories and individual CPX variables were analyzed using ANOVA or the Kruskal-Wallis test. Paired t tests were used to compare the initial and final values for each normally distributed CPX variable (Wilcoxon rank sum test for non-normal distributions). Given varying time between studies for each patient, we also analyzed relationships between predictors of interest and change in exercise test variables per year (e.g. Δ pVO2-perkg/year). We performed linear regression analysis, with change in an exercise parameter of interest (e.g. Δ pVO2-perkg or Δ pVO2-perkg/year) as the dependent variable, to assess the association between the level of PA during the interval between the visits, or the change in level of PhysAct reported at the two visits, and the dependent variables under analysis. Multivariable models were used to assess whether the observed associations were independent of potential confounders including age, sex, CHD diagnosis, time between tests, baseline pVO2-perkg(or other CPX variable), baseline height and weight (or BMI), weight change between studies, tobacco use, presence of a pacemaker, presence of systemic ventricular dysfunction by echocardiography (none, mild, moderate/severe), baseline heart rate, and use of specific cardiac medications (digoxin, beta-blocker, ACEI/ARB, diuretics). We also assessed the proportion of patients in each PhysAct group who changed from below to above median baseline pVO2-%pred categories (or vice versa) during the interval between exercise tests, and those who had an increase or decrease of pVO2-%pred by >1SD around the mean difference.
RESULTS
Patient characteristics
PhysAct was classifiable from clinical records at the time of the second test for 72.4%(n=147) of 203 patients who met study criteria. After examining distributions for percent change in pVO2-abs we excluded 1 outlier whose pVO2-abs increased 70.4% between the exercise tests (among the remaining subjects, ΔpVO2-abs ranged from−24.2% to +35.5%). Of the 146 subjects included, 145 had PhysAct classifiable at the time of the first test. Demographic, clinical and CPX data were collected on all patients. There were no important differences between those excluded (n=57) and those included in the analysis (e.g. age 32.8 vs. 33.5y, p=0.65; time between studies 13.3 vs. 13.6mos, p=0.63; baseline pVO2-abs 1.48 vs. 1.61 l/min, p=0.15; Δ pVO2-%pred +2.4 vs. +3.0%, p=0.76).
Baseline data
Of the 145 patients classifiable at the time of the first test, 42%(n=61) had low PhysAct, 32.4%(n=47) participated in occasional PhysAct and 25.5%(n=37) engaged in frequent PhysAct. BMI and systolic BP tended to be higher in the lowest PhysAct group while pVO2-perkg and O2 pulse were significantly higher (p<0.01) in patients who exercised more frequently (Table 1). Data for the 146 patients with classifiable data at follow-up were similar.
Table 1.
Baseline demographic and clinical description based on physical activity frequency classification at the 1st CPX
| Physical Activity Frequency | |||||
|---|---|---|---|---|---|
| Overall | Low | Occasional | Frequent | P | |
| N | 145 | 61 | 47 | 37 | |
| Age(y) | 33.5±10.2 | 34.1±11.4 | 35.2±9.9 | 30.3±7.8 | 0.09 |
| Male(%) | 49.6 | 50.8 | 55.3 | 40.5 | 0.39 |
| Height(cm) | 167.8±9.9 | 166.9±.9.9 | 167.6±7.7 | 169.5±12.2 | 0.53 |
| Weight(kg) | 73.9±17.8 | 77.1±20.1 | 72.4±13.5 | 70.7±18.1 | 0.21 |
| BMI, baseline(kg/m2) | 26.1±4.9 | 27.5±5.8 | 26±4.0 | 24±4.0 | 0.01 |
| Peak VO2-perkg, baseline(ml/min/kg) | 21.9±6.7 | 19.0±5.0 | 21.4±6.1 | 27.4±6.7 | <0.01 |
| O2 pulse, baseline(mL/beat) | 10.6±3.6 | 9.7±3.0 | 10.4±3.6 | 12.0±4.0 | <0.01 |
| Diabetes mellitus(%) | 1.5 | 3.3 | 0 | 0 | 0.27 |
| Tobacco(%) | 9.9 | 15.8 | 6.7 | 3.3 | 0.15 |
| Systolic BP(mmHg) | 122±15 | 125±16 | 119±14 | 119±13 | 0.04 |
| Diagnosis(%) | 0.49 | ||||
| Tetralogy of Fallot | 35.8 | 34.4 | 29.8 | 46.0 | |
| Fontan | 10.3 | 14.8 | 6.4 | 8.1 | |
| Systemic right ventricle | 22.8 | 16.4 | 29.8 | 24.3 | |
| Other | 31.0 | 34.4 | 34.0 | 21.6 | |
| Ventricular dysfunction(%) | 0.42 | ||||
| Moderate/severe | 13.1 | 18.0 | 12.8 | 5.4 | |
| Mild | 23.5 | 18.0 | 25.5 | 29.7 | |
| Normal | 61.4 | 60.6 | 61.7 | 62.1 | |
| Unknown | 2.1 | 3.2 | 0 | 2.7 | |
| Arrhythmia with exercise(%) | 0.62 | ||||
| None | 92.4 | 90.2 | 93.6 | 94.6 | |
| Atrial fibrillation/flutter/SVT | 2.1 | 3.3 | 2.1 | 0 | |
| Ventricular tachycardia | 0.7 | 0 | 2.1 | 0 | |
| Frequent APBs or VPBs | 4.8 | 6.6 | 2.1 | 5.4 | |
| Pacemaker(%) | 19.3 | 19.7 | 23.4 | 13.5 | 0.51 |
| Medication(%) | |||||
| ACEI | 33.8 | 37.7 | 34.0 | 27.0 | 0.58 |
| Beta blocker | 33.8 | 36.0 | 46.8 | 13.5 | <0.01 |
| Digoxin | 10.3 | 8.2 | 8.5 | 16.2 | 0.44 |
| Diuretic | 17.2 | 23.0 | 17.0 | 8.11 | 0.17 |
Descriptive statistics(mean±SD or %) based on physical activity frequency classification at the time of the first CPX, using ANOVA or Kruskal-Wallis and Fisher’s exact or Chi-squared tests for continuous and categorical variables respectively.
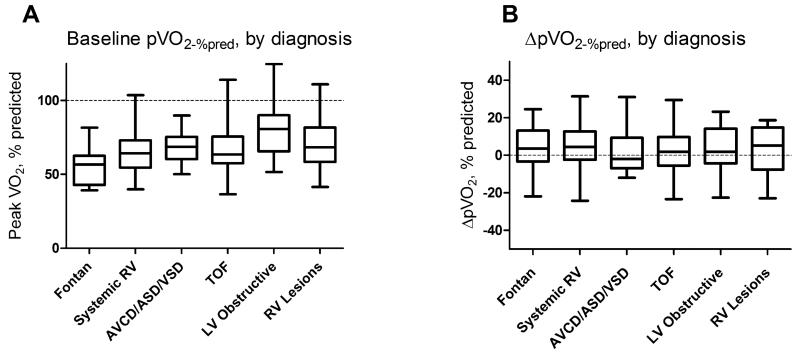
There were significant differences in baseline pVO2-%pred between CHD diagnoses (Figure 1a). Median pVO2-%pred was well below normal for all diagnoses, and more than 3/4ths of patients in all diagnostic groups fell below the predicted value. On the other hand, there was no difference between diagnostic groups with regard to ΔpVO2-%pred over the study (Figure 1b).
Figure 1. Baseline % predicted peak VO2(A) and change in % predicted peak VO2 between baseline and follow-up cardiopulmonary exercise tests(B) by congenital heart disease diagnostic category.
While baseline pVO2-%pred differed by diagnosis (Kruskal-Wallis, p=0.006), there was no such difference in ΔpVO2-%pred between diagnoses (Kruskal-Wallis, p=0.96). RV=right ventricular lesion, LV=left ventricular lesion, AVCD=atrioventricular canal defect, ASD/VSD=atrial/ventricular septal defect, TOF=tetralogy of Fallot
Predictors of change in peak VO2
The strongest predictor of % change in ΔpVO2-abs was BMI (Table 2). Patients with higher baseline BMI tended to have lower pVO2-abs at the second test (for +5 kg/m2 BMI, pVO2-abs on repeat testing was 3.0% lower, p=0.001, r2=0.07). Height was not associated with ΔpVO2-abs(p=0.26) while baseline weight was (for +10kg, pVO2-abs decreased by 1.6% between tests, p=0.002, r2=0.06). Those with higher baseline pVO2-abs tended to have slightly lower follow-up VO2-abs. For every 10% lower baseline pVO2-abs, pVO2-abs increased by 1.4%(p=0.01, r2=0.04). No other baseline clinical or demographic variable was associated with ΔpVO2-abs(Table 2). There was no relationship between chronic cardiac medication use of any class or initiation of a new cardiac medication during the study interval and ΔpVO2abs (beta-blockers n=10 p=0.27, ACEi/ARB n=5 p=0.21, digoxin n=2 p=0.82, diuretics n=7 p=0.22) were associated with ΔpVO2-abs. Patients who started a beta-blocker tended to have lower peak heart rate (Δ=−13.5bpm, p<0.001) on follow-up study but higher O2 pulse (Δ=+14ml/beat, p<0.001). None of the other medications were associated with changes in either peak heart rate or O2 pulse.
Table 2.
Predictors of % change in absolute peak VO2
| r2 | β | P | |
|---|---|---|---|
| Age(/y) | 0.012 | −0.12 | 0.19 |
| Sex(male) | <0.001 | 0.51 | 0.79 |
| BMI(/kg/m2) | 0.07 | −0.62 | 0.001 |
| Height(/cm) | 0.009 | −0.11 | 0.26 |
| Weight(/kg) | 0.063 | −0.16 | 0.002 |
| Tobacco | <0.001 | −0.35 | 0.92 |
| Diabetes mellitus | <0.001 | −0.76 | 0.93 |
| Pacemaker | <0.001 | −0.21 | 0.93 |
| Severe systemic ventricular dysfunction |
0.017 | 4.46 | 0.12 |
| Baseline pVO2- perkg(mL/kg/min) |
0.045 | −4.41 | 0.01 |
| MAP(mmHg) | 0.002 | 0.05 | 0.6 |
Univariate linear regression of various predictors of % change in absolute peak VO2.
Predictors of other measures of ΔpVO2 (i.e. Δ pVO2-%pred, Δ pVO2-perkg) were equivalent with similar magnitudes of association to that seen with ΔpVO2-abs.
Effect of season on exercise testing results
There was no significant difference in pVO2-%pred achieved on baseline exercise test by month (p=0.34) or season (October-March versus April-September, p=0.74) of testing. Season of baseline versus follow-up testing did not affect change in pVO2-%pred. Those who were re-tested in the same season as the baseline test (n=82, Δ pVO2-%pred =+1.8±1.1), those who had baseline testing in the winter and repeat in the summer (n=35, Δ pVO2-%pred =+2.0±1.8) and those who had the converse (n=29, Δ pVO2-%pred =+2.2±1.6) had the same mean change in pVO2-%pred (p=0.98).
Impact of physical activity on exercise parameters
No significant demographic and clinical differences were found between groups patients categorized according to their PhysAct levels reported at the time of the second CPX test (i.e., the activity level that they sustained during the interval between the exercise tests) except for BMI (p=0.02), peak VO2 (p<0.01) that were better in those who were more active and tobacco users that were more frequent in the moderate physical activity group (p<0.01). Fewer patients were classified as having low PhysAct at the follow-up visit, as compared with the baseline visit (n=42 vs. 61).
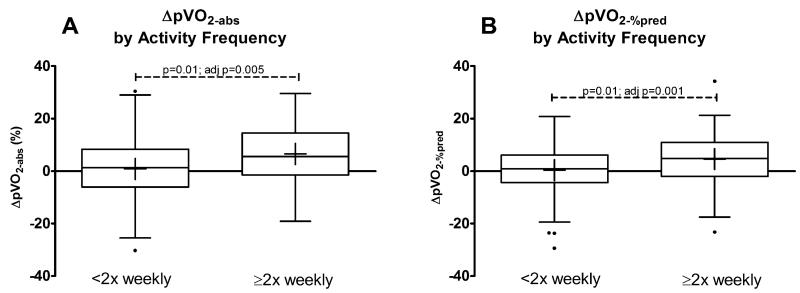
CPX data from the baseline test and change between tests, classified by PhysAct level sustained between tests, are presented in Table 3. Frequent PhysAct was associated with an improvement in both pVO2-perkg and pVO2%-pred(i.e., ΔpVO2-perkg and ΔpVO2%-pred were positive, p=0.003 and 0.04 respectively). There was a trend towards improved pVO2-abs(p=0.07), and a significant improvement in pVO2-abs when expressed on a per year basis(p=0.04). These relationships are shown graphically in Figure 2, comparing the lower 2 PhysAct frequency levels to those who engaged in frequent PhysAct.
Table 3.
Exercise data by physical activity frequency at the 2nd CPX
| Physical Activity Frequency | |||||
|---|---|---|---|---|---|
| Overall | Low | Occasional | Frequent | P | |
| N | 146 | 42 | 49 | 55 | |
|
Time between
tests(mos.) |
13.6±4.7 | 14.3±4.5 | 13.7±5.2 | 13.0±4.5 | 0.58 |
| BMI, baseline(kg/m2) | 26.1±4.9 | 27.7±5.6 | 26.1±4.8 | 24.9±4.0 | 0.03 |
| Weight(kg) | |||||
| baseline | 74.0±17.7 | 78.0±19.9 | 74.1±16.3 | 70.8±16.9 | 0.14 |
| Δ | −0.2±3.7 | 0.5±3.0 | 0.2±3.9 | −1.2±3.7 | 0.008 |
| Rest MAP(mmHg) | |||||
| baseline | 91.0±9.4 | 92.9±11.3 | 90.3±9.0 | 90.2±7.9 | 0.43 |
| Δ | 0.0±9.0 | −0.6±9.7 | 2.1±8.9 | −1.3±8.5 | 0.19 |
| Rest HR(bpm) | |||||
| baseline | 77.3±13.9 | 81.6±12.9 | 76.8±13.5 | 74.6±14.4 | 0.05 |
| Δ | −0.6±12.0 | 0.3±13.2 | −2.0±9.3 | 0.1±13.1 | 0.81 |
| Peak HR(bpm) | |||||
| baseline | 153.2±24.7 | 153.6±29.8 | 152.2±24.0 | 153.8±21.3 | 0.95 |
| Δ | 0.7±12.4 | −0.8±13.5 | −0.5±11.9 | 3.0±11.8 | 0.34 |
| Peak Work(W) | |||||
| baseline | 148.6±56.6 | 134.9±46.9 | 148.6±60.3 | 159.0±58.6 | 0.14 |
| Δ | 3.3±16.5 | −0.7±18.0 | 3.0±16.9 | 6.7±14.3 | 0.21 |
| pVO2-perkg(ml/kg/min) | |||||
| baseline | 22.0±6.7 | 19.3±5.7 | 22.0±6.1 | 24.0±7.3 | 0.005 |
| Δ | 0.6±2.7 | 0.07±2.1 | 0.0±3.0 | 1.6±2.7 | 0.004 |
| Δ(ml/min/kg/year) | 0.7±2.9 | 0.06±2.0 | −0.07±2.77 | 1.8±3.2 | 0.002 |
| pVO2-%pred (%) | |||||
| baseline | 67.3±16.7 | 61.2±13.1 | 67.3±12.8 | 71.9±20.6 | 0.02 |
| Δ | 1.9±9.9 | 0.5±8.6 | 0.2±9.7 | 4.6±10.5 | 0.04 |
| Δ(%pred/year) | 1.9±9.9 | 0.3±8.0 | −0.5±9.0 | 5.2±11.2 | 0.01 |
| pVO2-abs(L/min) | |||||
| baseline | 1.6±0.6 | 1.49±0.53 | 1.64±0.62 | 1.68±0.60 | 0.29 |
| Δ | 0.03±0.18 | 0.01±0.17 | 0.00±0.19 | 0.08±0.19 | 0.07 |
| Δ(%/year) | 2.3±14.4 | 1.6±10.1 | 0.2±12.2 | 6.6±13.3 | 0.04 |
| O2pulse(ml/beat) | |||||
| baseline | 10.6±3.6 | 9.7±2.9 | 10.9±4.0 | 10.9±3.6 | 0.19 |
| Δ | 0.1±1.3 | 0.1±1.4 | 0.0±1.4 | 0.2±1.2 | 0.62 |
| Δ(%/year) | 2.3±14.4 | 2.0±13.6 | 0.8±12.8 | 3.8±16.3 | 0.56 |
| VE/VCO2 Slope | |||||
| baseline | 28.2±4.6 | 29.5±4.4 | 27.9±4.2 | 27.5±5.0 | 0.02 |
| Δ | −0.01±4.16 | −0.1±5.4 | −0.4±3.0 | 0.3±4.0 | 0.37 |
CPX data at baseline and change in CPX data between both tests, based on PA classification at follow-up
Figure 2. Change in peak VO2 by frequency of moderate or strenuous physical activity, <2 vs. ≥2 times per week.
Panel A shows change in pVO2-abs while panel B shows change in pVO2-%pred between exercise tests. P values represent linear regression adjusting for baseline pVO2-%pred.
The association between exercise frequency and ΔpVO2-%pred was maintained despite multivariable adjustment for age, sex, and baseline pVO2-%pred(p=0.02) and additional factors including BMI, time between tests, weight change, diabetes, tobacco use, and presence of a pacemaker(p=0.03). The relationship between PhysAct frequency and ΔpVO2-perkg likewise persisted after equivalent adjustment for demographic and clinical data (p=0.002 and 0.02 respectively).
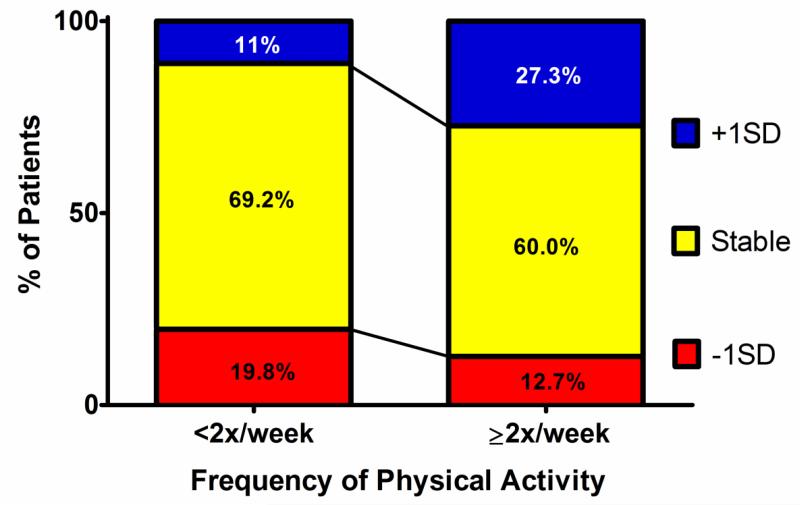
In order to better understand whether inter-group differences in ΔpVO2-abs were clinically significant, we assessed the proportion of patients who had either an increase or decrease in pVO2-abs of at least 1SD (mean change +3±11%, with +1SD defined as increase of 14% and −1SD as −8%; Figure 3). Patients with little or no PhysAct during the time interval between tests were more likely to sustain a decrease of at least 1SD in pVO2 (19.8%) than an increase (11.0%). Conversely, those who participated in frequent PhysAct during that time interval were more likely to improve pVO2-abs by at least 1 SD(27.3%), while in only 12.7% of those engaging in frequent PhysAct did pVO2-abs drop by >1SD(p=0.03). This finding was independent of age, BMI and baseline VO2-abs(multivariable logistic regression OR=7.4, 95%CI 1.5-35.7, for +1SD in pVO2-abs for frequent PhysAct).
Figure 3. Proportion of patients having >1SD(±11%) change in peak VO2-abs between exercise tests.
Most patients had stable pVO2, independent of PA frequency. However, approximately twice as many patients in the lower exercise frequency group had a >1SD decrease in pVO2 compared with a >1SD increase. Conversely, among those engaging in frequent PA, more than twice as many improved their pVO2 >1SD compared to the number that had equivalently decreased pVO2.
Change in physical activity frequency and pVO2
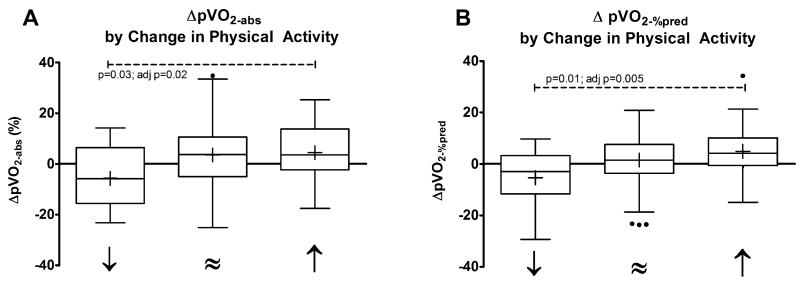
Most patients (61.4%) maintained the same level of PhysAct over the study period; 29.7% increased and 9.0% decreased PhysAct frequency. A statistically significant relationship existed between the Δ PhysAct between tests, the concomitant ΔpVO2-abs (l/min), and ΔpVO2-%pred(Figure 4). Decreasing PhysAct frequency was associated with a decrease in pVO2-perkg(−1.9±3.3ml/min/kg), while stable PhysAct frequency was associated with stable pVO2-perkg(+0.5±2.7 ml/min/kg) and increased PhysAct frequency was associated with improved pVO2-perkg(+1.6±2.1 ml/min/kg).
Figure 4. Change in peak VO2 by change in PA frequency.
(decreased=”↓”, stable=”≈”, increase=”↑”) between the baseline and follow-up CPX. Panel A shows %change pVO2-abs. Panel B shows ΔpVO2-%pred. P values represent linear regression adjusting for baseline pVO2-%pred.
DISCUSSION
We found frequent PhysAct of at least moderate intensity to be associated with maintenance of superior exercise capacity in ACHD patients. Patients who sustained a high level of PhysAct were more likely to maintain or improve their pVO2 and to lose weight during the interval between their exercise tests. In contrast, patients who participated in less frequent PhysAct were more likely to gain weight and their pVO2 was more likely to decline. Furthermore, not only did frequent baseline PhysAct correlate with a more favorable change in pVO2 over time, the subset of patients who increased physical activity frequency between tests appeared to derive benefit while those who became more sedentary saw a decline in pVO2. The change in peak VO2 was not attributable to weight loss per se, as adjustment for weight change in multiple regression models did not affect the relationship between PA and change in peak VO2.
Our data support the concept that the aerobic capacity of ACHD patients is not limited solely by fixed cardiac factors. They also imply that the time-related decline in aerobic capacity, frequently reported in past longitudinal studies of ACHD patients, is modifiable. For many ACHD patients, regular PhysAct appears to have the potential to attenuate or reverse this debilitating decline.
Regular aerobic activity has pleiotropic effects, a number of which could account for these results. These include peripheral factors(e.g., increased numbers of mitochondria and up-regulation of aerobic enzyme pathways within skeletal muscle cells, increased muscle size and capillary density[15], enhanced muscle pump function[16] and beneficial changes in peripheral vascular beds) which may enhance oxygen extraction and/or cardiac output during exercise[17], and central factors(e.g. increase in myocardial mass and improvements in myocardial systolic and diastolic function)[18] which also may enhance myocardial reserve and improve enhanced cardiac output during exercise.
Inadequate PhysAct is common among adults with CHD and >70% report moderate or extreme concern about participating in PhysAct.[19] This apprehension often stems from inappropriate advice or overprotection during childhood and adulthood. Historically, health-care providers restricted PhysAct in patients with CHD,[20] or did not provide specific exercise guidelines. A recent study of patients with aortic stenosis suggested, however, that exercise restriction does not prevent adverse outcomes, and the detrimental effects of exercise restriction on cardiovascular risk factors, exercise capacity and psychological well-being are often overlooked.[21]
Our observations are consistent with experience in other populations. In the general population, regular PhysAct is associated with lower mortality, improved quality of life and reduction in the incidence of primary [22, 23] and secondary [24] cardiovascular events. Interventions to increase PhysAct appears to provide benefit in a number of medical conditions including chronic heart failure,[25] obesity,[26] and diabetes [27].
The observed impact of frequent PhysAct on exercise capacity exceeds that reported for some ACHD surgical and interventional catheterization procedures.[28, 29] The few studies of cardiac rehabilitation in ACHD have reported promising results vis a vis exercise capacity and quality of life.[30, 31],[32] Cardiac rehabilitation programs have also been extensively studied in patients with acquired heart disease, and have been shown to increase exercise capacity, reduce morbidity, mortality, and medical costs.[33] The number of patients with ACHD is increasing, and the healthcare costs of these patients are increasing even more quickly.[34, 35] The impact of inexpensive lifestyle interventions such as providing an exercise prescription or referral to cardiac rehabilitation could improve the “natural history” and quality of life for many adults with CHD while at the same time lowering associated healthcare costs.
The finding that BMI and weight are significant clinical predictors of change in pVO2 suggests that, in conjunction with specific exercise training, educational, nutritional and behavioral interventions may have independent benefit.
Our data support the need for prospective clinical trials to assess the impact of various strategies to increase PhysAct and improve aerobic capacity in this population. Most critically, it remains unclear whether improving pVO2-%pred bestows a benefit in terms of quality of life, cardiovascular events, and mortality.
Limitations
Causality (i.e., frequent PhysAct causes ↑pVO2 over time) cannot be inferred from these data. The fact that a change in lifestyle in terms of PhysAct frequency was associated with a corresponding change in pVO2, provides some support for a causal relationship. It is also important to note that these data do not provide insights into the mechanisms by which frequent PhysAct results in ↑pVO2. PhysAct frequency was extracted from clinical notes and assessment is therefore somewhat imprecise. It is possible that some patients might provide inaccurate descriptions of PhysAct frequency. Any misclassification, however, would generally tend to bias our results to the null (e.g., if sedentary patients reported frequent PhysAct, this would tend to minimize the apparent effect of frequent PhysAct). The observed baseline variation in pVO2 and correlations between weight change and both PhysAct level and ΔpVO2 supports the accuracy of PhysAct classification.
Patient characteristics differed between PhysAct groups. There were differences in baseline resting heart rate, pVO2, and VE/VCO2 slope. These characteristics tended to be “better” in the frequent PhysAct group. One might expect a more prominent decline in patients with higher baseline pVO2 as a result of regression to the mean. Our data demonstrated this phenomenon; for each increase of 1mL/kg/min in baseline pVO2-perkg there was a 0.056 mL/kg/min decline in pVO2-rel between tests(p=0.07). The fact that we observed a strong relationship between PhysAct and ΔpVO2, despite this supports the validity of our findings.
CONCLUSION
Adults with CHD who engage in frequent PhysAct maintain higher pVO2 over time compared with sedentary patients. Given the strong prognostic significance of pVO2 in this population, strategies to increase the frequency of PhysAct in the ACHD population may have the potential to positively impact morbidity and mortality. Further studies are needed to determine the effect of specific interventions to modify PhysAct on pVO2 and clinical outcomes in adults with congenital heart disease.
Acknowledgements
We are grateful to Tracy J. Curran, Julie Ann ÓNeil, Jennifer Smith and Kathleen Solly for their technical expertise as well as to Lilamarie Moko for her assistance in drafting and revising the manuscript.
This work was supported by Fundación Alfonso Martin Escudero(AUT), National Institutes of Health 5-T32-HL07604-25(ARO) and the Dunlevie Fund(ARO/MJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
REFERENCES
- 1.Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–72. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 2.Muller J, Christov F, Schreiber C, et al. Exercise capacity, quality of life, and daily activity in the long-term follow-up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J. 2009;30:2915–20. doi: 10.1093/eurheartj/ehp305. [DOI] [PubMed] [Google Scholar]
- 3.Buys R, Cornelissen V, Van De Bruaene A, et al. Measures of exercise capacity in adults with congenital heart disease. Int J Cardiol. 2011;153:26–30. doi: 10.1016/j.ijcard.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Hager A, Hess J. Comparison of health related quality of life with cardiopulmonary exercise testing in adolescents and adults with congenital heart disease. Heart. 2005;91:517–20. doi: 10.1136/hrt.2003.032722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes SM, Alexander ME, Graham DA, et al. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis. 2011;6:294–303. doi: 10.1111/j.1747-0803.2011.00500.x. [DOI] [PubMed] [Google Scholar]
- 6.Kempny A, Dimopoulos K, Uebing A, et al. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life--single centre experience and review of published data. Eur Heart J. 2012;33:1386–96. doi: 10.1093/eurheartj/ehr461. [DOI] [PubMed] [Google Scholar]
- 7.Bird GL, Jeffries HE, Licht DJ, et al. Neurological complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young. 2008;18(Suppl 2):234–9. doi: 10.1017/S1047951108002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imms C. Occupational performance challenges for children with congenital heart disease: a literature review. Can J Occup Ther. 2004;71:161–72. doi: 10.1177/000841740407100306. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DS, Jacobs JP, Chai PJ, et al. Pulmonary complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young. 2008;18(Suppl 2):215–21. doi: 10.1017/S1047951108002941. [DOI] [PubMed] [Google Scholar]
- 10.Giardini A, Hager A, Pace Napoleone C, et al. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg. 2008;85:818–21. doi: 10.1016/j.athoracsur.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Swan L, Hillis WS. Exercise prescription in adults with congenital heart disease: a long way to go. Heart. 2000;83:685–7. doi: 10.1136/heart.83.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Department of Health and Human Services . Promotion OoDPaH, ed. 2009. Physical Activity Guidelines for Americans. [Google Scholar]
- 13.Muller J, Hess J, Hager A. Daily physical activity in adults with congenital heart disease is positively correlated with exercise capacity but not with quality of life. Clin Res Cardiol. 2012;101:55–61. doi: 10.1007/s00392-011-0364-6. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman K, Hansen J, Sue D. Principles of Exercise Testing and Interpretation. Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 15.McCardle WDKF, Katch VL. Exercise Physiology: Energy, nutrition and human performance. Baltimore Williams & Wilkins; 2007. [Google Scholar]
- 16.Rhodes J, Curran TJ, Camil L, et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–45. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 17.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 18.Pelliccia A, Maron MS, Maron BJ. Assessment of left ventricular hypertrophy in a trained athlete: differential diagnosis of physiologic athlete’s heart from pathologic hypertrophy. Prog Cardiovasc Dis. 2012;54:387–96. doi: 10.1016/j.pcad.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Harrison JL, Silversides CK, Oechslin EN, et al. Healthcare Needs of Adults With Congenital Heart Disease: Study of the Patient Perspective. J Cardiovasc Nurs. 2011;26:497–503. doi: 10.1097/JCN.0b013e31820984c9. [DOI] [PubMed] [Google Scholar]
- 20.Longmuir PE, McCrindle BW. Physical activity restrictions for children after the Fontan operation: disagreement between parent, cardiologist, and medical record reports. Am Heart J. 2009;157:853–9. doi: 10.1016/j.ahj.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Brown DW, Dipilato AE, Chong EC, et al. Sudden unexpected death after balloon valvuloplasty for congenital aortic stenosis. JACC. 2010;56:1939–46. doi: 10.1016/j.jacc.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 22.Paffenbarger RS, Jr., Hyde RT, Wing AL, et al. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 23.Blair SN, Kohl HW, 3rd, Barlow CE, et al. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–8. [PubMed] [Google Scholar]
- 24.Oldridge NB, Guyatt GH, Fischer ME, et al. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:945–50. [PubMed] [Google Scholar]
- 25.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144:23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 26.Heitmann BL, Hills AP, Frederiksen P, et al. Obesity, leanness, and mortality: effect modification by physical activity in men and women. Obesity (Silver Spring) 2009;17:136–42. doi: 10.1038/oby.2008.479. [DOI] [PubMed] [Google Scholar]
- 27.Chimen M, Kennedy A, Nirantharakumar K, et al. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia. 2012;55:542–51. doi: 10.1007/s00125-011-2403-2. [DOI] [PubMed] [Google Scholar]
- 28.Giardini A, Donti A, Specchia S, et al. Long-term impact of transcatheter atrial septal defect closure in adults on cardiac function and exercise capacity. Int J Cardiol. 2008;124:179–82. doi: 10.1016/j.ijcard.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Batra AS, McElhinney DB, Wang W, et al. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. Am Heart J. 2012;163:280–7. doi: 10.1016/j.ahj.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Winter MM, van der Bom T, de Vries LC, et al. Exercise training improves exercise capacity in adult patients with a systemic right ventricle: a randomized clinical trial. Eur Heart J. 2012;33:1378–85. doi: 10.1093/eurheartj/ehr396. [DOI] [PubMed] [Google Scholar]
- 31.Therrien J, Fredriksen P, Walker M, et al. A pilot study of exercise training in adult patients with repaired tetralogy of Fallot. Can J Cardiol. 2003;19:685–9. [PubMed] [Google Scholar]
- 32.Dua JS, Cooper AR, Fox KR, et al. Exercise training in adults with congenital heart disease: feasibility and benefits. Int J Cardiol. 2010;138:196–205. doi: 10.1016/j.ijcard.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111:369–76. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 34.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. JACC. 2009;54:460–7. doi: 10.1016/j.jacc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 35.O’Leary J, Siddiqi O, de Ferranti S, et al. The Changing Demographics of Congenital Heart Disease Hospitalizations in the United States, 1998 to 2010. JAMA. 2013;309:984–986. doi: 10.1001/jama.2013.564. [DOI] [PubMed] [Google Scholar]