Abstract
Insulin resistance is a hallmark of obesity, the cardiorenal metabolic syndrome and type 2 diabetes mellitus (T2DM). The progression of insulin resistance increases the risk for cardiovascular disease (CVD). The significance of insulin resistance is underscored by the alarming rise in the prevalence of obesity and its associated comorbidities in the Unites States and worldwide over the last 40-50 years. The incidence of obesity is also on the rise in adolescents. Furthermore, premenopausal women have lower CVD risk compared to men, but this protection is lost in the setting of obesity and insulin resistance. Although systemic and cardiovascular insulin resistance are associated with impaired insulin metabolic signaling and cardiovascular dysfunction, the mechanisms underlying insulin resistance and cardiovascular dysfunction remain poorly understood. Recent studies show that insulin resistance in obesity and diabetes is linked to a metabolic inflammatory response, a state of systemic and tissue specific chronic low grade inflammation. Evidence is also emerging that there is polarization of macrophages and lymphocytes towards a pro-inflammatory phenotype that contribute to progression of insulin resistance in obesity, cardiorenal metabolic syndrome and diabetes. In this review, we provide new insights into factors, such as, the renin-angiotensin-aldosterone system, sympathetic activation and incretin modulators (e.g., DPP-4) and immune responses that mediate this inflammatory state in obesity and other conditions characterized by insulin resistance.
Keywords: Obesity, DPP-4, immunity, uric acid, gender
1. Introduction
The prevalence of obesity and diabetes is increasing by alarming proportions in the United States and worldwide. Two-thirds of American adults are overweight or obese and 40% of overweight/obese individuals are diabetic. The prevalence of obesity has also increased considerably around the globe and more than 20% of the world population is overweight, while nearly 300 million are obese [1-4]. In addition, childhood-adolescent overweight and obesity, as well as obesity in premenopausal women are also emerging as major global public health concerns [5-6]. Driving forces for overweight and obesity include increasing sedentary lifestyles and consumption of a Western Diet (WD) high in fat, fructose and salt and their interaction with genetic factors and epigenetic processes [7-9]. The prevalence of hypertension in type 2 diabetes mellitus (T2DM) is increased 3-fold, and the coexistence of hypertension in diabetic patients greatly enhances the development of cardiovascular disease (CVD) and chronic kidney disease (CKD) [10]. It is estimated that 37% of the adult population has prehypertension and 40% of these people will progress to hypertension within a two year time frame [11]. Moreover, childhood obesity is associated with increased arterial stiffness as determined by pulse wave velocity [12]. Prehypertension is increasingly recognized as a risk factor for CVD. This is supported by studies demonstrating the association of increased diastolic dysfunction in a prehypertension state in genetic or diet-induced rodent models of obesity [13-15].
2. Central role of insulin resistance in the progression of cardiorenal metabolic syndrome
Overweight and obesity are associated with development of the cardiorenal metabolic syndrome which is a constellation of risk factors, such as insulin resistance, dyslipidemia, and high blood pressure that predispose affected individuals to well-characterized medical conditions such as diabetes, CVD and CKD [4, 5, 7]. Insulin resistance is one common underlying mechanism that contributes to the progression of CVD and renal injury in obesity and diabetes. Insulin resistance is also associated with vascular stiffness, which is an independent risk factor for CVD [12,16,17]. Although aging is associated with increased vascular stiffness, obesity and diabetes are associated with accelerated vascular stiffness [16, 17]. Insulin resistance is also associated with a metabolic (obesity) cardiomyopathy characterized by diastolic dysfunction independent of hypertension and hyperglycemia [18, 19]. The association of insulin resistance with cardiac dysfunction may also occur in diabetes independent of coronary heart disease or hypertension (diabetic or metabolic cardiomyopathy) [19, 20]. Insulin resistance is also the underlying pathophysiologic factor contributing to the development of hypertension [10]. Moreover, parental hypertension and insulin resistance may also contribute to elevations in blood pressure and insulin resistance in both male and female offspring [21, 22]. These findings suggest that that progression of insulin resistance has profound effects on cardiovascular dysfunction in obesity and diabetes.
3. Impairment of insulin signaling and CVD
Serine phosphorylation of insulin receptor substrate
Insulin signaling occurs through activation of the phosphatidylinsositol 3 kinase (PI3-K)/protein kinase B (Akt) signaling pathway linked to metabolic insulin signaling and extracellular regulated kinases ½ (ERK1/2) signaling with growth factor-like responses [4]. The major converging point contributing to insulin resistance is the docking protein insulin receptor substrate (IRS). The phosphorylation of serine residues of IRS by several kinases including protein Kinase C, C-Jun kinase (JNK), mammalian target of rapamycin (mTOR) and ribosomal p70 S6 kinase (S6K) is the major mechanism for regulation of IRS function [4, 18-20]. Phosphorylation of serine residues on IRS-1 attenuates IRS-1 tyrosine phosphorylation, association with p85 subunit of PI3-K, and triggers proteasome –dependent degradation. Proteasome degradation of IRS-1 can also occur by suppression of a cytokine signaling 3(SOC3-3) mediated mechanism that is independent of phosphorylation of IRS-1[4].
Impaired insulin metabolic signaling results in impaired glucose uptake, endothelial dysfunction, reduced coronary flow, impaired angiogenesis, cardiac lipotoxicity, and metabolic inflexibility, all of which contribute to cardiac diastolic function. Progression of insulin resistance and endothelial dysfunction also contribute to enhanced vascular stiffness, development of hypertension and atherosclerosis [4,10,16,18].
4. Cardiovascular insulin resistance at the cross roads of metabolism, immune and inflammatory response
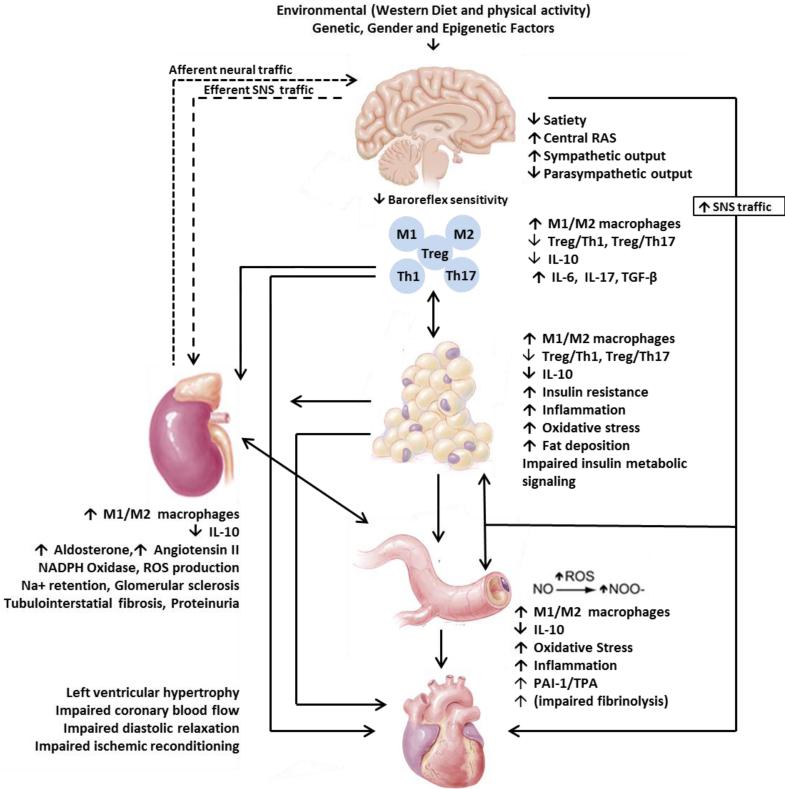
Adipose tissue dysfunction, systemic immune and inflammatory responses and insulin resistance (Fig.1)
Fig.1. Model for maladaptive immune and inflammatory responses leading to cardiovascular insulin resistance.
Environmental factors (sedentary life style, western diet), gender, genetic and epigenetic factors contribute to insulin resistance/ hyperinsulinemia, hyperuricemia, inappropriate activation of RAAS-SNS activation, increased DPP-4 activity and dysfunctional immune function and chronic low grade inflammation. These maladaptive immune and inflammatory responses lead to systemic and cardiovascular insulin resistance.
Although mechanisms and mediators of systemic insulin resistance are not clearly understood, recent studies link over-nutrition to a low grade systemic inflammatory response and this inflammatory response is distinct from an acute inflammatory response [23, 24]. Chronic over-nutrition results in white adipose tissue (WAT) immune and inflammatory responses that contribute significantly to low grade inflammation and this condition has been often referred to as metabolic inflammation or metaflammation [23, 24]. Although mechanisms underlying this inflammatory response are not well understood, endoplasmic reticular stress is one of the cellular stress events that activates inflammatory signaling pathways including activation of JNK, mTOR and S6K [23, 24]. These serine kinases not only mediate adipose tissue dysfunction but also phosphorylate serine residues of IRS-1, thereby mediating insulin resistance in adipose tissue. In addition to increased release of free fatty acids (FFAs), dysregulated adipocyte function results in increased secretion of cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and resistin and decreased secretion of adiponectin [4, 25]. TNF-α and IL-6 cause systemic insulin resistance through activation of mitogen activated protein kinases, protein kinase C (PKC), mTOR/S6K and SOCS-3 mediated proteasome degradation [4]. Resistin induces insulin resistance, whereas adiponectin improves insulin metabolic signaling and endothelial function [4, 26]. In addition to adipose tissue dysfunction, activation of Toll-like receptor 4 (TLR-4) and perhaps other TLRs by excess nutrients such as saturated fatty acid, gut derived lipopolysaccharide (LPS), uric acid and/or intestinal dysbiosis contribute significantly to hepatic and systemic inflammatory response [27,28].
Inappropriate activation of renin angiotensin aldosterone system (RAAS)
Inappropriate activation of RAAS is an important hormonal factor causing cardiovascular and renal injury in obesity and diabetes [29]. In this respect, adipose tissue expresses most of the components of the RAAS, including angiotensinogen, angiotensin II (Ang II) and angiotensin II type 1 receptor (AT1R) and increased expression and secretion of angiotensinogen by adipose tissue is seen in obesity [10,29,30]. A recent study utilizing adipocyte specific angiotensinogen knockout mice showed that increased secretion of angiotensinogen from adipose tissue in high-fat fed mice contributes to the development of hypertension in obesity [30]. High levels of plasma aldosterone have also been demonstrated in obesity [31]. Moreover, lipid soluble factors derived from adipose tissue stimulate adrenal aldosterone secretion [31,32]. Ang II- and aldosterone-mediated insulin resistance occur, in part, through activation of NADPH oxidase, generation of reactive oxygen species (ROS) and activation of redox-sensitive kinases [4, 26]. Ang II and aldosterone also cause insulin resistance indirectly through innate and acquired immune and inflammatory mediated oxidative stress [33, 34]. The impact of the RAAS on innate immunity is supported by the observation that Ang II infusion in rats triggers immune and inflammatory responses by the recruitment of helper T cells to cardiovascular tissues [33]. The effects of Ang II are blunted in Rag1−/− mice, which have absolute deficiency of T and B lymphocytes [33, 34].
Increased sympathetic nervous system (SNS) activation
Accumulating evidence suggests that the sympathetic nervous system (SNS) links central nervous system and immune systems [35,36]. Spleen and lymph nodes are highly innervated by the SNS and T cell activation is modulated by norepinephrine [35]. The role of increased SNS activity in insulin resistance and resistant hypertension is increasingly recognized [4,10]. Although enhanced activation of SNS is another component of insulin resistance, it is often related to activation of RAAS [4,26]. The RAAS system causes sustained sympathetic over-activity by modulating central neurons in the subfornical organ of the forebrain [35, 37]. This is supported by modulation of lymphocyte proliferation and spleen cytokine secretion by central administration of Ang II and suppression of Ang II effects by sympathetic denervation of the spleen [38]. In addition, Ang II also has a presynaptic potentiating effect on sympathetic neurotransmission in humans [39]. Administration of a low dose of Ang II that has a minimal or no effect on blood pressure, markedly increases blood pressure caused by central sympathetic outflow which is induced by specific deletion of superoxide dismutase (SOD)3 from the circumventricular organs (CVO) [40]. These manipulations are associated with modulation of peripheral T cell immune responses [35, 36] suggesting central regulation of systemic immune and inflammatory responses through brain Ang II signaling and resultant increased sympathetic nervous system outflow (Fig 1).
Local immune and inflammatory response and insulin resistance within cardiovascular tissue
Although systemic insulin resistance has been extensively studied in obesity, the importance of insulin resistance in the myocardium and its effect on cardiac dysfunction has also been demonstrated by using isolated perfused-heart preparations, cultured cardiomyocytes and positron emission tomography (PET) in human and animal hearts [4,18,41]. Moreover, insulin resistance develops in the heart of C57BL/6 mice as early as ten days after high-fat feeding, before the onset of insulin resistance in the skeletal muscle and liver which occur after three weeks of high-fat feeding [42]. Although several studies have shown that obesity is associated with systemic inflammatory response and insulin resistance [43], very little is known about the role of an inflammatory response in regulation of insulin resistance in the heart and vasculature in obesity and diabetes. In this respect, high-fat feeding has been shown to increase macrophage infiltration and cytokine production accompanied by impaired glucose utilization in the heart [44]. Elevated levels of nutrients such as plasma amino acids and fatty acids are known to contribute to cardiovascular insulin resistance [4,43]. However, a recent study demonstrated that accumulation of fat within cardiomyocytes can cause infiltration of macrophages leading to cardiac dysfunction in a model of lipotoxic cardiomyopathy [45].
Impaired insulin stimulated uptake of glucose and impaired vasodilatation have been shown to be early manifestations in insulin resistant models of obesity [4,18,19]. In this respect, increased activation of RAAS and enhanced oxidative stress within cardiovascular tissue in obesity is an important mediator of insulin resistance [26]. Ang II infusion and aldosterone induce inflammatory responses and oxidative stress in the heart [26, 46]. We have recently examined the signaling pathways by which enhanced tissue RAS contributes to insulin resistance in cardiovascular tissue [47]. Ang II increases serine phosphorylation of IRS-1 and inhibits insulin-stimulated phosphorylation of endothelial nitric oxide synthase (eNOS) through activation of S6K signaling pathway. An inhibitor of rapamycin (mTOR) attenuates Ang II-stimulated phosphorylation of p70S6K and IRS-1 and blocks the ability of Ang II to impair insulin-stimulated phosphorylation of eNOS and nitric oxide (NO) dependent-arteriole vasodilation. These results suggest that activation of mTOR/p70S6K by Ang II in vascular endothelium may contribute to the impairment of insulin-stimulated vasodilation through phosphorylation of IRS-1 and provide a biochemical basis to the insulin resistance in the development of vascular cell dysfunction [47].
Perivascular adipose tissue immune and inflammatory response and vascular dysfunction
Recent studies demonstrate the role of perivascular adipose tissue dysfunction in cardiovascular inflammation, oxidative stress and insulin resistance [48]. Perivascular tissue and vascular adventitia communicate with each other. Production of vasoactive factors and cytokines by perivascular fat has been shown to modulate vascular function by modulating oxidative stress, vascular relaxation and vascular stiffness [48,49]. In lean mice and people, perivascular fat exerts protective vasoregulatory action, and this protective effect of perivascular fat is lost in the setting of obesity [49]. Significant infiltration of macrophages and T cells has been demonstrated in perivascular adipose tissue in obesity and this was accompanied by endothelial dysfunction [49,50]. Decreased secretion of adiponectin and increased production of cytokines from dysfunctional adipose tissue may significantly contribute to vascular inflammation, insulin resistance, vascular stiffness and impaired relaxation [50,51]. Moreover, increased production of vascular Ang II by perivascular fat causes vascular inflammation and impairment of vascular function either directly or through modulation of endothelin or aldosterone effects [49-51].
Immune and inflammatory mediated renal damage and hypertension
Hypertension and albuminuria in several animal models of renal injury and are associated with dysregulation of innate and adaptive immunity in the kidney [33, 34 52]. Furthermore, immunosuppressive therapies using either adoptive transfer of immune cells or immunosuppressant drugs prevent renal injury and hypertension induced either by Ang II infusion or high salt in Dahl salt sensitive rats [33, 34, 35, 52, 53]. These studies highlight the importance of dysfunctional immunomodulation in the development of hypertension and renal disease in response to a WD (high in fructose and fat) and increased salt intake (Fig 1).
5. Mechanisms of maladaptive immune and inflammatory responses leading to cardiovascular insulin resistance: Immune cell polarization and cytokine imbalance
Accumulating evidence suggests the role of immune and inflammatory responses in the heart and vasculature in obesity and diabetes. Although initial studies were directed towards innate immunity, recent studies demonstrate a pivotal role played by adaptive immunity involving effector and regulatory T cells (Tregs) and associated macrophage polarization to a more inflammatory phenotype (Fig 1). Macrophage infiltration into adipose and cardiac tissue is associated with systemic and cardiac insulin resistance [23], however, recent studies show the role of different subsets of macrophages [25,54]. There are two major phenotypes of macrophage activation in tissues, in response to various immune and inflammatory responses [24,54,55]. Classical M1 activation is stimulated by TLR ligands and interferon gamma (IFN-γ) and characterized by the expression of high levels of pro-inflammatory cytokines, high production of reactive nitrogen and oxygen intermediates, promotion of a Th1 response, and strong microbicidal activity. The population of pro-inflammatory M1 macrophages is significantly increased in adipose tissue from obese mice fed high fat [54,55]. These pro-inflammatory macrophages secrete pro-inflammatory cytokines, such as TNF-α, and cause insulin resistance, thereby linking M1 macrophage polarization and insulin resistance. In contrast to M1 pro-inflammatory macrophage activation, alternative M2 activation is stimulated by IL-4/IL-13 [24,53,55]. M2 macrophages are characterized by expression of YM1, arginase-1 and IL-10. M2 macrophages are considered to be anti-inflammatory and are involved in promotion of normal tissue remodeling and immunoregulatory functions [24, 53-55].
Adaptive immunity and an imbalance of effector T cells and regulatory T cell responses
In addition to macrophages, T cells also accumulate in adipose tissues in obesity with a distinct pattern of inflammatory T cell and macrophage polarization [24, 53,55-57]. T helper Th1 cells (Th) and cytotoxic CD8+ T cells contribute to increased tissue M1 macrophage infiltration and insulin resistance and oxidative stress [54-57]. Tregs are a unique population of T-cells which play a crucial role in the maintenance of self-tolerance and suppression of potentially inflammatory T-cells [57]. The expression of forkhead/winged helix transcription factor 3 (FoxP3) is considered to be an essential factor for the proper development, maintenance, and function of CD+CD25+Tregs [55-57], one type of regulatory T cell. Chronic low-grade inflammation in obesity and impaired insulin sensitivity has been associated with fewer Tregs in adipose tissue, and reversal of insulin sensitivity following restoration has been demonstrated [55-57]. The mechanisms by which Tregs protect against insulin resistance are thought to be mediated, at least in part, by direct cell to cell interactions as well as through the secretion of soluble anti-inflammatory cytokines including Interleukin 10 (IL-10) and transforming growth factor beta (TGF-β) [54-57]. IL-10 has been shown to improve impaired insulin signaling caused by pro-inflammatory cytokines [58]. IL-10 prevents the development of IL-6 or lipid induced insulin resistance when administered in vivo [59]. Moreover, IL-10 inhibits NADPH oxidase and suppresses oxidative stress [60]. Since NADPH oxidase mediated oxidative stress has been shown to cause activation of serine kinases that phosphorylate insulin receptor substrate and blunt insulin metabolic signaling, these results imply that IL-10 plays an important role in modulation of cardiovascular insulin resistance [58-60]. In contrast to Tregs, T helper 17 cells (Th -17 cells) secrete IL-17 and they can be cytotoxic as well as protective [33,52, 61]. Th17 cells are elevated in obese and T2DM patients as well as obese mice [62]. Ang II infusion increases IL-17 levels and a decrease in IL-17 levels by Ang II receptor antagonism ameliorates Ang II induced insulin resistance [61]. In addition, cytotoxic CD8+ cells secreting IL-17 have also been shown to play a role in renal and vascular injury in hypertension [33, 37, 52, 61].
Inflammasome, dysfunctional immunity and insulin resistance in obesity and diabetes
Accumulating evidence suggests that inflammasome activation, through IL-1β activation, may contribute to insulin resistance and T2DM [63]. This response is seen after exposure to pathogens or activation of danger associated signals [63,64]. In obesity, the levels of palmitate and ceramide are elevated and these lipids activate inflammasomes [64]. Insulin sensitivity improves when mice deficient in central inflammasome molecules are fed high-fat diet and this improvement is accompanied by suppression of immune and inflammatory responses [64, 65].
6. Sex differences in modulation of immune function and cardiovascular insulin sensitivity
Enhanced insulin sensitivity in premenopausal women and loss of insulin sensitivity and increased CVD risk induced by obesity and sedentary life style
Although, non-diabetic and non-obese premenopausal women exhibit less incidence of CVD compared to age matched men, non-diabetic and diabetic obese women display increased risk for cardiac dysfunction [66-68]. Population based studies have documented higher ventricular and peripheral arterial stiffness in women, as a potential factor contributing to increased incidence of diastolic dysfunction in obese females even before the appearance of other CVD risk factors [69]. Left ventricular mass correlates positively with glucose intolerance and insulin resistance, especially in obese women [70]. Similar to obesity, a sedentary lifestyle in females predisposes to diastolic dysfunction. We recently reported impaired diastolic dysfunction in female rats with low aerobic capacity and this was associated with increased arterial and ventricular stiffness [13]. The sex-related differences in CVD incidence and severity are, in part, due to action of steroid hormones [71]. In the Framingham study, aldosterone levels were higher in women and higher aldosterone levels were associated with left ventricular wall thickness in females, but not in males [72].
Role of estrogen receptor alpha and GPR30 signaling in immune cells and inflammatory response
Estrogen is normally protective against CVD and estrogen receptor alpha and GPR-30 have been shown to exert an anti-inflammatory effect modulating T cell immune response [73,74]. In addition, a recent study demonstrated the role of estrogen receptor alpha-mediated signaling in macrophages contributing to enhanced insulin sensitivity [75]. The role of immune and inflammatory cell responses in mediating the cardioprotective effects of estrogens has been suggested by studies on viral-induced myocarditis. In coxsackievirus B3 (CVB3) induced myocarditis, immune activation of macrophages and T cells were more marked in male BALB/c mice compared to female mice [76]. This was accompanied by inhibition of Treg response. The immune mechanisms underlying abrogation of protective responses elicited by estrogen on insulin sensitivity or CVD risk in obese and diabetic females remains to be determined.
7. Targeting maladaptive immune and inflammatory responses leading to insulin resistance
In addition to diet and exercise, current therapeutic strategies for the prevention and management of progression of insulin resistance and hypertension and progression to cardiovascular and renal disease are not very effective. Therefore, therapeutic interventions targeting maladaptive immune and inflammatory responses leading to insulin resistance are intriguing. In this regard, drugs targeting immune inflammatory responses, such as recombinant human IL-1 receptor antagonists or non-acetylated salicylates such as sodium salicylate demonstrate improvement in insulin sensitivity [24, 77]. Drugs showing pleotropic effects modulating immune and inflammatory responses in addition to their classic effects, such as Ang II receptor blockers (ARBs), dipetidyl peptidase 4 (DPP-4) inhibitors and xanthine oxidase inhibitors may also prove effective [78-80]. Furthermore, targeting immune and inflammatory responses through adoptive transfer of immune cells and stem cells [81-82] or examining the immune modulatory effects of renal denervation [10] open up new avenues for immune mediated therapy for cardiovascular insulin resistance (Fig 1). Finally, accumulating evidence suggests the role of dysbiosis in metabolic dysregulation and insulin resistance [83] and manipulating gut microbiome is an attractive therapy for obesity and diabetes associated cardiovascular insulin resistance.
DPP-4 inhibitors
Glucagon like peptide-1 (GLP-1) and glucose-dependent insulinotrophic peptide (GIP) are gut derived hormones. They enhance glucose-stimulated insulin secretion and suppressing glucagon release thereby modulating both post-prandial and long-term glucose homeostasis [79,84, 85]. They are rapidly degraded by exopeptidase, DPP-4, which circulates in the plasma and limits the half-life of these hormones to about two minutes. Augmentation of GLP-1 utilizing GLP-1 analogs or DPP-4 inhibitors improves cardiovascular outcomes [84] whereas mice with genetic deletion of the GLP-1 receptor (GLP-1R) exhibit left ventricular hypertrophy and diastolic and systolic dysfunction [85]. We have recently shown blood pressure lowering and improvement in diastolic dysfunction by DPP-4 inhibition in an insulin resistant obese rat model [86] suggesting cardiovascular effects of DPP-4 inhibitors beyond glycemic control [85,86].
Because of the wide spread expression of the DPP-4 enzyme in CD4 and CD8+immune cells, the role of DPP-4 inhibitors in modulation of innate and adaptive immunity is an area of emerging importance [79]. In this regard, decreased accumulation of M1 macrophages and increased levels of M2 macrophages seen in adipose tissue or atherosclerotic lesions following DPP-4 inhibitor treatment [87, 88] is intriguing. These observations raise the possibility that observed improvements may occur because of attenuated DPP-4 mediated polarization of macrophages (decreases in M1 inflammatory macrophages). Since DPP-4 activity is markedly increased in obesity in humans and animal models [89, 90], inhibition of DPP-4 offers a novel approach for suppression of low-grade inflammation and associated tissue insulin resistance. Moreover, recent studies demonstrating Tregs promote M2 polarization [57] and GLP-1 enhances Treg function [91] raises the possibility that improvement of Treg function with DPP-4 inhibitor therapy may improve cardiac and vascular insulin metabolic signaling and associated cardiovascular insulin resistance and renal damage (Fig 1).
ARBs
Although AT1R antagonists are used for the management of hypertension, mounting evidence suggests the beneficial effects of ARBs are also due to their anti-inflammatory effects [78]. The effects of ARBs may also be contributed by lowering of uric acid or up regulation of PPAR gamma [92]. Recent studies showing increased GLP-1 by ARBs are suggestive of DPP-4 modulatory effects of ARBs [93].
Xanthine oxidase inhibitors
Obesity epidemics in the United States have paralleled the substantially increased consumption of high-fructose corn syrup (HFCS) which has increased dramatically in the past three decades [10-12]. High fructose (60%) only diet has also been shown to elevate plasma uric acid levels [94]. Soluble uric acid increases Ang II in the vasculature and renin expression in small animal models. Moreover, increased levels of renin are seen in humans with hyperuricemia [80]. Moreover, uric acid promotes inflammation [80,95]. Allopurinol treatment has been shown to suppress cardiac oxidative stress, vascular inflammation, renal inflammation, and hypertension in the setting of obesity or diabetes [80]. Therefore, combining allopurinol with ARB or DPP-4 inhibitor treatment may be beneficial in suppressing immune inflammatory responses and insulin resistance (Fig 1).
Renal denervation
The role of SNS activation is supported by studies on renal denervation as a therapeutic strategy for management of resistant hypertension [10,96]. Renal denervation comprises selective reduction of renal sympathetic afferent and efferent signaling that is accomplished by low-dose frequency energy to the renal artery endothelial surface [97]. This procedure is also associated with significant improvement of insulin sensitivity, and reductions in proteinuria in addition to sustained blood pressure lowering effects [98].
Tregs
Because of the critical importance of an appropriate balance between protective (Treg related) immunity and inflammatory related T helper cells, therapeutic targeting of Tregs is assuming a potential therapeutic importance. The beneficial effects of Tregs in lowering blood pressure and improving cardiac and renal dysfunction and inflammation has been demonstrated in Ang II and aldosterone infused models of cardiovascular and renal injury [33,34,37,52,53]. The improvement in insulin sensitivity and suppression of renal injury by adoptive transfer of Tregs in an animal model of diabetic nephropathy suggests the importance of targeting immune and inflammatory response in cardiovascular and renal insulin resistance [99]. Recently, the beneficial effects of stem cells targeting Tregs have been reported [81-82] and if successful provide an alternative approach to immune cell therapy for insulin resistance and hypertension (Fig 1).
Gut microbiome
The effects of altered gut microbiome and their effects on pattern recognition receptors, low grade inflammation and metabolic dysregulation and insulin resistance are increasingly recognized [27,28]. The beneficial effects of prebiotics on immune and inflammatory response and insulin resistance [83] offers novel approaches targeting progression of insulin resistance and CVD and CKD risk associated with cardiorenal metabolic syndrome.
8. Conclusion
In conclusion, maladaptive immune and inflammatory responses are central to over-nutrition-associated cardiovascular insulin resistance. Skewing of macrophage polarization and T cell polarization resulting in cytokine imbalance is one of the important underlying mechanisms in mediating cardiovascular insulin resistance. Inappropriate activation of RAAS, SNS and DPP-4 significantly contribute to this maladaptive immune and inflammatory response and progression of insulin resistance in obesity and diabetes. Drugs targeting these interactions offer promising therapy for the integrated control of glycemia and cardiovascular and renal outcomes of insulin resistance. Elevated uric acid and its link with cardiovascular dysfunction, activation of RAAS and immune dysfunction point to targeting uric acid metabolism as an attractive therapeutic modality. Renal denervation is an emerging therapeutic modality for resistant hypertension and SNS modulation of immune function may underlie the beneficial effects of insulin resistance, cardiovascular damage and renal injury. Therefore, studies directed toward understanding molecular and cellular mechanisms underlying maladaptive immune and inflammatory responses provide new insights to immune mediated drug targeting for insulin resistance and cardiovascular dysfunction (Fig 1).
9. Strengths and weaknesses
Immune regulation of insulin resistance and hypertension is the significant strength for the development of immune modulation therapy for the prevention and treatment of cardiovascular insulin resistance and hypertension. Drugs modulating polarization of macrophages and T regulatory cells are particularly intriguing. Indeed drugs such as DPP-4 inhibitors that affect immune cell polarization are promising in this regard. Moreover, drugs that show encouraging results in the improvement of insulin resistance or renal injury by drugs such as Ang II receptor blockers also modulate innate and adaptive immunity. Similarly, uric acid induced immune cell activation and inflammatory response is associated with high fructose diet and suppression of hyperuricemia by either allopurinol or olmesartan further strengthen the immune modulation as a therapeutic strategy. However, exaggerated and prolonged suppression of immune response or predominant modulation of selective macrophage or T cell polarization response may have deleterious effects either on suppression of immune response or immunological tolerance. Although renal denervation may modulate immune response and improvement in insulin resistance and cardiovascular dysfunction, long term studies on the effects of renal denervation are not well known at present. Therefore, these weaknesses should be addressed by developing drugs to tilt the imbalance of immune cell polarization and the proinflammatory response toward normal thereby improving cardiovascular insulin resistance in obesity and diabetes.
Acknowledgement
The authors would like to thank Brenda Hunter for her editorial assistance.
Funding
This research was supported by NIH (R01 HL73101-01A and R01 HL107910-01) and the Veterans Affairs Merit System (0018) for JRS.
Abbreviations
- WD
Western Diet
- T2DM
type 2 diabetes mellitus
- CVD
cardiovascular disease
- CKD
chronic kidney disease
- PI3-K
phosphatidylinsositol 3 kinase
- Akt
protein kinase B
- ERK1/2
extracellular regulated kinases ½
- IRS
insulin receptor substrate
- JNK
C-Jun kinase
- mTOR
mammalian target of rapamycin
- S6K
p70 S6 kinase
- SOC3-3
cytokine signaling 3
- WAT
white adipose tissue
- FFAs
free fatty acids
- TNF-α
tumor necrosis factor alpha
- IL-6
interleukin 6
- PKC
protein kinase C
- TLR-4
Toll-like receptor 4
- LPS
lipopolysaccharide
- RAAS
renin angiotensin aldosterone system
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- ROS
reactive oxygen species
- SNS
sympathetic nervous system
- SOD3
superoxide dismutase
- CVO
circumventricular organs
- PET
positron emission tomography
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- Tregs
regulatory T cells
- IFN-γ
interferon gamma
- FoxP3
forkhead/winged helix transcription factor 3
- IL-10
Interleukin 10
- TGF-β
transforming growth factor beta
- CVB3
coxsackievirus B3
- ARBs
Ang II receptor blockers
- DPP-4
dipetidyl peptidase 4
- GLP-1
glucagon like peptide-1
- GIP
glucose-dependent insulinotrophic peptide
- GLP-1R
GLP-1 receptor
- HFCS
high-fructose corn syrup
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions All authors contributed to the design and conduct of the study, data collection and analysis, data interpretation and manuscript writing.
Conflict of interest
The authors have no conflict of interest associated with this manuscript.
REFERENCES
- 1.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875–92. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Sowers JR, Whaley-Connell A, Hayden MR. The Role of Overweight and Obesity in the Cardiorenal Syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullough AJ. Epidemiology of the metabolic syndrome in the USA. J Dig Dis. 2011;12:333–40. doi: 10.1111/j.1751-2980.2010.00469.x. [DOI] [PubMed] [Google Scholar]
- 4.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–17. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allcock DM, Gardner JM, Sowers JR. Relation between childhood obesity and adult cardiovascular risk. Int J Pediatr Endocrinol. 2009;2009:108187. doi: 10.1155/2009/108187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo C, Jin Z, Palmieri V, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–8. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanhope KL. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med. 2012;63:329–43. doi: 10.1146/annurev-med-042010-113026. [DOI] [PubMed] [Google Scholar]
- 8.Garver WS, Newman SB, Gonzales-Pacheco DM, et al. The genetics of childhood obesity and interaction with dietary macronutrients. Genes Nutr. 2013;8:271–87. doi: 10.1007/s12263-013-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drong AW, Lindgren CM, McCarthy MI. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther. 2012;92:707–15. doi: 10.1038/clpt.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–7. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faselis C, Doumas M, Kokkinos JP, et al. Exercise capacity and progression from prehypertension to hypertension. Hypertension. 2012;60:333–338. doi: 10.1161/HYPERTENSIONAHA.112.196493. [DOI] [PubMed] [Google Scholar]
- 12.Sakuragi S, Abhayaratna K, Gravenmaker KJ, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53:611–616. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 13.DeMarco VG, Johnson MS, Ma L, et al. Overweight female rats selectively breed for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2012;302:H1667–H1682. doi: 10.1152/ajpheart.01027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majane OH, Vengethasamy L, du Toit EF, et al. Dietary-induced obesity hastens the progression from concentric cardiac hypertrophy to pump dysfunction in spontaneously hypertensive rats. Hypertension. 2009;54:1376–1383. doi: 10.1161/HYPERTENSIONAHA.108.127514. [DOI] [PubMed] [Google Scholar]
- 15.Demarco VG, Ford DA, Henriksen EJ, et al. Obesity-related alterations in cardiac lipid profile and nondipping blood pressure pattern during transition to diastolic dysfunction in male db/db mice. Endocrinology. 2013;154:159–71. doi: 10.1210/en.2012-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corden B, Keenan NG, de Marvao AS, et al. Body fat is associated with reduced aortic stiffness until middle age. Hypertension. 2013;61:1322–7. doi: 10.1161/HYPERTENSIONAHA.113.01177. [DOI] [PubMed] [Google Scholar]
- 17.Chen JY, Tsai PJ, Tai HC, et al. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol. 2013;33:839–46. doi: 10.1161/ATVBAHA.112.300036. [DOI] [PubMed] [Google Scholar]
- 18.Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068–76. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–43. doi: 10.1038/ncpcardio0943. [DOI] [PubMed] [Google Scholar]
- 20.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Mitsumata K, Saitoh S, Ohnishi H, et al. Effects of parental hypertension on longitudinal trends in blood pressure and plasma metabolic profile: mixed-effects model analysis. Hypertension. 2012;60:1124–1130. doi: 10.1161/HYPERTENSIONAHA.112.201129. [DOI] [PubMed] [Google Scholar]
- 22.Whaley-Connell A, Sowers JR. Indices of obesity and cardiometabolic risk. Hypertension. 2011;58:991–993. doi: 10.1161/HYPERTENSIONAHA.111.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–6. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha VZ, Folco EJ. Inflammatory concepts of obesity. Int J Inflam. 2011;2011:529061. doi: 10.4061/2011/529061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep. 2012;14:360–5. doi: 10.1007/s11906-012-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Brown K, DeCoffe D, Molcan E, et al. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender SB, McGraw AP, Jaffe IZ, et al. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62:313–9. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yiannikouris F, Gupte M, Putnam K, et al. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumagai E, Adachi H, Jacobs DR, Jr, et al. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension. 2011;58:1043–1048. doi: 10.1161/HYPERTENSIONAHA.111.180521. [DOI] [PubMed] [Google Scholar]
- 32.Whaley-Connell A, Sowers JR. Aldosterone and risk for insulin resistance. Hypertension. 2011;58:998–1000. doi: 10.1161/HYPERTENSIONAHA.111.182782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–7. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasal DA, Barhoumi T, Li MW, et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–30. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 35.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension. 2012;59:755–62. doi: 10.1161/HYPERTENSIONAHA.111.186833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dias da Silva VJ, Paton JF. Introduction: the interplay between the autonomic and immune systems. Exp Physiol. 2012;97:1143–5. doi: 10.1113/expphysiol.2011.061473. [DOI] [PubMed] [Google Scholar]
- 37.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganta CK, Lu N, Helwig BG, et al. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–91. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 39.Lu C, Su LY, Lee RM, et al. Superoxide anion mediates angiotensin II-induced potentiation of contractile response to sympathetic stimulation. Eur J Pharmacol. 2008;589:188–93. doi: 10.1016/j.ejphar.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 40.Lob HE, Marvar PJ, Guzik TJ, et al. Induction of hypertension and peripheral inflammation by reductionof extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–83. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohtake T, Yokoyama I, Watanabe T, et al. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–463. [PubMed] [Google Scholar]
- 42.Park SY, Cho YR, Kim HJ, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 43.Gray S, Kim JK. New insights into insulin resistance in the diabetic heart. Trends Endocrinol Metab. 2011;22:394–403. doi: 10.1016/j.tem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko HJ, Zhang Z, Jung DY, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schilling JD, Machkovech HM, Kim AH, et al. Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2012;303:H1366–73. doi: 10.1152/ajpheart.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azibani F, Fazal L, Chatziantoniou C, et al. Aldosterone mediates cardiac fibrosis in the setting of hypertension. Curr Hypertens Rep. 2013 May 18; doi: 10.1007/s11906-013-0354-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Kim JA, Jang HJ, Martinez-Lemus LA, et al. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;302:E201–8. doi: 10.1152/ajpendo.00497.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boydens C, Maenhaut N, Pauwels B, et al. Adipose tissue as regulator of vascular tone. Curr Hypertens Rep. 2012;14:270–8. doi: 10.1007/s11906-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 49.Eringa EC, Bakker W, van Hinsbergh VW. Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascul Pharmacol. 2012;56:204–9. doi: 10.1016/j.vph.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Mendizábal Y, Llorens S, Nava E. Hypertension in metabolic syndrome: vascular pathophysiology. Int J Hypertens. 2013;2013:230868. doi: 10.1155/2013/230868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag. 2013;9:105–16. doi: 10.2147/VHRM.S33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol. 29:543–8. doi: 10.1016/j.cjca.2012.06.009. 201 doi: 10.1016/j.cjca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Luft FC, Dechend R, Müller DN. Immune mechanisms in angiotensin II-induced target-organ damage. Ann Med. 2012;44(Suppl 1):S49–54. doi: 10.3109/07853890.2011.653396. [DOI] [PubMed] [Google Scholar]
- 54.Rocha VZ, Folco EJ. Inflammatory concepts of obesity. Int J Inflam. 2011;2011:529061. doi: 10.4061/2011/529061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun S, Ji Y, Kersten S, et al. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261–86. doi: 10.1146/annurev-nutr-071811-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu G, Ma H, Qiu L, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89:130–42. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 58.Hong EG, Ko HJ, Cho YR, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–35. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim HJ, Higashimori T, Park SY, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–7. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 60.Kassan M, Galan M, Partyka M, et al. Interleukin-10 releasedby CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–42. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohshima K, Mogi M, Jing F, et al. Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension. 2012;59:493–9. doi: 10.1161/HYPERTENSIONAHA.111.183178. [DOI] [PubMed] [Google Scholar]
- 62.Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–72. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stienstra R, Tack CJ, Kanneganti TD, et al. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15:10–8. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Akasheh RT, Pang J, York JM, et al. New pathways to control inflammatory responses in adipose tissue. Curr Opin Pharmacol. 2013 May 3; doi: 10.1016/j.coph.2013.04.008. doi:pii:S1471-4892(13)00057-X.10.1016/j.coph.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–2. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manrique C, Lastra G, Habibi J, et al. Loss of estrogen receptor α signaling leads to insulin resistance and obesity in young and adult female mice. Cardiorenal Med. 2012;2:200–210. doi: 10.1159/000339563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrett-Connor E, Giardina EG, Gitt AK, et al. Women and heart disease: the role of diabetes and hyperglycemia. Arch Intern Med. 2004;164:934–942. doi: 10.1001/archinte.164.9.934. [DOI] [PubMed] [Google Scholar]
- 68.Peterson LR, Waggoner AD, Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: Assessment by echocardiography and tissue doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 69.Russo C, Jin Z, Palmieri V, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Simone G, Devereux RB, Chinali M, et al. Sex differences in obesity-related changes in left ventricular morphology: the Strong Heart Study. J Hypertens. 2011;29:1431–1438. doi: 10.1097/HJH.0b013e328347a093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luther JM, Wang Z, Ma J, et al. Endogenous aldosterone contributes to acute angiotensin II-stimulated plasminogen activator inhibitor-1 and preproendothelin-1 expression in heart but not aorta. Endocrinology. 2009;150:2229–2236. doi: 10.1210/en.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasan RS, Evans JC, Benjamin EJ, et al. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957–62. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 73.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 2010;122(1-3):74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 74.Meyer MR, Clegg DJ, Prossnitz ER, et al. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203:259–69. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ribas V, Drew BG, Le JA, et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA. 2011;108:16457–62. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li K, Xu W, Guo Q, et al. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ Res. 2009;105:353–64. doi: 10.1161/CIRCRESAHA.109.195230. [DOI] [PubMed] [Google Scholar]
- 77.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–84. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 78.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–57. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009;30:600–7. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 80.Puddu P, Puddu GM, Cravero E, et al. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol. 2012;59:235–42. doi: 10.1016/j.jjcc.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 81.Kasal DA, Schiffrin EL. Angiotensin II, aldosterone, and anti-inflammatory lymphocytes: interplay and therapeutic opportunities. Int J Hypertens. 2012;2012:829786. doi: 10.1155/2012/829786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol. 2013;91:12–8. doi: 10.1038/icb.2012.60. [DOI] [PubMed] [Google Scholar]
- 83.Molinaro F, Paschetta E, Cassader M, et al. Probiotics, prebiotics, energy balance, and obesity: mechanistic insights and therapeutic implications. Gastroenterol Clin North Am. 2012;41:843–54. doi: 10.1016/j.gtc.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Lenski M, Kazakov A, Marx N, et al. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol. 2011;51:906–18. doi: 10.1016/j.yjmcc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Dicker D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care. 2011;34(Suppl 2):S276–8. doi: 10.2337/dc11-s229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aroor AR, Sowers JR, Bender SB, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin resistant male zucker obese rats. Endocrinology. 2013 May 7; doi: 10.1210/en.2013-1096. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shirakawa J, Fujii H, Ohnuma K, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60:1246–57. doi: 10.2337/db10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shah Z, Kampfrath T, Deiuliis JA, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–49. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J, Campitelli J, Hu G, et al. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci. 2007;81:272–9. doi: 10.1016/j.lfs.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 90.Lee SA, Kim YR, Yang EJ, et al. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes Mellitus. J Clin Endocrinol Metab. 2013;98:2553–61. doi: 10.1210/jc.2012-4288. [DOI] [PubMed] [Google Scholar]
- 91.Hadjiyanni I, Siminovitch KA, Danska JS, et al. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53:730–40. doi: 10.1007/s00125-009-1643-x. [DOI] [PubMed] [Google Scholar]
- 92.Smink PA, Bakker SJ, Laverman GD, et al. An initial reduction in serum uric acid during angiotensin receptor blocker treatment is associated with cardiovascular protection: a post-hoc analysis of the RENAAL and IDNT trials. J Hypertens. 2012;30:1022–8. doi: 10.1097/HJH.0b013e32835200f9. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez R, Viscarra JA, Minas JN, et al. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology. 2012;153:1684–95. doi: 10.1210/en.2011-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hosoya T, Kuriyama S, Yoshizawa T, et al. Effects of combined antihypertensive therapy with losartan/hydrochlorothiazide on uric acid metabolism. Intern Med. 2012;51:2509–14. doi: 10.2169/internalmedicine.51.7584. [DOI] [PubMed] [Google Scholar]
- 95.Conforti-Andreoni C, Spreafico R, Qian HL, et al. Uric acid-driven Th17 differentiation requires inflammasome-derived IL-1 and IL-18. J Immunol. 2011;187:5842–50. doi: 10.4049/jimmunol.1101408. [DOI] [PubMed] [Google Scholar]
- 96.Sobotka PA, Mahfoud F, Schlaich MP, et al. Sympatho-renal axis in chronic disease. Clin Res Cardiol. 2011;100:1049–57. doi: 10.1007/s00392-011-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 98.Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 99.Eller K, Kirsch A, Wolf AM, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–62. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]