Summary
Much of brain science is concerned with understanding the neural circuits that underlie specific behaviors. While the mouse has become a favorite experimental subject, the behaviors of this species are still poorly explored. For example, the mouse retina, like that of other mammals, contains ~20 different circuits that compute distinct features of the visual scene [1, 2]. By comparison, only a handful of innate visual behaviors are known in this species – the pupil reflex [3], phototaxis [4], the optomotor response [5], and the cliff response [6] – two of which are simple reflexes that require little visual processing. We explored the behavior of mice under a visual display that simulates an approaching object, which causes defensive reactions in some other species [7, 8]. We show that mice respond to this stimulus by either initiating escape within a second or by freezing for an extended period. The probability of these defensive behaviors is strongly dependent on the parameters of the visual stimulus. Directed experiments identify candidate retinal circuits underlying the behavior and lead the way into detailed study of these neural pathways. This response is a new addition to the repertoire of innate defensive behaviors in the mouse that allows the detection and avoidance of aerial predators.
Results
For the mouse, avoidance of aerial predators, such as hawks and owls, is a central survival function, likely supported by dedicated brain circuits. The only useful sensory modality for this purpose is vision. Thus we searched for innate visual behaviors that would support defense from overhead threats.
Visual display of an expanding dark disc triggers immediate flight or freezing while inhibiting rearing in mice
A wild-type mouse was placed into a behavioral arena with a display monitor covering most of the ceiling. An opaque nest in one corner of the arena offered a hiding place from visual stimuli (Fig. 1a). The mouse was allowed 10 min of acclimation in the arena with a plain gray monitor. In this period the animal commonly displayed exploratory postures such as rearing on the hind legs and sniffing. Then the “looming stimulus” was started: On a gray background a black disc appeared directly above the animal at a diameter of 2 degrees of visual angle, expanded to 20 degrees in 250 ms, and remained at that size for 250 ms (Fig. 1b). This stimulus was repeated 15 times with 500 ms pauses. This reliably triggered one of two behaviors: escape or freezing (Fig. 1d).
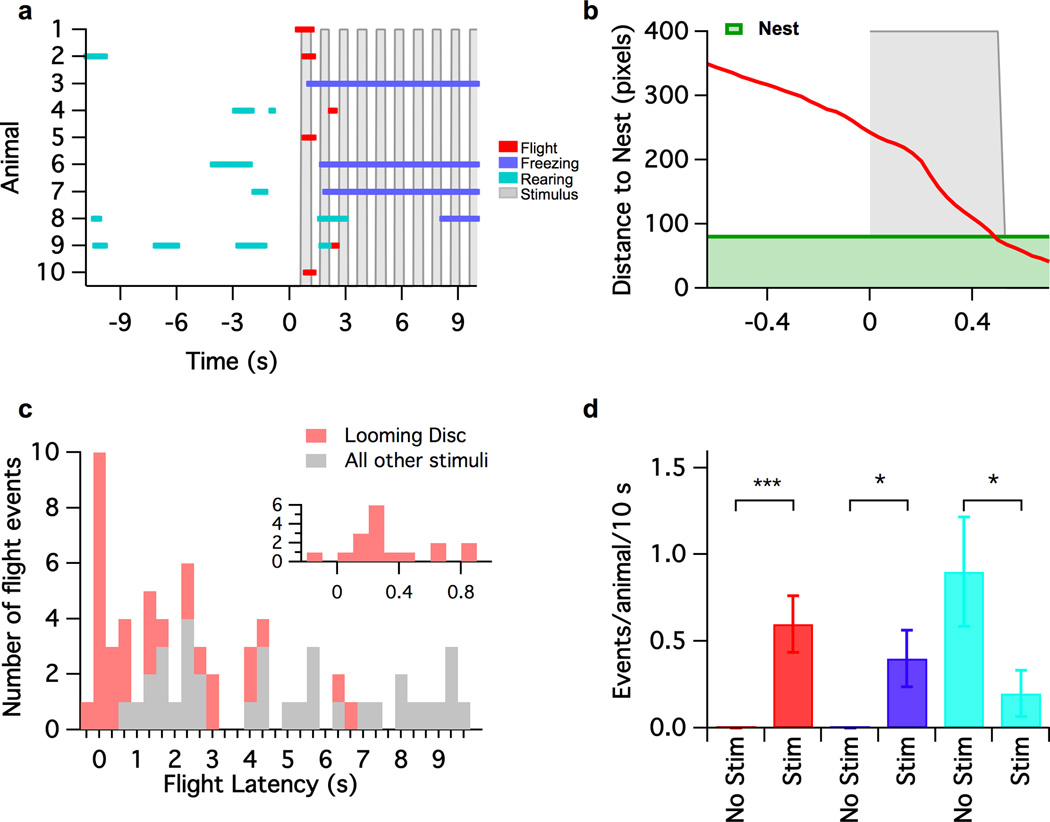
Figure 1. A dark expanding disc in the upper visual field triggers flight and freezing.
(a) Schematic of the experimental setup: a box with a display monitor (M) on the ceiling and an opaque nest (N) in a corner. Multiple cameras monitor the animal’s movements, from which one can measure the distance (D) to the nest. (b) Expansion of the looming stimulus in time from 2 degrees to 20 degrees. (c) An example trajectory of the mouse ~3 seconds before (green) and after (red) stimulus onset. The outline of the box shows the boundaries of the arena. Each tick is 33 ms. (d) Distance of the mouse from the nest before and during the stimulus. Example traces for flight (red) and freezing (purple) behaviors. Gray trace indicates the repetitions of the looming stimulus. See also Supplementary Movies 1 and 2. (e) Detail of the flight trace in (d) to emphasize the onset of the run to the nest in relationship to the disc expansion from 2 to 20 degrees.
Most animals initiated a rapid escape to the nest (Fig. 1c–e; Fig. 2a; p < 0.005; Supplementary Movie 1). Three of ten animals began their flight with a latency of less than 250 ms after stimulus onset, even before the disc reached its maximum size of 20 degrees (Fig. 1e; Fig. 2a). Such short-latency responses were observed repeatedly over many experiments (Fig. 2c, Fig. 3 a, i, j, k). In one case the animal had already initiated a run towards the nest prior to stimulus onset, but accelerated once the looming disc appeared (animal 1, Fig. 2a, b). The animals that did not flee responded by freezing, often for the remaining duration of the stimulus (Fig. 1d; Fig. 2a, d; p<0.02; Supplementary Movie 2). The looming display also suppressed the animal’s exploratory behavior, as observed by scoring the rearing events (Fig. 2a, d; p < 0.02). For the following report we focus on the analysis of rapid escapes – with a latency below 1 s – and upward rearing events.
Figure 2. Statistics of reactions to the looming stimulus.
(a) Occurrences of flight, freezing, and upward rearing behaviors 10 s before and after stimulus onset. (b) Distance of mouse 1 to the nest before and after the stimulus. The speed increases more than 2-fold at 0.2 s after stimulus onset. (c) Histogram of flight latencies for looming stimuli (red) and all other stimuli tested (gray). The inset shows the distribution of sub-second flight events. (d) Probability of each of the three behaviors before and after stimulus onset. Error bars show standard error of the mean.
Figure 3. The frequency and speed of defensive behaviors depend strongly on stimulus parameters.

(a–d) Comparison of four stimulus displays: black looming disc (a), white looming disc (b), white receding disc (c), dimming disc (d). For each condition an ethogram indicates occurrence of three behaviors after stimulus onset at time 0: flight (red), freezing (purple), and upward rearing (cyan). The experimental sequence was b-c-d-a. (e) Frequency of sub-second flight in each of the four disc displays from panels a-d. (f) Frequency of sub-second flight after addition of a patterned background, either static or moving. (g–h) Frequency of upward rearing events under the stimulus conditions of panels e-f. (i–k) Flight latencies observed in 10 animals under the four disc displays (i), as a function of expansion rate of a dark disc (j), and as a function of background pattern (k). Each trace is from a different animal. Error bars show standard error of the mean.
A looming dark disc is uniquely effective in driving sub-second flight and extended freezing behaviors
To investigate how different parameters of the looming stimulus influence the behavior, we tested five different stimulus conditions. First, when the same stimulus was presented in the lower visual field, with a display monitor below the floor, it caused no escapes or suppression of rearing (data not shown), suggesting that the looming response originates in the inferior retina. Stimulation from the top, but with a disc of reversed contrast (white on gray) produced no sub-second flight events (Fig. 3b). In the retina, the visual signal splits into ON and OFF channels that respond to a light increase and decrease respectively, and the above result points to a special role for the OFF channel in the looming response. However, a mere dimming of a disc of constant size, that matched the overall intensity change of the looming stimulus, failed to trigger rapid flight responses (Fig. 3d) suggesting that motion of a dark edge is essential. To test whether dark edge motion is sufficient, we displayed a bright receding disc, which has dark edges that move inward rather than outward. This stimulus also failed to evoke a flight response in less than 1 s (Fig. 3c). Under each of these alternative conditions, some animals did flee to the nest over an extended period of 10 s. However these events were less frequent and at much longer latency than under the dark expanding disc (Fig. 3b–d, i). Furthermore, the dark expanding disc was the only condition that fully suppressed exploratory rearing, or produced prolonged freezing (Fig. 3g). In summary the looming black disc is uniquely effective in triggering defensive responses in mice, even compared to closely related visual displays.
The speed of the expansion strongly influences the latency of flight
To explore visual stimulus space further, we took guidance from known retinal physiology. Several types of retinal ganglion cells (RGCs) are specialized for the detection of motion, but they differ in their tuning to motion velocity. The ON-DS cells are tuned to low speeds, ranging up to ~2 deg/s in the rabbit retina [9, 10], whereas the ON-OFF DS [9–11] and the OFF DS [12] cells respond at much higher speeds. We explored the dependence of the flight behavior on the expansion speed of the dark disc, ranging from 0.35 to 350 deg/s. Sub-second flight events were observed only at 35 deg/s. A ten-fold higher speed was moderately effective, though with longer latencies (Fig. 3j). Ten-fold lower speeds were ineffective. Therefore, if the ON-DS cells of the mouse resemble those of the rabbit in speed tuning, they are unlikely to drive the looming response, a conclusion reinforced by the weak effects of white disks (Fig. 3b).
Background motion on the retina inhibits flight
Further information was obtained from presenting the looming stimulus on a patterned background. Certain RGCs are strongly suppressed by image motion in the receptive field surround, whereas other types of RGC are unaffected [13–15]. To evoke this condition we surrounded the expanding disc with a stripe or checkerboard pattern. As the eye jitters during the locomotion of the animal this induces large-scale image motion on the retina; for good measure we also added a steady drift to the background pattern. These global motion stimuli significantly reduced the occurrence of sub-second flight events, increased the flight latencies (Fig. 3f, k), and increased the frequency of exploratory rearing events (Fig. 3h). This result favors the involvement of so-called “object motion sensitive” cells that are inhibited by global motion on the retina [13, 14].
Discussion
In conclusion, we found that mice execute robust flight and freezing behavior in response to the visual display of an approaching object. We showed that the probability of flight and rearing behaviors depends strongly on the parameters of the visual stimulus, suggesting that specialized visual channels are involved in transmitting the relevant information. The results are significant from multiple perspectives.
Firstly, they present a novel addition to the repertoire of visually guided behaviors in the laboratory mouse. This animal model is increasingly popular in visual neuroscience, including basic studies of processing at all levels of the visual system, and translational research on neural regeneration and recovery. However, the efforts to relate neural circuits to behavior are currently hampered by the scarcity of behavioral assays of visual function. Among the ~20 types of RGCs in the mouse retina some are known to support very specific behaviors. For example, melanopsin cells regulate circadian entrainment and the pupil constriction reflex [3], whereas ON-DS cells project specifically to nuclei of the accessory optic system, responsible for the optokinetic reflex [16]. The looming response described here can also be viewed as an essential reflex, presumably for avoiding aerial predators. It occurs on the animal’s very first exposure to the stimulus, and sports the same reliability and sub-second reaction time as the pupil and optokinetic reflex. This suggests that the looming response, too, may originate in a dedicated retinal module.
Second, our experiments suggest which may be the relevant retinal circuits. The comparison of dark and bright discs clearly points to a role for the OFF ganglion cells. Among these one distinguishes two prominent types with sustained or transient responses [17]. They both have receptive fields of ~10 deg diameter [18], so the stimulus at the optimal speed of 35 deg/s sweeps over the receptive field in ~0.3 s and the ineffective slower stimulus in 3 s (Fig. 3j). A sustained cell will fire under both conditions, whereas a transient cell prefers rapid changes, and will thus fire more weakly to the slow stimulus. Thus the observed speed-dependence of the looming response favors a transient OFF channel, such as the PV-5 neuron [19] whose firing is indeed strongly driven by dark expanding objects. Other candidate pathways include ganglion cells with ON-OFF responses, such as the ON-OFF direction-selective cell [11], or the W3 cell [13]. The latter has a transient response, stronger for OFF than for ON events; is concentrated in the inferior retina, which monitors events above the animal; and is strongly suppressed by surround motion, much like the looming response itself. All these candidates project to the superior colliculus, a central station that mediates approach and avoidance behaviors [20]. Of course it is possible that the stimulus-specificity instead arises from more central visual processing. Future experiments with genetically modified mice can test the involvement of specific neural circuits in this behavior.
Third, from a methods perspective, the behavior described here presents an interesting handle for the study of innate defensive responses. Predator avoidance in mice has been studied extensively using real predators [21] and their odors [22, 23]. By contrast the looming assay relies exclusively on visual cues and offers much greater control of the stimulus parameters. As documented here, one can systematically vary luminance, contrast, speed, and other features of the stimulus, and obtain a modulation of the defensive behavior. Taking advantage of accurate temporal control of the stimulus we observed innate flight responses with latencies as low as 250 ms, shorter than reported for noxious foot shock [24] or even for exposure of wild mice to real rats [21]. This short reaction time for the entire sensory-motor loop sets limits on the amounts of central processing involved in the behavior.
Finally, though it was not the focus of this study, we were intrigued by the observation of extended freezing in response to these visual displays (Fig. 2a; Supplementary Movie 2). Freezing in laboratory mice has been reported in response to aversive ultrasonic cues but only in highly stressed animals [25], or more commonly as a result of context conditioning after repetitive foot shocks [26]. Unconditioned exposure to a predator odor does not elicit extended freezing [22, 23]. Even exposure to real rats produces only temporary immobility interspersed with active movements [27]. One may speculate that freezing is uniquely effective as a defense from aerial predators, which can detect the mouse only by vision or audition. Freezing eliminates both the visual motion that distinguishes the target from the background, and the rustling that might give it away acoustically. By contrast, once a predator is close within olfactory range, escape may be the more effective strategy [28]. Future studies may illuminate what stimulus and environmental factors affect the animal’s choice between flight and freezing in the presence of an overhead visual threat.
Experimental Procedures
The behavioral arena was an open-top plexiglass box, 48 cm wide × 48 cm deep × 30 cm high. The floor and three walls were covered with a matte spray-on coating (Krylon) to prevent reflections of the stimulus. The nest was in the shape of a triangular prism 20 cm wide × 12 cm high. The arena had dim lighting from the gray screen of the monitor. Four bright LEDs (Marubeni America Corporation, L810N-66-60) provided infrared illumination for video recording that was invisible to the mouse. Two cameras, one from the top, one from the side (PointGrey, Flea3 firewire, monochrome) were triggered simultaneously to record the mouse’s movements at 30 fps. The stimulus was programmed in C++ using OpenGL libraries and was displayed on a LCD monitor (HPZR22w).
Mice of strain C57BL6/J from Jackson Labs aged 8–12 weeks were group-housed and maintained on a 13 h light / 11 h dark cycle. No significant difference in frequency or latency of flights was observed between males and females or between animals tested during day and night. Following these pilot tests, all experiments were performed on males and during the day, when the animals generally seemed less anxious.
The stimulus was triggered by the experimenter when the animal entered a black square in the middle of the arena. When multiple conditions were compared on the same animal (Fig 3), we conservatively presented the looming stimulus last. Thus any form of habituation would have its strongest effect on this reference condition. Each animal was used only once per experimental condition, and results are reported from a total of 40 animals: 10 for looming stimulus only (Fig. 2), 10 for comparison with the 3 disc display conditions, 10 for testing effects of background motion and 10 for testing the effect of different speeds (Fig. 3).
The video recordings were analyzed with a custom-written Matlab program that uses background subtraction to locate the mouse. The velocity was calculated and smoothed with a median filter. Freezing is defined as the episodes of 2 s or more where the velocity is less than 15% of its average value over the 10 second interval prior to the stimulus onset. Flight is defined as episodes where the velocity is greater than 4 times the average and the animal’s final position is in the nest. Upright rearing events were scored by hand as episodes when the mouse raises both front paws and puts them back on the ground.
The ethograms (Fig. 2a, 3a–d) mark episodes of flight, freezing or rearing as a function of time before or after stimulus onset. Statistical significance (Fig. 2d, 3e–k) is labeled as: * p<0.05, ** p<0.01, *** p< 0.005. These p-values were derived from a z-test for the equality of two proportions for flight and freezing (binomial distribution); z-test for the equality of two counts for upward rearing (Poisson distribution); one-sided sign test for latency comparisons. For animals that did not flee within 10 s of stimulus onset, a flight latency of 10 s was assigned.
Thanks to a move of the laboratory over the course of the study we could compare the behavior of mice housed in two different animal facilities: at Harvard and Caltech. Both sets of animals exhibited fast and robust defensive reactions to the looming disc, but with some significant differences. The Harvard animals reacted with escape every time, with no occurrence of freezing, unlike the animals housed at Caltech. Furthermore, the Harvard animals showed much weaker reactions still to the alternative disc displays (Fig. 3b–d). For a conservative and internally consistent report we only show data from the Caltech animals. We suspect that differences in the internal state of the animals owing to housing conditions in the two colonies underlie the different outcomes. This points to a need for standardization in animal care if one wants to compare behavioral results across laboratories.
Supplementary Material
Highlights.
Visual display of a looming dark disc triggers rapid escape or freezing in mice.
These reactions are strongly dependent on the parameters of the visual stimulus.
The selectivity of the behavior is consistent with specific retinal pathways.
Acknowledgements
We thank Xavier Burgos-Artizzu for advice on video analysis, and David Anderson, Joshua Sanes, Rachel Wilson for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melis Yilmaz, Email: myilmaz@caltech.edu.
Markus Meister, Email: meister@caltech.edu.
References
- 1.Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gollisch T, Meister M. Eye smarter than scientists believed: Neural computations in circuits of the retina. Neuron. 2010;65:150–164. doi: 10.1016/j.neuron.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 5.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 6.Fox MW. The visual cliff test for the study of visual depth perception in the mouse. Anim Behav. 1965;13:232–233. doi: 10.1016/0003-3472(65)90040-0. [DOI] [PubMed] [Google Scholar]
- 7.Card GM. Escape behaviors in insects. Curr Opin Neurobiol. 2012;22:180–186. doi: 10.1016/j.conb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto K, Nakata M, Nakagawa H. Input and output characteristics of collision avoidance behavior in the frog Rana catesbeiana. Brain Behav Evol. 2003;62:201–211. doi: 10.1159/000073272. [DOI] [PubMed] [Google Scholar]
- 9.Sivyer B, van Wyk M, Vaney DI, Taylor WR. Synaptic inputs and timing underlying the velocity tuning of direction-selective ganglion cells in rabbit retina. J Physiol. 2010;588:3243–3253. doi: 10.1113/jphysiol.2010.192716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyster CW. The analysis of image motion by the rabbit retina. J Physiol. 1968;199:613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng S, Sun W, He S. Identification of ON-OFF direction-selective ganglion cells in the mouse retina. J Physiol. 2005;562:915–923. doi: 10.1113/jphysiol.2004.076695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Kim IJ, Sanes JR, Meister M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc Natl Acad Sci U S A. 2012;109:E2391–E2398. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ölveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–408. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- 15.Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat Neurosci. 2003;6:600–608. doi: 10.1038/nn1061. [DOI] [PubMed] [Google Scholar]
- 16.Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji NL, Usui S, Noda M. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS ONE. 2009;4:e4320. doi: 10.1371/journal.pone.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- 19.Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- 20.Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci. 1986;6:723–733. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard RJ, Hebert MA, Ferrari PF, Palanza P, Figueira R, Blanchard DC, Parmigiani S. Defensive behaviors in wild and laboratory (Swiss) mice: the mouse defense test battery. Physiol Behav. 1998;65:201–209. doi: 10.1016/s0031-9384(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 22.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Sotnikov SV, Markt PO, Umriukhin AE, Landgraf R. Genetic predisposition to anxiety-related behavior predicts predator odor response. Behav Brain Res. 2011;225:230–234. doi: 10.1016/j.bbr.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Muller JM, Morelli E, Ansorge M, Gingrich JA. Serotonin transporter deficient mice are vulnerable to escape deficits following inescapable shocks. Genes Brain Behav. 2011;10:166–175. doi: 10.1111/j.1601-183X.2010.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mongeau R, Miller GA, Chiang E, Anderson DJ. Neural correlates of competing fear behaviors evoked by an innately aversive stimulus. J Neurosci. 2003;23:3855–3868. doi: 10.1523/JNEUROSCI.23-09-03855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS. Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav Neurosci. 2001;115:26–32. doi: 10.1037/0735-7044.115.1.26. [DOI] [PubMed] [Google Scholar]
- 27.Griebel G, Blanchard DC, Blanchard RJ. Evidence that the behaviors in the Mouse Defense Test Battery relate to different emotional states: a factor analytic study. Physiol Behav. 1996;60:1255–1260. doi: 10.1016/s0031-9384(96)00230-2. [DOI] [PubMed] [Google Scholar]
- 28.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.