Abstract
Health system responsiveness (HSR), a measure of patient healthcare experience, may influence adherence to HIV/AIDS care and be an important predictor of outcomes. We studied the relationship between HSR, patient factors and visit non-adherence in 16 PEPFAR-supported HIV/AIDS clinics in Dar es Salaam.
A HSR survey was administered in 2009 and all clinic visits one year following interviews were analyzed for 720 patients on antiretrovirals (ART). Definitions of visit non-adherence were: 1)low visit constancy (VC:no visit in ≥1 quarter), 2)gaps in care (>60 days between visits), 3)no visit in last quarter (VLQ). Relationships between factors were analyzed using multivariate analysis with adjusted odds ratio (AOR) and 95% confidence intervals reported. Few patients were non-adherent using VLQ (14%) and VC (28%). Gaps in care were more common (49.6%) and associated with younger age [AOR:3.86(2.02–7.40)], no explanation of side effects [AOR:2.21(1.49–3.28)], and shorter ART duration [0–3 months AOR:1.49(1.09–2.03); 3–6 months AOR:2.44(1.40–4.25)]. No VLQ was associated with younger age [AOR:3.40(1.63–7.07)], poor HCW communication [AOR:4.83(1.39–16.78)], and less time on ART [0–3 months AOR:5.04(2.47–10.30); 3–6 months AOR:3.09(1.72–5.57)]. Younger age, poor HCW communication and shorter ART duration also predicted lower VC, as did higher patient:HCW ratios.
Rates of visit non-adherence differed based on definitions used. Younger age, shorter time on ART and poor HCW communication predicted lower adherence regardless of definition. More work is needed to understand the relationship between HSR, patient factors and different patterns of visit non-adherence and their impact on ART outcomes.
BACKGROUND
Strong engagement and retention in care is critical to successful HIV treatment but remains a challenge for programs globally.1–3 While there has been increasing recognition of the importance of identifying risk factors for non-retention, when defined as loss to follow-up, understanding which patients remain in care but with weaker engagement is also important. Studies in resource richer settings have demonstrated associations between increased mortality and weaker engagement, as defined by missed visits in patients already successfully linked with care.4–8 However, there is limited research on this in resource-limited settings (RLS) and it is a critical area to address.9
Understanding patient and system factors associated with non-adherence to clinic visits is necessary to design and implement effective interventions that improve adherence to care. In RLS this work has focused on critical issues including healthcare access (distance, cost, wait time), duration of visits, health care worker attitudes, and patient factors including socioeconomic barriers, stigma, and issues of disclosure and knowledge related to HIV care and treatment.9–15 Barriers to access in these settings, defined as initiation and continuation of antiretroviral therapy (ART), include lack of knowledge about ART, perceived or actual cost of treatment, stigma, longer distance from home to the clinic, and lack of coordination of services.16 These risk factors are similar to those associated with poorer long term retention in care.7,14,17,18 However, there are still considerable gaps in understanding the full range of factors associated with lower rates of adherence to and retention in care.
Patient satisfaction and health system responsiveness (HSR) have been defined as an important component of quality of care and critical to ensuring that health systems meet patient needs and expectations.19,20 Responsiveness measures the patient’s experience with the health care system in eight domains: dignity, autonomy, confidentiality, communication, promptness of attention, social support, basic amenities, and choice of provider. Patient satisfaction, a more subjective measure of a number of domains of HSR, also captures the patient’s perspective of quality of care received. The importance of the patient’s perspective has been shown with high patient satisfaction correlated with increased compliance, decreased latency to care-seeking and improved understanding and retention of medical information.21,22 Not surprisingly, there is overlap between system factors associated with lower adherence to care and those associated with low patient satisfaction with care and low HSR. These include long wait times, long distance of clinic from the patient’s home, and health care worker shortages, which are incorporated elements of HSR.23,24 However the relationship between HSR and adherence to HIV care has not been well described in RLS.
Understanding the relationship between patient’s perspective of quality and HSR factors and adherence to care is critical as efforts are made to improve retention in care and HIV treatment outcomes. We report on a study to identify the association of patient factors and reported HSR on future adherence to care and retention in care for patients on ART at a network of HIV clinics in Dar es Salaam, Tanzania Sites supported by the PEPFAR1-supported Management and Development for Health (MDH). These Care and Treatment Centers (CTCs) are based in public sector hospitals or health centers and are staffed by a multidisciplinary team generally consisting of physicians, nurses, counselors and pharmacy staff who deliver comprehensive outpatient HIV care including ART, supportive services, outreach and outpatient management of opportunistic infections for adults and children.
METHODS
Patients
From March to July 2009, MDH implemented a cross sectional study in 16 CTCs that examined patient and site factors associated with patient reported health system responsiveness and satisfaction with care. The MDH survey was administered to non-pregnant adult patients at each clinical site in a systematic fashion. Patients were approached if they had been in care at the site for at least six months and were consented to the survey and its linkage to their clinical data. After obtaining verbal consent, the survey was administered by single trained research nurse.
A total of 809 patients completed the survey and had available clinical data one year following their survey date. Five patients who had died within 1 year of the survey date, 4 who did not have a clinic visit following the interview date, and 80 who were not on ART at the time of the survey were excluded from the analysis because of lower frequency of required visits (every six months versus every three months for patients on ART). In total, 720 patients were included in the final analysis.
Survey
The health system responsiveness survey was developed from other surveys measuring HSR 25 and structured on the WHO Health System responsiveness domains. It was then adapted based on focus groups with patients and staff. Patents were asked to rate services in areas including access, communication, confidentiality, and perceived quality. Responses were that a service was either received or not received and ratings were done on a 5-point Likert scale. Likert-scale ratings were further dichotomized into top 2 and lower 3 responses (i.e. Very Good/Good, versus Moderately Good/Fair/Poor). Surveys also collected sociodemographic information including age, household assets, education, distance from clinic, and health status.
Clinical data and site characteristics
Survey data were linked with clinical data from the MDH clinical care database using a unique patient identifier. Clinical data included date of enrollment at the clinic, date of starting ART, age, gender, serial CD4 counts, visit dates, and receipt of recommended care (TB screening, cotrimoxazole treatment if eligible, CD4 count monitoring and time to starting ART once eligible). Date of initial HIV diagnosis could not be reliably ascertained from the existing data.
Site factors included site size and staffing measured as number of patients to one full time equivalent (FTE). Site size was determined by average monthly patient volume, where small sites served <200 patients per month, medium sites 200–1,000 patients, and large sites >1,000 patients per month.
Outcome definitions
Adherence to care was defined through several approaches based on the data available within the MDH database as well as recent publications looking at different ways to measure adherence and engagement with care (Table 1).2 All outcomes were measured during the year following the survey administration. The outcomes included visit constancy, gaps in care and visit in the last quarter. Visit constancy was defined as the proportion of quarters (3 month periods) with at least one completed clinic visit in the year following the survey. Visit constancy was categorized into 3 groups: having at least one completed clinic visit in a) 0 or 1 quarter, b) 2 or 3 quarters, or c) all 4 quarters. Gaps in care was defined as the maximum time interval between two completed clinic visits in the year following the survey, which was dichotomized into two groups; less than 60 days or greater than or equal to 60 days. Visit in last quarter is the percentage or patients with a visit in the last quarter (3 month period) in the year following the survey. MDH requires that all patients on ARTs come to the clinic monthly.
Table 1.
Definition of Terms measuring adherence to care in year following interview
| Measure | Definition |
|---|---|
| Visit constancy | Proportion of quarters (3 month periods) with at least one completed clinic visit |
| Gaps in Care | Maximum time interval between completed clinic visits; either less than 60 days or greater than or equal to 60 days |
| Visit in Last Quarter | Percentage or patients with a visit in the last quarter (3 month period) |
Statistical analysis
The sample size was powered to estimated overall rates of satisfaction within a +/-− 8% confidence interval with an alpha of 0.05 and a beta of 90%. Bivariate logistic regressions were used to describe the association between patient sociodemographic factors and patient-reported ratings of health systems responsiveness with the 3 adherence to care outcomes. In order to determine independent predictors of adherence to care for each outcome, all covariates associated with adherence to care at a p-value <0.20 level were entered into a backwards elimination model using a stay criteria of 0.5. The final models included site factors, and accounted for clustering at the site level using generalized estimating equations. A p-value less than 0.05 was considered statistically significant. All analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, North Carolina)
Human subjects protection
The study protocol was reviewed and approved by IRBs at HSPH, HMS in the US and Muhimbili University of Health and Allied Sciences and the National Institute of Medical Research in Tanzania. All analysis was done using de-identified data.
RESULTS
Population
Baseline characteristics of the patients are shown in Table 2. Patients were predominantly female (74%), with a median age of 36, and 62.7% (450) had completed primary school education with 26.3% (189) having at least some secondary school education. All had been in care greater than 6 months and had been on ARTs for a median of 1.5 years (range 3 days–4.5 years), with over 50% with a CD4 count >350 cell/mm2 and 22% with CD4 count <200 cells/mm3 at the time of the interview.
Table 2.
Demographics of Patients included in the analysis
| Variable | % (n) |
|---|---|
| Female | 74.0% (533) |
| Age at Interview Date | (36.0 31.0, 43.0) |
| 0–24 | 6.9% (50) |
| 25–34 | 35.1% (253) |
| 35–44 | 37.1% (267) |
| 45+ | 20.8% (150) |
| Married or cohabitating | 36.8% (265) |
| Education level | |
| None | 11.0% (79) |
| Some – Completed Primary School (1–7 years) | 62.7% (450) |
| Secondary school or higher (≥ 8 years) | 26.3% (189) |
| Employment | |
| Formally or self-employed | 60.9% (438) |
| Mean Household Assets* | 3.4 |
| Time on ARTs (months) | |
| 0 to <3 Months | 16.9% (122) |
| 3 to <6 Months | 11.0% (79) |
| 6 to <9 Months | 8.3% (60) |
| 9+ Months | 63.8% (459) |
| CD4 count at time of interview | |
| ≤200 | 21.9%(155) |
| 201–349 | 25.2%(178) |
| ≥350 | 52.9%(373) |
one point for each of radio, bicycle, electricity, running water, television, car, owns house
Adherence to care
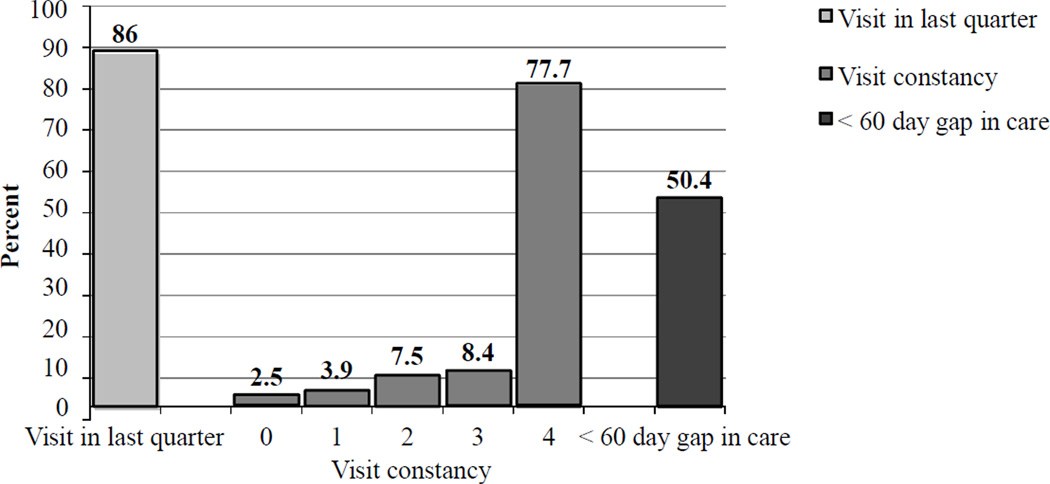
The majority of patients were adherent to care regardless of definitions used (Figure 1). Eighty-six percent had a visit in the last quarter, while slightly fewer (77.7%) had a visit in all 4 quarters. The lowest performance was in gap in care, with 49.6% having a maximum gap in care of 60 days or longer, of whom 21.9% had a gap between 60 and 90 days, 5.6% 90–120 days, and 22.3% 120 or longer. However we found that close to one-half of patients who had full visit constancy (visit every quarter), had a gap of 60 days or more between at least two or more of these visits..
Figure 1.
Percent adherence by various definitions
Factors associated with adherence to care
Factors significantly associated with decreased adherence to care defined as largest gap in care >60 days were younger age (≤24 years, AOR: 3.86 (95% CI: 2.02–7.40)), medication side effects not explained during visit (AOR: 2.21 (95% CI: 1.49–3.28)), and shorter time on ART (between 3–6 months, AOR: 2.44 (95% CI: 1.40–4.25); between 0–3 months, AOR: 1.49 (95% CI: 1.09–2.03)). Factors associated with having no visit in the last quarter included younger age (≤24 years, AOR: 3.40 (95% CI: 1.63–7.07)), HCW not having explained things in a way the patient understood (AOR: 4.83 (95% CI: 1.39–16.78)), and shorter time on ART (3–6 months, AOR: 3.09 (95% CI: 1.72–5.57) and 0–3 months, AOR: 5.04 (95% CI: 2.47–10.30)). Young age and shorter time on ART were also associated with lower visit constancy (at least one quarter with no visit) (≤24 years, AOR: 4.65 (95% CI: 2.36 – 9.17)), time on ARTs of 6–9 months (AOR: 2.73 (95% CI: 1.44 – 5.18)), 3–6 months (AOR: 5.26 (95% CI: 3.17 – 8.74)) and 0–3 months (AOR: 5.90 (95% CI: 3.18 – 10.96)). HCW not explaining things in a way the patient understood (AOR: 4.10 (95% CI: 1.54 – 10.89)) and higher patient to staff ratio (>200 patients per HCW, AOR: 1.46 (95% CI: 1.03 – 2.08)) were also associated with lower constancy. Higher comfort rating of the clinic (AOR: 1.66 (95% CI: 1.19 – 2.31)) was associated with lower constancy but not with other measures of lower care adherence (Table 3).
Table 3.
Adjusted odds ratios and confidence intervals for select patient and HSR factors by visit adherence definitions. Independent factors included if significant in univariate analysis at p≤ 0.2
| Outcome | Largest Gap Greater than 60 Days |
No Visit in the Last Quarter |
Low Visit Constancy | |
|---|---|---|---|---|
| Gender | ||||
| Female | reference | |||
| Male | 1.18(0.75–1.87) | ---- | ---- | |
| Age | ||||
| ≥45 | reference | reference | reference | |
| 35–44 | 0.98(0.70–1.37) | 1.46(0.82–2.60) | 1.18 (0.66–2.09) | |
| 25–34 | 1.17(0.81–1.70) | 1.92(0.97–3.78) | 1.57 (0.91–2.70) | |
| 24 and under | 3.86(2.02–7.40)*** | 3.40(1.63–7.07)*** | 4.65(2.36–9.17)*** | |
| Education | ||||
| None/Some Primary | reference | reference | reference | |
| Completed primary and greater | 1.22(0.83–1.80) | 1.35(0.74–2.48) | 1.32(0.80–2.18) | |
| Marital Status | ||||
| Married/Cohabitating | reference | |||
| Single/Divorced/Widowed | ---- | 1.36(0.89–2.09) | ---- | |
| Time to clinic | ||||
| ≥60 minutes | reference | |||
| 0–60 minutes | 1.27(0.86–1.88) | ---- | ---- | |
| HCW explained things in a way understood | ||||
| Yes | reference | reference | reference | |
| No | 2.23(0.81–6.10) | 4.83(1.39–16.78)* | 4.10(1.54–10.89)** | |
| Time to ask questions | ||||
| No | reference | |||
| Yes | ---- | ---- | 1.74(0.50–1.10) | |
| Side effects explained | ||||
| Yes | reference | |||
| No | 2.21(1.49–3.28)*** | ---- | ---- | |
| Confident can take medications | ||||
| Very Confident/confident | reference | |||
| Mod-Not at all | ---- | ---- | 1.79(0.85–3.77) | |
| Understood importance of labs | ||||
| Yes | reference | |||
| No | ---- | ---- | 0.67(0.43–1.05) | |
| Respect rating | ||||
| Very good/good | reference | |||
| Moderately Good/poor | 1.89(0.94–3.84) | ---- | ---- | |
| Comfort rating | ||||
| Uncomfortable | reference | reference | reference | |
| Very Comfortable/Comfortable | 1.48(1.07–2.05) | 1.55(0.93–2.56) | 1.66(1.19–2.31)** | |
| Overall Healthcare Rating | ||||
| Very Good/Good | reference | |||
| Moderately Good/Fair/Poor | 1.22(0.73–2.02) | ---- | ---- | |
| Time on ARTs | ||||
| ≥9 months | reference | reference | reference | |
| 6–9 months | 1.47(0.74–2.93) | 1.83(0.70–4.77) | 2.73(1.44–5.18)** | |
| 3–6 months | 2.44(1.40–4.25)** | 3.09(1.72–5.57)*** | 5.26(3.17–8.74)*** | |
| 0–3 months | 1.49(1.09–2.03)* | 5.04(2.47–10.30)*** | 5.90(3.18–10.96)*** | |
| Site Size | ||||
| Small | reference | reference | reference | |
| Medium | 1.14(0.59–2.20) | 0.67(0.42–1.07) | 1.01(0.63–1.63) | |
| Large | 1.05(0.54–2.07) | 0.72(0.48–1.10) | 0.88(0.57–1.37) | |
| Patient to Staff FTE ratio | ||||
| <200:1 | reference | reference | reference | |
| ≥200:1 | 1.35(0.71–2.54) | 1.40(0.91–2.18) | 1.46(1.03–2.08)* | |
HCW: Health care worker; ART: antiretroviral therapy; FTE: full time equivalent
p-value < 0.05,
p-value < 0.01,
p-value<0.001
DISCUSSION
We found relatively high rates of adherence to care for patients on ART in the setting of a network of PEPFAR-supported clinics in urban Tanzania as measured by having a recent visit and visit constancy, defined as a visit each quarter. However, one half of patients were not being seen at the intervals considered standard of care for ART patients at MDH-supported HIV Care and Treatment Clinics, which are monthly visits. Patient factors associated with decreased adherence to care were younger age and shorter time on ART. Among the HSR domains, only poor HCW communication was consistently associated with decreased adherence to care.
Rates of adherence in our cohort were higher than results from resource richer settings such as the United States. For example, in a study by Giordano,4 36% of patients were non-adherent as defined by visit constancy, with 36% having < 1 visit per quarter. Furthermore, in a cohort of 842, Torian26 found that only 45% had regular care, defined as at least one visit every 6 months. Most studies from RLS have focused on loss-to follow-up rather than adherence to care, although some definitions overlap.3,27 In Kenya, Ochieng-Ooko,27 reported that 4.3% were lost to follow-up as defined by a gap in care of 3 months or more. Other studies using gaps in visits to define lost to follow-up have reported rates of 15% in Malawi,12 and 16% in South Africa.17 While loss to follow-up has been associated with poor outcomes in RLS, there is only limited data on the impact of lower adherence to care among patients with weaker engagement but who remain in care in these settings.7
A number of patient factors were associated with adherence to care. Younger patients and those with a shorter time on ART had lower adherence to care across all definitions, similar to factors identified in studies from the United States,4,26,28 although an association between age and strength of engagement with care has not been reported in RLS. Differing from other studies in RLS, we did not find an association between gender, CD4 count, and education with adherence to care. Some may be related to the cohort. For example, 80.4% reported having completed an education level of primary school or higher, limiting the power to detect differences based on level of formal education achieved. While male gender has been associated with poorer outcomes in Tanzania, we found men and women were equally likely to maintain adherence to care, regardless of definition used, pointing to other reasons for this association.29 Similarly, other studies in Africa found competing work schedules as self-reported causes for non-adherence to care.27,30 We did not find an association between employment status and visit adherence in our cohort, although many of the patients were self-employed (39.2%) and so may have had more flexibility than those employed in the formal sector.
Poor HCW communication was the only domain of HSR associated with lower adherence to care using two of the three definitions. Provider communication has the potential to impact adherence to care in many ways. Previous studies have identified it as a critical component to ensuring patient understanding of the importance of regular visits. Furthermore, it has been associated with medication adherence and outcomes21,31 as well as a major determinant of patient satisfaction.22 A number of HSR domains were not associated with adherence to care in this study, but found to be important in other studies. Distance or difficulty in travel to the clinic were not associated with adherence to visits, factors which have been associated with missed visits in South Africa9,32 and Kenya.27 This may be in part due to the urban setting, where the range in travel time is not as broad as in more rural settings and the relatively small percent who had to travel longer than 60 minutes.
Patients seen at clinics with higher patient load per provider also had significantly lower adherence, as measured by visit constancy. There was an association with other non-adherence measures as well, although this did not reach statistical significance. While we were not able to measure wait time, increased patient load could result in longer wait times and decreased time with the provider, which have been found to be predictors of lower patient satisfaction in South Africa and the United States.23,24
The way in which non-adherence to care is measured will effect what factors predict patients most at risk for care non-adherence and how patients are categorized. For example, 50.2% of patients who were adherent based on visit constancy had a gap in care of 60 days or longer.(Table 3) Similarly, 8% of patients who were seen in the last quarter did not have a visit every quarter, so would have been classified as non-adherent using visit constancy but not by visit in the last quarter. Work to understand which definition or combination of definitions are correlated with outcomes of care is needed to identify which patterns of care non-adherence are of greatest concern to HIV care and treatment programs.
There were a number of limitations of this study. Our population had already been in care 6 months at the time of their survey, and so we were not able to capture patients who fall out of care soon after entry. Furthermore, we adapted measurements of HSR, which may not have captured certain components. Ratings were not anchored such that patient’s ratings were balanced against their expectations and high ratings across most measures of HSR limited power to identify predictors.33 We also relied on self-report for quantitative measures such as travel time and could not be verified. Finally, we did not track if patients transferred to other clinics or stopped ART, so what we defined as non-adherent but may actually been patients who were receiving appropriate care elsewhere or who had died.
Understanding the relative rates of adherence to care, the best method of measuring this, as well as identifying patient and HSR factors associated with stronger engagement and retention are increasingly critical to improving HIV treatment outcomes, especially as time in treatment has lengthened. Engagement with care requires a continuous relationship with providers and is associated with higher rates of medication adherence, better opportunistic infection prophylaxis, and overall improved outcomes in HIV patients.5,6,8,34 Furthermore, it’s been shown to decrease risky sexual behaviors that may reduce transmission to others.4 The potential benefit of HIV treatment programs is partially limited by strength of engagement with care, and more research is needed to characterize engagement with care to devise effective ways to strengthen it.35–37 Elements of HSR, such as HCW communication have been shown to promote adherence.21 However, there has been little research to explore this further, especially in RLS, and most studies have focused only on patient satisfaction.21–24 Understanding how factors of HSR affect adherence is important to implementing effective interventions. This would allow for identification of poor engagers so that care could be strengthened before they can be lost-to-follow-up, improving overall patient outcomes in HIV care and treatment programs.
ACKNOWLEDGEMENTS
The authors would like to thank the clinics patients, staff, site managers and supervisors of all the sites that participated in this study, Harvard University Center for AIDS Research (CFAR) which supported and funded the study through National Institutes of Health (NIH) funded program (P30AI060354), as well as the Doris Duke Charitable Foundation to Harvard University to fund Clinical Research Fellow Gabriela Poles. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the views of the aforementioned organizations.
Footnotes
Currently known as the Tom Lantos and Henry J. Hyde United States Global Leadership against HIV/AIDS, Tuberculosis and Malaria, Reauthorization Act of 2008
None of the authors have conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bulletin of the World Health Organization. 2008;86(7):559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care and STDs. 2010;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen S, Fox MP, Gill CJ. Sepulveda-Amor J, editor. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. [Accessed June 28, 2011];PLoS medicine. 2007 4(10):e298. doi: 10.1371/journal.pmed.0040298. Available at: http://dx.plos.org/10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed]
- 4.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clinical Trials. 2009;10(5):299–305. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- 5.Park WB, Choe PG, Kim S-H, et al. One-year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. Journal of Internal Medicine. 2007;261(3):268–275. doi: 10.1111/j.1365-2796.2006.01762.x. [DOI] [PubMed] [Google Scholar]
- 6.Berg MB, Safren S, Mimiaga MJ, et al. Nonadherence to medical appointments is associated with increased plasma HIV RNA and decreased CD4 cell counts in a community-based HIV primary care clinic. AIDS Care. 2005;17(7):902–907. doi: 10.1080/09540120500101658. [DOI] [PubMed] [Google Scholar]
- 7.Bajunirwe F, Arts EJ, Tisch DJ, et al. Adherence and treatment response among HIV-1-infected adults receiving antiretroviral therapy in a rural government hospital in Southwestern Uganda. Journal of the International Association of Physicians in AIDS Care. 2009;8(2):139–147. doi: 10.1177/1545109709332470. [DOI] [PubMed] [Google Scholar]
- 8.Mugavero MJ, Lin H-Y, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clinical Infectious Diseases. 2009;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Tropical Medicine & International Health. 2010;15(suppl 1):48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardon AP, Akurut D, Comoro C, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 11.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clinical Infectious Diseases. 2005;41(2):217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 12.Massaquoi M, Zachariah R, Manzi M, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. [Accessed September 19, 2011];Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009 103(6):594–600. doi: 10.1016/j.trstmh.2009.02.012. Available at: http://dx.doi.org/10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Medicine. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palombi L, Marazzi MC, Guidotti G, et al. Incidence and Predictors of Death, Retention, and Switch to Second-Line Regimens in Antiretroviral-Treated Patients in Sub-Saharan African Sites with Comprehensive Monitoring Availability. Clinical Infectious Diseases. 2009;48(1):115–122. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 15.Yu JK-L, Chen SC-C, Wang K-Y, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bulletin of the World Health Organization. 2007;85(7):550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posse M, Meheus F, van Asten H, van der Ven A, Baltussen R. Barriers to access to antiretroviral treatment in developing countries: a review. Tropical Medicine & International Health. 2008;13(7):904–913. doi: 10.1111/j.1365-3156.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 17.Dalal RP, Macphail C, Mqhayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. Journal of Acquired Immune Deficiency Syndromes. 2008;47(1):101–107. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 18.Cornell M, Myer L, Kaplan R, Bekker L-G, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Tropical Medicine & International Health. 2009;14(7):722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhardt UE, Cheng T-mei. The World Health Report 2000 - Health systems: improving performance. Bulletin of the World Health Organization. 2000;78(8):1064. [Google Scholar]
- 20.Ustin T, Chatterji S, Villanueva M, et al. [Accessed November 29, 2011];WHO Multi-country Survey Study on Health and Responsiveness 2000–2001: GPE Discussion Paper 37. 2001 Available at: http://www.who.int/responsiveness/papers/gpediscpaper37.pdf.
- 21.Roberts KJ. Physician-patient relationships, patient satisfaction, and antiretroviral medication Adherence among HIV-infected adults attending a public health clinic. AIDS Patient Care and STDs. 2002;16(1):43–50. doi: 10.1089/108729102753429398. [DOI] [PubMed] [Google Scholar]
- 22.Murphy-Cullen CL, Larsen LC. Interaction between the socio-demographic variables of physicians and their patients: Its impact upon patient satisfaction. Social Science & Medicine. 1984;19(2):163–166. doi: 10.1016/0277-9536(84)90283-1. [DOI] [PubMed] [Google Scholar]
- 23.Wouters E, Heunis C, van Rensburg D, Meulemans H. Patient satisfaction with antiretroviral services at primary health-care facilities in the Free State, South Africa--a two-year study using four waves of cross-sectional data. BMC Health Services Research. 2008;8(1):210. doi: 10.1186/1472-6963-8-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RT, Camacho FT, Balkrishnan R. Willing to wait?: the influence of patient wait time on satisfaction with primary care. BMC Health Services Research. 2007;7(1):31. doi: 10.1186/1472-6963-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Silva A. A framework for measuring responsiveness. GPE Discussion Paper Series: No 32. World Health Organization; 2000. [Accessed November 29, 2011]. Available at: http://www.who.int/responsiveness/papers/paper32.pdf. [Google Scholar]
- 26.Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City: 2005–2009: Do patients who initiate care stay in care? AIDS Patient Care and STDs. 2011;25(2):79–88. doi: 10.1089/apc.2010.0151. [DOI] [PubMed] [Google Scholar]
- 27.Ochieng-Ooko V, Ochieng D, Sidle JE, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bulletin of the World Health Organization. 2010;88(9):681–688. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howe CJ, Cole SR, Napravnik S, Eron JJ. Enrollment, retention, and visit attendance in the University of North Carolina Center for AIDS Research HIV clinical cohort: 2001–2007. AIDS Research and Human Retroviruses. 2010;26(8):875–881. doi: 10.1089/aid.2009.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins C, Chalamilla G, Okuma J, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. [Accessed October 4, 2011];AIDS. 2011 25(9):1189–1197. doi: 10.1097/QAD.0b013e3283471deb. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21505309. [DOI] [PubMed] [Google Scholar]
- 30.Park WB, Kim JY, Kim S-H, et al. Self-reported reasons among HIV-infected patients for missing clinic appointments. International Journal of STD & AIDS. 2008;19(2):125–126. doi: 10.1258/ijsa.2007.007101. [DOI] [PubMed] [Google Scholar]
- 31.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better Physician-Patient Relationships Are Associated with Higher Reported Adherence to Antiretroviral Therapy in Patients with HIV Infection. Journal of General Internal Medicine. 1998;19(11):1096–1103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maskew M, MacPhail P, Menezes C, Rubel D. Lost to follow up: contributing factors and challenges in South African patients on antiretroviral therapy. South African Medical Journal. 2007;97(9):853–857. [PubMed] [Google Scholar]
- 33.Lo M, Welty S, Steinfeld R, et al. Patient-reported health system responsiveness and experience with care at ARV clinics in Dar es Salaam, Tanzania. 5th IAS Conference on HIV Pathogenesis and Treatment.; Cape Town, South Africa. 2009. [Google Scholar]
- 34.Manosuthi W, Chaovavanich A, Tansuphaswadikul S, et al. Incidence and risk factors of major opportunistic infections after initiation of antiretroviral therapy among advanced HIV-infected patients in a resource-limited setting. The Journal of Infection. 2007;55(5):464–469. doi: 10.1016/j.jinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer KH. Linkage, engagement, and retention in HIV care: essential for optimal individual- and community-level outcomes in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2011;52(Suppl 2):S205–S207. doi: 10.1093/cid/ciq043. [DOI] [PubMed] [Google Scholar]
- 37.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clinical Infectious Diseases. 2011;52(Suppl 2):S238–S246. doi: 10.1093/cid/ciq048. [DOI] [PMC free article] [PubMed] [Google Scholar]