Abstract
Rationale
Compensation is a potential result of decreasing the available nicotine and tar dose in cigarettes. There is little published data linking compensation with cessation.
Objectives
We sought to examine whether compensation in response to restricted cigarette yield is associated with difficulty quitting smoking.
Methods
Questionnaires and blood samples were collected from 174 smokers interested in quitting smoking as part of a larger smoking cessation study. Participants were instructed to use a filter designed to remove 50% of tar and nicotine from the cigarette but otherwise smoke normally. Participants returned after three days of using the filter for follow up data collection.
Results
Nicotine levels and cigarettes per day decreased after use of the filter. Baseline nicotine and change in nicotine pre/post filter use, but not cigarettes per day or change in cigarettes per day, were associated with smoking abstinence at 30 days.
Conclusions
Smokers who demonstrate sensitivity to the biological or behavioral consequences of decreased nicotine content in tobacco smoke have greater difficulty quitting. These findings suggest the need for personalized cessation treatment linked to behavioral compensation.
Keywords: smoking cessation, nicotine, compensation, light cigarettes, FDA
INTRODUCTION
Compensation is a biological and behavioral response to the restriction of nicotine uptake (Scherer, 1999). Smokers may compensate for changes in nicotine yield by altering the amount and manner in which they smoke cigarettes, by changing the number of puffs they take, increasing the depth of the inhalation, and blocking cigarette paper ventilation holes to manipulate draw resistance and volume of smoke inhaled. Cigarette smokers are thought to maintain nicotine dosing within a “therapeutic” window, titrating nicotine intake to control symptoms of withdrawal and maintain rewarding effects while avoiding toxic effects at higher doses. (Kozlowski & Herman, 1984; Patterson et al., 2003) Smokers have been characterized as “peak seekers” or “trough avoiders” depending on their preference for the positive rewarding aspects of smoking or sensitivity to withdrawal states (Sutton, Feyerabend, Cole, & Russell, 1978).
Lab-based short-term studies of smokers’ reaction to nicotine restriction have yielded mixed evidence of compensation, with initial compensation in response to a single nicotine restricted cigarette, but decreased compensation over the course of further cigarettes (Strasser, Lerman, Sanborn, Pickworth, & Feldman, 2007; Hatsukami et al., 2010; Macqueen et al., 2012). Longer-term studies show small to moderate increases in compensation in the higher range of reduced nicotine yield cigarettes, but little evidence of compensation among smokers exposed to extremely low nicotine cigarettes (Benowitz, Jacob, & Herrera, 2006; Benowitz et al., 2007). Longer-term use of reduced nicotine cigarettes may lead to a gradual reduction in smoking and increased cessation success (Benowitz et al., 2007; Donny, Houtsmuller, & Stitzer, 2007; Hatsukami et al., 2010).
The current study utilized data collected by Niaura and colleagues (Niaura, Shadel, Abrams, Goldstein, & Hutchison, 2001) to examine whether compensation in response to short term use of a filter designed to restrict a cigarette’s nicotine yield is associated with difficulty quitting smoking. First, we explored how smokers’ cigarettes per day changed pre/post short-term use of a filter that reduced the amount of available nicotine in each cigarette. We hypothesized that greater compensation may signal increased sensitivity to plasma nicotine concentrations, its physiological consequences, or perhaps other smoke constituents. We also examined the degree to which compensation is associated with nicotine dependence and difficulty quitting. We posited that compensation is a biobehavioral marker of dependence, insofar as smokers who work harder to maintain a nicotine level (compensate) may find it harder to quit.
METHODS
The study sample consisted of 174 smokers who were interested in quitting smoking and participated in a smoking dependence study (Niaura et al., 2001). Conducted between 2000–2001, the purpose of the parent study was to investigate initial responses to nicotine after overnight abstinence and to determine to what degree participants’ responses conformed to theories of tolerance and sensitivity among smokers interested in quitting (Friedman, Lichtenstein, & Biglan, 1985; Pomerleau, Collins, Shiffman, & Pomerleau, 1993). Participants were recruited via local media and public service announcements. Due to procedural problems, data from 8 subjects were missing, leaving a final analytic sample of 166 with no missing data.
Detailed study procedures have been reported elsewhere (Niaura et al., 2001). An overview of the study schedule of sessions and assessments is presented in Figure 1. Briefly, participants provided informed consent, completed a battery of baseline questionnaires, and had their vital signs assessed and blood drawn at the first study session. This session was scheduled between 3:00 p.m. and 6:00 p.m. to allow plasma nicotine levels to plateau (Benowitz, Kuyt, & Jacob, 1982). Also during the first study session, participants were provided with cigarette filters designed to remove 50% of the nicotine and tar from their cigarettes (Teledyne Waterpik One Step At A Time ®, Step Two). This restriction manipulation was chosen to reduce nicotine yield, but not to the extent that smokers would discard the filters. Smokers were instructed to use the filters and to continue smoking their usual brand of cigarettes in their usual manner. As this portion of the study preceded the quit attempt, we instructed participants to refrain from cutting back on the number of cigarettes they would normally smoke each day. Participants returned for a second study session three days later, between 3:00 p.m. and 6:00 p.m., with each participant’s blood drawn the same time of day as in the first session. All participants returned the filters at this time and reported using the filters as instructed. Participants also reported the number of cigarettes they had smoked during each of the past two days.
Fig 1.
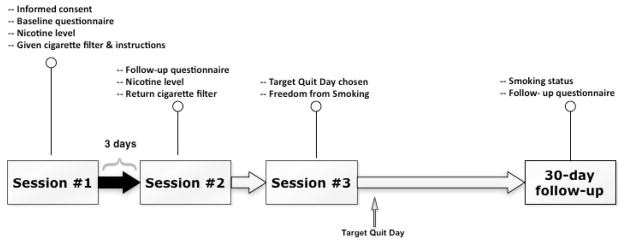
Overview of study schedule of sessions and assessments. Data for the current study were drawn from Sessions 1–2 and the 30-day follow-up.
At the third study session, participants received written self-help treatment materials as a cessation aid (“Freedom from Smoking for You and Your Family” from the American Lung Association) and instructions to quit smoking on their target quit day (TQD). A follow-up visit was scheduled for 30 days after their TQD, with smoking status confirmed with expired alveolar carbon monoxide (CO). Participants were compensated $170.00 for completing procedures associated with the parent smoking cessation intervention study.
Measures
At baseline, demographic information included age, years of education, and sex. Smoking history and dependence level were also assessed, including number of years smoking, age of first cigarette, number of prior quit attempts, average cigarettes consumed daily, and the Fagerstrom Tolerance Questionnaire (Fagerström, 1978). Nicotine concentrations were assessed at baseline and three days after the restriction manipulation by collecting whole blood samples that were stored on ice and centrifuged within 90 min of collection. Plasma was stored in a freezer at −90°C, and samples were shipped on dry ice to Dr. Neal Benowitz’s laboratory (University of California, San Francisco) for gas chromatographic assays of nicotine.
Abstinence was defined as a self-report of seven consecutive days of not smoking verified by expired alveolar carbon monoxide (CO) expired air CO (Bedfont Smokerlyzer) value of <8ppm at follow-up #4, 30 days post the participant’s chosen target quit date (Figure 1). Participants who did not meet this standard and those who missed the follow-up visit were considered smokers. For the purposes of this investigation, we defined compensatory smoking as minimal change in plasma nicotine levels post- versus pre-filter use.
Analyses
Standard methods were used to generate descriptive statistics and distribution displays. The binary abstinence outcome was analyzed using logistic regression procedures, with the log odds of abstinence modeled as a function of: 1) baseline nicotine level and change in plasma nicotine level (Model 1); 2) baseline cigarettes smoked per day, change in number of cigarettes smoked per day, and covariates from Model 1; and 3) sociodemographic information, smoking history, Fagerstrom score, and covariates from Models 1 and 2. In early analyses, we included carbon monoxide measures to control for time from the last cigarette smoked. This measurement was not significant and was not included in subsequent models. In addition to estimated regression coefficients, likelihood-ratio chi-square statistics, p-values, and the AIC (Akaike Information Criterion) and BIC (Bayes Information Criterion) model fit statistics are presented in Table 2. Both AIC and BIC values show that Model 2 was not a marked improvement upon Model 1. Thus, both baseline cigarettes smoked per day and change in cigarettes smoked per day were discarded from the final model because they were not predictive of smoking cessation in multivariable regression (Model 2). The AIC value for Models 1 and 3 are virtually the same, while the BIC value is higher because BIC penalizes model fit for the number of estimated parameters. We also examined time to first cigarette and cigarettes per day in separate analyses, but they did not predict outcomes either singly or in combination. Additionally, we examined the relationship between baseline plasma nicotine and change in plasma nicotine with a Spearman’s rho and multivariable regression. All statistical analyses and graphical displays were generated using JMP 9.0 statistical software. (SAS Institute Inc., 2012)
Table 2.
Plasma nicotine values (n/mL) at baseline, after three days of use of a cigarette filter that removed 50% of nicotine and tar from cigarettes (“post restriction”), and their difference (N=166).
| Baseline | Post restriction | Difference | |
|---|---|---|---|
| Mean | 16.4 | 12.5 | −3.7 |
| SD | 6.69 | 7.56 | 7.77 |
| SE Mean | 0.50 | 0.58 | 0.60 |
| 95% CI Mean | (17.3, 15.4) | (13.6, 11.3) | (−2.6, −4.9) |
| t-ratio | --- | --- | −6.25 |
| DF | --- | --- | 167 |
| Prob > |t| | --- | --- | <.0001 |
RESULTS
Summarized participant characteristic information by smoking status is presented in Table 1. The mean age was 42.8 years (SD = 11.7). Fifty-five percent of the participants were female and the majority (95%) was non-Hispanic white. On average, participants smoked 25.8 cigarettes per day (SD = 10.5) and reported regular smoking for a mean 24.6 years (SD = 11.4). Participants had an average of 3.3 (SD = 2.4) prior quit attempts, and reported being able to abstain from smoking for a median of six days (interquartile range: 2–50) during their most recent quit attempt. At 30 days after their target quit date, 28.3% (47/119) of smokers were abstinent.
Table 1.
Participant characteristics, means (sd) or number (%) (N=166)
| Abstinent (n=47) | Smoking (n=119) | t-ratio or chi-square | |
|---|---|---|---|
| Male/female % | 22/25 (46.8) | 52/67 (43.7) | 0.132 |
| Age (years) | 44.98(12.07) | 42.05(11.53) | −1.428 |
| Age first smoking (years) | 14.64(3.52) | 14.51(5.07) | −0.181 |
| Years smoking | 25.81(12.54) | 24.58(11.15) | −0.586 |
| Cigarettes/day | 24.81(9.96) | 25.95(10.5) | 0.655 |
| Quit attempts | 3.49(2.15) | 3.13(2.31) | −0.961 |
| FTQ | 6.39(1.89) | 6.54(1.69) | 0.464 |
| Education (years) | 14.51(3.40) | 13.54(2.12) | −1.826* |
p<.05
The distribution of the difference in plasma nicotine levels before and after manipulation is presented in Figure 2 and Table 2. The difference in means was statistically significant via paired t-test, corresponding to a 22.6% decrease in plasma nicotine concentrations following the restriction manipulation. The change was normally distributed about the mean, indicating that individuals varied in the degree and direction of compensation. Baseline plasma nicotine and the difference in plasma nicotine three days after use of the filter were negatively correlated (rho= −0.45, p<0.001) such that participants with lower baseline plasma nicotine showed smaller changes in plasma nicotine after filter use than participants with higher baseline plasma nicotine (Figure 3). In a multivariable regression including demographics (sex, education, age), years smoking, baseline plasma nicotine, CPD, and Fagerstrom score, only baseline plasma nicotine (β= −0.62, se = 0.08) and Fagerstrom score (β=0.88, se = 0.40) significantly predicted change in plasma nicotine score after use of the filter.
Fig 2.
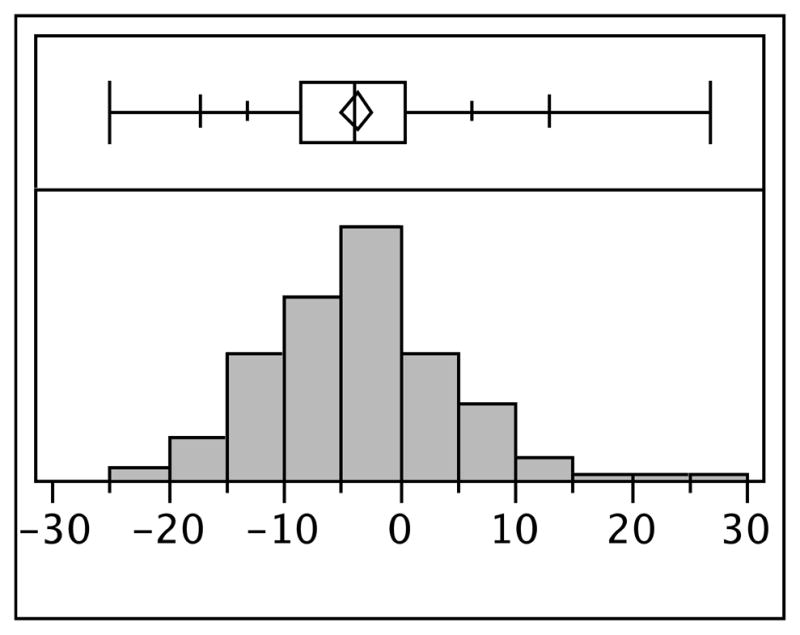
Distribution of the differences in plasma nicotine after use of a cigarette filter that restricted 50% of nicotine and tar in cigarettes among adult smokers interested in cessation (N=166).
Fig 3.
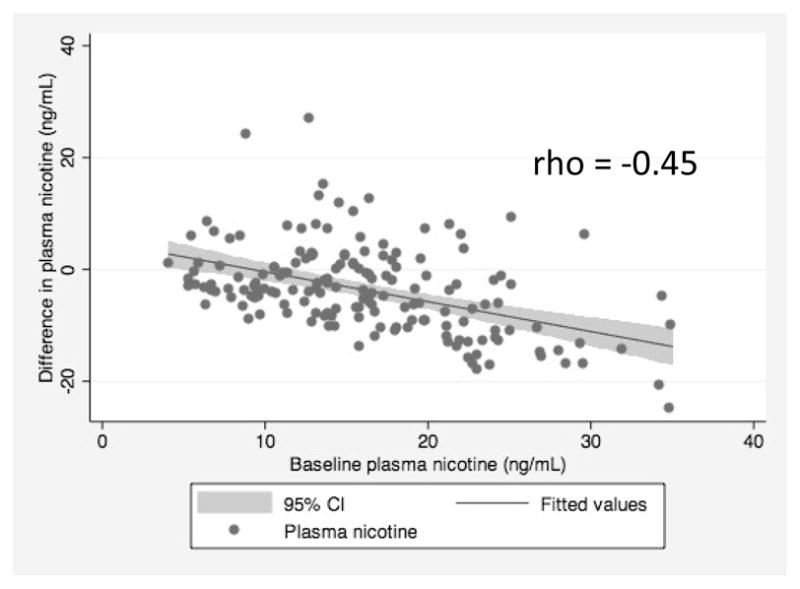
Negative correlation of baseline plasma nicotine with difference in plasma nicotine after three days of use of a filter that restricted 50% of the nicotine and tar in cigarettes among adult smokers interested in cessation (N=166).
Probability of abstinence at 30 days was modeled via binary logistic regression. Change in plasma nicotine levels from post- to pre-filter use was entered in the first step along with baseline nicotine level to control for baseline variability (see Table 3, Model 1). Both the baseline nicotine level and the change score significantly predicted abstinence such that the odds of abstinence decreased by 9% for every 1 ng/ml increase in baseline nicotine and decreased by 7% for every 1 ng/ml change in plasma nicotine after the restriction manipulation from negative (lower plasma nicotine after filter use) to positive (higher plasma nicotine after filter use). Thus, higher baseline plasma nicotine and higher plasma nicotine after filter use (less change, interpreted as a greater degree of compensation) predicted difficulty quitting at 30 days post TQD.
Table 3.
Logistic regression of abstinence on baseline nicotine concentration and difference after use of the cigarette filter (Model 1), adding cigarettes per day at baseline and change in cigarettes per day (Model 2), and adding demographic and smoking history variables (Model 3). (N=166)
| Model 1 (β, se) | Model 2 (β, se) | Model 3 (β, se) | |
|---|---|---|---|
| Intercept | 0.26 (0.48) | 0.53 (0.63) | −5.30 (1.91) |
| Plasma nicotine – baseline | −0.10 (0.03)* | −0.08 (0.04)* | −0.10 (0.04)* |
| Change in nicotine | −0.07 (0.03)* | −0.06 (0.03)* | −0.08 (0.03)* |
| CPD – baseline | --- | −0.03 (0.02) | --- |
| Change in CPD | --- | 0.05 (0.03) | --- |
| Female | --- | --- | 0.17(.20) |
| Education (years) | --- | --- | 0.15 (0.07)* |
| Age (years) | --- | --- | 0.09 (0.05) |
| Age of 1st cigarette | --- | --- | −0.05 (0.05) |
| Years smoking | --- | --- | −0.07 (0.05) |
| Previous quit attempts | --- | --- | 1.27 (0.12) |
| FTQ score | --- | --- | 0.16 (0.81) |
| AIC | 193.477 | 194.091 | 193.867 |
| BIC | 202.665 | 209.276 | 223.498 |
CPD = cigarettes per day
AIC = Akaike Information Criterion
BIC = Bayesian Information Criterion
p<.05
Relative to baseline, number of cigarettes smoked per day also decreased significantly during the restriction manipulation: baseline mean = 25.74 (10.33); mean during restriction = 20.76 (9.55), paired t-value (df = 167) = 7.129 (p<.0001), representing a 19.3% decrease. To examine whether change in number of cigarettes smoked could account for the effect of nicotine levels on abstinence, the baseline number of cigarettes and change from baseline were entered into a regression model predicting abstinence along with baseline and changes in nicotine concentrations. Neither baseline cigarettes per day nor change in cigarettes smoked per day were significantly associated with smoking abstinence (Table 3, Model 2). The predictive power of baseline and change in nicotine concentrations was weakened but retained statistical significance.
Along with baseline and nicotine changes score, sociodemographic information, smoking history and the total FTQ score were entered into a third model predicting abstinence (Model 3). Only years of education additionally predicted abstinence, with greater number of years increasing the odds of abstinence.
DISCUSSION
We found that high baseline plasma nicotine levels before administration of the filter predicted difficulty quitting 30-day post TQD. We also found that smokers with the highest baseline plasma nicotine were the least likely to respond to short-term nicotine restriction with compensatory behavior. This echoes conclusions from a recent animal study that found that rats with the highest nicotine self-administration demonstrated the lowest degree of compensation when their nicotine dose was restricted (Harris, Pentel, & LeSage, 2009). It is possible that those smokers who take in the most nicotine also have the most capacity to adapt to a decreased amount of nicotine over the short term. Smokers who had relatively lower baseline plasma nicotine levels may have been closer to a base plasma nicotine level below which they could not operate without significant discomfort, resulting in a greater degree of compensation.
After controlling for baseline plasma nicotine, CPD, Fagerstrom score, and sociodemographic characteristics, less compensation predicted abstinence. In other words, the more that a smoker adapted either his smoking topography (which was not measured in this study) or increased his cigarette consumption to account for the restricted nicotine content cigarettes, the less likely he was to be abstinent at the 30 day follow up. This suggests that smokers who demonstrate a greater degree of compensation are reacting to any number of consequences of nicotine deprivation, rather than dependence as typically measured by self-report symptoms and behaviors related to smoking and withdrawal. The measure of nicotine compensation demonstrated predictive effects that were independent of the self-report measures of dependence (FTQ) and smoking behavior (CPD and baseline cotinine). The latter may reflect smoking heaviness, while compensation behavior may reflect the response to smoking/nicotine restriction. These phenomena may represent different motivational mechanisms that sustain tobacco use, for example, smoking to stave off with withdrawal symptoms or smoking to attain a positive reward. More work is needed to address this issue. It could be that high compensation reflects the need of people already at their biological lower limit for nicotine intake to protect their nicotine yield. It is also worthwhile to note that plasma nicotine’s outperformance of the CPD measure may reflect weaker validity and reliability of the CPD measure, even over the short period of recall (three days) in this study.
Our findings also suggest that restricted nicotine content cigarettes may support cessation among smokers with a lower sensitivity to plasma nicotine concentrations, its physiological consequences, or other smoke constituents, and may encourage compensation in individuals with a greater sensitivity to plasma nicotine concentrations. Thus, one’s absolute level of dependence may not predict individual response to a reduction in cigarette nicotine yield. In fact, we found that a one-unit increase in baseline Fagerstrom score predicted a .88 greater difference in plasma nicotine after filter use, suggesting that more dependent smokers as measured by the Fagerstrom test are less likely to display compensatory behavior than less dependent smokers. This may have implications for a population-wide nicotine reduction policy for “hardcore” smokers, a hypothesized group of smokers who are resistant to quitting but who may be most likely to reduce CPD without compensation.
Limitations
These data were gathered after three days of nicotine and other cigarette constituent manipulation and are not a long-term assessment of smokers’ response to reduced cigarette nicotine and tar yield. Participants’ cigarettes per day over this three-day period were self-reported and retrospective, and thus open to recall bias. We did not measure all the parameters that allow smokers to vary their nicotine intake, such as depth of inhalation or puff frequency and duration (Scherer, 1999). We may only infer that the change in plasma nicotine and decrease in cigarettes per day translates to less compensation among some smokers. Additionally, although smokers reported compliance with the study protocol, we cannot confirm that participants actually used the cigarette filters. It is possible that more nicotine dependent smokers abandoned filter use during the experiment, differentially decreasing the difference in plasma nicotine before/after use of the filter in this population. Our study design also does not allow us to disentangle whether effects of sensitivity to changes in nicotine or to changes in other smoke constituents is associated with cessation. Our instruction to participants to refrain from cutting back on the number of cigarettes they would normally smoke likely also had an impact on the primary outcome variable of this study, as this instruction may have reduced the outcome variable’s ability to vary naturally. Finally, all participants self-identified as non-Hispanic white, and thus it is unclear whether the findings apply to other race and ethnic groups. Long-term longitudinal research on compensation among smokers interested in cessation is necessary to estimate the population level impact of any potential policy mandating nicotine reduction.
CONCLUSIONS
Our findings suggest that sensitivity to the biological or behavioral consequences of decreased nicotine in tobacco smoke is associated with difficultly quitting smoking. Smokers with higher baseline plasma nicotine and lower Fagerstrom scores were less likely to compensate after three days of filter use. We also cannot rule out other effects of smoking, such as orosensory effects of smoke or the extent to which smoking quelled craving. The mechanisms behind these findings await further study; however, they suggest the need for personalized cessation treatment linked to behavioral compensation (Abrams et al., 1996; Institute of Medicine, 2007).
Acknowledgments
This research was supported by HL32318 from the NIH Heart, Lung, and Blood Institute.
Footnotes
Conflicts of interest
None to disclose.
BIBLIOGRAPHY
- Abrams DB, Orleans CT, Niaura RS, Goldstein MG, Prochaska JO, Velicer W. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: A combined stepped-care and matching model. Ann Behav Med. 1996;18(4):290–304. doi: 10.1007/BF02895291. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidem Biomar. 2007;16(11):2479–2485. doi: 10.1158/1055-9965.epi-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther. 2006;80(6):703–14. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P. Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982;32(6):758–764. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- Burns D, Anderson C, Gray N. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Cause Control. 2011;22(1):13–22. doi: 10.1007/s10552-010-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer I. 2000;92(2):106–11. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–34. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3–4):235–41. doi: 10.1037/1064-1297.9.4.355. [DOI] [PubMed] [Google Scholar]
- Friedman LS, Lichtenstein E, Biglan A. Smoking onset among teens: An empirical analysis of initial situations. Addict Behav. 1985;10(1):1–13. doi: 10.1016/0306-4603(85)90048-6. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Correlates of individual differences in compensatory nicotine self-administration in rats following a decrease in nicotine unit dose. Psychopharmacology. 2009;205(4):599–611. doi: 10.1007/s00213-009-1567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard Hecht SS, et al. Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Ending the tobacco problem: A blueprint for the nation. Washington, DC: National Academies Press; 2007. [Google Scholar]
- Kozlowski LT, Herman CP. The interaction of psychosocial and biological determinants of tobacco use: more on the boundary model. J Appl Soc Psychol. 1984;14(3):244–256. doi: 10.1111/j.1559-1816.1984.tb02234.x. [DOI] [Google Scholar]
- MacQueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Goldstein MG, Hutchison K. Individual differences in responses to the first cigarette following overnight abstinence in regular smokers. Nicotine Tob Res. 2001;3(1):37–44. doi: 10.1080/14622200124231. [DOI] [PubMed] [Google Scholar]
- Patterson F, Benowitz N, Shields P, Lerman C, et al. Individual differences in nicotine intake per cigarette. Cancer Epidem Biomar. 2003;12(5):468–71. doi: 10.1093/ntr/ntq166. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS. Why some people smoke and others do not: New perspectives. J Consult Clin Psych. 1993;61(5):723. doi: 10.1037/0022-006X.61.5.723. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. JMP, version 9 [Computer Software] Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- Scherer G. Smoking behaviour and compensation: A review of the literature. Psychopharmacology. 1999;145(1):1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depen. 2007;86(2–3):294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Sutton SR, Feyerabend C, Cole PV, Russell MA. Adjustment of smokers to dilution of tobacco smoke by ventilated cigarette holders. Clin Pharmacol Ther. 1978;24(4):395–405. doi: 10.1002/cpt1978244395. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Burns DM. Health impact of “reduced yield” cigarettes: A critical assessment of the epidemiological evidence. Tob Control. 2001;10(90001):i4–11. doi: 10.1136/tc.10.suppl_1.i4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer I. 1997;89 (21):1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]


