Abstract
Objective
Adipose tissue inflammation is a cause of obesity-related metabolic disease. Natural killer (NK) cells are an understudied cell type in the context of obesity. The goal of this study was to determine the phenotype of human adipose tissue NK cells.
Methods
We used flow cytometry phenotyping to study adipose tissue and peripheral blood NK cells from obese and lean humans.
Results
Human adipose tissue NK cells, relative to peripheral blood NK cells, express increased levels of activation markers. Adipose tissue NK cells also demonstrate an activated phenotype in obese relative to lean subjects, with increased expression of the activating receptor NKG2D.
Conclusions
These data are the first detailed phenotypic characterization of human adipose tissue NK cells, and suggest a role for NK cells in adipose tissue inflammation in obesity.
Keywords: Inflammation, NKG2D, CD56
Introduction
Obesity is associated with a state of systemic inflammation that is based in adipose tissue and underlies the pathogenesis of metabolic disease. While adipose tissue macrophages (ATM) are central mediators of this inflammatory process, other leukocytes are involved, including T-cells and NKT cells [1–4]. Others have reported aberrations in peripheral blood NK cells (PBNK) in obesity [5–7]. Few published data study human adipose tissue NK cells (ATNK). The goal of this research was to describe the phenotype of human ATNK. We demonstrate that human ATNK manifest an activated phenotype relative to PBNK and in obese relative to lean subjects.
Methods
Subjects, tissue
Obese (BMI>=30 kg/m2) and lean (BMI<=25) subjects undergoing abdominal surgery were enrolled and consented with Institutional Review Board approval consistent with institutional and governmental regulations. Peripheral blood (PB) and visceral (greater omentum) adipose tissue (VAT) were collected and processed immediately. Subcutaneous (abdominal wall) adipose tissue (SAT) was collected from obese subjects. Thirteen obese subjects undergoing laparoscopic bariatric surgery and 7 lean subjects undergoing laparoscopic abdominal surgery for benign disease (6, fundoplication for gastroesophageal reflux disease, 1, cholecystectomy for gallstones) were enrolled. For obese subjects, mean age was 51 years (standard deviation (SD), 12), mean BMI 52 (SD, 6), 85% were female, the prevalence of diabetes, hypertension, sleep apnea, and hyperlipidemia were 46%, 69%, 62%, and 62% respectively,and medications included angiotensin converting enzyme inhibitor (31%), statin (31%), proton pump inhibitor (23%), beta blocker (38%), metformin (38%), and aspirin (23%). For lean subjects, mean age was 52 years (SD, 18), mean BMI 24 (SD, 6), 71% were female, and 86% (n=6) had gastroesophageal reflux disease, and 14% ( n=1) had biliary colic. No lean subjects had diabetes, sleep apnea, or hyperlipidemia, one (14%) had hypertension, and medications included angiotensin converting enzyme inhibitor (14%), proton pump inhibitor (86%), and aspirin (29%).
Cell isolation
Media and reagents were certified to have endotoxin levels less than 0.030 EU/ml. Vessels were dissected from adipose tissue, which was washed in PBS + 2% BSA, minced, and digested with Type II collagenase (175 units/ml in PBS + 2% BSA, Life Technologies Inc., Carlsbad, CA, USA) for 60 minutes at 37°C followed by centrifugation at 200g. The stromovascular cell fraction (SVF) cell pellet was retrieved and washed in PBS. Peripheral blood mononuclear cells (PBMC) were isolated from blood with Ficoll gradient, treated with ammonium chloride, and washed in PBS..
Flow cytometry
Cells were incubated with antibodies (CD158a/h-FITC (clone HP-MA4), CD16-PE (clone 3G8), NKp46-PE (clone 9E2), NKG2D-PE/Cy7 (clone 1D11), CD56-APC (clone HCD56), CD3-APC/Cy7(clone UCHT1) (Biolegend, SanDiego, CA, USA); CD45-PE/Cy5.5(clone HI30), CD27-PE/Cy7 (clone 0323) (eBiosciences Inc., San Diego, CA, USA) for 30 minutes, washed with PBS, 0.5%, BSA, 0.1% NaN3, fixed with Cytofix/Perm solution and analyzed on an LSR II flow cytometer (Becton Dickinson Inc., Franklin Lakes, NJ, USA). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA). Post-acquisition compensation, isotype controls, and fluorescence minus one gating were used to determine gates. After exclusion of doublets and nonviable cells using viable dye, a large forward and side scatter gate was used to include all viable cells, followed by gating on the pan-leukocyte marker CD45, followed by gating on cell populations of interest.
Results
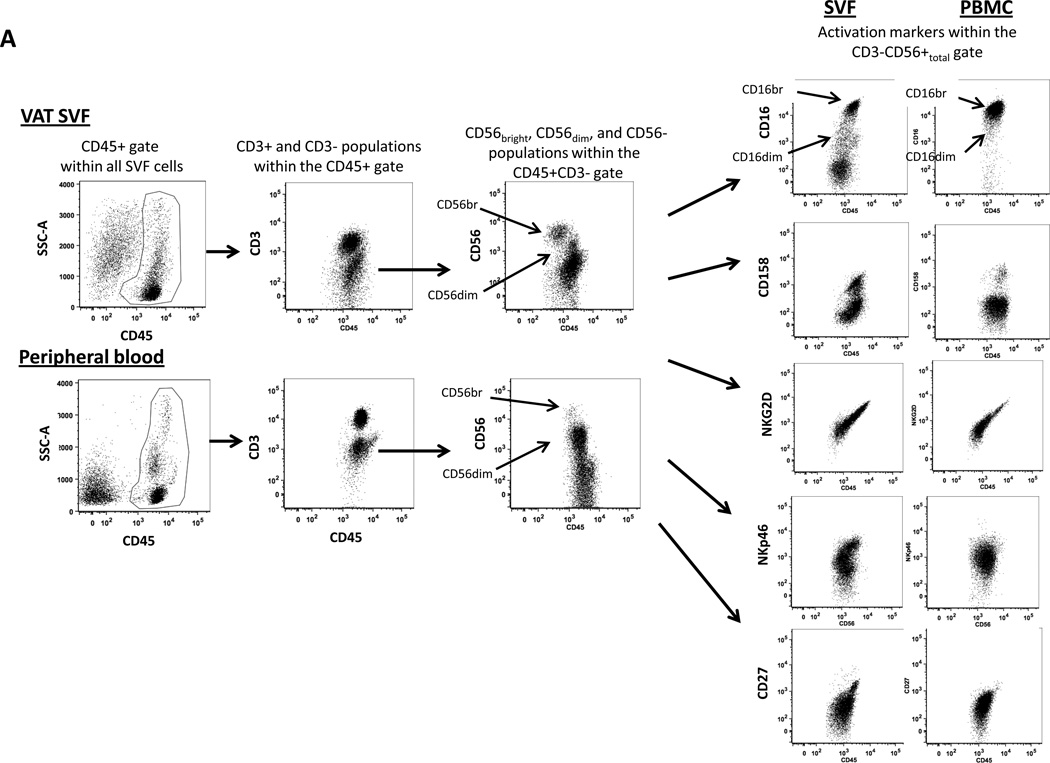
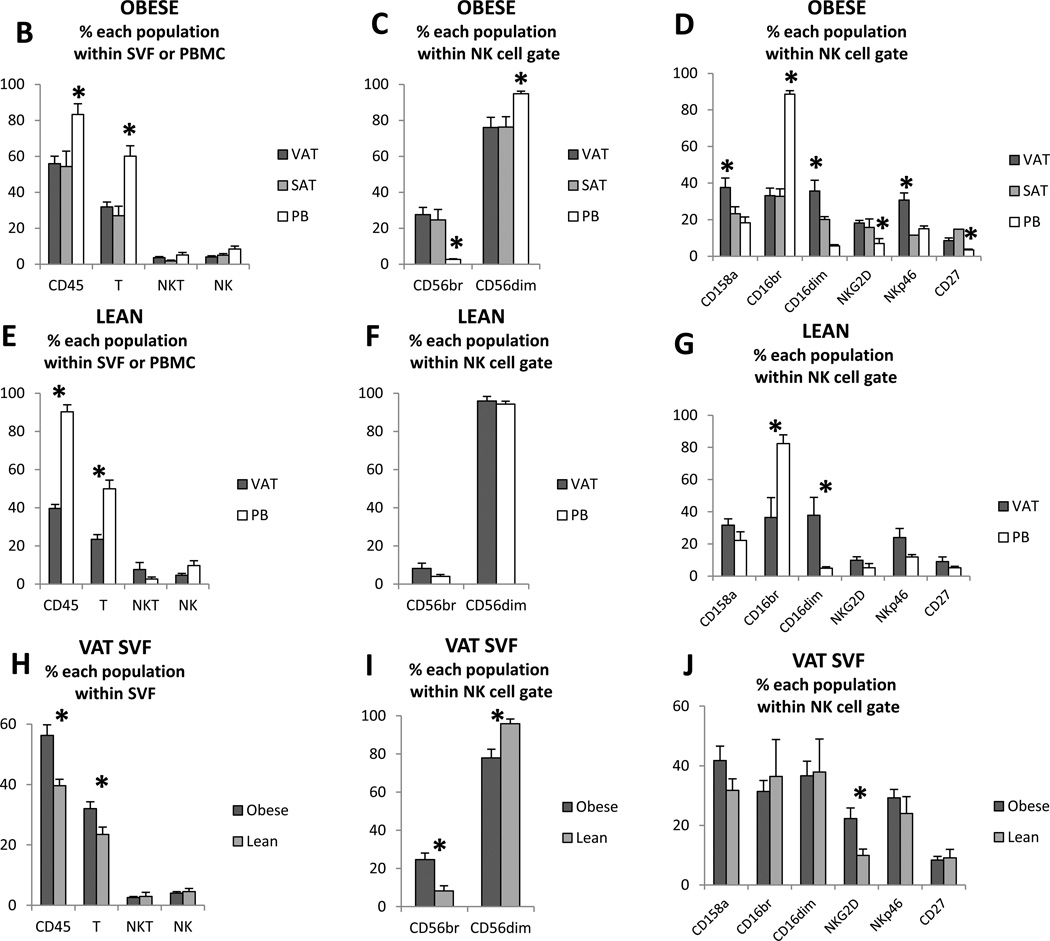
Flow cytometry was used to characterize the phenotype of human ATNK and PBNK (Figure 1A). In obese and lean subjects, CD45+ cells and CD3+ cells (T-cells) comprised a greater percentage of total PBMC than of VAT or SAT SVF, while frequencies of CD3+CD56+ (NKT) cells and CD3−CD56+ (NK cells) were similar between VAT, SAT, and PBMC (Figure 1B, E). The frequency of CD56bright cells within the total NK cell population was markedly higher, while the frequency of CD56dim cells was lower, in VAT and SAT SVF relative to PBMC from obese subjects (Figure 1C) but not in lean subjects (Figure 1F). The frequencies of CD158+, NKG2D+, CD16dim, NKp46, and CD27+ cells within the NK cell population were increased, while the frequency of CD16bright cells was decreased, in VAT SVF relative to PBMC in obese subjects (Figure 1D). In lean subjects, the frequencies of CD16bright and CD16dim populations within the total NK cell population were similarly decreased and increased respectively in VAT SVF compared to PBMC, but, in contrast to obese subjects, no differences were observed between VAT SVF and PBMC with respect to expression of CD158, NKG2d, NKp46, or CD27 within the NK cell population (Figure 1G).
Figure 1. Flow cytometry phenotype of human ATNK.
1A: Representative flow cytometry scatter-plots of NK cell-related subpopulations in human visceral adipose tissue SVF and peripheral blood mononuclear cells from an obese subject. T-cells were defined as CD45+CD3+. NK cells were defined as CD45+CD3−CD56+ cells. After exclusion of doublets and non-viable cells, CD45+ cells were gated and then divided into CD3+ and CD3− gates. The CD3− gate was further divided into CD56bright and CD56dim gates, as well as a CD56total gate that encompassed all CD3−CD56+ cells (bright and dim). Further phenotyping was performed on the CD56total population (CD56bright + CD56dim) for NK cell activation markers.
1B–J:Frequencies of named subpopulations (CD45 cells i.e. leukocytes, T-cells (CD45+CD3+), NKT cells (CD45+CD3+CD56+), and NK cells(CD45+CD3−CD56+)) within total SVF or PBMC populations (B, E, H), and of NK cell subpopulations within the total NK cell gate (C, D, F, G, I, J) in VAT, SAT, and PB from obese (B–D, H–J) and lean (E–G, H–J) subjects. Asterisks denote p<0.050 for the indicated data groups using paired t-test (comparing VAT, SAT, and PB within obese or lean subject groups, Figures 1B–G) or independent t-test (comparing lean and obese groups, Figure H–J).
The frequency of CD45+ cells and T-cells was increased in obese compared to lean subjects in VAT SVF, while no such differences were observed for NKT or NK cells (Figure 1H). The frequency of CD56bright cells within the total NK cell population was higher, while the frequency of CD56dim cells was lower in VAT SVF from obese compared to lean subjects (Figure 1I). Expression levels of the NK cell activation marker NKG2D were increased within the NK cell population in VAT SVF from obese compared to lean subjects (Figure 1J).
Discussion
Much attention has focused on ATM, T-cells, and NKT cells in obesity-associated adipose tissue inflammation, but the role of NK cells in this inflammatory process remains unknown. PBNK cytotoxic function is attenuated in obesity and these changes are reversed with weight loss [5, 7] while PBNK from obese rats demonstrate signaling defects [8, 9]. Few studies of human ATNK exist. We present the first detailed phenotypic characterization of human adipose tissue NK cells.
CD56dim NK cells predisposed to cytotoxicity predominate in peripheral blood. CD56bright cells, in contrast, are a minority of human PBNK cells, express tissue homing molecules, are increased in tissues, and are predisposed to IFN-γ secretion [10–11]. Expression of the Fc receptor CD16 inversely correlates with CD56 expression in humans, with CD56brCD16dim and CD56dimCD16br subpopulations described. We confirm a similar reciprocity of expression of these markers in human ATNK in lean and obese patients. Furthermore, we demonstrate that in obese subjects, ATNK, compared to PBNK, are comprised of a greater percent of CD56br cells, consistent with prior reports demonstrating a similar predominance of CD56br NK cells in other tissues [12, 13]. We also demonstrate that ATNK in obese subjects, compared to PBNK, express higher levels of activation markers, including CD158, NKG2D, NKp46, and CD27. In contrast, in lean subjects, expression levels of CD158, NKG2D, NKp46, and CD27 were similar between ATNK and PBNK. Furthermore, expression of the NK cell activating marker NKG2D was increased in ATNK from obese compared to lean subjects, suggesting that the inflammatory state in obese adipose tissue promotes NK cell activation. Finally, the observed relative increased expression of the NK cell markers CD158 and NKp46, the latter an activation marker, in VAT relative to SAT within obese subjects is consistent with the established increased inflammatory state of the former depot [14], and further suggests a link between ATNK cells and inflammation.
Prior data by our group demonstrate that IFN-γ promotes ATM inflammation and insulin resistance in obesity, and that ATNK are a source of IFN-γ [15, 16]. Our present data demonstrate increased of CD56bright NK cells within adipose tissue, a cell population prone to cytokine secretion [10–11]. Others demonstrate that NK cells regulate macrophage inflammatory responses in murine obesity and other inflammatory diseases [17, 18]. Taken together these observations suggest a role for ATNK in promoting adipose tissue inflammation. Precedent exists for this concept, as others have implicated IFN-γ-expressing CD56bright NK cells in other inflammatory diseases [13, 18].
Our data contribute to increasing literature demonstrating that adaptive immune cells, including canonical and regulatory T-cells, NKT cells, and B-cells, contribute to inflammation in obesity (2, 4, 19). Crosstalk occurs between regulatory T-cells and B-cells in adipose tissue (19), and between NK cells and macrophages in the liver (20). Our data suggest that NK cells contribute to similar complex leukocyte interactions in obese adipose tissue.
Limitations in tissues and cell yield, exacerbated by the fact that NK cells represent a small fraction of SVF and PBMC, precluded functional analysis or correlation of NK cell phenotype with clinical variables such as diabetes. These data nevertheless provide the first detailed phenotypic characterization of human adipose tissue NK cells, and demonstrate that ATNK manifest an activated phenotype in obesity, suggesting a role of NK cells in adipose tissue inflammation in metabolic disease and providing avenues for further study.
Acknowledgments
Funding: This work is supported b y National Institutes of Health Grants K08DK074397 (RWO), R03DK095050 (RWO), R01DK097449 (RWO), and R01DK070333 (DLM).
Abbreviations
- ATNK
adipose tissue NK cells
- ATM
adipose tissue macrophages
- PB
peripheral blood
- PBMC
peripheral blood mononuclear cells
- PBNK
peripheral blood NK cells
- SAT
subcutaneous adipose tissue
- SVF
stromovascular cell fraction
- VAT
visceral adipose tissue
Footnotes
Disclosure statement: The authors have no relevant financial conflicts of interest to report.
Author contributions: RWO conceived, designed, and oversaw experiments and wrote the manuscript. DLM contributed to conception and oversight of experiments, and drafting and critical review of the manuscript. GG, KAM, and AEW carried out experiments, contributed to experimental planning, and provided critical review of the manuscript.
References
- 1.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 2.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 5.Dovio A, Caramello V, Masera RG, et al. Natural killer cell activity and sensitivity to positive and negative modulation in uncomplicated obese subjects: relationships to leptin and diet composition. Int J Obes Relat Metab Disord. 2004;28(7):894–901. doi: 10.1038/sj.ijo.0802639. [DOI] [PubMed] [Google Scholar]
- 6.Lynch LA, O'Connell JM, Kwasnik AK, et al. Are natural killer cells protecting the metabolically healthy obese patient? Obesity. 2009;17(3):601–605. doi: 10.1038/oby.2008.565. [DOI] [PubMed] [Google Scholar]
- 7.Moulin CM, Marguti I, Peron JP, et al. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes Surg. 2011;21(1):112–118. doi: 10.1007/s11695-010-0250-8. [DOI] [PubMed] [Google Scholar]
- 8.Lautenbach A, Wrann CD, Jacobs R, et al. Altered phenotype of NK cells from obese rats can be normalized by transfer into lean animals. Obesity (Silver Spring) 2009;17(10):1848–1855. doi: 10.1038/oby.2009.140. [DOI] [PubMed] [Google Scholar]
- 9.Nave H, Mueller G, Siegmund B, et al. Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology. 2008;149:3370–3378. doi: 10.1210/en.2007-1516. [DOI] [PubMed] [Google Scholar]
- 10.Basu S, Eriksson M, Pioli PA, et al. Human uterine NK cells interact with uterine macrophages via NKG2D upon stimulation with PAMPs. Am J Reprod Immunol. 2009;61(1):52–61. doi: 10.1111/j.1600-0897.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferlazzo G, Thomas D, Lin SL, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 12.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198(8):1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottaviani C, Nasorri F, Bedini C, et al. CD56brightCD16– NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 14.Vohl MC, Sladek R, Robitaille J, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12(8):1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 15.O'Rourke RW, Metcalf MD, White AE, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-γin inflammation in human adipose tissue. Int J Obes (Lond) 2009;33(9):978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Rourke RW, White AE, Metcalf MD, Winters B, Diggs BS, Zhu X, Marks DL. Systemic inflammation and insulin resistance in obese IFN-γ knockout mice. Metabolism. 2012;61(8):1152–1161. doi: 10.1016/j.metabol.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellora F, Castriconi R, Dondero A, et al. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc Natl Acad Sci USA. 2010;107(50):21659–21664. doi: 10.1073/pnas.1007654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayama T, Kamada N, Chinen H, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(−)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology. 2010;139(3):882–889. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 19.DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110(13):5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tu Z, Bozorgzadeh A, Pierce RH, et al. TLR-dependent crosstalk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]