Abstract
We previously reported that 3-hydroxyphthalic anhydride-modified human serum albumin (HP-HSA) as an anti-HIV microbicide could potently inhibit infection by a broad spectrum of HIV-1 strains; however, its mechanism of action is still elusive. Here, we aimed to identify the target(s) of HP-HSA. HIV-1 envelope glycoprotein (Env)-mediated cell–cell fusion assays were conducted using noninfectious CHO-WT cells or infectious HIV-1IIIB-infected H9 cells as effector cells and MT-2 as target cells. The cell-to-cell transmission and single-round HIV-1 infection assays were performed by measuring luciferase activity. Binding of HP-HSA to CD4 or gp120 was determined by enzyme-linked immunosorbent assay (ELISA) and flow cytometry, while binding of HP-HSA to the coreceptor CXCR4 or CCR5 was detected by cell-based ELISA. HP-HSA strongly inhibited HIV-1 Env-mediated cell–cell fusion and blocked infection by HIV-1 pseudoviruses bearing Env of HIV-1HXB2 (X4 strain) or HIV-1SF162 (R5 strain). HP-HSA was also effective in blocking HIV-1BaL transmission from infected to uninfected cells. HP-HSA could strongly bind to HIV-1 Env gp120 and cellular receptor CD4. These results suggest that HP-HSA inhibits HIV-1 entry into the target cell by interacting with viral Env gp120 and/or the cellular CD4 receptor, making it a promising microbicide candidate for preventing HIV-1 sexual transmission.
Introduction
The human immunodeficiency virus (HIV) has continuously spread around the world, causing one of the most severe global epidemics in modern times. Currently, the main route of HIV transmission is attributed to unprotected sexual contact, especially for females. The use of condoms has high efficacy in preventing the sexual transmission of HIV, but most women living in developing countries are unable to protect themselves by persuading their partners to use a condom.1 Therefore, an alternate strategy to prevent sexually transmitted infections (STIs) and decrease HIV infection rates is to develop female-controlled microbicides.2–4
Up to now, almost two decades of research on microbicide candidates for the prevention of sexual HIV transmission have resulted in limited success.5 The reports of early-generation microbicides tested clinically have shown them to be ineffective in protecting against HIV infection because of their low in vivo anti-HIV activities or high toxicity on vaginal epithelium. Based on those clinical trial reports, further studies should be conducted to develop new microbicides.
Our previous studies demonstrated that 3-hydroxyphthalic anhydride-modified human serum albumin (HP-HSA) had strong anti-HIV-1 activity on infection by most of the tested HIV-1 strains, especially the HIV-1 R5 virus.6 Because human serum albumin (HSA) comprises about half of the human blood serum proteins, HP-HSA, as a topically applied microbicide, is expected to have more advantages than anhydrate-modified animal proteins, including lower cytotoxicity and stronger anti-HIV activity, as well as little or no immunogenic/allergenic effects.
It is well known that three key steps are involved in HIV-1 entry into the target cell.7,8 First, the HIV-1 Env surface subunit gp120 interacts with the CD4 receptor on the host cell. Second, induced by CD4 binding, gp120 changes conformation and further binds to a chemokine receptor, CCR5 or CXCR4. Third, when triggered by gp120-coreceptor binding, gp41 changes conformation, resulting in gp41-mediated membrane fusion. Each of these steps can serve as a target for developing anti-HIV-1 drugs or microbicides.
Although we previously demonstrated that HP-HSA may function as an HIV-1 entry inhibitor, its detailed mechanism of action is still elusive. Here, we evaluated the effect of HP-HSA on each step of the HIV-1 fusion/entry process and determined its binding to the viral Env and the cellular receptors. We found that HP-HSA inhibited HIV-1 fusion/entry by binding to HIV-1 Env gp120 and/or cellular receptor CD4, suggesting that it has sufficient potency for development as an effective and safe anti-HIV-1 microbicide.
Materials and Methods
Reagents
MT-2 cells, CHO-WT cells, CHO-EE cells, HIV-1IIIB-infected H9 cells (H9/HIV-1IIIB), H9 cells, TZM-bl cells, U87-CD4-CCR5 cells, mouse anti-CXCR4 MAb 12G5, mouse anti-CCR5 MAb 17b, pNL4-3E–R–Luc plasmid, HIV-1 Env-encoding plasmids, pVSV-G plasmid, and gp120 from HIV-1BaL were obtained from the AIDS Research and Reference Reagent Program of the U.S. National Institutes of Health (NIH). HEK-293T cells were purchased from ATCC (Manassas, VA). Lymphoid cell line CEMX174 5.25M7 expressing CD4 and coreceptors CCR5 and CXCR4,9 kindly provided by Dr. C. Cheng-Mayer, were stably transduced with an HIV-1 long terminal repeat (LTR)-green fluorescent protein (GFP) reporter and LTR-luciferase reporter construct cassette. 3-Hydroxyphthalic anhydride (HP), human serum albumin (HSA), bovine serum albumin (BSA), rabbit antialbumin antibody, gelatin, and polyethyleneimine (PEI) were purchased from Sigma (St. Louis, MO). p-Hydroxyphenylglyoxal (p-HPG) was purchased from Fisher Scientific (Valley Park, VA). Calcein-AM was purchased from Molecular Probes (Eugene, OR). Recombinant soluble CD4 (sCD4), biotinylated sCD4, and gp120 from HIV-1IIIB and HIV-1MN were obtained from Immunodiagnostics Inc. (Woburn, MA). Peptides N36 and biotinylated C34 were synthesized by GL Biochem (Shanghai, China).10,11 Mouse MAb NC-1 specific for the gp41 six-helix bundle was prepared and characterized as previously described.12
Measurement of inhibition of HP-HSA on HIV-1 Env-mediated cell–cell fusion
Two methods were used to measure the inhibitory activities of HP-HSA on cell–cell fusion. A noninfectious syncytium formation assay was determined as previously described.13,14 Briefly, CHO-WT cells (2×105/ml, 50 μl/well), which were stably transfected with HIV-1 Env gp160, were incubated with MT-2 (1×105/ml, 100 μl/well) cells expressing CD4 and CXCR4 in the absence or presence of HP-HSA at graded concentrations at 37°C for 48 h. The numbers of syncytia were counted under an inverted fluorescent microscope (Zeiss, Germany) with an eyepiece micrometer discs (10×10 mm2) and a 10×objective. Four fields per well were examined. The percentage of inhibition of syncytium formation was calculated using the following formula: [1 – (No. of syncytia in experiment – No. of syncytia in negative control)/(No. of syncytia in positive control – No. of syncytia in negative control)]×100%. The positive or negative controls were the wells that were added with CHO-WT cells expressing gp120/gp41 or CHO-EE cells expressing no gp120/gp41, respectively, in the absence of inhibitors.
A dye transfer assay was performed using MT-2 cells as the target cells and HIV-1IIIB-infected H9 (H9/HIV-1IIIB) cells as the effector cells, as previously described.15,16 Briefly, H9/HIV-1IIIB cells (2×105/ml) were labeled with calcein-AM (1 mM), a fluorescent reagent, at 37°C for 30 min and then incubated with MT-2 cells (1×106/ml) at 37°C for 2 h in the presence or absence of HP-HSA. The calcein-AM-labeled H9/HIV-1IIIB cells, both fused and unfused with MT-2 cells, were counted under an inverted fluorescent microscope as described above. The fused cell is much larger (at least 2-fold) than the unfused cell and the intensity of fluorescence in the fused cell is weaker than in the unfused cell due to the diffusion of fluorescence from one cell to two or more cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). The average percentage of cell fusion was calculated by the following formula: fused cells/(fused+unfused cells)×100%. The percentage of inhibition of cell fusion by antiviral agents was calculated using the following formula: [1 – (% fusion in experiment – % fusion in negative control)/(% fusion in positive control – % fusion in negative control)]×100%. The positive or negative controls were the wells that were added with H9/HIV-1IIIB cells or H9 cells, respectively, in the absence of inhibitors. In some experiments, unmodified HSA was included as a control of the chemically modified HSA. The effective concentration for 50% inhibition (EC50) values were calculated using CalcuSyn software,17 kindly provided by Dr. T.C. Chou at Sloan-Kettering Cancer Center (New York, NY).
Detection of the inhibition of HP-HSA on HIV-1BaL transmission from PBMCs to CEMx174 5.25M7 cells
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors at the New York Blood Center by standard density gradient centrifugation by using Histopaque-1077 (Sigma). The cells were then incubated at 37°C for 2 h. The nonadherent cells were collected and resuspended at 5×106/ml in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 5 μg/ml of phytohemagglutinin (PHA) (Sigma), and 100 U/ml of interleukin-2 (Sigma), followed by incubation at 37°C for 3 days. Thereafter, PHA-stimulated PBMCs were infected by HIV-1BaL with a multiplicity of infection (MOI) of 0.01 for 7 days. After three washes with phosphate-buffered saline (PBS) buffer, HIV-1BaL-infected PBMCs were collected and mixed with 5% Triton X-100 for 1 h at room temperature (RT). Then those virus lysates were tested for p24 production by enzyme-linked immunosorbent assay (ELISA) as previously described.13,14 Subsequently, 50 μl of HIV-1BaL-infected PBMCs (1×105/ml) was incubated with 50 μl of HP-HSA at graded concentrations at 37°C for 30 min. Then 100 μl of CEMx174 5.25M7 cells (2×105/ml) was added and cocultured at 37°C for 3 days. The cells were collected and lysed for analysis of luciferase activity as described above. HSA was used as a negative control.
Determination of the activities of HP-HSA on a single-round HIV-1 infection
HEC-293T cells were transiently cotransfected with pNL4-3E-R-Luc and HIV-1 Env-encoding plasmids derived from HIV-1 HXB2 (X4 strain) and SF162 (R5 strain) using a PEI transfection reagent.18 Vesicular stomatitis virus-G (VSV-G) plasmid was used as a control. The HIV-1 Env or VSV-G pseudotyped viruses were produced as single-round infectious viral particles as previously described.14,19 Briefly, 1×104/ml TZM-bl or U87-CD4-CCR5 cells were seeded in a 96-well plate and challenged with HIV-1 or VSV-G env-pseudotyped viruses, which were preincubated with HP-HSA or unmodified HSA at graded concentrations for 1 h at 37°C. The culture supernatants were replaced with fresh media 24 h postinfection. The cells were collected 72 h postinfection and the luciferase activity was detected using a luciferase assay kit (Promega, Madison, WI). The EC50 values were calculated using CalcuSyn software.17
Assessment of the binding of HP-HSA to gp120 or sCD4 by ELISA
The binding effect of HP-HSA on gp120 or sCD4 was determined as previously described.20,21 Briefly, wells of 96-well plates were coated with 5 μg/ml of gp120 from HIV-1IIIB, gp120 from HIV-1BaL, or sCD4 in 0.1 M Tris buffer (pH 8.8) at 4°C overnight, followed by washing with TS buffer (0.14 M NaCl, 0.01 M Tris, pH 7.0). The wells were then blocked with 1 mg/ml BSA and 0.1 mg/ml gelatin in TS buffer. HP-HSA and unmodified HSA at the indicated concentrations in PBS containing 100 μg/ml BSA were added to wells coated with gp120 or sCD4 for 1 h at RT. The unmodified HSA was used as the negative control of HP-HSA and PBS containing 100 μg/ml BSA was used as the background control. Rabbit anti-HSA antibody, HRP goat antirabbit IgG (Sigma), TMB, and 1 N H2SO4 were added sequentially.
Measurement of the inhibition of HP-HSA on the binding of sCD4 and gp120 by ELISA
The interactions between sCD4 and the HIV-1 Env (gp120 from HIV-1IIIB, HIV-1BaL, and HIV-1MN) were also determined using the methods described before.20,21 Briefly, wells of 96-well plates were coated with 5 μg/ml HIV-1 Env proteins in 0.1 M Tris buffer (pH 8.8) at 4°C overnight, followed by washing with TS buffer. Then the wells were blocked for 1 h at RT with 1 mg/ml BSA and 0.1 mg/ml gelatin in TS buffer. Biotinylated sCD4 (1 μg/ml) was preincubated with HP-HSA or unmodified HSA at the indicated concentrations in PBS containing 100 μg/ml BSA for 18 h at 4°C. SA-HRP, TMB, and 1 N H2SO4 were added sequentially. The absorbance at 450 nm was measured by using an ELISA reader, and the EC50 values were calculated as described above. Previous studies showed that 3-hydroxyphthalic anhydride-modified β-lactoglobulin (3HP-β-LG) inhibited the binding between gp120 and sCD4.20 Therefore, 3HP-β-LG was included as a positive control.
Flow cytometric analysis of the binding of HP-HSA to cells expressing HIV-1 Env or CD4 receptor
The binding of HP-HSA with CHO-WT cells that express the HIV-1 Env or HeLa-CD4-LTR-β-gal cells that express CD4 (CHO-EE and HeLa cells bearing neither HIV-1 Env nor CD4 as controls) was determined by flow cytometry as previously described.14,22,23 In brief, 100 μl of the cells (1×107/ml) suspended in PBS containing 10% goat serum (PBS-GS) was incubated at 4°C for 1 h before the addition of 100 μl of HP-HSA (2 μM). After incubation at 4°C for 1 h, cells were washed three times with PBS-GS. Rabbit anti-HSA serum and FITC goat antirabbit IgG were added sequentially. After incubation at 4°C for 1 h, the cells were washed and resuspended in 500 μl of wash buffer, followed by analysis by flow cytometry. Unmodified HSA (2 μM) was used as the negative control of HP-HSA.
Analysis the binding of HP-HSA and coreceptor-expressing cells by ELISA
Then 100 μl of TZM-bl cells (1×106/ml) was cultured in a 96-well cell plate at 37°C overnight, followed by fixing with 5% formaldehyde at RT for 15 min. After washing three times with pH 7.4 PBS buffer and blocking with 1% dry fat-free milk at 37°C for 1 h, 5 μg/ml mouse anti-CXCR4 MAb 12G5 or anti-CCR5 MAb 17b was added into each well and incubated for another 1 h in the presence or absence of HP-HSA at a graded concentration at 37°C for 1 h. Biotin-labeled goat antimouse IgG (Sigma), SA-HRP, and TMB were added into the reaction system successively. Then 1 N H2SO4 was used to terminate the reaction, and the absorbance at 450 nm was measured by using an ELISA reader.24
Detection of the inhibition of gp41 six-helix bundle formation
A model system mimicking gp41 core (six-helix bundle, 6-HB) formation by mixing the gp41 N- and C-peptides (N36 and C34 peptides) at equal molar concentrations in vitro was performed as reported previously.11,25 The inhibitory activity of HP-HSA on 6-HB formation between N36 and biotinylated C34 was determined by a sandwich ELISA as previously described.11,15 Briefly, 2 μM peptide N36 was preincubated with HP-HSA at a graded concentration at 37°C for 30 min, followed by the addition of 2 μM biotinylated C34. After another 30 min, the mixtures were added to wells of a 96-well polystyrene plate (Costar; Corning, Inc., Corning, NY), which were precoated with MAb NC-1 IgG (2 μg/ml) and blocked with 2% nonfat milk in PBS. Then MAb NC-1, biotin-labeled goat antimouse IgG (Sigma), SA-HRP, TMB, and 1 N H2SO4 were added sequentially. The A450 was measured by using an ELISA reader and the EC50 values were calculated as described above.
Results
The antiviral activity of HP-HSA was attributed to blocking HIV-1 entry into the target cell
As described before, HP-HSA exhibited significantly decreased inhibitory activity when it was added to target cells 0.5–2 h post-HIV-1 infection by time of addition assay.6 While such results indicated that HP-HSA was an HIV-1 entry/fusion inhibitor, the potential targets of HP-HSA remained unknown. Therefore, to systematically search for these targets, we examined the effect of HP-HSA on the early steps of the HIV-1 replication cycle, i.e., HIV-1 fusion/entry, using cell–cell fusion and virus–cell fusion assays.
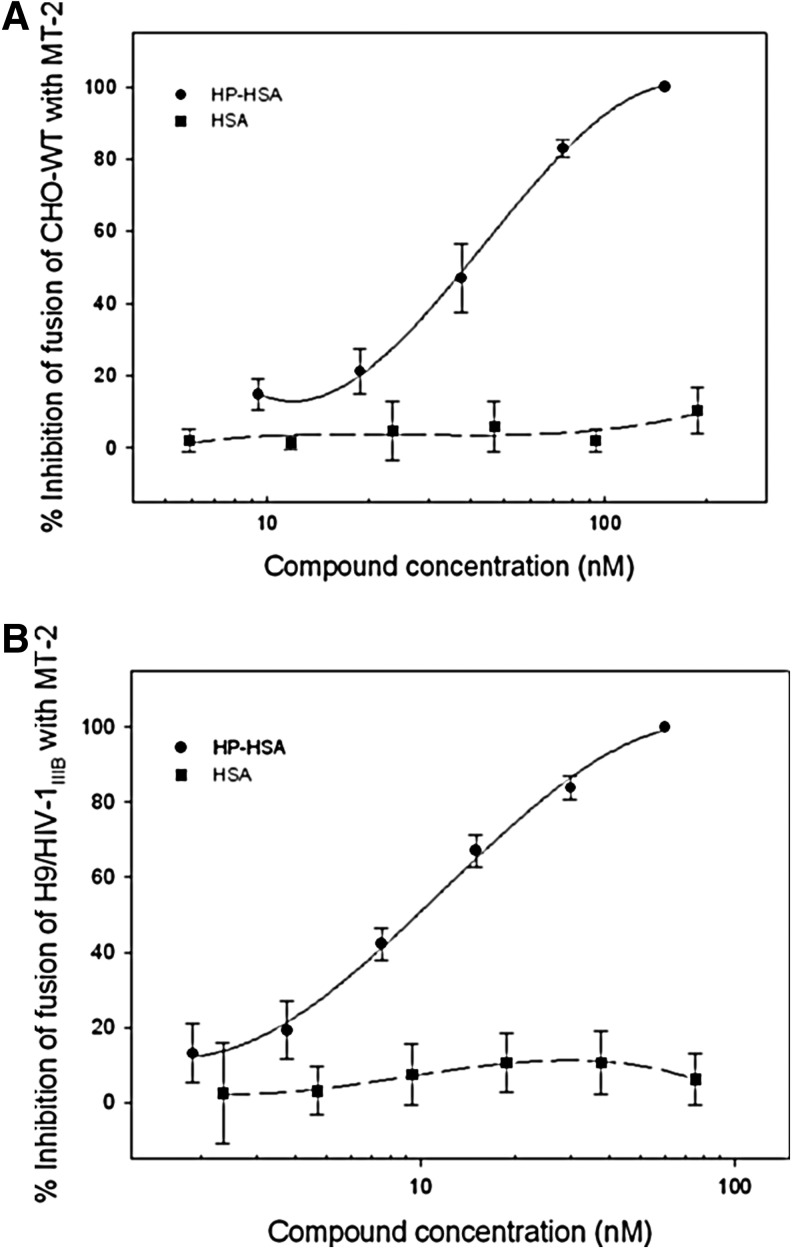
HIV-1 Env-mediated cell–cell fusion was detected by a syncytium-formation assay using the noninfectious CHO-WT cells and MT-2 cells or by a dye transfer assay using HIV-1IIIB-infected H9 cells (H9/HIV-1IIIB cells) and MT-2 cells as the effector and target cells, respectively, as described in detail in Materials and Methods. In a syncytium-formation assay, the size of a syncytium is usually≥2-fold larger than that of a normal cell (Supplementary Fig. S1A). In the dye transfer assay, the fused and unfused cells were counted under an inverted fluorescence microscope (Supplementary Fig. S1B). The numbers of syncytia and fluorescence-labeled fused cells were counted under an inverted microscope. As shown in Supplementary Fig. S2A and B, HP-HSA could significantly inhibit HIV-1 Env-mediated cell–cell fusion, resulting in the reduction of syncytium formation and dye transfer in a dose-dependent manner. The percentages of inhibition of cell–cell fusion of HP-HSA and HSA were calculated according to the formula described above (Fig. 1). Results showed that HP-HSA inhibited both kinds of HIV-1 Env-mediated cell–cell fusion, with EC50 about 27.82 nM in a noninfectious system and 7.45 nM in an infectious system, respectively (Fig. 1). In the above-described assays, unmodified HSA demonstrated no ability to inhibit cell–cell fusion (Fig. 1 and Supplementary Fig. S2).
FIG. 1.
Inhibitory activity of 3-hydroxyphthalic anhydride-modified human serum albumin (HP-HSA)on HIV-1-mediated cell–cell fusion. (A) Cell–cell fusion between CHO-WT cells and MT-2 cells; (B) cell–cell fusion between calcein-labeled HIV-1IIIB-infected H9 cells (H9/HIV-1IIIB) and MT-2 cells. Data are presented as means±SD.
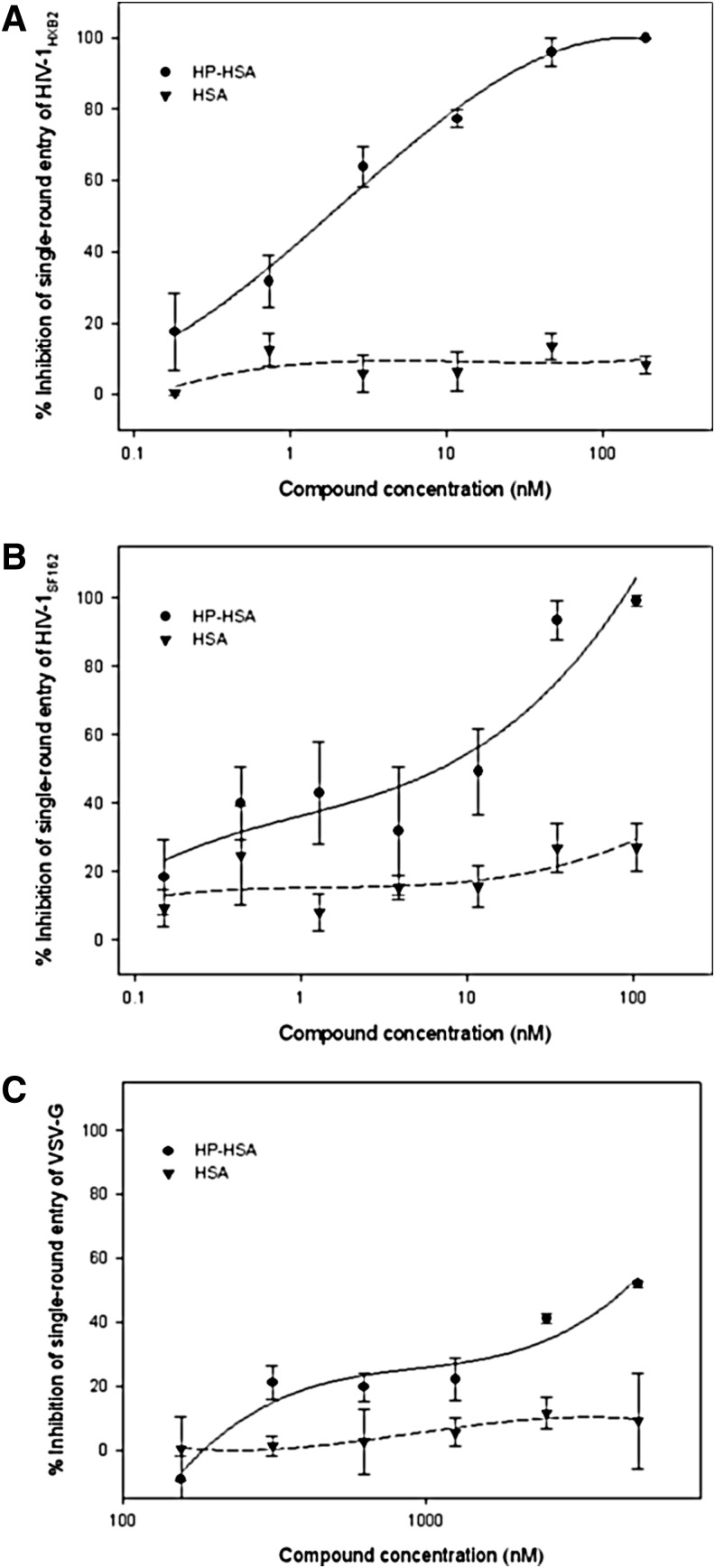
A single-round entry assay was chosen to detect the inhibition of HP-HSA on direct virus–cell fusion by using pseudotyped viruses expressing HIV-1 HXB2 (X4 strain) and SF162 (R5 strain) Env. Similar results were observed in this assay. HP-HSA inhibited infection by both X4 and R5 Env pseudotyped viruses, with EC50 about 1.51 and 1.54 nM, respectively (Fig. 2A and B). VSV-G pseudovirus was produced by cotransfecting VSV envelope G-protein plasmid and pNL4-3E–R–Luc plasmid. Therefore, VSV-G pseudovirus could be used to evaluate the specific effects of HP-HSA on HIV-1 envelope proteins. Results showed that VSV-G pseudovirus infection could not be inhibited by HP-HSA, even at a concentration as high as 1.25 μM (Fig. 2C).
FIG. 2.
Inhibition of HP-HSA on single-round infection of HIV-1HXB2 (A), HIV-1SF162 (B), and vesicular stomatitis virus-G (VSV-G) (C). Data are presented as means±SD.
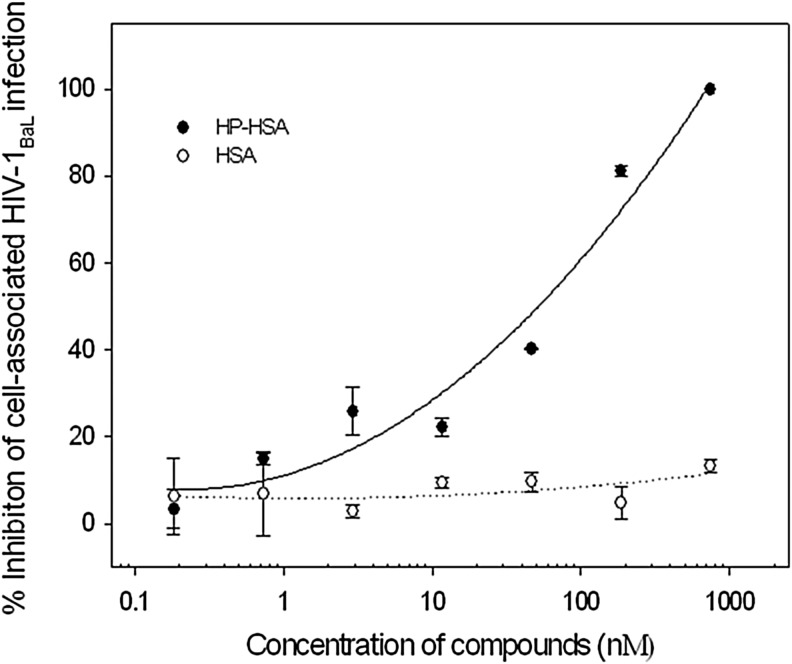
Cell-to-cell transmission is the major route for the spread of HIV-1. Here, we detected the inhibition of HP-HSA on the transmission of cell-associated HIV-1 virions from PBMCs to CEMx174 5.25M7 cells. As shown in Fig. 3, HP-HSA blocked the cell–cell HIV-1BaL transmission between those two kinds of virus target cells, which suggests that it could prevent the transmission of cell-associated HIV-1BaL isolates. The EC50 of cell–cell transmission was 31.01 nM. Unmodified HSA had no inhibitory activity on cell–cell transmission.
FIG. 3.
HP-HSA-mediated inhibition of transmission from peripheral blood mononuclear cells (PBMCs) to CEMx174 5.25M7 cells. Data are presented as means±SD.
All of these results indicate that HP-HSA can inhibit HIV-1 infection by blocking the virus entry step. HIV-1 entry into a CD4+ T cell is mediated by viral envelopes, which comprise the surface subunit gp120 and transmembrane subunit gp41.8 Similar to HP-OVA and ML-OVA, one of the probable mechanisms of HP-HSA might be interference with the viral envelope glycoprotein or the receptor on the target cell membrane.
HP-HSA blocked gp120-CD4 interaction by binding both gp120 and CD4 molecules
The gp120 recognition of cellular receptor CD4 is the first step during HIV-1 entry. Interfering with the binding of gp120 and CD4 might be one of the mechanisms of HIV-1 entry inhibitors. Therefore, we first tested the potential inhibitory effect of HP-HSA against the interaction between sCD4 and gp120 using ELISA. As indicated in Table 1, HP-HSA could block the interaction between sCD4 and gp120 from HIV-1IIIB, HIV-1MN, and HIV-1BaL, with EC50s at low nM levels. Unmodified HSA exhibited no inhibition at a concentration up to 100 nM. The positive control, 3HP-β-LG, could significantly inhibit the binding of sCD4 to gp120 from HIV-1IIIB (Table 1 and Supplementary Fig. S3A), HIV-1MN (Table 1 and Supplementary Fig. S3B), or HIV-1BaL (Table 1 and Supplementary Fig. S3C). But it is less effective than HP-HSA (Table 1 and Supplementary Fig. S3).
Table 1.
Inhibitory Activity of 3-Hydroxyphthalic Anhydride-Modified Human Serum Albumin on the Association Between Soluble CD4 and Distinct HIV-1 Envelope Proteins
| |
|
HIV-1 gp120 from |
||
|---|---|---|---|---|
| Compound | Inhibitorya concentration (nM) | HIV-1IIIB | HIV-1MN | HIV-1BaL |
| HP-HSA | EC50 | 8.00±1.04 | 15.60±3.90 | 8.52±0.21 |
| EC90 | 31.56±0.42 | 45.26±1.76 | 34.76±4.96 | |
| HSA | EC50 | >100.000 | >100.000 | >100.000 |
| EC90 | >100.000 | >100.000 | >100.000 | |
| 3HP-β-LG | EC50 | 50.49±10.32 | 137.83±14.40 | 53.51±2.44 |
| EC90 | 254.25±37.20 | 527.87±193.20 | 290.77±2.05 | |
The measurements were performed in triplicate, and the experiment was repeated twice. Data are presented as means±SD.
HP-HSA, 3-hydroxyphthalic anhydride-modified human serum albumin; HSA, human serum albumin; 3HP-β-LG, 3-hydroxyphthalic anhydride-modified β-lactoglobulin.
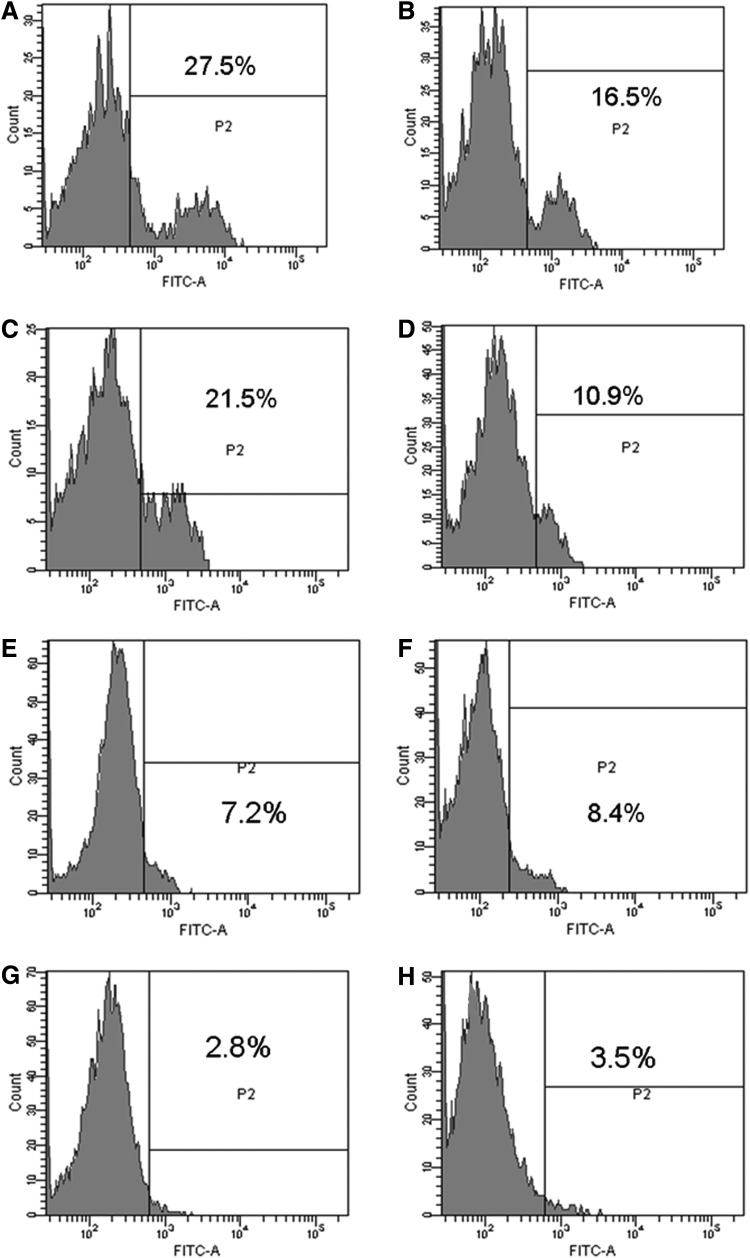
To further confirm the specific targets, the binding of HP-HSA to gp120 or sCD4 molecules was subsequently determined by ELISA. Results showed that HP-HSA could bind to both sCD4 (Fig. 4A) and gp120 from HIV-1 IIIB and BaL (Fig. 4B and C) in a dose-dependent manner. These results indicated that the targets of HP-HSA might be both gp120 and CD4. This deduction was also verified by flow cytometry using CHO-WT cells that express HIV-1 gp160 Env or HeLa-CD4-LTR-β-gal cells that express the CD4 receptor. CHO-EE and HeLa cells that express neither HIV-1 Env nor CD4 were used as negative controls. HP-HSA could bind with both CHO-WT and HeLa-CD4-LTR-β-gal cells (Fig. 5A and C), while it had only background binding to CHO-EE and HeLa cells (Fig. 5B and D). The background binding was also observed when unmodified HSA was used as a negative control of the chemically modified HSA, HP-HSA (Fig. 5E–H). These results indicate that HP-HSA is able to interact with both HIV-1 Env and the cell-surface receptor CD4.
FIG. 4.
The binding of HP-HSA to soluble CD4 (sCD4) and gp120, as assessed by enzyme-linked immunosorbent assay (ELISA). (A) Dose-dependent binding of HP-HSA to sCD4; (B) dose-dependent binding of HP-HSA to gp120 from HIV-1IIIB; (C) dose-dependent binding of HP-HSA to gp120 from HIV-1BaL. Data are presented as means±SD.
FIG. 5.
Flow cytometric analysis of HP-HSA binding to cells expressing HIV-1 Env or CD4 molecule. (A) HP-HSA+CHO-WT cells; (B) HP-HSA+CHO-EE cells; (C) HP-HSA+HeLa-CD4-LTR-β-gal cells; (D) HP-HSA+HeLa cells; (E) HSA+CHO-WT cells; (F) HSA+CHO-EE cells; (G) HSA+HeLa-CD4-LTR-β-gal cells; and (H) HSA+HeLa cells.
HP-HSA had no significant effects on coreceptor-expressing cells by ELISA
Based on the above results, we reasoned that HP-HSA might bind to HIV-1 gp120 or the CD4 receptor on the host cell surface during HIV-1 entry. Thus, we needed to address whether HP-HSA could affect the HIV-1 chemokine coreceptors, including CXCR4 and CCR5, expressed on the surface of CD4+ T lymphocytes. HP-HSA could bind neither CXCR4 nor CCR5 at the concentration of 1.25 μM (data not shown).
HP-HSA did not interfere with gp41 six-helix bundle formation
The binding to the coreceptors is followed by fusion of the viral and target cell membranes mediated by gp41. During HIV-1 entry, gp41 6-HB formation is a critical conformational change. Here a model system mimicking gp41 core (6-HB) formation by mixing the gp41 N- and C-peptides at equal molar concentrations in vitro was performed.11,25 The formation of 6-HB could be detected by a sandwich ELISA assay using a conformation-specific MAb, NC-1, as reported earlier.12,26 Similar to its effects on binding to coreceptor-expressing cells, HP-HSA could not inhibit 6-HB formation significantly, even at a relatively high concentration (1.25 μM) (Supplementary Fig. S4).
Discussion
In 2010, a 1% tenofovir [TFV, a nucleotide reverse transcriptase inhibitor (NRTI)] vaginal gel formulation was reported to be effective in preventing HIV transmission in its Phase IIb study in South Africa (CAPRISA 004 trial).27,28 However, it, like most topical microbicides evaluated in clinical trials, showed no significant effects in reducing the risk of acquiring HIV-1 infection in the further larger-scale clinical trial due to low adherence to the products (VOICE study).29 Therefore, further studies should continue to search for new candidate microbicides or microbicide combinations.
We previously demonstrated that a series of anhydride-modified bovine proteins, including 3-hydrophthalic anhydride-modified β-lactoglobulin (3HP-β-LG),20 3-hydrophthalic anhydride-modified ovalbumin (HP-OVA), and maleic-modified ovalbumin (ML-OVA), exhibited potent anti-HIV-1 activity.13,14 Most recently, we reported that HP-HSA could also potently inhibit infection by a broad spectrum of HIV-1 strains of different subtypes, including laboratory-adapted and primary HIV-1 strains, as well as drug-resistant strains.6 HSA is one of the most common human blood proteins, constituting about half of the total blood serum protein. In contrast to animal proteins, it may therefore present a more appropriate pathway to bypass further unpredictable complications of the human immune system upon the introduction of a vaginal microbicide. In addition, HP-HSA had much stronger anti-HIV-1 activity on infection by both laboratory-adapted HIV-1 X4 and R5 strains than other anhydride-modified OVAs, especially on HIV-1BaL virus. These results indicated that HP-HSA might be an ideal microbicide candidate for preventing HIV-1 sexual transmission.
In our previous study, we found that HP-HSA might be an HIV-1 entry/fusion inhibitor.6 At that time, however, the specific mechanism of HP-HSA inhibition was unknown. Therefore, in this study, we attempted to search for the exact targets of HP-HSA. To accomplish this, the early effect of HP-HSA on a cell–cell fusion assay was first analyzed. As shown in Fig. 1, HP-HSA had strong inhibitory activity on both HIV-1-induced and HIV-1 Env-mediated cell–cell membrane fusion.
A pseudovirus-based assay was then used to detect the inhibition of HP-HSA on a single-round virus–cell fusion and to analyze the function of the HIV-1 envelope. Figure 2 shows that HP-HSA inhibited infection by HIV-1 pseudoviruses bearing Env of HIV-1HBX2 (X4 strain) and HIV-1SF162 (R5 strain). VSV-G pseudovirus infection could not be inhibited by HP-HSA, even at a concentration of 1.25 μM (Fig. 2C). These results further confirmed that HP-HSA was an HIV-1 entry/fusion inhibitor and that the specific target of HP-HSA might be HIV-1 envelope proteins.
In addition to virus–cell transmission, cell–cell transmission has been regarded as the main route for the spread of HIV. A further advantage of microbicide candidates is their inhibition of cell-associated virus.30,31 Cell-associated HIV-1 in seminal fluid may contribute to the initial HIV-1 infection.32 As shown in Fig. 3, HP-HSA had potent inhibitory activity on the transmission of cell-associated HIV-1BaL virus from PBMCs to CEMx174 5.25M7 cells, which means that HP-HSA could block HIV-1 infection between infected and uninfected target cells. The ability to block HIV-1 transmission at the initial stage of viral infection makes HP-HSA an excellent candidate for prophylactic use as an anti-HIV-1 microbicide.
Binding of HIV-1 gp120 envelope protein to the cellular CD4 receptor is the first step leading to the fusion of viral and cell membranes. Interference with the binding of gp120 and CD4 might block HIV-1 entry. Our previous studies demonstrated that 3HP-β-LG was mainly targeted to the CD4 receptor on the surface of T lymphocytes.20 At higher concentrations, 3HP-β-LG could also bind to HIV-1 Env glycoprotein gp120 from HIV-1 IIIB and MN strains.33 In addition, other anhydride-modified proteins, such as HP-OVA and ML-OVA, could also inhibit the interaction between gp120 and CD4.13,14 Based on our previous studies, we also detected the binding of HP-HSA to gp120 or sCD4. Results showed that HP-HSA could bind HIV-1 gp120, as well as soluble CD4 molecules (Fig. 4), thus inhibiting the interaction between HIV-1 gp120 (from X4 or R5 virus) and sCD4 (Table 1). The results of HP-HSA binding to the HIV-1 Env and CD4 receptor were also verified by a flow cytometry assay using CHO-WT cells that express HIV-1 gp160 and HeLa-CD4-LTR-β-gal cells that express the CD4 receptor (Fig. 5). The results suggest that HP-HSA can inhibit HIV-1 entry/fusion through its interaction with gp120 and CD4 molecules. The binding ability with both gp120 and CD4 may arise from the modification of positively charged residues of HSA by 3-hydroxyphthalic anhydride. Similar to 3HP-β-LG, HP-OVA binds preferably to CD4, rather than to gp120, as demonstrated by using a surface plasmon resonance (SPR) assay.13 However, HP-HSA did not show such binding preference as proven by ELISA and flow cytometric analysis. It is worth noting that the EC50 value (96 nM) of 3HP-β-LG for inhibiting HIV-1-mediated cell–cell fusion was higher than the EC50 value (about 25 nM) for inhibiting the binding between gp120 and CD4. There are 59 lysines and 27 arginines in an HSA protein. OVA contains 20 lysines and 15 arginines, while β-LG has only 16 lysines and 3 arginines. It is possible that more modified lysines and arginines in HP-HSA than those in 3HP-β-LG may contribute to the higher binding of HP-HSA to gp120. But more experiments to further clarify the binding target of HP-HSA are warranted.
Normal human CD4+ T lymphocytes constitute an essential part of the immune system. They can regulate the function of the antigen-presenting cells through cell-to-cell interaction or through their released cytokines.34 As described above, there has been a concern that HP-HSA may affect the function of CD4+ T lymphocytes when HP-HSA binds to CD4 molecules on these cells.35 Our previous studies have demonstrated that HP-HSA had no significant effect on the proliferation of T lymphocytes or on the production of interferon-gamma (IFN-γ) in either PHA-stimulated or unstimulated PBMCs. HSA, a protein in human sera, is not expected to have any significant effect on the function of CD4+ T cells, especially for those circulating in the bloodstream. Nonetheless, the long-term use of a CD4 blocker like HP-HSA may still suppress the function of CD4+ T cells located in vaginal mucosa. Therefore, long-term observation of the potential harmful effect of HP-HSA on the mucosal immune system is warranted.
The second step of HIV-1 entry is mediated by HIV-1 chemokine coreceptors, including CXCR4 and CCR5 coreceptors expressed on the surface of CD4+ T lymphocytes. Thus, in the present work, we attempted to analyze the blocking effects of HP-HSA on coreceptor-expressing cells by cell-based ELISA. The monoclonal antibodies specifically recognize CXCR4 or CCR5 coreceptors and block the HIV-1 infection of CXCR4+ or CCR5+ T cells. Results showed that neither HP-HSA nor HSA could bind to CXCR4 or CCR5 coreceptors, though more precise tests should be implemented because of the limitation of anti-CXCR4 and anti-CCR5 monoclonal antibodies in these assays.
During HIV-1 entry, gp41 6-HB formation is a critical conformational change. Here we also tested the effect of HP-HSA on the formation of the fusion-active gp41 6-HB by ELISA and found that it had no inhibition, even at 1.25 μM concentration. It is difficult to understand the exact reasons why those two similar anhydride-modified proteins, HP-OVA and HP-HSA, had different effects on gp41 6-HB formation. In our previous studies, we found that only higher concentrations of HP-OVA (15 and 7.5 μM) showed certain inhibitory activities by using a convenient biophysical method, the FN-PAGE assay.13 It is possible that the inhibition of HP-OVA on gp41 6-HB formation is a result of nonspecific binding. More extensive studies that explore the difference between different anhydride-modified proteins are warranted.
Similar to HP-OVA, HP-HSA inhibits HIV-1 entry/fusion through binding to both gp120 and CD4 due to the fact that positively charged side chains of lysine and arginine residues in HSA were modified so that the HSA molecule carries net negative charges. Previous studies showed that HP-OVA and some negatively charged polymeric microbicide candidates, such as Carrageenan (Carraguard), cellulose sulfate (CS), and poly(naphthalene sulfonate) (PRO 2000), inhibited HIV fusion/entry by targeting multiple sites in the viral envelope glycoproteins, including gp120 and gp41 and receptors, such as CD4.13,36,37
It was reported that succinylated human serum albumin (Suc-HSA) also had anti-HIV-1 activities in vitro with an EC50 of 1.0 μg/ml,38 which is about 15 nM when calculated as a mole concentration. The mechanism of Suc-HSA, as an HIV-1 fusion inhibitor, is through binding the positively charged V3-loop of gp120.39 A Phase I/IIa study showed that adequate antiviral plasma levels of Suc-HSA administered intravenously could not be maintained and that the increases of liver transaminases prohibit further dose escalation,40 possibly because the accumulated Suc-HSA in the liver affects the function of the hepatocytes. In our study, we chose a different anhydride to modify HSA, and HP-HSA was shown to have more potent anti-HIV-1 activities than Suc-HSA in vitro. Most importantly, our study aimed to develop HP-HSA as a topical microbicide candidate, rather than as a therapeutic agent through intravenous injection. Microbicides are compounds designed for application inside the vagina or rectum to protect against STIs, including HIV-1. Therefore, HP-HAS, which will be formulated in a gel and applied inside the vagina or rectum, is unlikely to get into the blood and cause liver damage.
In general, HP-HSA inhibited HIV-1 entry/fusion by binding to viral Env gp120 and/or the cellular receptor CD4 through the negatively charged residues of HP-HSA resulting from the chemical modification of the positively charged residues in HSA. Since HSA is a human protein, HP-HSA can be further developed as an effective and safe topical microbicide for preventing HIV-1 sexual transmission.
Supplementary Material
Acknowledgments
This study was supported by the Natural Science Foundation of China (81273560 to L.L. and 81173098 to S.J.), the National Key Science and Technology Special Project (2012ZX10001-007-009) to L.L., and by the National 973 Project (2012CB519001) to S.J.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Buckheit RW., Jr Watson KM. Morrow KM, et al. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Res. 2010;85:142–158. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friend DR. Pharmaceutical development of microbicide drug products. Pharm Dev Technol. 2010;15:562–581. doi: 10.3109/10837450903369879. [DOI] [PubMed] [Google Scholar]
- 3.McElrath MJ. Ballweber L. Terker A, et al. Ex vivo comparison of microbicide efficacies for preventing HIV-1 genomic integration in intraepithelial vaginal cells. Antimicrob Agents Chemother. 2010;54:763–772. doi: 10.1128/AAC.00891-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen JS. Easterhoff D. Dewhurst S. Advances in HIV microbicide development. Future Med Chem. 2011;3:2101–2116. doi: 10.4155/fmc.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obiero J. Mwethera PG. Wiysonge CS. Topical microbicides for prevention of sexually transmitted infections. Cochrane Database Syst Rev. 2012;6:CD007961. doi: 10.1002/14651858.CD007961.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Li L. Qiu J. Lu L, et al. 3-Hydroxyphthalic anhydride-modified human serum albumin as a microbicide candidate inhibits HIV infection by blocking viral entry. J Antimicrob Chemother. 2013;68:573–576. doi: 10.1093/jac/dks458. [DOI] [PubMed] [Google Scholar]
- 7.Wilen CB. Tilton JC. Doms RW. Molecular mechanisms of HIV entry. Adv Exp Med Biol. 2012;726:223–242. doi: 10.1007/978-1-4614-0980-9_10. [DOI] [PubMed] [Google Scholar]
- 8.Chan DC. Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 9.Hsu M. Harouse JM. Gettie A, et al. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIV(SF162P3) maps to envelope gp120. J Virol. 2003;77:989–998. doi: 10.1128/JVI.77.2.989-998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan DC. Fass D. Berger JM, et al. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 11.Lu M. Kim PS. A trimeric structural subdomain of the HIV-1 transmembrane glycoprotein. J Biomol Struct Dyn. 1997;15:465–471. doi: 10.1080/07391102.1997.10508958. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S. Lin K. Lu M. A conformation-specific monoclonal antibody reacting with fusion-active gp41 from the HIV-1 envelope glycoprotein. J Virol. 1998;72:10213–10217. doi: 10.1128/jvi.72.12.10213-10217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L. He L. Tan S, et al. 3-Hydroxyphthalic anhydride-modified chicken ovalbumin exhibits potent and broad anti-HIV-1 activity: A potential microbicide for preventing sexual transmission of HIV-1. Antimicrob Agents Chemother. 2010;54:1700–1711. doi: 10.1128/AAC.01046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L. Qiao P. Yang J, et al. Maleic anhydride-modified chicken ovalbumin as an effective and inexpensive anti-HIV microbicide candidate for prevention of HIV sexual transmission. Retrovirology. 2010;7:37. doi: 10.1186/1742-4690-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S. Lu H. Liu S, et al. N-substituted pyrrole derivatives as novel human immunodeficiency virus type 1 entry inhibitors that interfere with the gp41 six-helix bundle formation and block virus fusion. Antimicrob Agents Chemother. 2004;48:4349–4359. doi: 10.1128/AAC.48.11.4349-4359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang S. Debnath AK. Development of HIV entry inhibitors targeted to the coiled coil regions of gp41. Biochem Biophys Res Commun. 2000;269:641–646. doi: 10.1006/bbrc.1999.1972. [DOI] [PubMed] [Google Scholar]
- 17.Chou TC. Hayball MP. Ferguson, MO 63135, USA: BIOSOFT; 1991. CalcuSyn: Windows software for dose effect analysis. [Google Scholar]
- 18.Demirkhanyan LH. Marin M. Padilla-Parra S, et al. Multifaceted mechanisms of HIV-1 entry inhibition by human alpha-defensin. J Biol Chem. 2012;287:28821–28838. doi: 10.1074/jbc.M112.375949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geuenich S. Goffinet C. Venzke S, et al. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology. 2008;5:27. doi: 10.1186/1742-4690-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neurath AR. Jiang S. Strick N, et al. Bovine β-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptors for HIV-1. Nature Med. 1996;2:230–234. doi: 10.1038/nm0296-230. [DOI] [PubMed] [Google Scholar]
- 21.Neurath AR. Strick N. Li YY, et al. Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect Dis. 2001;1:17. doi: 10.1186/1471-2334-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanCott TC. Mascola JR. Kaminski RW, et al. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J. Swiderski P. Li H, et al. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009;37:3094–3109. doi: 10.1093/nar/gkp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q. Lu H. Schols D, et al. Development of a cell-based enzyme-linked immunosorbent assay for high-throughput screening of HIV type 1 entry inhibitors targeting the coreceptor CXCR4. AIDS Res Hum Retroviruses. 2003;19:947–955. doi: 10.1089/088922203322588297. [DOI] [PubMed] [Google Scholar]
- 25.Liu S. Zhao Q. Jiang S. Determination of the HIV-1 gp41 postfusion conformation modeled by synthetic peptides: Applicable for identification of the HIV-1 fusion inhibitors. Peptide. 2003;24:1303–1313. doi: 10.1016/j.peptides.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Jiang S. Lin K. Zhang L, et al. A screening assay for antiviral compounds targeted to the HIV-1 gp41 core structure using a conformation-specific monoclonal antibody. J Virol Methods. 1999;80:85–96. doi: 10.1016/s0166-0934(99)00041-5. [DOI] [PubMed] [Google Scholar]
- 27.Berger RE. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. J Urol. 2011;185:1729. doi: 10.1016/S0022-5347(11)60197-3. [DOI] [PubMed] [Google Scholar]
- 28.Abdool KQ. Abdool Karim SS. Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEnery R. Oral tenofovir arm of VOICE trial discontinued early. IAVI Rep. 2011;15:21. [PubMed] [Google Scholar]
- 30.Tachet A. Dulioust E. Salmon D, et al. Detection and quantification of HIV-1 in semen: Identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS. 1999;13:823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 31.Kelly CG. Shattock RJ. Specific microbicides in the prevention of HIV infection. J Intern Med. 2011;270:509–519. doi: 10.1111/j.1365-2796.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaizu M. Weiler AM. Weisgrau KL, et al. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis. 2006;194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 33.Neurath AR. Debnath AK. Strick N, et al. 3-Hydroxyphthaloyl-β-lactoglobulin: I. Optimization of production and comparison with other compounds considered for chemoprophylaxis of mucosally transmitted HIV. Antiv Chem Chemother. 1997;8:53–61. [Google Scholar]
- 34.Luckheeram RV. Zhou R. Verma AD, et al. CD4(+)T cells: Differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 36.Rusconi S. Moonis M. Merrill DP, et al. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob Agents Chemother. 1996;40:234–236. doi: 10.1128/aac.40.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parish CR. Low L. Warren HS, et al. A polyanion binding site on the CD4 molecule. Proximity to the HIV-gp120 binding region. J Immunol. 1990;145:1188–1195. [PubMed] [Google Scholar]
- 38.Jansen RW. Schols D. Pauwels R, et al. Novel, negatively charged, human serum albumins display potent and selective in vitro anti-human immunodeficiency virus type 1 activity. Mol Pharmacol. 1993;44:1003–1007. [PubMed] [Google Scholar]
- 39.Kuipers ME. vd Berg BM. Swart PJ, et al. Mechanism of anti-HIV activity of succinylated human serum albumin. Biochem Pharmacol. 1999;57:889–898. doi: 10.1016/s0006-2952(98)00369-4. [DOI] [PubMed] [Google Scholar]
- 40.Vermeulen JN. Meijer DK. Over J, et al. A phase I/IIa study with succinylated human serum albumin (Suc-HSA), a candidate HIV-1 fusion inhibitor. Antivir Ther. 2007;12:273–278. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.